Abstract
BACKGROUND
Matrix Gla protein (MGP) is a vitamin K dependent peptide which has an established role in suppression of vascular calcification. Recent studies have pointed to a possible link between immunomodulatory effect of MGP and inflammatory bowel disease (IBD).
AIM
To compare plasma levels of dephosphorylated and uncarboxylated MGP (dp-ucMGP) between IBD patients and controls.
METHODS
This cross-sectional study was conducted on 70 patients with IBD (30 patients with ulcerative colitis and 40 patients with Crohn’s disease) and 60 age and gender matching healthy controls. Plasma dp-ucMGP levels were analyzed from blood samples by CLIA method using IDS-iSYS InaKtif MGP (Immunodiagnostic Systems, Frankfurt, Germany) according to the manufacturer's instructions. fecal calprotectin (FC) levels were determined from stool samples by turbidimetric immunoassay method using Bühlmann fecal calprotectin turbo assay (Bühlmann Laboratories Aktiengesellschaft, Schonenbuch, Switzerland). Other parameters were analyzed according to the standard laboratory procedures.
RESULTS
Plasma levels of dp-ucMGP were significantly higher in patients with IBD compared to the healthy control group (629.83 ± 124.20 pmol/mL vs 546.7 ± 122.09 pmol/mL, P < 0.001), and there was no significant difference between patients with Crohn’s disease and patients with ulcerative colitis (640.02 ± 131.88 pmol/mL vs 616.23 ± 113.92 pmol/mL, P = 0.432). Furthermore, a significant positive correlation of plasma dp-ucMGP levels was found with both FC levels (r = 0.396, P < 0.001) and high sensitivity C-reactive protein (hsCRP) levels (r = 0.477, P < 0.001). Moreover, in the total study population a significant positive correlation was found between dp-ucMGP with age (r = 0.210, P = 0.016) and waist circumference (r = 0.264, P = 0.002). Multiple linear regression analysis showed that dp-ucMGP levels retained significant association with FC (β ± SE, 0.06 ± 0.02, P = 0.003).
CONCLUSION
Study results support experimental data of MGP immunomodulatory IBD effect and indicate potential involvement in the pathophysiology of the disease, and possibly extraintestinal manifestations.
Keywords: Matrix Gla protein, Inflammatory bowel disease, Fecal calprotectin, Ulcerative colitis, Crohn's disease
Core tip: Matrix Gla protein (MGP) is well-established as a protector against vascular calcification. Recent studies pointed to a possible link between MGP and inflammatory bowel disease (IBD). Our study found significantly higher inactive plasma MGP levels in patients with IBD compared to the healthy controls and there were no differences between patients with ulcerative colitis and Crohn’s disease. Furthermore, there was a significant positive correlation between inactive plasma MGP levels with both fecal calprotectin and high sensitivity C-reactive protein levels. These results imply that MGP is somehow involved in complex IBD pathophysiology.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic, intermittent inflammatory disorder with an unstable course[1]. While the exact etiology of the disease is unknown, it is currently considered to be a complex interaction between genetics, environmental factors, immunological disorders and microbial disturbances[2]. Although the globally highest prevalence of IBD has been reported to be in Europe and North America, incidence in these regions appears to be stable or even on the decline. Yet it is important to stress that the incidence of IBD is rising in newly developing countries in Africa, South America and Asia[3]. A phenotypical classification approach put forward by the Montreal Working Group in 2003 encompasses the forms and the extents of IBD and classifies them as ulcerative colitis (UC), Crohn´s disease (CD) and indeterminate colitis[4]. It is well established that IBD has numerous extraintestinal manifestations which can affect the joints, eyes, kidneys, liver, skin, blood circulation and neurocognitive performance[5,6]. Furthermore, there is an increased risk of ischemic heart disease that had been reported in patients with IBD[7,8]. Previous studies compared endothelial-dependent dilatation assessed via reactive hyperemia and compared it to endothelial-independent dilatation elicited by sublingual isosorbide dinatrate in a set of UC patients with variable disease severity. The endothelial dependent dilatation was significantly worse in patients with severe UC compared to its milder forms. It can be assumed that the systemic inflammatory state evoked by IBD drives the impairment of endothelial function[9,10].
Matrix Gla protein (MGP) is a 12-kDa vitamin K–dependent extracellular matrix protein synthesized mostly by vascular smooth muscle cells and chondrocytes. With high binding affinity to hydroxyapatite crystals, it is considered as one of the most important biological agents in the prevention of vascular calcification[11-13]. Furthermore, increased levels of its inactive dephosphorylated and uncarboxylated form, designated as dp-ucMGP, have been associated with number of different cardiovascular consequences and complications, including endothelial dysfunction, retinal arteriolar narrowing, atherosclerosis, increased aortic stiffness, and higher overall cardiovascular, non-cancer and total mortality[14-17].
However, aside from its cardiovascular importance, experimental studies indicate that MGP might be a novel important mediator of mesenchymal stromal cell-mediated immunomodulation in treating experimental Crohn´s disease in mice. In a recent experiment, lentiviral transfected short hairpin RNA (shRNA) targeting the MGP gene in mesenchymal stromal cells (MSC) of mice was introduced, decreasing MGP secretion compared to insert-free mice. MSCs are known to inhibit the secretion mediated by T-cells of proinflammatory cytokines such as TNF-α and INF-γ. By comparing the inhibition proinflammatory cytokine secretion of T-cells in insert-free mice MSCs to the shRNA modified MSCs, it has been proposed that higher levels of MSC-derived MGP are likely to be the reason for suppressed cytokine production and a possible alleviation of experimental colitis in mice[18].
The possible immunomodulatory role of MGP and its inactive counterpart stresses the importance of further necessary research in this field. However, while experimental studies showed the potential that MGP could bring in managing IBD, clinical studies are still lacking. Therefore, the aim of this study was to investigate plasma dp-ucMGP levels in patients with IBD in comparison with the control group and evaluate the difference of dp-ucMGP levels between UC and CD patients. The additional goal was to investigate the association of plasma dp-ucMGP levels with the anthropometric, clinical and laboratory parameters.
MATERIALS AND METHODS
Study design and ethical considerations
This cross-sectional study was performed at the University Hospital of Split during the period from January 1, 2018 to September 1, 2018.
The study was approved by the Ethics Committee of University Hospital of Split, and was conducted in accordance with all ethical principles of the Helsinki Declaration from 2013. Prior to the commencement of the study, every participant was informed about the procedures, course and purpose of this research, and they individually signed an informed written agreement.
Subjects
The study included 70 adult patients with pre-diagnosed IBD (30 patients with ulcerative colitis and 40 patients with Crohn’s disease) and 60 healthy control subjects. IBD was diagnosed in accordance with the European Consensus on Crohn's Disease and Ulcerative Colitis (ECCO)[19]. The control group consisted of healthy volunteers, matched with the age and gender of the investigated group. Inclusion criteria for participation were: At least one year of disease duration and age between 18 and 65 years. Exclusion criteria were: Malignancies; diabetes mellitus; chronic cardiovascular, renal, endocrine and pulmonary diseases; therapy with corticosteroids during 3 mo prior to study onset. Detailed medical records of the control subjects were checked relating to gastrointestinal conditions and additional screening was performed regarding the presence of symptoms according to Rome IV criteria for irritable bowel syndrome[20], as well as any other indications which could suggest gluten and lactose intolerance. If any of these disorders were found, the subject was excluded from the control group.
Clinical and laboratory evaluation
All participants were subjected to detailed physical examination and measurements of anthropometric traits while relevant clinical information was collected from patient’s medical records while anamnestic data were taken directly from all study participants.
Disease severity was evaluated by the same experienced gastroenterologist according to the latest recommendations and the colonoscopy used for the assessment was performed within 2 wk of blood sampling. Disease activity in patients with UC was assessed using Ulcerative colitis endoscopic index of severity (UCEIS) while disease activity in patients with CD was assessed using Simple endoscopic score for Crohn’s disease (SES-CD)[21-23].
UCEIS is a grading system used for scoring mucosal inflammation based on the specific endoscopic findings. Three parameters are graded: Vascular pattern; bleeding; erosions and ulcers. Depending on the score there are four possible grades for disease activity: ≤ 1 remission; 2-4 mild activity; 5-6 moderate activity; and ≥ 7 severe disease[22].
SES-CD is a grading system used for endoscopic evaluation of CD activity based on four parameters: Ulcers; extent of affected surface; extent of ulcerated surface; presence and type of narrowing. According to the majority of studies the threshold values for interpretation of the results are: ≤ 2 remission; 3-7 mild activity; 7-15 moderate activity; and ≥ 16 severe disease[23].
Blood samples were collected after 12-h fasting in test tubes with anticoagulant and after extraction were centrifuged and stored at -80°C for further analysis. Blood samples were handled by the same, qualified medical biochemist, who was blinded to the subject’s group in the study. Plasma dp-ucMGP levels were analyzed by CLIA method using IDS-iSYS InaKtif MGP (Immunodiagnostic Systems, Frankfurt, Germany) according to the manufacturer's instructions. Minimum limit of detection was 200 pmol/L. Intra-assay coefficient of variability (CV) was 4.5% and inter-assay CV of 7.9%. Plasma high sensitivity C-reactive protein (hs-CRP) levels were determined using a latex turbidimetric method (Abbott Laboratories, Chicago, USA).
Stool samples were collected within 3 d of sampling. FC levels were determined by turbidimetric immunoassay method using Bühlmann fecal calprotectin turbo assay (Bühlmann Laboratories Aktiengesellschaft, Schonenbuch, Switzerland).
Statistical analysis
Statistical analysis was performed using MedCalc for Microsoft Windows (MedCalc Software, Ostend, Belgium, version 17.4.1). Data distribution was analyzed with D'Agostino-Pearson test, and according to results quantitative data was expressed as mean ± standard deviation or median and interquartile range. Qualitative data was expressed as whole number and percentage, with Chi-squared test used for comparison between such variables. Comparison of dp-ucMGP levels and anthropometrics between groups was performed with t-test for independent samples, while Mann-Whitney U test was used to test differences in hsCRP concentrations between IBD and control group. Correlation analysis of dp-ucMGP levels with FC, disease duration and hsCRP was estimated with Spearman rank correlation, while Pearson’s correlation coefficient was used to test association between dp-ucMGP levels and age, BMI and waist circumference. Furthermore, multiple linear regression analysis adjusted for age and anthropometric measurements was used to determine significant independent predictors of plasma dp-ucMGP levels. From these analyses, we reported respective P values with unstandardized β-coefficients, standard error and t-values. In addition, independent predictors for positive IBD status were analyzed with multivariable logistic regression, with OR (odds ratio), 95%CI (95% confidence interval) and P value reported. Finally, FC and hsCRP concentrations were divided into tertiles, and according dp-ucMGP levels were calculated and compared using one-way ANOVA with post hoc Sceffé test. Statistical significance was set at P value < 0.05.
RESULTS
There was no statistically significant difference regarding age, gender and anthropometric features between the patients with IBD and the control group (P > 0.05; for all analysis) (Table 1). Endoscopic scores determined that 8 patients were in remission, 9 had mild disease, 33 moderate and 20 severe form of disease. Laboratory analysis showed significantly higher hsCRP levels in IBD patients compared to the control group [2.65 (1.0-7.8) mg/L vs 0.75 (0.4-1.75) mg/L, P < 0.001] (Table 1).
Table 1.
Baseline characteristics of inflammatory bowel disease patients and healthy controls
| Parameter | IBD group (n = 70) | Control group (n = 60) | P value |
| Men (n, %) | 46 (65.7) | 38 (63.3) | 0.7781 |
| Women (n, %) | 24 (34.3) | 22 (36.7) | |
| Age (yr) | 40.8 ± 14.9 | 38.7 ± 12.6 | 0.3902 |
| Body weight (kg) | 77.7 ± 12.5 | 81.3 ± 13.2 | 0.1112 |
| Body height (cm) | 177.7 ± 8.2 | 180.1 ± 9.1 | 0.1322 |
| Body mass index (kg/m2) | 24.6 ± 3.5 | 25.0 ± 2.8 | 0.4932 |
| Waist circumference (cm) | 88.1 ± 12.6 | 88.0 ± 12.1 | 0.9972 |
| hsCRP (mg/L) | 2.65 (1.0-7.8) | 0.75 (0.4-1.75) | < 0.0013 |
| Fecal calprotectin (μg/g) | 235 (56.0-658) | - | - |
| Disease duration (yr) | 8.5 (3.5-13.0) | - | - |
| UCEIS score | 6.0 (5.0-7.0) | - | - |
| SES-CD | 10.3 (5.2-13.6) | - | - |
| mAb therapy (n, %) | 48 (68.5%) | - | - |
Data are presented as mean ± standard deviation or median or n (%).
Chi-squared test.
t-test for independent samples.
Mann-Whitney U test. UCEIS: Ulcerative Colitis Endoscopic Index of Severity; SES-CD: Simple Endoscopic Score for Crohn's Disease; mAb: Monoclonal antibody; hsCRP: High sensitivity C-reactive protein.
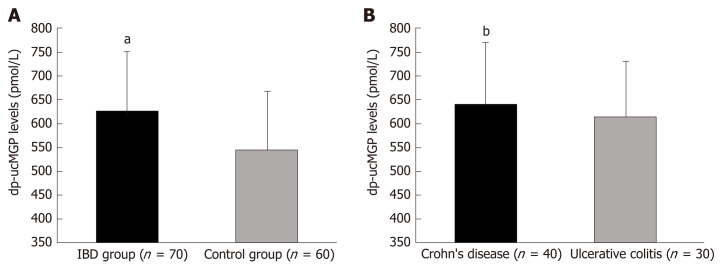
Plasma dp-ucMGP levels were significantly higher in patients with IBD compared with the control group (629.83 ± 124.20 pmol/mL vs 546.7 ± 122.09 pmol/mL, P < 0.001) and there was no significant difference between patients with CD and patients with UC (640.02 ± 131.88 pmol/mL vs 616.23 ± 113.92 pmol/mL, P = 0.432) (Figure 1).
Figure 1.
Plasma dephosphorylated and uncarboxylated matrix Gla protein levels in inflammatory bowel disease patients and control group (A) and between patients with Crohn’s disease and ulcerative colitis (B). aP < 0.001 vs control group; bP = 0.432 vs ulcerative colitis group. dp-ucMGP: Dephosphorylated and uncarboxylated matrix Gla protein.
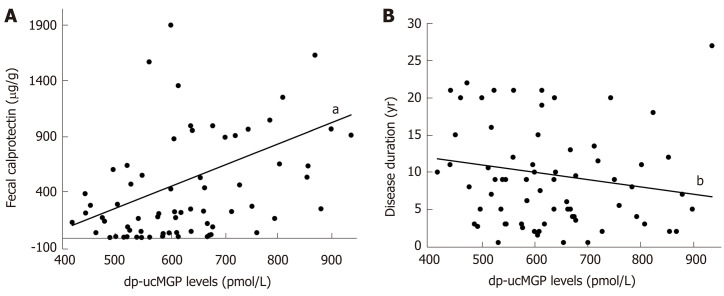
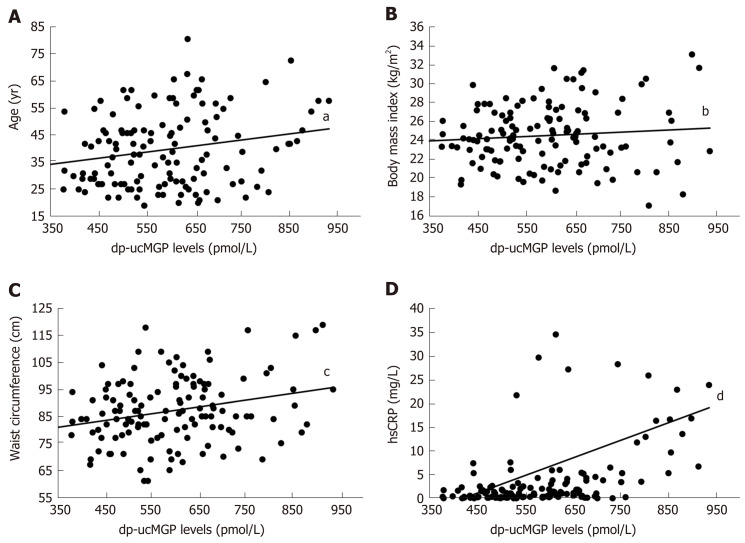
A significant positive correlation was found in patients with IBD between dp-ucMGP and fecal calprotectin (FC) levels (r = 0.396, P < 0.001), but there was no significant correlation between dp-ucMGP and the disease duration (r = -0.190, P = 0.115) (Figure 2). Moreover, in the total study population a significant positive correlation was found between dp-ucMGP and hsCRP (r = 0.477, P < 0.001), age (r = 0.210, P = 0.016) and waist circumference (r = 0.264, P = 0.002) (Figure 3).
Figure 2.
Correlation analysis between plasma dephosphorylated and uncarboxylated matrix Gla protein levels and fecal calprotectin (A) and disease duration (B) in patients with inflammatory bowel disease (n = 70). aP < 0.001, r = 0.396; bP = 0.115, r = -0.190. dp-ucMGP: Dephosphorylated and uncarboxylated matrix Gla protein.
Figure 3.
Correlation analysis between plasma dephosphorylated and uncarboxylated matrix Gla protein levels and age (A), body Mass Index (B) waist circumference (C) and hsCRP (D) in total study population (n = 130). aP = 0.016, r = 0.210; bP = 0.289, r = 0.094; cP = 0.002, r = 0.264; dP < 0.001, r = 0.477.
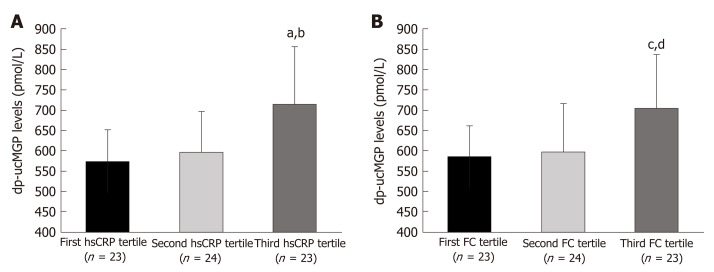
Comparison of hsCRP tertiles in patients with IBD (F-ratio = 11.22, P = 0.004) showed a significantly higher dp-ucMGP plasma levels in the 3rd tertile compared to both the 2nd and the 1st tertiles (P < 0.05) (Figure 4). Moreover, comparison of FC tertiles in patients with IBD (F-ratio = 7.81, P = 0.014) also showed a significantly higher levels of plasma dp-ucMGP in the 3rd tertile compared to both the 2nd and the 1st tertiles (P < 0.05) (Figure 4).
Figure 4.
Dephosphorylated and uncarboxylated matrix Gla protein concentrations between tertiles of high sensitivity C-reactive protein (A) and FC levels (B). Tested with one-way analysis of variance with post hoc Sceffé test to examine differences between each of the groups; aP < 0.05 vs first hsCRP tertile; bP < 0.05 vs second hsCRP tertile ; cP < 0.05 vs first FC tertile; dP < 0.05 vs second FC tertile; hsCRP: High sensitivity C-reactive protein; FC: Fecal calprotectin.
Multiple linear regression analysis showed that dp-ucMGP levels retained significant association with FC (β ± SE, 0.06 ± 0.02, P = 0.003) and waist circumference (4.35 ± 1.72, P = 0.013) after model adjustment for BMI, age and disease duration, with plasma dp-ucMGP levels as a dependent variable (Table 2). Furthermore, multivariable logistic regression showed that plasma dp-ucMGP was a significant predictor of positive IBD status when computed along with baseline characteristics (OR 1.006, 95%CI: 1.002-1.009, P < 0.001). There was no significant difference in plasma dp-ucMGP levels between patients with IBD who are using mAb therapy and those who are not using mAb therapy (621.37 ± 115.03 pmol/L vs 648.27 ± 143.36 pmol/L, P = 0.404).
Table 2.
Multiple linear regression model of independent predictors for dephosphorylated and uncarboxylated matrix Gla protein concentration
| Variable | β1 | SE | t value | P value |
| Fecal calprotectin (μg/g) | 0.06 | 0.02 | 3.08 | 0.003 |
| hsCRP (mg/L) | 0.66 | 0.47 | 1.41 | 0.162 |
| Body mass index (kg/m2) | -11.15 | 5.82 | -1.92 | 0.058 |
| Age (years) | 1.87 | 1.13 | 1.65 | 0.102 |
| Waist circumference (cm) | 4.35 | 1.72 | 2.54 | 0.013 |
| Disease duration (yr) | -3.96 | 1.81 | -1.84 | 0.062 |
Unstandardized coefficient β. SE: Standard error; hsCRP: High sensitivity C-reactive protein.
DISCUSSION
This cross-sectional study showed that plasma dp-ucMGP levels are significantly higher in patients with IBD compared to the healthy control group, while there was no significant difference between UC and CD patients. Furthermore, there was a significant positive correlation between dp-ucMGP with both hsCRP and FC levels. As far as we know, this is the first clinical study that investigated plasma dp-ucMGP levels in patients with IBD.
Given results are in line with the outcomes of a recent study conducted on human patients with UC and on mice with dextran sulfate sodium (DSS) induced colitis[24]. They showed that MGP mRNA levels are significantly higher in both UC patients and DSS-induced colitis mice compared to their control groups. Additionally, they showed that the expression of MGP mRNA increases with the disease severity while patients in remission had a similar MGP mRNA expression as the healthy controls. Feng Y et al[18] in their study conducted on MSC reported an immunomodulatory effect of MGP by showing that in vitro MGP reduces proliferation and production of proinflammatory cytokines in T-cells. Furthermore, by using mouse models with experimentally induced colitis, they compared results from intraperitoneal injection of MSC with knockout MGP and those with insert free MSC. The results showed that MGP improved the clinical and histopathological severity of colonic inflammation, alleviated the T-cells infiltration and suppressed the production of pro-inflammatory cytokines in colon tissues. These results indicate that MGP could play an important role in IBD inflammation and it could even have a therapeutic effect.
Another common feature that could link higher dp-ucMGP in IBD patients and positive association with inflammatory markers is endothelial dysfunction. It is well-established that intestinal inflammation leads to functional and structural changes of the vascular endothelium, which can consequently lead to increased cardiovascular risk in IBD patients[25,26]. Moreover, as disease severity and inflammation rise, greater endothelial dysfunction accompanies these changes. On the other hand, studies have also linked higher dp-ucMGP concentrations with markers of endothelial dysfunction in patients on hemodialysis via brachial artery flow-mediated dilation (FMD)[27], and in healthy postmenopausal women via biochemical markers (Vascular Cell Adhesion Molecule, VCAM; E-selectin; Advanced Glycation Endproducts, AGEs)[28]. According to these data, it is evident that dp-ucMGP concentration could easily be increased due to combined features of its ameliorative effect on active inflammatory process, increased endothelial dysfunction present in such process, but also due to vitamin K deficiency as well.
It is known that MGP depends on vitamin K for its full activation through carboxylation of Gla residues, followed by phosphorylation of serine residues. Furthermore, its inactive form has been acknowledged as a direct marker of vitamin K deficiency, due to its inability to be contained in artery wall, and consequent plasmatic accumulation[29]. Moreover, there is a well-established connection between IBD and vitamin K deficiency, with malnutrition stated as a leading cause of disorder[30,31]. Due to exacerbation of abdominal pain, patients tend to avoid food entirely, or they are using parenteral nutrition without administrating proper supplements[32]. Additionally, due to ulcerations, erosions and surgical resections they have a reduction of the intestinal absorptive surface and consequently vitamin K malabsorption[33]. However, in a recent RCT study, IBD patients were given daily supplementation of 1000 µg of vitamin K1 (phylloquinone) during a 12-mo period, with results showing no significant effect of the indices of their bone health[34]. Those results imply that although the patients were taking a large supplementation dose of vitamin K1, there was no significant beneficial effect coming along with the improvement of their vitamin K status. It is possible that vitamin K2 (menaquinone) deficiency is the main reason for such results due to a recent study showing vitamin K2 as a more potent and effective bone and vascular protector than vitamin K1[35]. Furthermore, two large scale studies showed that an adequate dietary intake of vitamin K2 significantly correlates with better cardiovascular and bone health, while they failed to prove that vitamin K1 demonstrates any protective value against vascular calcification and osteoporosis[36,37]. Interestingly, patients with IBD have a higher risk of coronary heart disease and heart failure[38], while the prevalence of high BMI, diabetes, hyperlipidemia, obesity, hypertension, lack of physical activity and other traditional cardiovascular risk factors is fairly lower in comparison with the general population[39,40]. Possible reason for cumulative increase in cardiovascular risks could lie in accelerated promotion of vascular calcification, one of the most prominent risk factors for cardiovascular diseases, due to inability of dp-ucMGP to fully activate in vitamin K2 deficiency. Furthermore, several studies already confirmed significant association of elevated dp-ucMGP concentrations with promotion of vascular calcification[41,42]. Knapen et al[28] conducted an RCT study that further confirms these results. They showed that three years of vitamin K2 supplementation in healthy postmenopausal women significantly improved arterial stiffness parameters, and decreased dp-ucMGP concentrations by 50% in comparison with placebo. Additionally, a recent study conducted on mice with DSS induced colitis reported that mice supplemented with vitamin K2 showed reduced symptoms of colitis along with a decrease of IL-6 expression[43]. Nevertheless, MGP and vitamin K2 are tightly related, and all these evidence stresses the need of addressing their involvement and possible therapeutic admission for IBD in future studies.
Another interesting finding of this study is the positive correlation of plasma dp-ucMGP levels with both FC and hsCRP. A recent study showed that MGP is synthesized and carboxylated in the majority of human immune system cells which are either involved in innate or adaptive immune reactions[44]. Furthermore, it showed that stimulation of monocytes and macrophages with LPS or hydroxyapatite triggered an inflammatory response which up regulated MGP expression following the up-regulation pattern of IL-1β. These results indicate that MGP is somehow involved in the inflammatory response mechanisms of monocytes and macrophages. Inflammation is considered as a potential leading cause in promotion of vascular calcification, and it is closely associated with endothelial dysfunction, with both conditions being hallmarks of IBD. Both FC and CRP are established and used as markers of IBD activity and severity[45] while several studies showed a significant correlation between CRP and coronary artery calcification[46-48]. Our findings could be implying that increased IBD activity up regulates MGP through complex inflammatory mechanisms, but due to possible vitamin K deficiency, the peptide cannot be fully activated and therefore loses its inhibitory effect on vascular calcification. This would be in line with the results of a Danish cohort study, which reported that flares and persistent IBD activity increases the risk of myocardial infarction, stroke and cardiovascular death[49], and the previously mentioned study on UC patients which showed that the expression of MGP mRNA increases with the disease severity[24]. Moreover, after division of patients with IBD into tertiles depending on hsCRP levels and FC levels our study showed in both cases significantly higher plasma levels of dp-ucMGP in the 3rd tertile compared to the 1st and 2nd tertiles which further indicates the possible association of dp-ucMGP with the IBD activity. However, additional research is needed to give us a better insight of the relationship between MGP and IBD activity.
The limitation of our study was its cross-sectional design and a relatively small sample size. Additionally, due to technical reasons we were not able to determine vitamin K2 levels in patients with IBD.
CONCLUSION
This is the first study that proved higher plasma dp-ucMGP levels in patients with IBD and showed a significant positive correlation between dp-ucMGP with both hsCRP and FC levels. Overall, our results support previous experimental data of MGP involvement in IBD pathophysiology through inflammation process and disease activity. However, these findings should be addressed in future larger studies.
ARTICLE HIGHLIGHTS
Research background
Several recent studies have pointed to a possible link between immunomodulatory effect of matrix Gla protein (MGP) and inflammatory bowel disease (IBD). Their results showed that MGP improved the clinical and histopathological severity of colonic inflammation, alleviated the T-cells infiltration and suppressed the production of pro-inflammatory cytokines in colon tissues.
Research motivation
While experimental studies showed the potential that MGP could bring in managing IBD, clinical studies are still lacking.
Research objectives
The aim of this study was to compare plasma levels of inactive dephosphorylated and uncarboxylated (dp-ucMGP) between patients with IBD and healthy controls. The additional goal was to investigate the association of plasma dp-ucMGP levels with the anthropometric, clinical and laboratory parameters.
Research methods
This cross-sectional study was conducted on 70 patients with IBD (30 patients with ulcerative colitis and 40 patients with Crohn’s disease) and 60 age and gender matching healthy controls. Plasma dp-ucMGP levels were analyzed from blood samples by CLIA method using IDS-iSYS InaKtif MGP (Immunodiagnostic Systems, Frankfurt, Germany) according to the manufacturer's instructions. Other parameters were analyzed according to the standard laboratory procedures.
Research results
Plasma levels of dp-ucMGP were significantly higher in patients with IBD compared to the control group (P < 0.001), and there was no significant difference between patients with Crohn’s disease and patients with ulcerative colitis (P = 0.432). Furthermore, a significant positive correlation of plasma dp-ucMGP levels was found with both fecal calprotectin levels (P < 0.001) and hsCRP levels (P < 0.001).
Research conclusions
Our clinical results support previous experimental data of MGP involvement in IBD pathophysiology through inflammation process and disease activity.
Research perspectives
The potential MGP immunomodulatory effect should be addressed with larger scale studies in the future.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the University Hospital of Split Institutional Review Board (Approval No. 2181-147-01/06/M.S.-17-2).
Conflict-of-interest statement: There are no conflicts of interest to report.
Corresponding Author's Membership in Professional Societies: ECCO, 42093.
Peer-review started: May 30, 2020
First decision: June 12, 2020
Article in press: August 15, 2020
P-Reviewer: Poullis A S-Editor: Wang DM L-Editor: A P-Editor: Zhang YL
Contributor Information
Darko Brnic, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Dinko Martinovic, Department of Emergency Medicine, Institute of Emergency Medicine of Split-Dalmatia County, Split 21000, Croatia.
Piero Marin Zivkovic, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Daria Tokic, Department of Emergency Medicine, Institute of Emergency Medicine of Split-Dalmatia County, Split 21000, Croatia.
Marino Vilovic, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia.
Doris Rusic, Department of Pharmacy, University of Split School of Medicine, Split 21000, Croatia.
Ivana Tadin Hadjina, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Christian Libers, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia.
Sandro Glumac, Department of Anesthesiology and Intensive Care, University Hospital of Split, Split 21000, Croatia.
Daniela Supe-Domic, Department of Medical Laboratory Diagnostics, University Hospital of Split, Split 21000, Croatia.
Ante Tonkic, Department of Internal Medicine, University of Split School of Medicine, Split 21000, Croatia.
Josko Bozic, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia. josko.bozic@mefst.hr.
Data sharing statement
Data set is available from the corresponding author at email: josko.bozic@mefst.hr.
References
- 1.Kim DH, Cheon JH. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17:25–40. doi: 10.4110/in.2017.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 4.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982–1992. doi: 10.1097/MIB.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadin Hadjina I, Zivkovic PM, Matetic A, Rusic D, Vilovic M, Bajo D, Puljiz Z, Tonkic A, Bozic J. Impaired neurocognitive and psychomotor performance in patients with inflammatory bowel disease. Sci Rep. 2019;9:13740. doi: 10.1038/s41598-019-50192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Singh H, Loftus EV, Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382–93.e1: quiz e22. doi: 10.1016/j.cgh.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Schicho R, Marsche G, Storr M. Cardiovascular complications in inflammatory bowel disease. Curr Drug Targets. 2015;16:181–188. doi: 10.2174/1389450116666150202161500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocaman O, Sahin T, Aygun C, Senturk O, Hulagu S. Endothelial dysfunction in patients with ulcerative colitis. Inflamm Bowel Dis. 2006;12:166–171. doi: 10.1097/01.MIB.0000217764.88980.74. [DOI] [PubMed] [Google Scholar]
- 10.Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P, Bucci A, Zito A, Caputo P, Albano F, Ierardi E, Di Leo A, Ciccone MM. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427–e433. doi: 10.1016/j.crohns.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Hackeng TM, Rosing J, Spronk HM, Vermeer C. Total chemical synthesis of human matrix Gla protein. Protein Sci. 2001;10:864–870. doi: 10.1110/ps.44701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett H, O'Keeffe M, Kavanagh E, Walsh M, O'Connor EM. Is Matrix Gla Protein Associated with Vascular Calcification? A Systematic Review. Nutrients. 2018;10 doi: 10.3390/nu10040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilovic M, Dogas Z, Ticinovic Kurir T, Borovac JA, Supe-Domic D, Vilovic T, Ivkovic N, Rusic D, Novak A, Bozic J. Bone metabolism parameters and inactive matrix Gla protein in patients with obstructive sleep apnea†. Sleep. 2020;43 doi: 10.1093/sleep/zsz243. [DOI] [PubMed] [Google Scholar]
- 14.Wei FF, Huang QF, Zhang ZY, Van Keer K, Thijs L, Trenson S, Yang WY, Cauwenberghs N, Mujaj B, Kuznetsova T, Allegaert K, Struijker-Boudier HAJ, Verhamme P, Vermeer C, Staessen JA. Inactive matrix Gla protein is a novel circulating biomarker predicting retinal arteriolar narrowing in humans. Sci Rep. 2018;8:15088. doi: 10.1038/s41598-018-33257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YP, Gu YM, Thijs L, Knapen MH, Salvi E, Citterio L, Petit T, Carpini SD, Zhang Z, Jacobs L, Jin Y, Barlassina C, Manunta P, Kuznetsova T, Verhamme P, Struijker-Boudier HA, Cusi D, Vermeer C, Staessen JA. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension. 2015;65:463–470. doi: 10.1161/HYPERTENSIONAHA.114.04494. [DOI] [PubMed] [Google Scholar]
- 16.Mayer O, Jr, Seidlerová J, Wohlfahrt P, Filipovský J, Vaněk J, Cífková R, Windrichová J, Topolčan O, Knapen MH, Drummen NE, Vermeer C. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30:418–423. doi: 10.1038/jhh.2015.55. [DOI] [PubMed] [Google Scholar]
- 17.Yao J, Guihard PJ, Blazquez-Medela AM, Guo Y, Liu T, Boström KI, Yao Y. Matrix Gla protein regulates differentiation of endothelial cells derived from mouse embryonic stem cells. Angiogenesis. 2016;19:1–7. doi: 10.1007/s10456-015-9484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y, Liao Y, Huang W, Lai X, Luo J, Du C, Lin J, Zhang Z, Qiu D, Liu Q, Shen H, Xiang AP, Zhang Q. Mesenchymal stromal cells-derived matrix Gla protein contribute to the alleviation of experimental colitis. Cell Death Dis. 2018;9:691. doi: 10.1038/s41419-018-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagorowicz E, Raine T, Harbord M, Rieder F, European Crohn’s and Colitis Organisation [ECCO] Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 20.Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustins E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, Gonzalez Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J, European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 22.Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987–995. doi: 10.1053/j.gastro.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 24.Dong XY, Wu MX, Zhang HM, Lyu H, Qian JM, Yang H. Association between matrix Gla protein and ulcerative colitis according to DNA microarray data. Gastroenterol Rep (Oxf) 2020;8:66–75. doi: 10.1093/gastro/goz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cibor D, Domagala-Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067–1077. doi: 10.3748/wjg.v22.i3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zivkovic PM, Matetic A, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Borovac JA, Mudnic I, Tonkic A, Bozic J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J Clin Med. 2020;9 doi: 10.3390/jcm9030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fain ME, Kapuku GK, Paulson WD, Williams CF, Raed A, Dong Y, Knapen MHJ, Vermeer C, Pollock NK. Inactive Matrix Gla Protein, Arterial Stiffness, and Endothelial Function in African American Hemodialysis Patients. Am J Hypertens. 2018;31:735–741. doi: 10.1093/ajh/hpy049. [DOI] [PubMed] [Google Scholar]
- 28.Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113:1135–1144. doi: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]
- 29.Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JW. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225:397–402. doi: 10.1016/j.atherosclerosis.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Fabisiak N, Fabisiak A, Watala C, Fichna J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51:878–889. doi: 10.1097/MCG.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara A, Tanaka K, Tsugawa N, Nakase H, Tsuji H, Shide K, Kamao M, Chiba T, Inagaki N, Okano T, Kido S. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int. 2009;20:935–942. doi: 10.1007/s00198-008-0764-2. [DOI] [PubMed] [Google Scholar]
- 32.Zallot C, Quilliot D, Chevaux JB, Peyrin-Biroulet C, Guéant-Rodriguez RM, Freling E, Collet-Fenetrier B, Williet N, Ziegler O, Bigard MA, Guéant JL, Peyrin-Biroulet L. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:66–72. doi: 10.1002/ibd.22965. [DOI] [PubMed] [Google Scholar]
- 33.Lucendo AJ, De Rezende LC. Importance of nutrition in inflammatory bowel disease. World J Gastroenterol. 2009;15:2081–2088. doi: 10.3748/wjg.15.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor EM, Grealy G, McCarthy J, Desmond A, Craig O, Shanahan F, Cashman KD. Effect of phylloquinone (vitamin K1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn's disease. Br J Nutr. 2014;112:1163–1174. doi: 10.1017/S0007114514001913. [DOI] [PubMed] [Google Scholar]
- 35.van Ballegooijen AJ, Beulens JW. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr Nutr Rep. 2017;6:197–205. doi: 10.1007/s13668-017-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 37.Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19:504–510. doi: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 39.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556–1562. doi: 10.1111/j.1572-0241.2003.07520.x. [DOI] [PubMed] [Google Scholar]
- 40.Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514–521. doi: 10.1038/sj.ejcn.1601049. [DOI] [PubMed] [Google Scholar]
- 41.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 2013;24:624–628. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 43.Shiraishi E, Iijima H, Shinzaki S, Nakajima S, Inoue T, Hiyama S, Kawai S, Araki M, Yamaguchi T, Hayashi Y, Fujii H, Nishida T, Tsujii M, Takehara T. Vitamin K deficiency leads to exacerbation of murine dextran sulfate sodium-induced colitis. J Gastroenterol. 2016;51:346–356. doi: 10.1007/s00535-015-1112-x. [DOI] [PubMed] [Google Scholar]
- 44.Viegas CSB, Costa RM, Santos L, Videira PA, Silva Z, Araújo N, Macedo AL, Matos AP, Vermeer C, Simes DC. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases. PLoS One. 2017;12:e0177829. doi: 10.1371/journal.pone.0177829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norouzinia M, Chaleshi V, Alizadeh AHM, Zali MR. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench. 2017;10:155–167. [PMC free article] [PubMed] [Google Scholar]
- 46.Li JJ, Zhu CG, Yu B, Liu YX, Yu MY. The role of inflammation in coronary artery calcification. Ageing Res Rev. 2007;6:263–270. doi: 10.1016/j.arr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Stompór T, Pasowicz M, SulÅ‚owicz W, DembiÅ„ska-Kieć A, Janda K, Wójcik K, Tracz W, Zdzienicka A, Klimeczek P, Janusz-Grzybowska E. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41:203–211. doi: 10.1053/ajkd.2003.50005. [DOI] [PubMed] [Google Scholar]
- 48.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D'Agostino RB, Wilson PW, O'Donnell CJ. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation. 2002;106:1189–1191. doi: 10.1161/01.cir.0000032135.98011.c4. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. doi: 10.1371/journal.pone.0056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data set is available from the corresponding author at email: josko.bozic@mefst.hr.