Abstract
AIM
To evaluate detailed anterior segment parameters of patients with idiopathic inflammatory myopathies (IIM), including polymyositis (PM), and dermatomyositis (DM), and to clarify the associations between these data and clinical variables of IIM.
METHODS
Totally 57 PM, 41 DM patients and 62 controls were enrolled in this cross-sectional, observational, case-control study. All study participants underwent Pentacam evaluation. Laboratory investigations consisted of different antibody assays, while extramuscular clinical assessments included Raynaud's phenomenon, dysphagia, interstitial lung disease, arthritis/arthralgia, and weight loss. Objective signs and subjective symptoms of dry eye disease (DED) were also evaluated.
RESULTS
All pachymetric parameters [center, apex, thinnest and maximal keratometry (Kmax)] and corneal volume (CV) of both sides of PM patients proved to be significantly lower. Some pachymetric data were also noticed as significantly decreased compared to those of controls. Several significant differences were traced between anterior segment values and extramuscular manifestations of myositis, largely in case of arthritis/arthralgia and weight loss, whereas associations between anterior segment parameters and antibodies were weak. Objective clinical tests of DED were also significantly decreased in IIM patients.
CONCLUSION
The results suggest that all IIM patients have thinner corneas compared with those of controls, and decreased corneal parameters are significantly associated with the occurrence of some extramuscular manifestations. In addition, IIM patients tend to develop objective signs of DED.
Keywords: dry eye, extramuscular manifestations, dermatomyositis, polymyositis, Scheimpflug imaging
INTRODUCTION
The group of idiopathic inflammatory myopathies (IIM), also accepted as autoimmune myositis, represents diverse, systemic rheumatic diseases identified by chronic weakness and inflammatory cell infiltrates in the skeletal muscles[1]. IIM incorporate various subgroups, including polymyositis (PM), dermatomyositis (DM), inclusion body myositis (IBM), and amyopathic dermatomyositis (ADM) in adults, and juvenile dermatomyositis (JDM) in children, while three other subgroups of small sample sizes have also been classified: juvenile PM, immune-mediated necrotizing myopathy (IMNM), and hypomyopathic DM[2]. IIM affects multiple organs and systems, including principally the muscles, but also often the skin, joints, and lungs, and even the eyes. Ocular involvement of IIM encompasses a broad spectrum of complications of adjacent structures and diverse anterior and posterior segment damage to the eyes. There is only scant evidence published in the literature regarding ocular manifestations of IIM and most of them come from limited case studies and reports. Previously corneal changes in IIM have been extenuated despite the fact that cornea is especially predisposed to intra-and postoperative complications during corneal laser interventions, cataract surgeries and keratoplasties in autoimmune diseases[3]–[5]. Recently ophthalmic investigations in connection with immune-related and connective tissue disorders have become more prominent since ocular impairments can be traced as part of various systemic immune-based disorders.
As far as is known, there has been no other, but our own previous study that dealt with corneal involvement of PM and DM patients[6], but no investigation of detailed anterior segment values of the eye has been presented so far in patients with IIM, consequently our primary aim was to obtain comprehensive data about anterior segment of IIM patients with Scheimpflug imaging. Our secondary aim was to elucidate relationship between these quantities and clinical parameters of IIM.
SUBJECTS AND METHODS
Ethical Approval
The study protocol was approved by the local ethics committee (HBR/052/00204/2014; DE OEC RKEB/IKEB 4071-2013) and was in full compliance with Good Clinical Practices (GCP) guidelines of the European Union, and the Declaration of Helsinki. Informed consent was collected from all patients and controls.
Characteristics of Patients
Consecutive PM and DM patients diagnosed according to the corresponding international criteria[7] were selected into the study. All patients visited the Department of Immunology of the University of Debrecen, and none of them suffered from secondary Sjögren's syndrome, because all of them has normal ranged anti-Sjögren's syndrome related antigen A, and B (SS-A and SS-B) test values. Ocular examinations, containing noncycloplegic best spectacle-corrected visual acuity (BCVA) in Snellen's chart, a regular slit lamp investigation in order to exclude any irregularities of the ocular surface, tear film or adnexa, and intraocular pressure determination, were also performed for all contributors.
Features of Controls
Controls were recruited out of the participants who entered the Department of Ophthalmology of the University of Debrecen for routine ophthalmological check-ups, and had minor refractive errors (±1.0 diopter), without any known systemic diseases such as diabetes mellitus, Sjögren's syndrome, rheumatic diseases, connective tissue diseases (Sharp syndrome, mixed connective-tissue disease, undifferentiated connective tissue disease, Ehlers-Danlos syndrome, epidermolysis bullosa, etc.) at the same time. The same ophthalmological examinations were carried out as in patients.
Exclusion Criteria
Eye drop administration within two weeks preceding examinations was an exclusion criterion for all participants, and no eye drops were allowed during the study period. Regarding further exclusion criteria irregular eyelid position and closure, contact lens wearing, history of ocular surgery, presence of ocular infection, inflammation of sclera, episcleral layer or uvea, trauma of the eye, corneal haze, peripheral or central corneal melting.
Ophthalmological and Clinical Tests
To examine dry eye disease (DED) both tear break-up time (tBUT) and Schirmer-I tests (STI) were used to analyze tear film stability and tear production. For the former a strip of fluorescein (Haag-Streit, Koenitz, Switzerland) wetted with a drop of unpreserved, sterile saline solution 0.9% was used. This strip was touched to the lower part of the bulbar conjunctiva causing minimal stimulus and followed by some blinking the tear film was inspected under cobalt blue light of a slit lamp. The tBUT value was determined as the interval between the final entire blink and the first appearance of a dry spot and was given in seconds. Three evaluations were done in each eye of every subject and the average of the three quantities was considered as mean value.
To investigate tear production un-anesthetized Schirmer test, i.e. the STI was used. In the course of this procedure standardized strips of filter paper (Alcon Laboratory, Fort Worth, Texas, USA) were placed at the lower-lid margin at the junction of the middle and temporal third of each eye, and during the maneuver special care was taken not to touch the cornea. Participants were requested to avoid eye and eyelid movements for 5min followed by a careful eye closure, next the strip was extracted. STI was determined by measuring the wet part of the strip (mm/5min). Regarding the subjective symptoms of DED the Ocular Surface Disease Index (OSDI) questionnaire (provided by Allergan, Inc., Irvine, CA, USA)[8] was adopted since this is highly recommended by the U.S. Food and Drug Administration (FDA) for use in clinical studies[9]. As for IOP quantification Huvitz HNT-1P (Huvitz, Dongan-gu, Republic of Korea) noncontact tonometer was applied, and three evaluations per eye were performed and the average of them was determined as the mean value. Furthermore, ages at the time of diagnosis, disease duration of patients were also taken into consideration. Regarding laboratory quantities anti-histidyl-tRNA synthetase antibody (Jo-1), anti-nuclear factor (ANF), beta-2-glycoprotein (β2GPI), both anti-Sjögren's-syndrome-related antigen A (SS-A), and B (SS-B), metaphase chromosomes, anti-cyclic citrullinated peptide (CCP), and out of specific antigens anti-transcription intermediary factor 1-gamma (TIF1-γ), anti-Mi-2, anti-Pm/Scl (polymyositis/scleroderma), and extractable nuclear antigen (ENA) were measured. As for clinical parameters the assessment of Raynaud's phenomenon, dysphagia, mechanic's hands, interstitial lung disease (ILD), arthritis/arthralgia, muscle pain, and weight loss were examined.
Pentacam Examinations
Regarding anterior segment evaluations a Pentacam (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany) was applied and undilated pupils were used in order to avoid fluorescein dying.
Participants were requested to fix their chin on the chinrest while their foreheads touched the headband.
Corneal power of both flat (K1) and steep (K2) axes, also mean corneal power (Km), and pachymetric measurements [pachy center, pachy apex, thinnest location and maximal keratometry (Kmax)] were noted. In addition, corneal volume (CV), anterior chamber volume (ACV), and anterior chamber depth (ACD), anterior chamber angle width (ACA) were determined besides pupil size. The averages of three consecutive quantifications were accepted for the study.
To exclude the issue of diurnal alterations all classifications were completed between 9:00 a.m. and 11:00 a.m.
All measurements were performed on successive days, and constant temperature, light, and humidity conditions were applied to eliminate any ocular surface stress.
Statistical Analyses
Continuous variables were characterized as the mean with standard deviation (SD), while categorical variables were defined as frequencies and their percentages. The distribution of the data was monitored by applying the Kolmogorov-Smirnov test, while Mann-Whitney U test was utilized assuming nonparametric distribution. To compare categorical data, Chi-square test and Fisher's exact test were applied. Correlation coefficients between variables were counted using Spearman's method. P values less than 0.05 were considered significant. For the statistical analysis IBM SPSS 25 statistical software (GraphPad Software Inc., San Diego, CA, USA) was employed.
RESULTS
Characteristics of Patients and Controls
A total of 57 patients with PM (45 females and 12 males), mean age 57.70±9.19y and 41 patients with DM (26 female and 15 male, mean age 45.39±11.23y, were recruited into our study. The mean disease duration was 12.74±9.91 and 10.20±7.37y, respectively. None of the patients received immunomodulatory therapy, including cyclosporine A (CsA). During examinations, the basic disease was inactive, only a maintenance treatment with methylprednisolone (4 mg/die) was used.
Totally 62 gender-, age-, and refraction-matched controls (50 females and 12 males, mean age 58.32±9.41y were also enrolled. All participants were of Caucasian origin. No statistically significant differences in age and gender between the two groups were detected. Data are presented in Table 1.
Table 1. Demographic data and clinical parameters of patients with PM and DM and control persons.
| Parameters | PM (n=57) | DM (n=41) | Control (n=62) | P (PM vs control) | P (DM vs control) | P (PM vs DM) |
| Age (y) | 57.70±9.19 | 45.39±11.23 | 58.32±9.41 | 0.697 | 0.303 | 0.308 |
| Age at diagnosis (y) | 45.39±11.23 | 44.66±12.72 | - | - | - | 0.894 |
| Disease duration (y) | 12.74±9.91 | 10.20±7.37 | - | - | - | 0.270 |
| OSDI | 23.00±21.54 | 15.62±11.83 | 14.94±9.60 | 0.237 | 0.827 | 0.346 |
| tBUT (s) | ||||||
| R | 6.81±3.51 | 7.84±5.18 | 9.90±2.81 | <0.001 | 0.002 | 0.514 |
| L | 7.08±3.75 | 8.14±5.25 | 9.90±2.75 | <0.001 | 0.013 | 0.427 |
| Schirmer-I (mm/5min) | ||||||
| R | 7.46±3.85 | 8.68±5.38 | 10.52±2.49 | <0.001 | 0.013 | 0.406 |
| L | 6.85±3.58 | 8.35±5.45 | 10.61±2.49 | <0.001 | 0.024 | 0.292 |
| BCVA | ||||||
| R | 0.770±0.28 | 0.819±0.18 | 0.790±0.17 | 0.613 | 0.380 | 0.983 |
| L | 0.776±0.25 | 0.782±0.22 | 0.781±0.23 | 0.931 | 0.840 | 0.878 |
| IOP (mm Hg) | ||||||
| R | 15.81±2.56 | 15.26±3.73 | 15.84±2.85 | 0.544 | 0.432 | 0.221 |
| L | 16.14±2.77 | 15.97±5.36 | 15.23±2.32 | 0.208 | 0.946 | 0.199 |
PM: Polymyositis; DM: Dermatomyositis; OSDI: Ocular Surface Disease Index; tBUT: Tear breakup time; BCVA: Best spectacle-corrected visual acuity; IOP: Intraocular pressure.
mean±SD
Outcome of Ocular Examinations
Regarding subjective symptoms of DED the average OSDI score was 23.00±21.54 for PM patients, 15.62±11.83 for DM patients, and 14.94±9.60 for control subjects. There was no significant difference in OSDI score values between different groups. Considering objective signs of DED both Schirmer-I and tBUT test results were found to be significantly lower as compared to those of healthy controls. No significant difference was found in BCVA and IOP values between IIM patients and healthy volunteers. Data are also represented in Table 1.
Pentacam Data
Regarding Pentacam data, significantly lower parameters were detected between each pachymetric measurement (center, apex, thinnest, Kmax) and CV values for PM patients in both sides and some pachymetric data were also traced to be significantly reduced compared to those of the control group, moreover, except for Kmax differences of pachymetric quantities between PM and DM patients were significant (Table 2).
Table 2. Anterior segment data of PM and DM patients and controls.
| Parameters | PM (n=57) | DM (n=41) | Control (n=62) | P (PM vs control) | P (DM vs control) | P (PM vs DM) |
| Pachy center (µm) | ||||||
| R | 548.18±28.50 | 558.37±27.54 | 570.94±28.37 | 0.001 | 0.039 | 0.038 |
| L | 550.02±29.91 | 561.24±26.46 | 571.58±26.49 | 0.002 | 0.116 | 0.034 |
| Pachy apex (µm) | ||||||
| R | 550.65±29.30 | 560.44±27.27 | 556.58±97.29 | 0.005 | 0.094 | 0.049 |
| L | 552.00±30.59 | 563.10±26.48 | 572.71±27.20 | 0.003 | 0.174 | 0.028 |
| Pachy thinnest (µm) | ||||||
| R | 543.00±29.08 | 552.17±26.45 | 566.35±28.15 | 0.001 | 0.019 | 0.046 |
| L | 544.63±30.62 | 554.63±27.00 | 567.68±26.93 | 0.001 | 0.065 | 0.044 |
| Kmax (D) | ||||||
| R | 45.39±2.14 | 44.55±1.78 | 43.96±1.16 | <0.001 | 0.195 | 0.051 |
| L | 45.28±1.70 | 44.68±1.71 | 44.04±1.14 | <0.001 | 0.107 | 0.105 |
| CV (mm3) | ||||||
| R | 59.70±2.83 | 60.31±3.15 | 61.68±3.57 | 0.006 | 0.084 | 0.187 |
| L | 60.12±3.39 | 61.12±4.38 | 61.78±3.13 | 0.022 | 0.172 | 0.306 |
| ACV (mm3) | ||||||
| R | 132.26±32.10 | 137.22±32.87 | 135.42±28.39 | 0.429 | 0.842 | 0.336 |
| L | 134.54±34.22 | 140.15±33.57 | 140.03±28.38 | 0.378 | 0.982 | 0.347 |
| ACD (mm) | ||||||
| R | 4.05±5.63 | 3.46±0.73 | 3.31±0.49 | 0.339 | 0.716 | 0.306 |
| L | 3.41±0.73 | 3.41±0.66 | 3.37±0.52 | 0.510 | 0.941 | 0.562 |
| KPD | ||||||
| R | 1.23±0.13 | 1.17±0.19 | 1.22±0.15 | 0.678 | 0.279 | 0.078 |
| L | 1.26±0.19 | 1.18±0.15 | 1.22±0.15 | 0.386 | 0.443 | 0.082 |
| ACA (o) | ||||||
| R | 32.74±7.39 | 34.85±7.73 | 31.84±6.61 | 0.892 | 0.088 | 0.107 |
| L | 33.91±8.12 | 34.34±7.88 | 32.95±6.12 | 0.865 | 0.528 | 0.687 |
PM: Polymyositis; DM: Dermatomyositis; Kmax: Maximal keratometry; CV: Corneal volume; ACV: Anterior chamber volume; ACD: Anterior chamber depth; KPD: Keratometric power deviation; ACA: Anterior chamber angle width.
mean±SD
Outcome of Clinical and Laboratory Examinations
Raynaud's phenomenon occurred in 28 (49%) of 57 PM and 15 (37%) of 41 DM patients, mechanic's hand was found in 7 (12%) and 6 (15%), dysphagia was present in 7 (12%) and 19 (46%), ILD developed in 18 (32%) and 14 (34%), arthritis/arthralgia in 35 (61%) and 22 (54%), muscle pain in 32 (56%) and 25 (61%), and weight loss in 3 (5%) and 5 (12%) patients, respectively. Regarding occurrence of extramuscular manifestations only dysphagia was represented significantly in DM patients, as shown in Table 3. As for antibodies, ANF occurred in 21 PM patients and 22 DM patients, β2GPI in 7 and 4, anti-CCP in 3 and also 3, anti-Jo-1 in 10 and 3, ENA in 6 and 1, metaphase chromosomes in 9 and 10, and anti-Pm/Scl in 2 and 3 patients, respectively.
Table 3. Extramuscular findings in PM and DM patients.
| Parameters | PM (n=57) | DM (n=41) | P |
| Raynaud + | 28 (49%) | 15 (37%) | 0.217 |
| Raynaud - | 29 (51%) | 26 (63%) | |
| Mechanic's hands + | 7 (12%) | 6 (15%) | 0.735 |
| Mechanic's hands - | 50 (88%) | 35 (85%) | |
| Dysphagia + | 7 (12%) | 19 (46%) | <0.001 |
| Dysphagia - | 50 (88%) | 22 (54%) | |
| ILD + | 18 (32%) | 14 (34%) | 0.789 |
| ILD - | 39 (68%) | 27 (66%) | |
| Arthritis/arthralgia + | 35 (61%) | 22 (54%) | 0.443 |
| Arthritis/arthralgia - | 22 (39%) | 19 (46%) | |
| Muscle pain + | 32 (56%) | 25 (61%) | 0.632 |
| Muscle pain - | 25 (44%) | 16 (39%) | |
| Weight loss + | 3 (5%) | 5 (12%) | 0.273 |
| Weight loss - | 54 (95%) | 36 (88%) |
ILD: Interstitial lung disease.
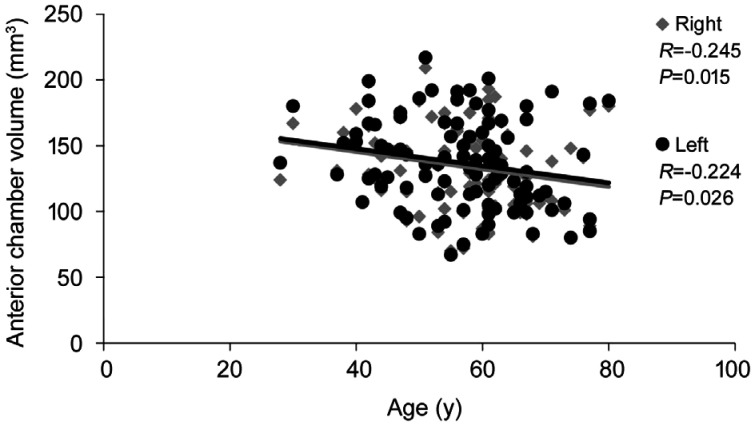
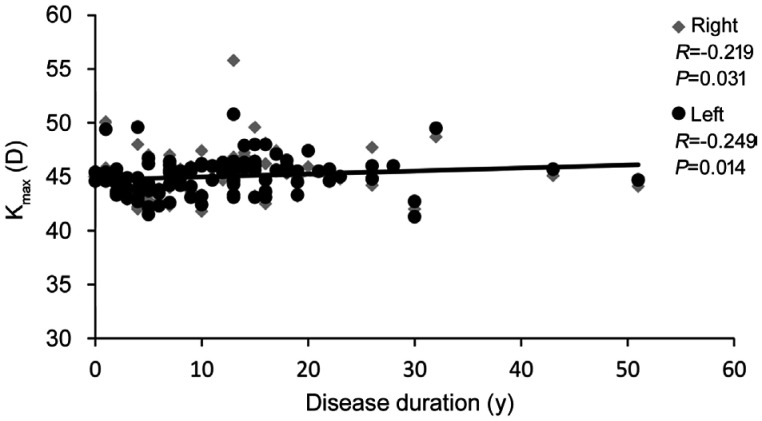
Table 4 shows the results of the associations between anterior segment data and antibodies when patients were investigated together. In general, the associations were weak, with only sporadic significant differences traced. However, numerous significant differences were traced between anterior segment values and extramuscular manifestations of myositis, largely in case of arthritis/arthralgia and weight loss; data are given in Table 5. Concerning correlation between Pentacam data and age or disease duration of IIM patients a significant negative correlation was only found between ACV and age of patients, while a significant positive correlation was observed between Kmax and disease duration. Data are presented in Figures 1 and 2.
Table 4. Association between anterior segment data and different antibodies of IIM patients.
| Parameters | Jo-1 |
P | ANF |
P | β2GPI |
P | Metaphase chromosomes |
P | ||||
| + | - | + | - | + | - | + | - | |||||
| Pachy center (µm) | ||||||||||||
| R | 551.15±31.35 | 552.64±28.13 | 0.586 | 548.95±24.63 | 551.16±31.00 | 0.278 | 543.09±31.89 | 553.62±27.92 | 0.143 | 557.21±25.17 | 551.29±29.16 | 0.434 |
| L | 548.38±29.48 | 555.68±28.88 | 0.274 | 552.84±27.27 | 556.18±30.31 | 0.704 | 554.73±37.98 | 554.71±27.85 | 0.955 | 564.26±24.36 | 552.42±29.59 | 0.059 |
| Pachy apex (µm) | ||||||||||||
| R | 553.54±32.20 | 554.93±28.38 | 0.568 | 551.67±24.94 | 557.15±31.41 | 0.363 | 545.64±31.55 | 555.90±28.35 | 0.161 | 561.63±26.16 | 553.09±29.24 | 0.235 |
| L | 549.77±29.79 | 557.69±29.29 | 0.245 | 555.19±28.08 | 557.78±30.47 | 0.781 | 556.36±39.25 | 556.68±28.12 | 0.955 | 567.63±24.02 | 554.00±29.99 | 0.031 |
| Pachy thinnest (µm) | ||||||||||||
| R | 544.85±29.51 | 547.14±28.21 | 0.476 | 542.86±24.08 | 549.95±30.97 | 0.200 | 536.82±29.75 | 548.10±27.97 | 0.151 | 551.26±25.61 | 545.77±28.89 | 0.556 |
| L | 544.08±29.55 | 549.54±29.53 | 0.362 | 545.77±27.83 | 551.20±30.68 | 0.574 | 545.36±38.82 | 549.25±28.29 | 0.698 | 557.16±24.02 | 546.81±30.35 | 0.112 |
| Kmax (D) | ||||||||||||
| R | 44.59±1.44 | 45.11±2.10 | 0.414 | 45.20±1.79 | 44.91±2.20 | 0.323 | 46.20±1.96 | 44.89±2.00 | 0.024 | 45.15±1.77 | 45.01±2.09 | 0.480 |
| L | 44.56±1.31 | 45.10±1.77 | 0.271 | 45.28±1.69 | 44.84±1.73 | 0.140 | 46.14±1.85 | 44.89±1.66 | 0.012 | 45.05±1.87 | 45.03±1.69 | 0.847 |
| CV (mm3) | ||||||||||||
| R | 59.82±2.82 | 59.98±3.00 | 0.706 | 59.99±2.85 | 59.98±3.08 | 0.957 | 60.89±3.76 | 59.84±2.86 | 0.461 | 61.04±2.48 | 59.70±3.03 | 0.102 |
| L | 59.42±2.87 | 60.71±3.96 | 0.320 | 60.90±4.35 | 60.25±3.41 | 0.847 | 64.24±7.01 | 60.07±3.00 | 0.055 | 62.68±5.04 | 60.02±3.34 | 0.023 |
| ACV (mm3) | ||||||||||||
| R | 131.23±24.13 | 134.81±33.52 | 0.769 | 139.91±34.97 | 129.98±29.73 | 0.176 | 126.36±41.92 | 135.34±31.09 | 0.256 | 133.32±30.65 | 134.58±32.92 | 0.911 |
| L | 131.62±21.52 | 137.69±35.43 | 0.537 | 145.05±35.07 | 130.51±31.82 | 0.043 | 137.55±41.18 | 136.80±33.14 | 0.955 | 140.37±29.39 | 136.05±35.01 | 0.559 |
| ACD (mm) | ||||||||||||
| R | 3.25±0.37 | 3.89±4.63 | 0.745 | 3.53±0.78 | 4.01±5.74 | 0.175 | 3.25±0.65 | 3.87±4.57 | 0.298 | 3.38±0.67 | 3.90±4.80 | 0.939 |
| L | 3.25±0.37 | 3.44±0.73 | 0.597 | 3.62±0.82 | 3.25±0.54 | 0.036 | 3.59±0.96 | 3.39±0.66 | 0.800 | 3.49±0.72 | 3.39±0.69 | 0.489 |
Kmax: Maximal keratometry; CV: Corneal volume; ACV: Anterior chamber volume; ACD: Anterior chamber depth; Jo-1: Histidyl-tRNA synthetase antibody; ANF: Anti-nuclear factor; β2GPI: Anti-β2 glycoprotein.
mean±SD
Table 5. Association between anterior segment parameters and extramuscular features of IIM patients.
| Parameters | Dysphagia |
P | Arthritis/arthralgia |
P | Weight loss |
P | |||
| + | - | + | - | + | - | ||||
| Pachy center (µm) | |||||||||
| R | 554.77±27.76 | 551.60±28.78 | 0.398 | 547.96±25.66 | 558.66±31.10 | 0.044 | 527.75±29.36 | 554.63±27.43 | 0.024 |
| L | 559.23±29.24 | 557.08±28.83 | 0.250 | 549.79±26.42 | 561.56±31.12 | 0.036 | 534.25±27.87 | 556.53±28.45 | 0.049 |
| Pachy apex (µm) | |||||||||
| R | 557.77±28.40 | 553.65±28.98 | 0.398 | 550.46±25.65 | 560.71±31.93 | 0.043 | 531.12±30.97 | 556.84±27.75 | 0.037 |
| L | 561.81±29.16 | 554.78±29.31 | 0.175 | 551.70±26.48 | 563.51±31.94 | 0.039 | 536.38±28.45 | 558.44±28.87 | 0.052 |
| Pachy thinnest (µm) | |||||||||
| R | 549.15±28.17 | 546.00±28.41 | 0.452 | 542.74±25.50 | 552.54±31.08 | 0.041 | 523.25±30.41 | 548.93±27.24 | 0.044 |
| L | 552.00±30.13 | 547.67±29.31 | 0.346 | 543.81±27.57 | 555.78±31.94 | 0.042 | 525.25±28.21 | 550.91±28.77 | 0.032 |
| Kmax (D) | |||||||||
| R | 44.28±1.68 | 45.31±2.08 | 0.025 | 45.15±1.78 | 44.89±2.35 | 0.136 | 44.40±1.83 | 45.09±2.04 | 0.512 |
| L | 44.47±1.72 | 45.23±1.68 | 0.035 | 45.13±1.67 | 44.89±1.80 | 0.240 | 44.45±1.55 | 45.08±1.76 | 0.428 |
| CV (mm3) | |||||||||
| R | 59.50±3.29 | 60.12±2.85 | 0.590 | 59.51±2.55 | 60.58±3.40 | 0.031 | 56.92±3.97 | 60.23±2.73 | 0.018 |
| L | 60.12±3.82 | 60.69±3.87 | 0.626 | 59.98±3.02 | 61.31±4.70 | 0.048 | 57.76±3.99 | 60.78±3.76 | 0.065 |
| ACV (mm3) | |||||||||
| R | 138.19±32.51 | 132.94±32.40 | 0.338 | 130.07±33.55 | 140.27±30.00 | 0.120 | 129.12±34.70 | 134.80±32.30 | 0.746 |
| L | 138.96±34.61 | 136.14±33.84 | 0.590 | 132.21±35.85 | 143.39±30.19 | 0.095 | 127.88±34.73 | 137.69±33.90 | 0.521 |
| ACD (mm) | |||||||||
| R | 3.46±0.76 | 3.92±5.02 | 0.593 | 4.03±5.64 | 3.48±0.65 | 0.067 | 3.23±0.76 | 3.85±4.50 | 0.321 |
| L | 3.42±0.74 | 3.41±0.68 | 0.878 | 3.35±0.72 | 3.50±0.66 | 0.095 | 3.15±0.72 | 3.43±0.69 | 0.157 |
Kmax: Maximal keratometry; CV: Corneal volume; ACV: Anterior chamber volume; ACD: Anterior chamber depth.
mean±SD
Figure 1. Correlation between anterior chamber volume and age of IIM patients.
IIM: Idiopathic inflammatory myopathies.
Figure 2. Correlation between maximum corneal power and disease duration of IIM patients.
IIM: Idiopathic inflammatory myopathies; Kmax: Maximum corneal power.
DISCUSSION
IIM is an uncommon acquired, systemic disease, the etiopathogenesis of which is unknown as yet, but genes within the major histocompatibility complex (MHC), the most significant genetic risk factor and the interferon pathway have been identified in its formation[10]. Incidence rates range from 1.16 to 19/1000 000 per year with prevalence rates between 2.4 and 33.8/100 000 inhabitants[11]. There is only an insufficient number of papers regarding ocular involvement in IIM, principally manifestations of the adnexa or posterior segment changes. Heliotrope rash, a characteristic skin finding in DM has been the most widely discussed finding[12]–[15]. In addition, diverse unlimited inflammations in the anterior segment, such as conjunctivitis, iritis, and episcleritis have also been described[16]–[17]. In a study carried out among patients with rheumatic diseases DED was also delineated in PM patients[18]. Regarding posterior segment of the eye, DM related retinopathy was first described by Bruce[19] in 1938, followed by several case reports later on[20]–[24], while Purtscher-like retinopathies in DM were depicted not long ago[25]–[26]. In a survey asymptomatic retinopathy was noticed in 14% of patients with PM and DM[27]. Lowder et al[28] described a 49-year-old Japanese DM patient presenting initially with unilateral retinal pigment epithelial (RPE) detachment, and later with the fellow eye's serous retinal detachment (SRD) at the macular area as well. Furthermore, his visual acuities and SRD fluctuated with exacerbations and remissions of the underlying disease. Ocular myositis associated with DM is a rare phenomenon in the course of the disease and two publications point out that it might be missed by clinicians[29]–[30]. Based on the abovementioned papers, the eye is expected to be involved in IIM. However, anterior segment lesions mainly corneal impairments have been unfairly put into the shade even if corneal structure and function considerably determined by highly ordered hierarchical organization of collagen fibrils can vary in immune-related and connective tissue diseases[31]. To date, there is still no study evaluating anterior segment findings in detail in IIM patients and their associations with clinical parameters have not been investigated either. The assessment of anterior segment values including corneal quantities is of vital importance for various diagnostic and therapeutic approaches and accordingly different modern imaging technologies have been developed. In the present study accurate anterior segment values of PM and DM patients were quantified, and additionally, objective signs and subjective symptoms of DED were also evaluated, since DED and corneal involvement are usual ocular findings in patients with rheumatic diseases[32]–[34].
Based on our investigations PM patients rather than DM tend to develop thinner and lower volume corneas, since in terms of Pentacam data the former has more impaired corneal values. Concerning DED both PM and DM patients were found to have significantly reduced measured objective clinical test values, however, there was no significant difference for OSDI scores between patients and controls. Investigating IIM patients together only few significant differences were noticed between distinct anterior segment quantities and antibodies. However, numerous significant differences were discovered between anterior segment parameters and extramuscular manifestations of IIM. A close connection was revealed between pachymetric data for both sides and arthritis/arthralgia and weight loss, moreover, sporadic associations were revealed in case of other arguments. Assaying organ specific autoantibodies in autoimmune disorders can have both diagnostic and prognostic relevance. To the best of our knowledge the first publication of circulating immune complexes in IIM was by Whitaker and Engel[35]. Nowadays, laboratory testing usually shows elevated myositis-specific antibody (MSA) levels, with a diagnostic specificity exceeding 90%, and as such generally accepted to be characteristic antibodies found in PM and DM, but they can also be present in other autoimmune diseases. Anti-aminoacyl-tRNA (e.g., anti-Jo-1, anti-PL-7), anti-Mi-2 (chromodomain-helicase-DNA binding protein 4), anti-signal recognition particle (SRP), anti-transcriptional intermediary factor 1 gamma (TIF-1 γ), and anti-nuclear matrix protein 2 (NXP-2) antibodies are taken into account in this group. On the contrary anti-Sjögren's-syndrome-related antigen A (anti-Ro60/SS-A), anti-polymyositis/scleroderma (anti-PM/Scl), anti-Ku, and anti-U1 ribonucleoprotein (U1RNP) antibodies are reckoned among the myositis-associated antibodies (MAAs) that are not disease-specific, but related to other connective tissue disorders, mostly myositis overlap syndromes[36]. These antibodies have not been used as IIM classification criteria yet, thus maintaining a clinical-serologic gap[37].
Ocular findings in IIM are expected to be the manifestations of vascular anomalies, immune-related dysregulations, and some genetic predispositions[38]. Since cornea is an avascular tissue the role of vascular irregularity can be excluded. As cornea has an integrated ultrastructural morphology containing Langerhans cells and thymic stromal lymphopoietin in the peripheral epithelium, DCs in the peripheral anterior stroma and a wide variety of immunoregulatory factors in the epithelium as a consequence auto-reactive T-cell mediated immune processes should definitely bear on the formation of corneal lesions in IIM[39]. Various corneal findings in IIM may be due to diverse cellular and molecular mechanisms and a weak association between anterior segment parameters and autoantibodies thus clinical parameters could be attributed to the heterogeneity of the underlying disease. Since different mechanisms contribute to the etiopathogenesis of PM and DM, diverse corneal and DED manifestations may be explained by these molecular and cellular events.
Albeit most connective tissue diseases are immune-related and broadly identified as relative contraindications to laser refractive interventions, cataract surgeries or keratoplasties as they may be accompanied by an increased rate of intra- and postoperative complications and wound healing problems, these procedures can be accomplished when there is no active ocular interference with the inherent disease or its treatment i.e. the underlying disease is well-controlled or inactive.
The group of IIM is considered a multisystem disease that affects several organs, and by default different specialists are involved. Our data could be essential for both ophthalmologists, who deal with ophthalmic conditions of autoimmune patients, and for rheumatologists and immunologists, who treat IIM patients most likely suffering from ocular lesions as well, and accordingly, ocular examinations should be included in their routine. IIM is regarded as a rare condition, however, varied incidence and/or prevalence rates may put a considerable emphasis on this kind of autoimmune disease.
Acknowledgments
The authors would like to thank Katalin Hodosi for her technical contribution to this manuscript.
Conflicts of Interest: Griger Z, None; Danko K, None; Nemeth G, None; Hassan Z, None; Aszalos Z, None; Szabo K, None; Bodoki L, None; Gesztelyi R, None; Zsuga J, None; Szodoray P, None; Kemeny-Beke A, None.
REFERENCES
- 1.Cavazzana I, Fredi M, Selmi C, Tincani A, Franceschini F. The clinical and histological spectrum of idiopathic inflammatory myopathies. Clin Rev Allergy Immunol. 2017;52(1):88–98. doi: 10.1007/s12016-015-8517-4. [DOI] [PubMed] [Google Scholar]
- 2.Oldroyd A, Chinoy H. Recent developments in classification criteria and diagnosis guidelines for idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2018;30(6):606–613. doi: 10.1097/BOR.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez VL, Azar DT, Foster CS. Sterile corneal melting and necrotizing scleritis after cataract surgery in patients with rheumatoid arthritis and collagen vascular disease. Semin Ophthalmol. 2002;17(3-4):124–130. doi: 10.1076/soph.17.3.124.14786. [DOI] [PubMed] [Google Scholar]
- 4.Shan SJ, Wu EI, Akpek EK. Sterile corneal melt after descemet stripping endothelial keratoplasty in patients with previously undiagnosed Sjogren syndrome. Arch Ophthalmol. 2009;127(2):219–220. doi: 10.1001/archophthalmol.2008.601. [DOI] [PubMed] [Google Scholar]
- 5.Schallhorn JM, Schallhorn SC, Hettinger KA, Venter JA, Pelouskova M, Teenan D, Hannan SJ. Outcomes and complications of excimer laser surgery in patients with collagen vascular and other immune-mediated inflammatory diseases. J Cataract Refract Surg. 2016;42(12):1742–1752. doi: 10.1016/j.jcrs.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Griger Z, Danko K, Bodoki L, Aszalos Z, Nemeth G, Ziad H, Gesztelyi R, Zsuga J, Szodoray P, Kemeny-Beke A. Corneal involvement of patients with polymyositis and dermatomyositis. Ocul Immunol Inflamm. 2020;28(1):58–66. doi: 10.1080/09273948.2018.1547407. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, Asbell PA, Pflugfelder SC. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinal-Fernandez I, Mammen AL. Dermatomyositis etiopathogenesis: a rebel soldier in the muscle. Curr Opin Rheumatol. 2018;30(6):623–629. doi: 10.1097/BOR.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer A, Meyer N, Schaeffer M, Gottenberg JE, Geny B, Sibilia J. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford) 2015;54(1):50–63. doi: 10.1093/rheumatology/keu289. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Huang L, Yang Y, Chen H, Liu YJ, Liu K, Liu MD, Xiao YZ, Luo H, Zuo XX, Li YS, Xiao XZ, Zhang HL. Calcinosis and malignancy are rare in Chinese adult patients with myositis and nuclear matrix protein 2 antibodies identified by an unlabeled immunoprecipitation assay. Clin Rheumatol. 2018;37(10):2731–2739. doi: 10.1007/s10067-018-4216-x. [DOI] [PubMed] [Google Scholar]
- 13.Fang YF, Wu YJ, Kuo CF, Luo SF, Yu KH. Malignancy in dermatomyositis and polymyositis: analysis of 192 patients. Clin Rheumatol. 2016;35(8):1977–1984. doi: 10.1007/s10067-016-3296-8. [DOI] [PubMed] [Google Scholar]
- 14.Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features-a comprehensive review. Clin Rev Allergy Immunol. 2016;51(3):293–302. doi: 10.1007/s12016-015-8496-5. [DOI] [PubMed] [Google Scholar]
- 15.Iaccarino L, Ghirardello A, Bettio S, Zen M, Gatto M, Punzi L, Doria A. The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun. 2014;48-49:122–127. doi: 10.1016/j.jaut.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheim H. Lehrbuch der Nervenkrankhetien fur Arzte und Studierende. 7th ed. Berlin: Karger; 1923. p. 840. [Google Scholar]
- 17.Cohen BH, Sedwick LA, Burde RM. Retinopathy of dermatomyositis. J Clin Neuroophthalmol. 1985;5(3):177–179. [PubMed] [Google Scholar]
- 18.Ausayakhun S, Louthrenoo W, Aupapong S. Ocular diseases in patients with rheumatic diseases. J Med Assoc Thail. 2002;85(8):855–862. [PubMed] [Google Scholar]
- 19.Bruce GM. Retinitis in dermatomyositis. Trans Am Ophthalmol Soc. 1938;36:282–297. [PMC free article] [PubMed] [Google Scholar]
- 20.Lisman JV. Dermatomyositis with retinopathy. Arch Ophthalmol. 1947;37(2):155. doi: 10.1001/archopht.1947.00890220164005. [DOI] [PubMed] [Google Scholar]
- 21.de Vries S. Retinopathy in dermatomyositis. AMA Arch Ophthalmol. 1951;46(4):432–435. doi: 10.1001/archopht.1951.01700020443009. [DOI] [PubMed] [Google Scholar]
- 22.Munro S. Fundus appearances in a case of acute dermatomyositis. Br J Ophthalmol. 1959;43:548–558. doi: 10.1136/bjo.43.9.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klien BA. Comments on the cotton-wool lesion of the retina. Am J Ophthalmol. 1965;59:17–23. doi: 10.1016/0002-9394(65)95012-9. [DOI] [PubMed] [Google Scholar]
- 24.Liebman S, Cook C. Dermatomyositis with retinopathy. Arch Ophthalmol. 1965;74(5):704–705. [Google Scholar]
- 25.Barreiro-González A, Cerdà-Ibáñez M, Barranco González H, Harto Castaño MÁ, Calvo Penadés I, Senent Peris ML, Azorín Villena I. Purtscher-like retinopathy associated with dermatomyositis and hemophagocytic lymphohistiocytosis. Arch Soc Esp Oftalmol. 2018;93(4):202–205. doi: 10.1016/j.oftal.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Yan Y, Shen X. Purtscher-like retinopathy associated with dermatomyositis. BMC Ophthalmol. 2013;13:36. doi: 10.1186/1471-2415-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migliaresi S, Ambrosone L, Tirri G. Eye involvement in dermatomyositis/polymyositis. J Rheumatol. 1996;23(11):2006–2007. [PubMed] [Google Scholar]
- 28.Lowder CY, Gutman FA, Zegarra H, Zakov ZN, Lowder JN, Clough JD. Macular and paramacular detachment of the neurosensory retina associated with systemic diseases. Trans Am Ophthalmol Soc. 1981;79:347–370. [PMC free article] [PubMed] [Google Scholar]
- 29.Kokotis P, Theodossiadis P, Bouros C, Sfikakis PP. Bilateral ocular myositis as a late complication of dermatomyositis. J Rheumatol. 2005;32(2):379–381. [PubMed] [Google Scholar]
- 30.Liu DT, Chan AY. Bilateral ocular myositis as a late complication of dermatomyositis. J Rheumatol. 2006;33(3):637. [PubMed] [Google Scholar]
- 31.Zhou HY, Cao Y, Wu J, Zhang WS. Role of corneal collagen fibrils in corneal disorders and related pathological conditions. Int J Ophthalmol. 2017;10(5):803–811. doi: 10.18240/ijo.2017.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Shereef RR, Mohamed AS, Hamdy L. Ocular manifestation of systemic lupus erythematosus. Rheumatol Int. 2013;33(6):1637–1642. doi: 10.1007/s00296-011-2296-x. [DOI] [PubMed] [Google Scholar]
- 33.Rentka A, Nagy A, Harsfalvi J, Szucs G, Szekanecz Z, Gesztelyi R, Szodoray P, Kemeny-Beke A. Association between objective signs and subjective symptoms of dry eye disease in patients with systemic sclerosis. Rheumatol Int. 2017;37(11):1835–1845. doi: 10.1007/s00296-017-3794-2. [DOI] [PubMed] [Google Scholar]
- 34.Murray PI, Rauz S. The eye and inflammatory rheumatic diseases: The eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract Res Clin Rheumatol. 2016;30(5):802–825. doi: 10.1016/j.berh.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Whitaker JN, Engel WK. Vascular deposits of immunoglobulin and complement in inflammatory myopathy. Trans Am Neurol Assoc. 1971;96:24–28. [PubMed] [Google Scholar]
- 36.Palterer B, Vitiello G, Carraresi A, Giudizi MG, Cammelli D, Parronchi P. Bench to bedside review of myositis autoantibodies. Clin Mol Allergy. 2018;16:5. doi: 10.1186/s12948-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selva-O'Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17(9):816–828. doi: 10.1016/S1474-4422(18)30254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garlepp MJ, Laing B, Zilko PJ, Ollier W, Mastaglia FL. HLA associations with inclusion body myositis. Clin Exp Immunol. 1994;98(1):40–45. doi: 10.1111/j.1365-2249.1994.tb06604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Hanlon TP, Miller FW. T cell-mediated immune mechanisms in myositis. Curr Opin Rheumatol. 1995;7(6):503–509. doi: 10.1097/00002281-199511000-00007. [DOI] [PubMed] [Google Scholar]