Abstract
AIM
To observe the efficacy and safety of pars plana vitrectomy (PPV) with eye position guided fluid-air exchange (FAX) and air tamponade in the treatment of rhegmatogenous retinal detachment (RRD).
METHODS
RRD patients without severe proliferative vitreoretinopathy (PVR) C1 or more were enrolled. All patients underwent PPV combining with air tamponade. During operation, the primary retinal break(s) were placed at lower site and subretinal fluid was aspirated through the break(s) at the same time when eye position guided FAX was proceeding. Sufficient laser spots were made to seal the retinal break(s) after FAX, and filtered air was left in vitreous cavity as tamponade agent finally. The main outcomes were primary and final success rates, best corrected visual acuity (BCVA), and the secondary outcomes were rate of postoperative cataract surgery and high intraocular pressure.
RESULTS
A total of 37 eyes (20 males and 17 females) with a follow-up time of ≥6mo were included. The range of RRD was 5.6±1.8h, and the number of retinal breaks was 1.9±1.2. The breaks located at inferior quadrants (between 3:00 and 9:00) in 5 cases (13.5%), and both superior and inferior breaks were found in 3 cases (8.1%). A total of 25 cases (67.6%) with macular detached involvement, 9 cases (24.3%) with intraocular lens, and 8 patients (21.6%) were treated with phacoemulsification and intraocular lens implantation together. The success rate of primary retinal reattachment was 100% (37/37). At 6mo postoperatively, BCVA (logMAR) was increased from 1.13±1.07 to 0.23±0.15 (P<0.001). Phacoemulsification combined with intraocular lens implantation was performed in 2 patients (5.4%), and one of them underwent macular epiretinal membrane peeling in addition (2.7%). Furthermore, high intraocular pressure was found in 4 cases (10.8%).
CONCLUSION
PPV with air tamponade by eye position guided FAX can achieve a high reattachment success rate in the management of patients with RRD, and it has the advantages of short postoperative prone time and fewer operative complications.
Keywords: pars plana vitrectomy, eye position guided FAX, air tamponade, rhegmatogenous retinal detachment
INTRODUCTION
Rhegmatogenous retinal detachment (RRD) is a kind of ophthalmic emergency. The clinical symptom is shadow in front of the eyes and suddenly painless loss of vision[1]. In recent years, scleral buckling and pars plana vitrectomy (PPV) are still the mainstream surgical methods for the treatment of RRD[2]. With the continuous development and improvement of surgical instruments and equipment, minimally invasive vitrectomy has shown more advantages, including minimum surgical trauma, higher surgical success rate and less surgical complications[3]–[4]. Nowadays, more and more surgeons regard vitrectomy as the preferred surgical procedure for RRD repair[5].
Current views believe that subretinal fluid should be expelled as completely as possible in vitrectomy before intraocular tamponade in order to make vitreous cavity be fully filled[6]–[8]. Therefore, it is necessary to use heavy liquid to expel subretinal fluid thoroughly as most of the primary break(s) located at the peripheral area of the fundus in detached retina[9]. However, heavy liquid has some well-known complications, and the injection and removal of heavy liquid may complicate the surgery and prolong the operation time[10]–[11]. For intraocular tamponade, silicone oil and long-acting gas are most commonly used in PPV for RRD at present[12]. In experimental animals, the strength of chorioretinal adhesion produced by laser photocoagulation exceed that in normal retina 24h after laser retinopexy[13]–[15]. In RRD patients, we have observed that the laser spots could produce a certain amount of immediate chorioretinal adhesion force, and with the surface tension of intraocular gas, residual subretinal fluid will be rapidly absorbed by RPE layer within a few hours. Therefore, we tried to use eye position guided fluid-air exchange (FAX) to expel the subretinal fluid from primary retinal break(s) as much as possible in PPV for the treatment of RRD with endolaser photocoagulation retinopexy and air tamponade, without usage of heavy liquid. A thorough review found no similar reports about the technique. The present study retrospectively reviewed 37 patients with RRD in order to analyze the efficacy and safety of this modified technique.
SUBJECTS AND METHODS
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and was approval by the Research Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University (No.w12). All patients had been fully informed of the purpose and methods of the present study and provided written informed consent from themselves or their guardians.
Subjects
The medical records of all patients at the First Affiliated Hospital of Xi'an Jiaotong University who suffered with primary RRD and received 23- or 25-gauge PPV combined with air tamponade from September 1, 2017 to July 1, 2019 were analyzed retrospectively. The inclusion criteria were eyes with RRD caused by either superior breaks or inferior breaks that underwent 23- or 25-gauge PPV with air tamponade agent. The exclusion criteria were: 1) previous vitreoretinal surgery; 2) proliferative vitreous retinopathy (PVR) grade C1 or greater; 3) retinal detachment caused by macular hole/giant retina tears/dialysis of ora serrata; 4) follow-up of less than six months; 5) incomplete data and other severe eye disease. The main outcomes were success rate of retinal reattachment and best corrected visual acuity (BCVA), the secondary outcomes were rate of postoperative cataract surgery and high intraocular pressure (IOP).
During the follow-up period, all enrolled patients received ophthalmologic examinations including non-contact tonometry, Snellen BCVA, slit lamp microscopy and dilated funduscopic examination. Additional information was recorded including, disease course, retinal detachment range, size and number of retinal breaks. During operation, the peripheral retina was examined again for any undetected pathological changes. The intraoperative information was also collected.
Surgical Procedure
All surgeries were performed with retrobulbar anesthesia by an experienced surgeon. A standard three-port PPV (23/25-gauge, Constellation Vitrectomy System; Alcon Laboratories, Fort Worth, TX, USA) was performed. The core vitrectomy and peripheral vitreous was cut off step by step with the wide-angle viewing system (the Resight 700, Carl Zeiss Meditec AG, Jena, Germany). The peripheral retina was examined by scleral indentation to confirm whether there were any pathological changes. The primary retinal breaks were placed at the lower site with eye position guided FAX performing by vitrectomy probe and light fiber, and subretinal fluid was drained off at the same time using Vit probe or flute needle. Then put the flute needle onto the optic disc and fully drained off the liquid of vitreous cavity. Little residual subretinal fluid at the posterior pole did not need to be completely expelled unless it affected the endolaser retinopexy. Make sure that the subretinal fluid around the breaks was completely drained. Then endolaser photocoagulation was applied to seal around degenerative areas and the retinal breaks for three rows. At last, the remaining balanced salt solution in the vitreous cavity was drained off and left filtered air as intraocular tamponade agent (Figure 1). Finally, the cannulae were removed and 8-0 absorbable suture was used if necessary, to watertight the incision. Patients who had severe cataracts were scheduled to receive concurrent cataract extraction and intraocular lens (IOL) implantation.
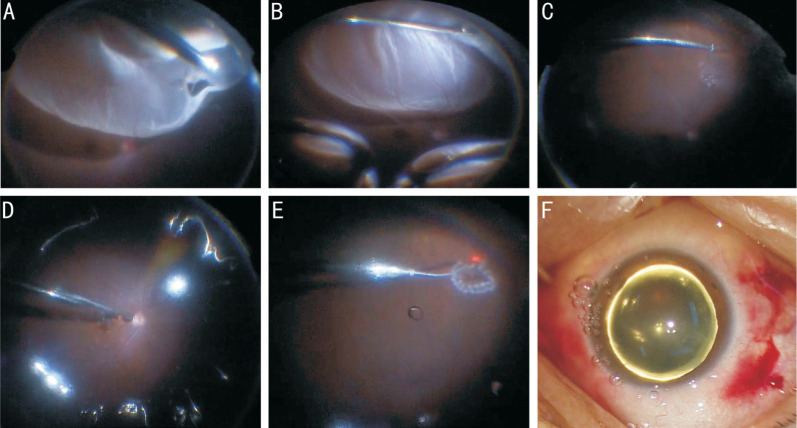
Figure 1. RRD caused by superior retinal break locating at peripheral fundus.
A: Vitreous around the break was cut off as completely as possible; B: Retinal break were placed at lower position by eye position guided FAX, and subretinal fluid was aspirated at the same time; C, D: Subretinal fluid around the breaks was fully drained off, and a small amount of subretinal fluid remains; E: Sufficient laser spots were accomplished to seal the retinal break; F: Filtered air was left in the vitreous cavity as tamponade agent.
Postoperative Follow-up
The patients were required to maintain a prone position for at least 24h post operation. On the first postoperative day, the patients were received the same ophthalmologic examinations as before surgery. Follow-up examinations for patients were scheduled at 1, 2wk, 1, 3, and 6mo postoperative. Additional follow-up examinations may be performed at any time as required. During the follow-ups, all phakic eyes with deterioration or new-onset of lens opacification should receive cataract extraction and IOL implantation once there was visual significance was performed. Complete absorption of subretinal fluid and firm adhesion of neuroretina to retinal pigment epithelium was defined as anatomic success.
Statistical Analysis
The Snellen visual acuity values were converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Counting fingers was assigned 2.3, hand movements was assigned 2.6, and light perception was assigned 2.9[16]. All of the continuous data were expressed as mean±standard deviation (SD). SPSS 23.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Mann-Whitney test and t-test were used as needed. Statistical significance was defined if the P value <0.05.
RESULTS
Thirty-seven patients (37 eyes) who qualified for inclusion criteria were included in this study from all of the 42 RRD patients who underwent this modified surgery during the study period. Most of the retinal breaks (n=32, 86.5%) located in equator or peripheral fundus and few others (n=5, 13.5%) located in posterior fundus. The breaks were located at inferior quadrants (between 3:00 and 9:00 clock hours) in 5 cases (13.5%), and both superior and inferior breaks were found in 3 cases (8.1%). There were 8 (21.6%) eyes had inferior breaks in all. The mean number breaks were 2.1±1.1, and the total area was 2.1±1.0 papilla diameters (PDs). The mean disease duration was 15.0±8.7d. The mean range of RRD was 5.6±1.8 clock hours. The macula was detached preoperatively in 25 eyes (67.6%) and 5 eyes (13.5%) had a whole RRD. The 9 eyes (24.3%) were pseudophakic, and 2 eyes (5.4%) had vitreous hemorrhage. Table 1 shows the baseline characteristics of all of the enrolled patients.
Table 1. Baseline characteristics of included patients.
| Variables | Data |
| Gender, male (%) | 20 (54.1) |
| Age (y) | 50.9±13.9 |
| Disease duration (d) | 15.0±8.7 |
| Follow-up duration (mo) | 11.9±4.7 |
| Preoperative BCVA, logMAR (Snellen) | 1.13±1.07 (21/299) |
| IOP (mean±SD, mm Hg) | 11.1±3.1 |
| Axial length (mean±SD, mm) | 25.58±2.68 |
| Range of RD (clock hours) | 5.6±1.8 |
| Macula on, n (%) | 25 (67.6%) |
| Retinal breaks | |
| Number (mean±SD) | 2.1±1.1 |
| Total area (PD) | 2.1±1.0 |
| Location (posterior/peripheral) | 5/32 |
| Vitreous hemorrhage, n (%) | 2 (5.4) |
| Lens status (pseudophakic), n (%) | 9 (24.3) |
| Presence of inferior retinal breaks, n (%) | 8 (21.6) |
BCVA: Best corrected visual acuity; logMAR: Logarithm of minimal angle of resolution; IOP: Intraocular pressure; RD: Retinal detachment; PD: Papilla diameter.
Cataract extraction and IOL implantation were performed concurrently in 8 (21.6%) eyes. Two eyes (5.4%) had phacoemulsification and IOL implantation 3mo after initial PPV, and one of them underwent macular epiretinal membrane peeling surgery further (2.7%). No intraoperative complication was observed. The mean follow-up time was 11.9±4.7mo. The overall BCVA (logMAR) improved from 1.13±1.07 preoperatively to 0.39±0.27 postoperatively (P<0.001) at the last follow up time. The primary anatomic success rate was 100% (37/37) in enrolled patients without any additional measures. A total of 35 patients (94.6%) had their BCVA improved, and there was no patient with declined BCVA. Four (10.8%) eyes with high IOP ranging from 24 to 38 mm Hg occurred in 3d post operation. All the elevated IOP became normal by siding anti-glaucoma medication within 3d. Table 2 shows the intraoperative and postoperative parameters of all enrolled patients.
Table 2. Intraoperative and postoperative parameters collected from all enrolled patients.
| Variables | Data |
| Phacovitrectomy (yes/no) | 8/29 |
| Laser photocoagulation number (shots) | 536.3±231.1 |
| Primary anatomic success, n (%) | 37 (100) |
| Subretinal fluid residue, n (%) | 20 (54.1) |
| Final anatomic success, n (%) | 37 (100) |
| Postoperative cataract surgery, n | |
| Phakic after primary RD repair | 20 |
| Underwent secondary cataract surgery | 2 |
| Phakic until the final follow-up | 18 |
| Elevated IOP, n | 4 |
| Postoperative ERM peeling surgery, n | 1 |
| Postoperative BCVA, logMAR (Snellen) | |
| Day 1 | 2.55±0.33 (20/5999) |
| Week 1 | 1.21±0.41 (20/343) |
| Week 2 | 1.11±0.39 (20/278) |
| Month 1 | 0.98±0.29 (20/199) |
| Month 3 | 0.77±0.30 (20/113) |
| Month 6 | 0.51±0.25 (20/72) |
| Final follow-up | 0.39±0.27 (20/51) |
RD: Retinal detachment; IOP: Intraocular pressure; ERM: Macular epiretinal membrane; logMAR: Logarithm of minimal angle of resolution; BCVA: Best corrected visual acuity.
DISCUSSION
Current views believe that vitreoretinal traction should be released completely by exhaustive vitrectomy, so that the retinal breaks can be well sealed and the vitreous cavity can be fully filled in the treatment of RRD by PPV[6]–[7],[17]. In order to drain off the subretinal fluid as completely as possible, heavy liquid was often used to make subretinal fluid moving toward to peripheral fundus around retinal breaks. The usage of heavy liquid not only increased surgical procedure and prolonged the operation time, but also increased expenses and surgical complications, such as heavy liquid remaining in vitreous cavity or entering into the space under retina or macula, which often need to be removed by an additional surgery[10]. At present, silicone oil and long-acting gas are most commonly used as intraocular tamponade agent in PPV[18]. However, silicone oil needs to be removed after firmly retinal reattachment by a secondary surgery. Long-acting gases such as octafluoropropane (C3F8) or sulfur hexafluoride (SF6) was often selected as tamponade for the repair of RRD without severe proliferative vitreoretinopathy[5],[12]. The absorption time of long-acting gas usually last from 2wk to 2mo, and during this period patients may always suffer defection of visual field and restriction of altitude travel. Furthermore, the usage of gas often leads to aggravation of cataract, and it also has a higher risk of causing high IOP which often need to be treated with a combination of anti-glaucoma medication. In addition, patients are often demanded to keep a prolonged time of prone position postoperatively. Due to the short half-life and nonexpanding property of air, which may reduce the operative complications of silicone oil and long-acting gas, there has been a trend of broadening its application in vitreoretinal surgeries for RRD repair, with reduction of heavy liquid usage[12].
Previous studies have shown the safety and efficacy of PPV with air tamponade for RRD patients caused by superior or inferior retinal breaks. Zhang et al[19] shown that PPV with only partial air tamponade can get a high anatomic success rate in RRD caused by superior retinal breaks. Martinez-Castillo et al[6] revealed that PPV combined with air tamponade was effective in the management of pseudo phakic RRD with inferior breaks even without facedown position. These studies emphasized the necessity of thorough drainage of subretinal fluid for adequate tamponade of vitreous cavity. Therefore, the usage of heavy liquid was inevitable in most patients in order to drain off the subretinal fluid completely. Unlike those previous studies, we believe that adequate drainage of subretinal fluid was necessary for patients who underwent silicone oil tamponade, due to that if the vitreous cavity was not filled adequately by silicone oil, a higher oil-liquid interface will be generated, and then various fibroblasts may gather in the surface of retina under the oil-liquid interface which may cause the occurrence of PVR and even recurrence of retinal detachment[20]. It is well known that if vitreoretinal traction was fully released by PPV, little subretinal fluid would be quickly absorbed by the retinal pigment epithelium layer within a few hours on the effect of the surface tension of intraocular tamponade. From then on, once the chorioretinal adhesion produced by laser photocoagulation develops completely, intraocular fluid could not easily enter into the subretinal space again through the breaks[6],[21]–[22].
In our research, residual subretinal fluid was completely absorbed within a few hours post operation promoted by the surface tension of intraocular air in all patients. So, we emphasized that all patients must keep a prone position for at least 24h and did not insist on complete drainage of subretinal fluid. We have achieved a satisfactory result by operative use of this modified surgical skills, with quick recovery of postoperative visual acuity, less postoperative discomfort, shorten operation time and reduction of medical costs. Among our enrolled patients, there were 20 patients with varying amounts of subretinal fluid residue at the end of surgery, and 7 of them had relatively large amount. However, according to our observation residual subretinal fluid had been completely absorbed in all patients within 24h after the surgery. It took at least 3d for the intraocular air tamponade decreasing postoperative about 1/2 of the vitreous cavity. The time for complete absorption of air was between 7 and 10d. A total of 37 eyes of 37 RRD patients were reattachment by using eye position guided in-situ FAX technique combined with air tamponade in our study. The success rate of primary retinal reattachment in enrolled patients was 100%. The high anatomic success rate indicates that PPV with laser retinopexy and air tamponade may be enough to seal retinal breaks for the establishment of strong chorioretinal adhesion.
We took the following measures to strive for the satisfactory surgical outcomes. Strict case selection and careful operative examination of the fundus were the first important step. The exclusion of the following cases was important for successful endolaser retinopexy, such as patients with PVR C1 or more, RRD caused by macular hole, giant retina tears, dialysis of ora serrata and previous vitreoretinal surgery. Complete PVD and vitreous shaving was an important guarantee to interrupt the occurrence of PVR for successful operation. Vitreous around the breaks must be shaved completely to avoid reopening of the break caused by contraction of residual vitreous gel which may lead recurrent retinal detachment. To make sure subretinal fluid around the breaks can be fully drained off by placing the retinal breaks at lower position during FAX, sufficient laser spots can be made to seal the break firmly. Above measures would lead to a successful endolaser photocoagulation retinopexy. We sealed the retinal breaks by photocoagulation under air rather than under heavy liquid, so that the neuroretina around breaks were precisely aligned with retinal pigment epithelium. With this step, the possible leakage of break which may cause by dislocation of break edge could be avoided after the heavy liquid was removed. And keeping a prone position strictly for at least 24h after surgery was a critical guarantee for fast absorption of residual subretinal fluid and sufficient adhesion of laser spot around the breaks.
Another advantage of air is the less incidence rate of postoperative cataract. Previous studies have reported that there was an acceleration of nuclear sclerosis after lens-sparing vitrectomy[23]–[25]. It was also well known that intraocular gas could lead a temporary feathery posterior subcapsular opacity after vitreoretinal surgery. Holekamp et al[26] found that exposure of the crystalline lens to abnormally high oxygen levels could lead to nuclear formation and sclerosis in vitrectomized eyes. In our study, only two patients with concurrent cataracts required cataract surgery, and both of them were combined with the posterior capsule opacity. One of the eyes with single cataract had combined phacoemulsification and IOL implantation, and the other eye was also combination of epiretinal macular membrane who received additional macular membrane peeling surgery. After surgery, both of them get better visual acuity with well retinal reattachment. No intraoperative complication was observed. There were 4 cases suffered secondary glaucoma with high intraocular pressure ranging from 24 to 38 mm Hg post operation, however, all the elevated IOP became normal within 3d by using anti-glaucoma medication.
There were several limitations in our study. First, the study was retrospective and there was no control group. Second, all the enrolled patients were from a single center, and all surgeries were performed by one single surgeon, so it may have selection bias and prejudice its generalization. Finally, the study was imperfect because it was carried out with a small sample size and short duration of follow-ups.
In conclusion, PPV with air tamponade by eye position guided in-situ FAX can achieve a high anatomic success rate in the management of RRD patients without severe PVR, with short time of prone position and a less incidence rate of postoperative complications. Further prospective randomized multicenter studies with a larger sample size and long period of follow-ups should be undertaken.
Acknowledgments
Foundation: Supported by the Natural Science Basic Research Project of Shaanxi Province (No.2019JM-578).
Conflicts of Interest: Cheng YH, None; Wang H, None; Li B, None; Ji M, None; Shi Q, None; Qi Y, None; Hu YG, None; Xie AM, None; Pei C, None.
REFERENCES
- 1.Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100(2):289–292. doi: 10.1001/archopht.1982.01030030291012. [DOI] [PubMed] [Google Scholar]
- 2.Lv Z, Li Y, Wu YZ, Qu Y. Surgical complications of primary rhegmatogenous retinal detachment: a meta-analysis. PLoS One. 2015;10(3):e0116493. doi: 10.1371/journal.pone.0116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duvdevan N, Mimouni M, Feigin E, Barak Y. 25-gauge pars plana vitrectomy and SF6 gas for the repair of primary inferior rhegmatogenous retinal detachment. Retina. 2016;36(6):1064–1069. doi: 10.1097/IAE.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 4.Antoun J, Azar G, Jabbour E, Kourie HR, Slim E, Schakal A, Jalkh A. Vitreoretinal surgery with silicone oil tamponade in primary uncomplicated rhegmatogenous retinal detachment: clinical outcomes and complications. Retina. 2016;36(10):1906–1912. doi: 10.1097/IAE.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 5.Mandelcorn ED, Mandelcorn MS, Manusow JS. Update on pneumatic retinopexy. Curr Opin Ophthalmol. 2015;26(3):194–199. doi: 10.1097/ICU.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Castillo VJ, García-Arumí J, Boixadera A. Pars Plana vitrectomy alone for the management of pseudophakic rhegmatogenous retinal detachment with only inferior breaks. Ophthalmology. 2016;123(7):1563–1569. doi: 10.1016/j.ophtha.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Zhou CD, Qiu QH, Zheng Z. Air versus gas tamponade in rhegmatogenous retinal detachment with inferior breaks after 23-gauge pars plana vitrectomy: a prospective, randomized comparative interventional study. Retina. 2015;35(5):886–891. doi: 10.1097/IAE.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Liang QH, Lin K, Hu ZX, Chen TY, Wu RH, Moonasar N. Air tamponade and without heavy liquid usage in pars plana vitrectomy for rhegmatogenous retinal detachment repair. Int J Ophthalmol. 2018;11(11):1779–1783. doi: 10.18240/ijo.2018.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan HS, Oberstein SY, Mura M, Bijl HM. Air versus gas tamponade in retinal detachment surgery. Br J Ophthalmol. 2013;97(1):80–82. doi: 10.1136/bjophthalmol-2012-302140. [DOI] [PubMed] [Google Scholar]
- 10.Mackiewicz J, Szczesny P, Zagórski Z. Histopathologic studies of the rabbit retina after use of perfluorodecalin. Klin Oczna. 1998;100(1):5–8. [PubMed] [Google Scholar]
- 11.Park SW, Kwon HJ, Kim HY, Byon IS, Lee JE, Oum BS. Comparison of scleral buckling and vitrectomy using wide angle viewing system for rhegmatogenous retinal detachment in patients older than 35 years. BMC Ophthalmol. 2015;15:121. doi: 10.1186/s12886-015-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaziri K, Schwartz SG, Kishor KS, Flynn HW., Jr Tamponade in the surgical management of retinal detachment. Clin Ophthalmol. 2016;10:471–476. doi: 10.2147/OPTH.S98529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology. 1988;95(10):1385–1388. doi: 10.1016/s0161-6420(88)33000-9. [DOI] [PubMed] [Google Scholar]
- 14.Folk JC, Sneed SR, Folberg R, Coonan P, Pulido JS. Early retinal adhesion from laser photocoagulation. Ophthalmology. 1989;96(10):1523–1525. doi: 10.1016/s0161-6420(89)32696-0. [DOI] [PubMed] [Google Scholar]
- 15.Kita M, Negi A, Kawano S, Honda Y. Photothermal, cryogenic, and diathermic effects of retinal adhesive force in vivo. Retina. 1991;11(4):441–444. doi: 10.1097/00006982-199111040-00015. [DOI] [PubMed] [Google Scholar]
- 16.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Hadden B. Pioneering surgery for retinal detachment in Australasia: a review. Clin Exp Ophthalmol. 2016;44(7):618–623. doi: 10.1111/ceo.12776. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Cheung N, Jia L, Zhang H, Liu N. Surgical outcomes of 25-gauge pars plana vitrectomy using air as an internal tamponade for primary rhegmatogenous retinal detachment. Retina. 2020 doi: 10.1097/IAE.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZT, Peng MJ, Wei YT, Jiang XT, Zhang SC. Pars plana vitrectomy with partial tamponade of filtered air in rhegmatogenous retinal detachment caused by superior retinal breaks. BMC Ophthalmol. 2017;17(1):64. doi: 10.1186/s12886-017-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ, Kwon HJ, Park KY, Park SW, Byon IS, Lee JE. Prognostic factors of anatomical success in microincisional vitrectomy for rhegmatogenous retinal detachment. J Korean Ophthalmol Soc. 2016;57(10):1613. [Google Scholar]
- 21.Martínez-Castillo V, Verdugo A, Boixadera A, García-Arumí J, Corcóstegui B. Management of inferior breaks in pseudophakic rhegmatogenous retinal detachment with pars plana vitrectomy and air. Arch Ophthalmol. 2005;123(8):1078–1081. doi: 10.1001/archopht.123.8.1078. [DOI] [PubMed] [Google Scholar]
- 22.Mateo-Montoya A, de Smet MD. Air as tamponade for retinal detachments. Eur J Ophthalmol. 2014;24(2):242–246. doi: 10.5301/ejo.5000373. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto Y, Okamoto F, Hiraoka T, Oshika T. Refractive changes after lens-sparing vitrectomy for rhegmatogenous retinal detachment. Am J Ophthalmol. 2014;158(3):544–549.e1. doi: 10.1016/j.ajo.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Rahman R, Briffa BV, Gupta A, Chinn DJ. Factors contributing to posterior capsule opacification following 23-gauge transconjunctival phacovitrectomy. Ophthalmic Surg Lasers Imaging. 2011;42(3):229–233. doi: 10.3928/15428877-20110420-02. [DOI] [PubMed] [Google Scholar]
- 25.Banker AS, Freeman WR, Kim JW, Munguia D, Azen SP. Vision-threatening complications of surgery for full-thickness macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology. 1997;104(9):1442–1452. discussion 1452–1453. doi: 10.1016/s0161-6420(97)30118-3. [DOI] [PubMed] [Google Scholar]
- 26.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]