Abstract
AIM
To explore the influencing factors of diabetes type 2 patients with mild non-proliferative diabetic retinopathy (NPDR) in the Kailuan area of Tangshan, Hebei Province, China.
METHODS
In this non-interventional, retrospective study, 683 patients with type 2 diabetes were included in the Kailuan Diabetic Retinopathy Study involving participants with diabetes in the community-based longitudinal Kailuan Study. Based on the undilated ultra-wide field (200°; UWF) images and partial dilated digital fundus images, the diabetic retinopathy (DR) of the surveyed population was graded. Interobserver agreement was estimated by using Cohen's Kappa statistics. The main outcome indicators included gender, age, weight, height, body mass index, blood pressure, circumferences of neck, waist and hip, current smoking, levels of fasting plasma glucose (FPG), hypersensitive C-reactive protein, creatinine, and cholesterol, etc. According to different lesions' locations of patients with mild NPDR, logistic regression models were used to estimate the odds ratios (ORs) and their 95%CIs of each risk factor.
RESULTS
The study group of 683 patients included 570 males and 113 females. The mean age of the patients was 62.18±9.41y. Compared with dilated fundus examinations, there was fair agreement with the level of DR identified on UWF images in 63.91% of eyes (k=0.369, 95%CI, 0.00-0.00). Detected by UWF images, there were 98 patients with mild NPDR having peripheral retinal lesions, 35 patients with mild NPDR having posterior lesions, 44 patients with mild NPDR whose lesions were detected both in and out the standard two fields area, and 336 patients with non obvious DR. Parameters that conferred a statistically significant increased risks for mild NPDR with having peripheral retinal lesions were neck circumstance (OR, 1.124; 95%CI, 1.044-1.211), and with posterior lesions were FPG (OR, 1.052; 95%CI, 1.007-1.099).
CONCLUSION
UWF is an effectiveness means of DR screening. Moreover, it is necessary to evaluate peripheral diabetic retinal lesions which can help to estimate the severity of DR. The phenomenon that nonuniform and inhomogeneous distribution of DR lesions has been found. And the influencing factors in mild NPDR are differing by different lesions' locations.
Keywords: diabetic retinopathy, ultra-wide field image, neck circumference, peripheral lesions, fasting plasma glucose, posterior pole lesions
INTRODUCTION
Diabetes mellitus (DM) has been impacting on worse general health and poor quality of life, and it has emerged as an important clinical and public health problem throughout the world. Since last decade, the prevalence of DM has reached an epidemic proportion[1]–[3]. In China, the weighted prevalence of total diagnosed diabetes has been increasing from 9.7% in 2007 and 2010, to 10.4% in 2013, and to 11.2% in 2017[4], and the total number of patients with type 2 DM reaches almost 114 million which accounts one in three globally[5]. Diabetic retinopathy (DR) is the most common microvascular complications of diabetes and is prior to bring about blindness in diabetic patients. Without treatment, 50% of patients with proliferative diabeticretinopathy (PDR) will be blinded within 5y[6]. In the past 10y, the prevalence of DR in China has increased significantly. In a multi-hospital-based population across China, the prevalence of DR was 27.9%[7]. Shanghai Community Survey showed that the cumulative incidence rate and progression rate of DR was 46.89% and 32.23% respectively between 5y. In recent years, a large number of studies reveal that the severity of peripheral retinopathy in diabetic patients has a major impact on the extent as much as progress of DR. Furthermore, the early control of DR is of great significance. Silva et al[8] revealed the increased risk of DR progression were associated with the presence and growing extent of predominantly peripheral lesions over 4y. So the peripheral lesions for the severity of DR should not be ignored. This study is a cross-sectional, prospective, based on epidemiological investigations, and assess the lesions of DR by color fundus photographs of two fields (macular and disc field) and ultra-wide field (200°; UWF) imaging.
SUBJECTS AND METHODS
Ethical Approval
The Kailuan Diabetic Retinopathy Study is a retrospective cohort study which is based on the participants of the Kailuan Study. The Medical Ethics Committee of the Beijing Tongren Hospital approved the study protocol and all participants gave informed written consent.
A total of 683 patients with type 2 DM were included in the Kailuan rehabilitation hospital service center in Tangshan City, Hebei Province, China between October 2016 and December 2016. Detailed history was taken using a standardized questionnaire. Age, gender, current smoking (no/yes) were elicited from the administrated standardized questionnaire. The diagnostic criterion for diabetes was any measurement of the fasting plasma glucose (FPG) concentration of ≥7.0 mmol/L, or a self-reported history of diabetes, or a history of medication with hypoglycemic agents. A venous blood collection was drawn on the day of each examiner's examination to estimate several levels of assay index, including FPG, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, total cholesterol, uric acid, hypersensitive C-reactive protein, glutamate pyruvate transaminase, total bilirubin, creatinine, urea, red blood cell count, white blood cell count, blood platelet count, hemoglobin, neutrophil cell count, and urinary protein. Other physical examinations include heart rate, blood pressure, weight, height, waist circumference, hip circumference, and neck circumference. Body weight and height were measured using standard technique and body mass index (BMI) was calculated. BMI was calculated as weight (kg)/height (m) squared, and waist-hip ratio was calculated as waist circumference (m)/hip circumference (m). Glomerular filtration rate (GFR) was assessed by taking the modified Modification of Diet in Renal Disease (MDRD) formula for Chinese patients[9]: eGFR (mL/min/1.73m2)=175 × Scr−1.234 × age−0.179 × 0.79 (if female).
Screening Processand Grading
All patients underwent an anterior segment examination of eyeball, dilated fundus examination, and measurement of intraocular pressure (IOP). IOP was tested by non-contact tonomter. An ophthalmic examination was performed by an experienced ophthalmologist with a holding type slit-lamp in the clinic. The patients with abnormal IOP (more than 21 mm Hg) or shallow anterior chamber judged by slip-lamp would not be dilated the pupils. Patients underwent a nonmydriatic UWF laser photography and two standard horizontal field mydriatic digital photography (one centered on the fovea and the other on the disc), using a 200 degree UWF scanning laser ophthalmoscope in United Kingdom and a 45 degree Canon CR6 retinal camera in Norwich respectively. The certified imager repeated imaging up to two times during the imaging session if the imager considered the images to be of suboptimal quality. The imager stored all images using proprietary software and images were available for review using proprietary Optos image review software (Optos V2 Vantage Dx Review version 2.5.0.135; Optos plc, Dunfermline, Scotland, United Kingdom).
All two methods of examination for DR were graded independently. The ophthalmoscopic assessments were completed twice at the time of examination by two experienced ophthalmologists, respectively. The digital and film graders were masked regarding both each subject's history of DM and visual acuity, noting the underlying cause of the discrepancy and explanation (grader error, image quality, or field coverage). Image quality for the two-field digital photography was determined using the following criteria: Assessable—possible to see the small vessels of the temporal arcades with reasonable clarity. Not assessable—the large vessels of the temporal arcades are blurred or more than one third of the picture is blurred unless sight threatening retinopathy is detected in the remainder. Insufficient image quality for UWF images was stated as laying over at most the central 60° and without both the optic disc and the macula in good quality.
Grading was performed to determine the presence and severity of the following lesions: microaneurysm (Ma), blot or flame shaped hemorrhage (H), hard exudate, cotton wool spot, intraretinal microvascular abnormalities (IRMA), new vessel (NV) formation or evidence of laser treatment for DR at baseline. Images that were classified as ungradable were excluded from the analysis. The International Clinical Diabetic Retinopathy (ICDR) severity scale was used for grading[10]. DR was categorized as non-obvious retinopathy, mild non-proliferative diabetic retinopathy (NPDR), moderate NPDR, severe NPDR, PDR and after laser photocoagulation. By using UWF imaging in this report, we sought to explore the issue in a case-control study to find the risk factors for early DR in Kailuan area of Tangshan City in China. In our study, lesions locating outside the standard two-field photograph fields simulated without the dashed line on UWF images were defined as peripheral retinal lesions. Besides, lesions locating in the two-field retina area simulated within the dashed line on UWF images were defined as posterior pole lesions.
Statistical Analysis
Inter-observer agreement was evaluated by using Cohen's Kappa statistics[11]: <0.20 poor, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 good, and 0.81-1.00 excellent agreement.
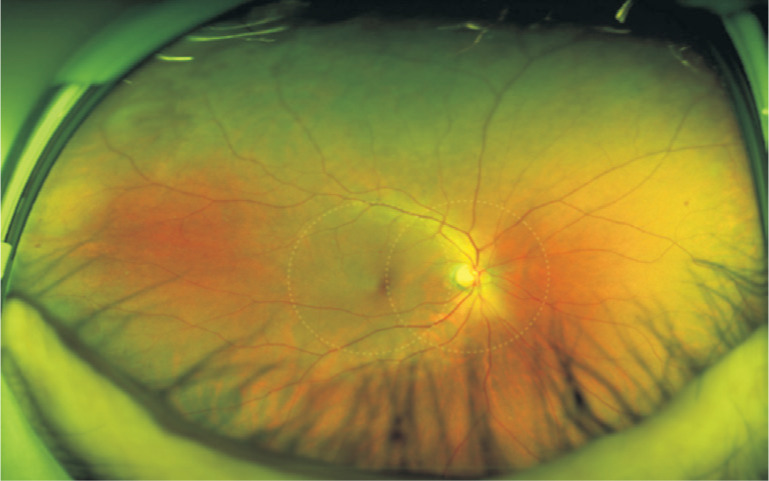
The results were expressed as mean±standard deviation or as mean and the 95% confidential interval (CI). All subjects consisted three groups. With mild NPDR, ones only having peripheral retinal lesions which locate outside the two-field retina area simulated within the dashed line (Figure 1) or only having posterior pole lesions were defined as cases respectively and subjects with no DR were defined as controls. The Fisher's exact, Chi-squared and Student's t-tests were used to compare means and percentages between each independent groups. And the first two independent groups participants with mild NPDR were analyzed with all of the predictors together in a multivariate logistic regression model using stepwise forward selection procedure with an entry probability of 0.05 and a removal probability of 0.10 respectively. Logistic regression models were used to estimate the odds ratios (ORs) and their 95% CIs of each risk factor. A P-value smaller than 0.05 was considered significant. All analyses were performed by SPSS software version 22.0 (SPSS Inc., IBM, NC, USA).
Figure 1. The UWF image of the right eye whose lesion (H/Ma) locate outside the two-field retina area (simulated within the dashed line) with mild NPDR.
RESULTS
Characteristics of the Study Population
Retinal images from 1366 eyes (683 patients) were evaluated, including 27 eyes with photocoagulation. Among these 683 patients, 570 were males (83.5%) and 113 were females (16.5%). Meanwhile, the range of age was from 28 to 83, with the average age was 62.18±9.41 years old.
Characteristics of Diabetic Retinopathy
Compared with dilated fundus examinations, there was fair agreement with the level of DR identified on UWF images in 63.91% of eyes (k=0.369, 95%CI, 0.00-0.00; Table 1).
Table 1. Severity level of diabetic retinopathy in ultra-wide field images and two standard 45° photographs (eyes).
| Parameters | Two-field images | UWF images |
| No obvious DR | 944 (69.1) | 760 (55.6) |
| Mild NPDR | 158 (11.6) | 304 (22.3) |
| Moderate NPDR | 151 (11.1) | 180 (13.2) |
| Sever NPDR | 69 (5.0) | 73 (5.3) |
| PDR | 17 (1.2) | 22 (1.6) |
| Treated by laser | 27 (2.0) | 27 (2.0) |
| Total | 1366 (100) | 1366 (100) |
Simple kappa statistic: 0.369 (95%CI, 0.00-0.00); Perfect agreement: 63.9%.
n (%)
Approximately a half of H/Ma, IRMA and NVs were located predominantly outside the two-field photograph fields. The distribution of DR lesions revealed inhomogeneous on the UWF images. Imaginary vertical lines through the center of the optic disc divide the image into the nasal side and the temporal side. It was found that all kinds of DR lesions [H/Ma, IRMA, venous beading (VB) and NVs] were nearly less predominant in the nasal half of the retina than in the temporal fields. In the visible range, there were 74.0% of H/Ma, 73.1% of IRMA, 65.0% of VB and 64.2% of NVs in the temporal fields respectively (Figure 2).
Figure 2. DR lesions in nasal and temporal retinal fields.
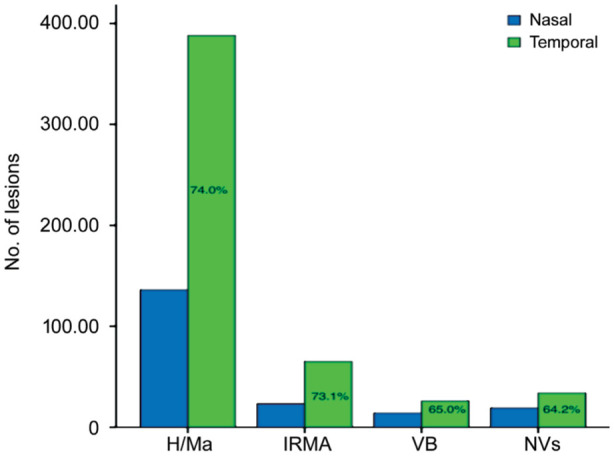
H/Ma: Hemorrhage and/or microaneurysm; IRMA: Intraretinal microvascular abnormality; VB: Venous beading; NVs:New vessels.
In the study cohorts, there were 672 eyes of 336 patients (49.2% of total patients) with non-obvious retinopathy and 304 eyes of 152 patients (11.1% of total patients) with mild NPDR. There were 68 eyes of 35 patients (2.6% of total patients, 22.4% of total eyes with mild NPDR) with mild NPDR just having posterior pole lesions. In contrast, 197 eyes of 98 patients (7.2% of total patients) with mild NPDR just having peripheral lesions. In addition, 44 patients with mild NPDR having both peripheral lesions and posterior pole lesions.
Multivariate Analysis of the Risk of Mild NPDR
Table 2 shows descriptive statistics and the frequencies of the potential prognosticators, along with P-values summarizing the univariate associations of each prognosticator for the DR cases stratified by DR type respectively (non-obvious DR vs mild NPDR with peripheral lesions and non-obvious DR vs mild NPDR with posterior pole lesions).
Table 2. Differences between the group of diabetic participants with mild NPDR having peripheral retinal lesions and posterior pole lesions compared respectively with the group of diabetic participants with no obvious diabetic retinopathy in the Kailuan Diabetic Retinopathy Study.
| Parameters | No obvious DR | Mild NPDR with peripheral retinal lesions | Mild NPDR with posterior lesions |
| n | 336 (49.2%) | 98 (14.3%) | 35 (5.1%) |
| Gender | |||
| Male | 268 (39.2%) | 80 (11.7%) | 29 (10.0%) |
| Female | 68 (10.0%) | 18 (2.6%) | 6 (0.9%) |
| Age (y) | 59.85±10.38 | 59.37±9.55 | 59.14±8.06 |
| Smoking | |||
| No | 248 (36.3%) | 62 (9.1%)a | 26 (3.8%) |
| Yes | 84 (12.3%) | 35 (5.1%) | 8 (1.2%) |
| Mean systolic blood pressure (mm Hg) | 142.02±18.33 | 143.43±18.83 | 149.82±21.93 |
| Mean diastolic blood pressure (mm Hg) | 83.26±9.95 | 83.97±10.59 | 85.10±13.91b,c |
| Height (cm) | 168.72±6.78 | 169.24±7.84 | 167.51±7.33 |
| Body weight (kg) | 74.55±11.66 | 75.99±10.77 | 73.77±13.91 |
| Waist circumference (mm) | 89.13±10.07 | 91.56±9.81 | 90.57±9.35 |
| Neck circumference (mm) | 38.49±2.26 | 39.09±3.18a | 38.53±1.99 |
| Hip circumference (mm) | 99.26±10.67 | 101.18±11.20 | 99.17±11.02 |
| Mean ventricular rate (beats/min) | 75.19±12.98 | 75.12±11.55 | 76.60±12.16 |
| Mean fasting blood glucose (mmol/L) | 9.31±6.53 | 9.66±6.92 | 12.62±2.24 |
| Mean high-density lipoprotein cholesterol concentration (mmol/L) | 1.32±0.44 | 1.36±0.49 | 1.25±0.23 |
| Mean low-density lipoprotein cholesterol concentration (mmol/L) | 2.84±0.77 | 2.76±0.83 | 2.54±0.84 |
| Mean triglyceride (mmol/L) | 2.47±5.72 | 2.18±2.06 | 2.67±3.27c |
| Mean total cholesterol concentration (mmol/L) | 5.54±1.18 | 5.48±1.34 | 5.61±1.47 |
| Mean uric acid concentration (µmol/L) | 324.52±102.18 | 333.16±91.57 | 336.23±92.84 |
| Mean hypersensitive C-reactive protein concentration (mg/L) | 3.63±6.60 | 4.08± 10.28 | 4.02±5.17 |
| Mean total protein concentration (g/L) | 79.07±43.39 | 75.69±5.44 | 76.54±4.34 |
| Mean glutamate pyruvate transaminase (U/L) | 25.51±21.09 | 26.63±23.83 | 20.83±9.81 |
| Mean total bilirubin (µmol/L) | 16.08±5.79 | 16.71±5.48 | 16.81±5.39 |
| Mean direct bilirubin (µmol/L) | 4.73±2.86 | 4.90±1.95 | 4.90±1.53 |
| Mean creatinine (mmol/L) | 77.83±57.79 | 67.41±15.22 | 69.25±24.70 |
| Mean urea (mmol/L) | 6.18±4.20 | 6.10±1.77 | 6.35±1.58 |
| Mean red blood cell count (1012/L) | 4.85±0.44 | 4.81±0.40 | 4.89±0.43 |
| Mean white blood cell count (109/L) | 6.70±1.58 | 6.45±1.58 | 6.86±1.95 |
| Mean blood platelet count (109/L) | 242.43±66.28 | 239.24±67.73 | 218.00±48.22c |
| Mean hemoglobin (g/L) | 151.00±16.74 | 151.19±15.61 | 153.23±13.30 |
| Mean neutrophil cell count (109/L) | 4.49±5.17 | 4.20±3.95 | 4.20±1.68 |
| Mean waist-hip ratio | 0.90±0.11 | 0.91±0.52 | 0.92±0.05 |
| Meanbody mass index (kg/m2) | 26.17±3.52 | 26.55±2.89 | 26.22±4.15c |
| Urinary protein | |||
| - | 292 (42.8%) | 84 (12.9%) | 27 (4.0%) |
| Trace | 13 (1.9%) | 4 (0.6%) | 1 (0.1%) |
| +- | 76 (1.0%) | 0 | 1 (0.1%) |
| + | 10 (1.5%) | 6 (0.9%) | 1 (0.1%) |
| ++ | 12 (1.8%) | 3 (0.4%) | 3 (0.4%) |
| +++ | 2 (0.3%) | 1 (0.1%) | 2 (0.3%) |
| Fatty liver | |||
| No | 109 (16.0%) | 33 (4.8%) | 12 (1.8%) |
| Light | 104 (15.2%) | 27 (4.0%) | 12 (1.8%) |
| Moderate | 97 (14.2%) | 31 (4.5%) | 10 (1.5%) |
| Heavy | 26 (3.8%) | 7 (1.0%) | 1 (0.1%) |
| eGFR (mL/min/1.73 m2) | 48.10±35.52 | 48.08±15.05 | 45.19±13.04 |
aP<0.05 vs no obvious DR; bP<0.05 vs no obvious DR; cP<0.05 vs mild NPDR with peripheral retinal lesions.
In multivariable logistic regression analysis, neck circumference (P=0.026; OR:1.124; 95%CI:1.044, 1.211) seemed to have a significantly associated with mild NPDR just having peripheral lesions. By contrast, FPG (P=0.022; OR:1.052; 95%CI: 1.007, 1.099) seemed to have a significantly associated with mild NPDR just having posterior polar lesions (Table 3).
Table 3. Associations (multivariable logistic regression analysis) of biologic risk factors with mild NPDR having peripheral and posterior retinal lesions respectively.
| Parameters | B | OR | 95%CI for OR | P |
| Neck circumference (mm) | 0.117 | 1.124 | 1.044, 1.211 | 0.002 |
| FPG (mmol/L) | 0.051 | 1.052 | 1.007, 1.099 | 0.022 |
FPG: Fasting plasma glucose.
DISCUSSION
DM is a large public health problem which is reported to affect one in 11 adults worldwide, with significant morbidity and mortality worldwide[12]. World Health Organization (WHO) projects that diabetes will be the seventh leading of cause death by 2030 and this would increase disproportionately more in developing countries[13]. Moreover, DM is estimated to be the cause of blindness in 4.8% of the 37 million people who are blind throughout the world[14].
Type 2 diabetes is a fast-growing public health challenge in China. Nationally in China, the representative survey of adults in 2013 was estimated that 10.9% of Chinese adults have diabetes[15]. In addition to the deleterious effects of the disease itself, its long-term complications can conspicuously decrease the quality of life of diabetes patients. So, the impact on the health care system for the patient with DM is an urgent public health matter. And the sooner the disease is discovered, the better the complications can be controlled.
It has been found that the microcirculatory system of the retina has the same anatomical and physiological characteristics as the microcirculatory system of the brain, coronary artery and other organs[16]. There are a number of population-based epidemiological surveys showing that retinal vascular abnormalities are closely related to systemic blood vessels, such as the heart, brain, and kidney vessels[17]–[20], suggesting that retinal vascular abnormalities can be considered as a crucial clinical indicator in a risk assessment for systemic diseases[21]–[22]. Therefore, before the serious complications of diabetes take place, the slight changes of retinal blood vessels may predict the transformation of microcirculation. So to discover diabetic retinopathy at early stage is meaningful for control of diabetes and systemic microcirculation.
Over the decades of years, a variety of techniques can be used to detect and grade DR, including direct and indirect ophthalmoscopy, stereoscopic color film fundus photography, fluorescein angiography, and mydriatic or nonmydriatic digital color or monochromatic photography. It has proven to be beneficial to use a screening tool like digital non-mydriatic fundus imaging for detection of early DR changes by an endocrinologist, or any physician treating diabetes who can refer to an ophthalmologist for screening[23]–[25]. It is known that many primary care physicians are used to screen and monitor DR by two-field fundus photographic pictures in China. However, the peripheral lesions are easy to be ignored. Although it has been known that the Early Treatment Diabetic Retinopathy Study (ETDRS) 7-field photography is the gold standard for DR in terms of diagnosis and classification[26], it is seems time consuming to realize the large number of community screening for DR in a limit time and hard for both patient and photographer through the clinical practices.
Recent technological advantages have also resulted in the availability of UWF scanning laser ophthalmoscopy techniques for imaging, which can obtain a 200° UWF image at a time, covering about 82% of the retina range. Meanwhile, taking into account the advantages of it such as nonmydriatic, fast, safety and non-contact, it is convenient for physicians to detect community-based DR in large populations. So it has become the most effective and reliable way to observe peripheral retinopathy. In this study we used the UWF scanning laser ophthalmoscopy (Optos 200TX) to screen for DR in a large sample population with type 2 DM of Kailuan area of Tangshan City. UWF images were evaluated to impact on the grading of DR (36.09% of eyes with DR) in 493 eyes, not only because of inadequate two-field photograph quality and just almost having peripheral lesions, also because it can obtain a better retinal image for a patient with a certain degree of cataract, for the red or green laser of the UWF scanning laser ophthalmoscope system can reduce the light scattering when entering the anterior segment of the eye. Neither UWF image nor two-field fundus photographic picture ungradable was included in our study. However, we found that compared with UWF images, there were almost 2 times number of two-field fundus pictures ungradable because of the opaque refractive medium. Except for heavy-cloudy cataracts, uncooperative and eyelashes and eyelids blocking were the main reasons for ungradable UWF images.
The two technologies showed consistent for DR grading (Kappa=0.369, P<0.001). So it has an advantage for detecting DR by UWF images over by two-field fundus photographic pictures. Throughout all UWF images with DR lesions, peripheral lesions in the nasal half of the retina were less than in the temporal half. The phenomenon that nonuiform and inhomogeneous distribution of DR lesions has been found by some experimental research[27]–[28]. Furthermore, Silva et al[29] demonstrated that peripheral DR pathology primarily in the temporal and superior quadrants by using Optomap UWF scanning laser ophthalmoscope images.
In our study, interestingly, patients with mild NPDR whose lesions visibly sited in different retina area with inhomogeneous distribution. Some lesions were found outside the two-field retina area rather than in the posterior pole area, vice versa. The implied implications and pathogenesis are not clear. It may be associated with the the protective mechanism of retinal oxygenation, which was unavailable in the peripheral retinal arterioles rather than in the posterior pole retinal areas. Owing to the regionally different disturbance of diameter caused by increased blood pressure, distribution of DR lesions are regionally different[30].
As we known, the occurrence and advancement of DR is known to be caused by the combined effects of multiple factors in the whole body, including duration of diabetes[31]–[33], systolic blood pressure[34], anemia[35]–[36], hyperlipidemia[37]–[39], renal dysfunction[40], high HbAlc[41] etc. The results of each study are not completely consistent, and are related to the research objects and geographical differences. However, what made the nonuiform distribution of lesions with mild NPDR happen?
Interestingly, we found that the influnerence factors for patient with mild NPDR were different in the light of different lesions' location. On one hand, we carried out an analysis of the risk factors for these ones with mild NPDR who are often easily neglected and not taken seriously on account of being misdiagnosis as usual. As far as we known, there are few reports on the factors affecting the peripheral lesions preceded at the early-stage of DR: mild NPDR. On the other hand, we did an analysis of the risk factors for those ones whose lesions chiefly and obviously located in the posterior pole area. Compared with the group of mild NPDR with peripheral lesions in our study, the group of mild NPDR with posterior lesions had higher diastolic blood pressure, higher triglyceride, lower blood platelet count and lower BMI. Also, these patients were more smoking and had longer neck circumference compared with no DR patients. Statistically, neck circumstance was the independent risk factor in the multivariable model for the mild NPDR that lesions located obviously outside posterior pole area. On contrary, FPG was the independent risk factor in the multivariable model for the mild NPDR with lesions occurred predominantly in the two-field retina area. It was found that BMI and age were not the significant risks. The result is similar with a multi-hospital-based cross-sectional study[42], although several previous studies presented the two had certain relationship[43]–[45].
Previously, several studies revealed neck circumference as a cardiometabolic possiblerisk factor[46]–[47]. Neck circumference were showed positively related to the metabolic syndrome risk factors, such as insulin resistance and abdominal visceral fat, manifesed a positively mutual relationship with triglycerides, FPG, fasting insulin, and showed a moderate negative correlation with insulin sensitivity[46],[48]. Further investigations with prospective design are required to confirm these findings. Although more and more research pay close attention to the relationship between neck circumference with DM, the relationship between DR and neck circumference was rare reported. Some research[49]–[50] provided a positive correlation of neck circumference with metabolic syndrome in patients with type 2 diabetes. The result from a study from Khalangot et al[51] revealed neck circumference was a novel risk factor of diabetes during screen-based research of glycaemia and glucose tolerance. Our study showed positively relation of neck circumference with mild NPDR having peripheral lesions. Ischemia has been known as a critical component of DR, which has been associated with the advancement and progress of both microvascular and macrovascular complications[52]. In a study[53] based on population of Amerindians, smaller neck circumference was associated with lower carotid intima-media thickness but BMI was associated. These with mild obstructive sleep apnea (OSA) had shorter neck circumference and longer ocular axial length than those with either moderate or severe OSAS[54]. These findings support the result of our study that an increase of neck circumference may lead to an aggravation of retinal hypoxia and ischemia especially in peripheral area for the patients with diabetic. The results from Korean indicated a negative impact on large neck circumference (the mean±SD of neck circumference; ranges) was 37.6±2.0 (31.8-45.3) cm in men and 32.9±1.8 (23.0-40.0) cm in women in the development of DM and the mean±SD of neck circumference (ranges) was 37.6±2.0 (31.8-45.3) cm in men and 32.9±1.8 (23.0-40.0) cm in women[55]. In our study, compared with the mean±SD of neck circumference was 38.49±2.26 cm in patient with non-obvious DR, 39.09±3.18 cm in the patient with mild NPDR having peripheral retinal lesions obviously (F=4.227, P=0.40). It revealed the patients with early stage of DR whose lesions (H/Ma) located mainly outside the posterior pole retina might have larger neck circumference than the ones suffered from DM with non-obvious DR. We deemed that neck circumference may be an important predictor of early stage of DR and H/Ma located outside posterior pole retina might be a sign of cardiometabolic alter, which is a novel, easily measured index.
Excessive high glucose levels were widely considered by researchers as a big risk of vascular diseases and all-cause mortality[56]–[57]. Additionally, the prevalence of retinopathy was found having an association with FPG[58]–[61]. In China, some studies' conclusion was analogous to those results[60],[62]. Similarly, in our study, the patients with mild NPDR whose lesions located in the posterior pole area have higher FPG. However, FPG was not the risk factor for the patients with mild NPDR having peripheral retinal lesions. It is possible that presence of H/Ma in posterior pole identifies an individual with an increased microvascular susceptibility to glycemia. The presence of retinopathy lesions at baseline was related to be a two-fold increased risk of progression of FPG and suggested that an assessment of retinopathy could be of value in initial assessments of risk of metabolic worsening in early diabetes, adding information beyond the risk associated with age and glycemia[63]. And retinopathy in newly diagnosed type 2 diabetes was associated with elevated fasting and postprandial glucose[64]. It is deemed that detection of early DR, especially in posterior pole retinal area, may beneficial to prevent progressive metabolic worsening and promote individualized therapy in early diabetes further. Clinically, in patients with DR, we also found the ones with high FPG whose lesions of DR located in the posterior pole retina are more severe than those patients with normal FPG controlled.
Our study has some limitations. Although the designed project actually contains nearly 1200 people, the complete data were not available for a significant proportion of the subjects (almost 30%) who were thus not included in these analyses, which might cause some systematic error. Furthermore, some indicators (such as glycosylated hemoglobin, duration of DM) were not recorded. In addition, some data are not included (such as peripheral neuropathy, urinary albumin excretion and time of insulin intervention). The missing information of ex-smoker or never smoked was too much to include into the statistical analysis, although those who reported not current smoking were further classified as ex-smoker or never smoked in our questionnaire. Compared with other studies, our population had little prevalence of microvascular complications[65]–[67]. It might because the subjects of our study were health-examined individuals, who were different from the hospital patients. On the analysis side, the presence of goiter and quantitative parameters of thyroid gland were not considered in our study, which affected the results of neck circumference. The selection criteria could limit the generalisability of the study. All above may create a potential selection bias. Despite these limitations, our study has many strengths, including the large sample size of individuals with DM, standardized laboratory assessment of serum samples and imaging data. To the best of our knowledge, this was the first time that a wide-angle laser retinal imaging system had been used in the screening of DR in community of China, and this is the first reporting the risk factors for patients with mild NPDR in light of different lesions' locationin a Chinese population. These observations may be of value in future investigations.
In summary, the present study has shown the effectiveness of DR screening using UWF scanning laser ophthalmoscope system, especially for reducing the burden of DR screening and enhancing early detection of treatable DR in large group people suffered from DM. As we known, clinically, retina outside the two-field area is often easily overlooked, and it has been found that lesions in peripheral retina could affect the grading of DR. The phenomenon that nonuiform and inhomogeneous distribution of DR lesions has been found. Meanwhile our data showed different influence factors for patient with mild NPDR on the basis of different lesions' location. In view of current study, we deem that the pathogenesis of retinopathy is dissimilar from distribution of lesions for patients with NPDR. In detail, the patients with peripheral lesions might have larger neck circumference and should pay more attention to cardiometabolic or macrovascular complications, while the patients with posterior pole lesions might get along with higher FPG in advanced stage DM. Clinically, they all could give tips for the analysis of the severity of a patient's systematic diseases. Further investigations with prospective design are required to confirm these findings.
Acknowledgments
Conflicts of Interest: Yang MC, None; Zhu XB, None; Wang YX, None; Wu SL, None; Wang Q, None; Yan YN, None; Yang X, None; Yang JY, None; Chen MX, None; Lei YH, None; Wei WB, None.
REFERENCES
- 1.Yang LL, Shao J, Bian YY, Wu HQ, Shi LL, Zeng L, Li WL, Dong JC. Prevalence of type 2 diabetes mellitus among inland residents in China (2000-2014): a meta-analysis. J Diabetes Investig. 2016;7(6):845–852. doi: 10.1111/jdi.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao ZH, Huo LL, Wang LY, Wang LJ, Fu ZD, Li YF, Wu XH. Survival of Chinese people with type 2 diabetes and diabetic kidney disease: a cohort of 12-year follow-up. BMC Public Health. 2019;19(1):1498. doi: 10.1186/s12889-019-7859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu LN, Wu SM, Zhao SL, Zhou HX, Zhang SF, Gao M, Qu ZY, Zhang WJ, Tian DH. Association of social support and medication adherence in Chinese patients with type 2 diabetes mellitus. Int J Environ Res Public Health. 2017;14(12):E1522. doi: 10.3390/ijerph14121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YZ, Teng D, Shi XG, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. IDF Diabetes Atlas. 8th ed. 2017. [Google Scholar]
- 6.Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi: 10.2337/dc15-2171. [DOI] [PubMed] [Google Scholar]
- 7.Zhang GH, Chen HY, Chen WQ, Zhang MZ. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol. 2017;101(12):1591–1595. doi: 10.1136/bjophthalmol-2017-310316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva PS, Cavallerano JD, Haddad NM, Kwak H, Dyer KH, Omar AF, Shikari H, Aiello LM, Sun JK, Aiello LP. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949–956. doi: 10.1016/j.ophtha.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 10.International Clinical Diabetic Retinopathy (ICDR) severity scale. International Council of Ophthalmology. 2002. [Accessed on January 4, 2015]. www.icoph.org/pdf/Diabetic-Retinopathy-Scale.pdf.
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 12.Ong SE, Koh JJK, Toh SES, Chia KS, Balabanova D, McKee M, Perel P, Legido-Quigley H. Assessing the influence of health systems on type 2 diabetes mellitus awareness, treatment, adherence, and control: a systematic review. PLoS One. 2018;13(3):e0195086. doi: 10.1371/journal.pone.0195086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maa AY, Wojciechowski B, Hunt KJ, Dismuke C, Shyu J, Janjua R, Lu XQ, Medert CM, Lynch MG. Early experience with technology-based eye care services (TECS): a novel ophthalmologic telemedicine initiative. Ophthalmology. 2017;124(4):539–546. doi: 10.1016/j.ophtha.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Drake L. Prevention of blindness from diabetes mellitus - report of a WHO Consultation Prevention of blindness from diabetes mellitus - report of a WHO consultation. Nurs Stand. 2007;21(32):30. [Google Scholar]
- 15.Wang LM, Gao P, Zhang M, Huang ZJ, Zhang DD, Deng Q, Li YC, Zhao ZP, Qin XY, Jin DY, Zhou MG, Tang X, Hu YH, Wang LH. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Li SM, Kang MT, Liu LR, Li H, Wei SF, Ran AR, Wang NL, Anyang Childhood Eye Study Group Association between blood pressure and retinal arteriolar and venular diameters in Chinese early adolescent children, and whether the association has gender difference: a cross-sectional study. BMC Ophthalmol. 2018;18(1):133. doi: 10.1186/s12886-018-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SB, Mitchell P, Plant AJ, Phan K, Liew G, Thiagalingam A, Burlutsky G, Gopinath B. Metabolic syndrome and retinal microvascular calibre in a high cardiovascular disease risk cohort. Br J Ophthalmol. 2016;100(8):1041–1046. doi: 10.1136/bjophthalmol-2015-307637. [DOI] [PubMed] [Google Scholar]
- 18.Braun G, Hafner B, Königstein K, Infanger D, Klenk C, Rossmeissl A, Schmidt-Trucksäss A, Hanssen H. Association of cardiorespiratory fitness with retinal vessel diameters as a biomarker of cardiovascular risk. Microvasc Res. 2018;120:36–40. doi: 10.1016/j.mvr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Jinnouchi H, Kitamura A, Yamagishi K, Kiyama M, Imano H, Okada T, Cui RZ, Umesawa M, Muraki I, Hayama-Terada M, Kawasaki R, Sankai T, Ohira T, Iso H, CIRCS Investigators Retinal vascular changes and prospective risk of disabling dementia: the circulatory risk in communities study (CIRCS) J Atheroscler Thromb. 2017;24(7):687–695. doi: 10.5551/jat.37291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yip W, Ong PG, Teo BW, Cheung CY, Tai ES, Cheng CY, Lamoureux E, Wong TY, Sabanayagam C. Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci Rep. 2017;7(1):9374. doi: 10.1038/s41598-017-09204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daien V, Granados L, Kawasaki R, Villain M, Ribstein J, Du Cailar G, Mimran A, Fesler P. Retinal vascular caliber associated with cardiac and renal target organ damage in never-treated hypertensive patients. Microcirculation. 2017;24(4) doi: 10.1111/micc.12344. [DOI] [PubMed] [Google Scholar]
- 22.Arichika S, Uji A, Murakami T, Suzuma K, Gotoh N, Yoshimura N. Correlation of retinal arterial wall thickness with atherosclerosis predictors in type 2 diabetes without clinical retinopathy. Br J Ophthalmol. 2017;101(1):69–74. doi: 10.1136/bjophthalmol-2016-309612. [DOI] [PubMed] [Google Scholar]
- 23.Avidor D, Loewenstein A, Waisbourd M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: a systematic review. Cost Eff Resour Alloc. 2020;18:16. doi: 10.1186/s12962-020-00211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzetta P, Sarao V, Scanlon PH, Barratt J, Porta M, Bandello F, Loewenstein A, Vision Academy Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020;57(7):785–798. doi: 10.1007/s00592-020-01506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quellec G, Bazin L, Cazuguel G, Delafoy I, Cochener B, Lamard M. Suitability of a low-cost, handheld, nonmydriatic retinograph for diabetic retinopathy diagnosis. Trans Vis Sci Tech. 2016;5(2):16. doi: 10.1167/tvst.5.2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the modified airlie house classification: ETDRS report number 10. Ophthalmology. 2020;127(4S):S99–S119. doi: 10.1016/j.ophtha.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Jørgensen CM, Bek T. Lack of differences in the regional variation of oxygen saturation in larger retinal vessels in diabetic maculopathy and proliferative diabetic retinopathy. Br J Ophthalmol. 2017;101(6):752–757. doi: 10.1136/bjophthalmol-2016-308894. [DOI] [PubMed] [Google Scholar]
- 28.Munuera-Gifre E, Saez M, Juvinyà-Canals D, Rodríguez-Poncelas A, Barrot-de-la-Puente JF, Franch-Nadal J, Romero-Aroca P, Barceló MA, Coll-de-Tuero G. Analysis of the location of retinal lesions in central retinographies of patients with type 2 diabetes. Acta Ophthalmol. 2020;98(1):e13–e21. doi: 10.1111/aos.14223. [DOI] [PubMed] [Google Scholar]
- 29.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587–2595. doi: 10.1016/j.ophtha.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.El Dabagh Y, Petersen L, Pedersen M, Bek T. Reduced baseline diameter and contraction of peripheral retinal arterioles immediately after remote ischemia in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2095–2101. doi: 10.1007/s00417-019-04407-x. [DOI] [PubMed] [Google Scholar]
- 31.Voigt M, Schmidt S, Lehmann T, Köhler B, Kloos C, Voigt UA, Meller D, Wolf G, Müller UA, Müller N. Prevalence and progression rate of diabetic retinopathy in type 2 diabetes patients in correlation with the duration of diabetes. Exp Clin Endocrinol Diabetes. 2018;126(9):570–576. doi: 10.1055/s-0043-120570. [DOI] [PubMed] [Google Scholar]
- 32.Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62(4):211–217. doi: 10.1159/000499541. [DOI] [PubMed] [Google Scholar]
- 33.Graue-Hernandez EO, Rivera-De-La-Parra D, Hernandez-Jimenez S, Aguilar-Salinas CA, Kershenobich-Stalnikowitz D, Jimenez-Corona A. Prevalence and associated risk factors of diabetic retinopathy and macular oedema in patients recently diagnosed with type 2 diabetes. BMJ Open Ophthalmol. 2020;5(1):e000304. doi: 10.1136/bmjophth-2019-000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foo V, Quah J, Cheung G, Tan NC, Ma Zar KL, Chan CM, Lamoureux E, Tien Yin W, Tan G, Sabanayagam C. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics. J Diabetes. 2017;9(2):200–207. doi: 10.1111/1753-0407.12403. [DOI] [PubMed] [Google Scholar]
- 35.Traveset A, Rubinat E, Ortega E, Alcubierre N, Vazquez B, Hernández M, Jurjo C, Espinet R, Ezpeleta JA, Mauricio D. Lower hemoglobin concentration is associated with retinal ischemia and the severity of diabetic retinopathy in type 2 diabetes. J Diabetes Res. 2016;2016:3674946. doi: 10.1155/2016/3674946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung JO, Park SY, Chung DJ, Chung MY. Relationship between anemia, serum bilirubin concentrations, and diabetic retinopathy in individuals with type 2 diabetes. Medicine (Baltimore) 2019;98(43):e17693. doi: 10.1097/MD.0000000000017693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan S, Raman R, Kulothungan V, Swaminathan G, Sharma T. Influence of serum lipids on the incidence and progression of diabetic retinopathy and macular oedema: Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular genetics Study-II. Clin Exp Ophthalmol. 2017;45(9):894–900. doi: 10.1111/ceo.12990. [DOI] [PubMed] [Google Scholar]
- 38.Chung YR, Park SW, Choi SY, Kim SW, Moon KY, Kim JH, Lee K. Association of statin use and hypertriglyceridemia with diabetic macular edema in patients with type 2 diabetes and diabetic retinopathy. Cardiovasc Diabetol. 2017;16(1):4. doi: 10.1186/s12933-016-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papavasileiou E, Davoudi S, Roohipoor R, Cho H, Kudrimoti S, Hancock H, Wilson JG, Andreoli C, Husain D, James M, Penman, Chen CJ, Sobrin L. Association of serum lipid levels with retinal hard exudate area in African Americans with type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):509–517. doi: 10.1007/s00417-016-3493-9. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YT, Tsai MJ, Tu ST, Hsieh MC. Association of abnormal renal profiles and proliferative diabetic retinopathy and diabetic macular edema in an Asian population with type 2 diabetes. JAMA Ophthalmol. 2018;136(1):68–74. doi: 10.1001/jamaophthalmol.2017.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rozing MP, Møller A, Aabenhus R, Siersma V, Rasmussen K, Køster-Rasmussen R. Changes in HbA1c during the first six years after the diagnosis of type 2 diabetes mellitus predict long-term microvascular outcomes. PLoS One. 2019;14(11):e0225230. doi: 10.1371/journal.pone.0225230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Yang JR, Tao LY, Lv H, Jiang XD, Zhang MZ, Li XM. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7(9):e016280. doi: 10.1136/bmjopen-2017-016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YH, Sheu WH, Lee IT. Influence of diabetic retinopathy on the relationship between body mass index and mortality in patients with poorly controlled type 2 diabetes. Diabetes Metab Syndr Obes. 2020;13:907–914. doi: 10.2147/DMSO.S246032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarrafan-Chaharsoughi Z, Manaviat MR, Namiranian N, Yazdian-Anari P, Rahmanian M. Is there a relationship between body mass index and diabetic retinopathy in type II diabetic patients? A cross sectional study. J Diabetes Metab Disord. 2018;17(1):63–69. doi: 10.1007/s40200-018-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JCY, Chee, Tan NYQ, Cheng CY, Wong TY, Sabanayagam C. Differential effect of body mass index on the incidence of diabetes and diabetic retinopathy in two Asian populations. Nutr Diabetes. 2018;8(1):16. doi: 10.1038/s41387-018-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morais AA, Morais UAB, Soares MMS, Romano MCC, Lamounier JA. Neck circumference in adolescents and cardiometabolic risk: a sistematic review. Rev Assoc Med Bras (1992) 2018;64(1):54–62. doi: 10.1590/1806-9282.64.01.54. [DOI] [PubMed] [Google Scholar]
- 47.Yang GR, Yuan MX, Wan G, Zhang XL, Fu HJ, Yuan SY, Zhu LX, Xie RR, Zhang JD, Li YL, Sun YH, Dai QF, Gao DY, Cui XL, Gao JQ, Wang ZM, Chen YJ, Hu DM, Gao J, Bai LY. Association between neck circumference and the occurrence of cardiovascular events in type 2 diabetes: Beijing community diabetes study 20 (BCDS-20) Biomed Res Int. 2019;2019:4242304. doi: 10.1155/2019/4242304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saneei P, Shahdadian F, Moradi S, Ghavami A, Mohammadi H, Rouhani MH. Neck circumference in relation to glycemic parameters: a systematic review and meta-analysis of observational studies. Diabetol Metab Syndr. 2019;11:50. doi: 10.1186/s13098-019-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozkaya I, Yardimci B, Tunckale A. Appropriate neck circumference cut-off points for metabolic syndrome in Turkish patients with type 2 diabetes. Endocrinol Diabetes Nutr. 2017;64(10):517–523. doi: 10.1016/j.endinu.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Ting MK, Liao PJ, Wu IW, Chen SW, Yang NI, Lin TY, Hsu KH. Predicting type 2 diabetes mellitus occurrence using three-dimensional anthropometric body surface scanning measurements: a prospective cohort study. J Diabetes Res. 2018;2018:6742384. doi: 10.1155/2018/6742384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalangot M, Gurianov V, Okhrimenko N, Luzanchuk I, Kravchenko V. Neck circumference as a risk factor of screen-detected diabetes mellitus: community-based study. Diabetol Metab Syndr. 2016;8:12. doi: 10.1186/s13098-016-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, Casellas A, Barrot-De la Puente JF, Franch-Nadal J, Coll-de Tuero G. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS One. 2016;11(2):e0149448. doi: 10.1371/journal.pone.0149448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Brutto OH, Mera RM, Nader JA, Zambrano M, Castillo PR, Matcha G, Simon LV. The relationship between the neck circumference and the carotid intima-media thickness in Amerindians. Potential links to health risks? Pathophysiology. 2018;25(4):427–431. doi: 10.1016/j.pathophys.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Chuang LH, Koh YY, Chen HSL, Lo YL, Yu CC, Yeung L, Lai CC. Normal tension glaucoma in obstructive sleep apnea syndrome: a structural and functional study. Medicine (Baltimore) 2020;99(13):e19468. doi: 10.1097/MD.0000000000019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho NH, Oh TJ, Kim KM, Choi SH, Lee JH, Park KS, Jang HC, Kim JY, Lee HK, Lim S. Neck circumference and incidence of diabetes mellitus over 10 years in the Korean genome and epidemiology study (KoGES) Sci Rep. 2015;5:18565. doi: 10.1038/srep18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Leeuw J, Visseren FL, Woodward M, van der Graaf Y, Grobbee DE, Harrap S, Heller S, Mancia G, Marre M, Poulter N, Zoungas S, Chalmers J. Estimation of individual beneficial and adverse effects of intensive glucose control for patients with type 2 diabetes. Diabetologia. 2016;59(12):2603–2612. doi: 10.1007/s00125-016-4082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian JY, Ohkuma T, Cooper M, Harrap S, Mancia G, Poulter N, Wang JG, Zoungas S, Woodward M, Chalmers J. Effects of intensive glycemic control on clinical outcomes among patients with type 2 diabetes with different levels of cardiovascular risk and hemoglobin A1c in the ADVANCE trial. Diabetes Care. 2020;43(6):1293–1299. doi: 10.2337/dc19-1817. [DOI] [PubMed] [Google Scholar]
- 58.Samadi Aidenloo N, Mehdizadeh A, Valizadeh N, Abbaszadeh M, Qarequran S, Khalkhali H. Optimal glycemic and hemoglobin A1c thresholds for diagnosing diabetes based on prevalence of retinopathy in an Iranian population. Iran Red Crescent Med J. 2016;18(8):e31254. doi: 10.5812/ircmj.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagami T, Takahashi K, Suto C, Oya J, Tanaka Y, Kurita M, Isago C, Hasegawa Y, Ito A, Uchigata Y. Diabetes diagnostic thresholds of the glycated hemoglobin A1c and fasting plasma glucose levels considering the 5-year incidence of retinopathy. Diabetes Res Clin Pract. 2017;124:20–29. doi: 10.1016/j.diabres.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R, Li YF, Zhang SM, Cai XL, Zhou XH, Ji LN. The association of retinopathy and plasma glucose and HbA1c: a validation of diabetes diagnostic criteria in a Chinese population. J Diabetes Res. 2016;2016:4034129. doi: 10.1155/2016/4034129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takao T, Inoue K, Suka, Yanagisawa H, Iwamoto Y. Optimal cutoff values of fasting plasma glucose (FPG) variability for detecting retinopathy and the threshold of FPG levels for predicting the risk of retinopathy in type 2 diabetes: a longitudinal study over 27 years. Diabetes Res Clin Pract. 2018;140:228–235. doi: 10.1016/j.diabres.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 62.Cui J, Ren JP, Chen DN, Xin Z, Yuan MX, Xu J, You QS, Yang JK. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ Open. 2017;7(8):e015473. doi: 10.1136/bmjopen-2016-015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel YR, Kirkman MS, Considine RV, Hannon TS, Mather KJ. Retinopathy predicts progression of fasting plasma glucose: an Early Diabetes Intervention Program (EDIP) analysis. J Diabetes Complicat. 2017;31(3):605–610. doi: 10.1016/j.jdiacomp.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy Chowdhury S, Thomas RL, Dunseath GJ, Peter R, Rees DA, North RV, Luzio SD, Owens DR. Diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus: contribution of β-cell function. J Clin Endocrinol Metab. 2016;101(2):572–580. doi: 10.1210/jc.2015-2203. [DOI] [PubMed] [Google Scholar]
- 65.Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, Sardu C, Rinaldi L, Galiero R, Acierno C, Caturano A, de Sio C, de Nicola L, Salvatore T, Nevola R, Adinolfi LE, Minutolo R, NO BLIND Study Group Relationship between albuminuric CKD and diabetic retinopathy in a real-world setting of type 2 diabetes: Findings from No blind study. Nutr Metab Cardiovasc Dis. 2019;29(9):923–930. doi: 10.1016/j.numecd.2019.05.065. [DOI] [PubMed] [Google Scholar]
- 66.Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 67.Gupta R, Misra A. Epidemiology of microvascular complications of diabetes in South Asians and comparison with other ethnicities. J Diabetes. 2016;8(4):470–482. doi: 10.1111/1753-0407.12378. [DOI] [PubMed] [Google Scholar]


