Abstract
Inflammatory bowel disease (IBD) includes inflammation of the gastrointestinal (GI) tract and is characterized by periods of acute inflammation and remission. Therapeutic management of IBD is still problematic, because of incomplete understanding its pathogenesis. This study focuses on the effect of 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis on changes in enteric neuronal subpopulations in adult zebrafish. These changes are suggested to be related to the altered neuro-immune interactions and GI motility, and in IBD pathogenesis. New insights into neuroplasticity will be instrumental in finding appropriate therapeutic treatments. TNBS was intraluminally administered in the distal intestine (DI) of anesthetized adult zebrafish. A histological time course of the intestinal inflammatory response was created to establish optimal TNBS concentration and acute inflammation phase. Using double immunolabelling on whole mounts, the effect of inflammation on neuronal populations was analyzed. Based on intestinal wall thickening, epithelial fold disruption, reduced goblet cell number, and eosinophil infiltration, our analysis indicated that the optimal TNBS concentration (320 mM in 25% ethanol) inducing non-lethal inflammation reached a peak at 6 h post-induction. The inflammatory response returned to baseline values at 3 days post-induction. At the acute inflammation phase, no influence on the distribution or proportion of nitrergic neurons was observed, while only the proportion of cholinergic neurons was significantly reduced in the DI. The proportion of serotonergic neurons was significantly increased in the entire intestine during inflammation. This study describes a method of TNBS-induced colitis in the adult zebrafish. Given that the acute inflammation phase is accompanied by neuroplasticity comparable to changes observed in IBD patients, and the unique and versatile characteristics of the zebrafish, allows this model to be used alongside IBD animal models to unravel IBD pathology and to test new IBD therapies.
Key words: TNBS-induced colitis, zebrafish, enteric nervous system, serotonergic neurons, cholinergic neurons, nitrergic neurons
Introduction
Inflammatory bowel disease (IBD) is characterized by chronic immune activation and inflammation within the gastrointestinal (GI) tract periods of acute inflammation and remission. Crohn’s disease (CD) and ulcerative colitis (UC) are the two main phenotypes of IBD. Considerable knowledge concerning IBD is revealed in recent years. However, the pathogenesis of IBD remains incompletely understood. What we do know so far is that a complex interplay among genetic, environmental, microbial and immune factors is involved. Patients experience cramping, fecal urgency, diarrhea, abdominal pain and weight loss. IBD or GI inflammation is well known to affect GI functions, including motility, secretion, absorption, the intestinal barrier function, and sensation.1-4
GI functions are controlled by the autonomic nervous system, including the enteric nervous system (ENS). The ENS consists of all neurons present within the wall of the GI tract, and is divided into two major ganglionated plexuses, the myenteric and submucous plexus. The ENS contains a diverse set of functional neuron types, including sensory neurons, interneurons and motor neurons, synaptically linked to each other in microcircuits.5 Results of previous studies in animal models and patients have revealed that inflammation causes structural and architectural changes, including neurodegeneration, in enteric ganglia and plexuses, and modifies the expression of neurotransmitters, neuropeptides and receptors in the ENS.1
Although animal models of colitis do not exactly mimic IBD in man, much data concerning the pathophysiology of IBD has been acquired by using 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis in animals.1,6 In recent years, zebrafish have been increasingly used for unraveling of human disease patterns - including that of IBD.7,8 Zebrafish have some unique features as a model organism, e.g., large numbers of offspring, lower maintenance costs, a completely sequenced genome, the availability of numerous genetic tools, an easy to control environment and the presence of innate and adaptive immune responses.9,10 Chemical-based experiments of colitis induction similar to those in mice have been performed in larval and adult zebrafish, using immersion, oral microgavage, or intrarectal administration.11 Subsequent immunological response, genetic factors and environmental factors seem to be comparable to those observed in mammalian animal models for enterocolitis.12-14 These studies indicate that the induction of enterocolitis is dependent on a specific microbial environment and that each laboratory has to optimize its own TNBS model.7 The present study focuses on the effect of TNBSinduced colitis on the ENS in adult zebrafish. Changes within distinct enteric neuronal populations are believed to be associated with the altered neuro-immune interactions and GI motility and even with the pathogenesis of IBD. A better insight into the neuroplasticity will be instrumental in finding appropriate therapeutic treatments. 1-3,15-17
The zebrafish intestine has only a non-ganglionated myenteric plexus of dispersed enteric neurons and no submucosal plexus.18 Zebrafish lack a stomach, but the proximal intestine (PI) serves as a site for storage and mixing of food.18,19 Next is the mid-intestine (MI) which contains mucin-producing goblet cells and enterocytes, and primarily functions in nutrient absorption. The distal portion of the MI contains some specialized enterocytes assumed to play a role in intestinal immunity.18 The architecture of the short distal intestine (DI) suggests a role analogous to the colon in mammals. 19,20 Previous studies have shown the presence of different neuronal subpopulations in the adult zebrafish intestine, which may be grouped into three populations: serotonergic, cholinergic and nitrergic neurons.21,22
The aims of the present study were to optimize the adult TNBS-induced colitis model for our laboratory conditions, by determining the optimal dose and acute phase of inflammation, and to investigate putative neuronal changes of the major enteric populations present in the zebrafish intestine.
Materials and Methods
Fish stock
Adult zebrafish were bred and kept at the Laboratory of Human Anatomy and Embryology, University of Antwerp (LA1100125). Zebrafish maintenance and breeding were performed as previously described.23,24 Briefly, the animals were kept in colonies in aerated fresh water at 26-28.5°C in a 14/10 h light/dark cycle. The water was continuously filtered, both chemically and biologically. Fish were daily fed with commercial aquarium fish food. All experimental procedures were approved by the ethical committee of the University of Antwerp (ECD2012-67). A pain-scoring system was set up to measure the discomfort of the fish. These scores ranged from P0 (= no pain, normal behavior and eating habits), P1 (= limited discomfort, normal to slightly agitated swimming behavior and sometimes a change in color to orange), P2 (= moderate discomfort, agitated or slightly lethargic swimming behavior, decreased appetite, possible change of color to orange and possible increased movement of the gills) to P3 (= severe discomfort, very agitated swimming behavior or very lethargic, refusing food, pale color, very fast gill movement).
Induction of enterocolitis in adult zebrafish
Female zebrafish (6-8 months) were anesthetized in 0.016% MS222 (ethyl-m-aminobenzoate methanesulphonate; Western Chemical Inc., Ferndale, WA, USA) for 3 min, weighed, measured and placed on a wet tissue. The belly was gently squeezed to enhance the visualization of the rectal opening. The fish were rectally injected with the appropriate volume (10 μL/g body weight), using a flexible Eppendorf GELoader tip (Eppendorf AG, Hamburg, Germany) with an outer diameter of 0.3 mm. To assess the optimal dose, the fish were injected with different concentrations of TNBS (Sigma-Aldrich Corp., St. Louis, MO, USA): 5 mM, 40 mM, 80 mM, 160 mM, 320 mM and 640 mM dissolved in 25% ethanol, and subsequently placed separately in well aerated stand-alone tanks for individual observation and survival analysis. Control groups included a vehicle group, receiving only 25% ethanol (10 μL/g body weight), and a handling group (control), receiving only the operational procedure. Each experimental group in this part of the study always comprised 15 adult zebrafish, while both control groups contained each 5 animals.
After 6 h, zebrafish were euthanized in 0.04% MS222, decapitated and the GI tract was dissected in block. The intestines were fixed overnight in 4% paraformaldehyde (PFA) in 10 mM phosphate- buffered saline (PBS; pH 7.4) and, after rinsing in PBS, further processed for embedding in paraffin. Serial sections of 7 μm were made of the posterior part of the MI and the DI on a HM340E microtome (Microm, Walldorf, Germany). It was previously demonstrated that the posterior part of the GI tract of the adult zebrafish is affected in chemical-based induction of enterocolitis.13 The sections were alternately processed for a standard hematoxylin and eosin (H&E) staining to visualize the general morphology of the intestinal wall, or for an Alcian blue/periodic acid Schiff (PAS) reaction to detect mucus-producing goblet cells and infiltrating eosinophilic granulocytes. Each analyzed section was photographed using an Olympus DP70 camera (Olympus Belgium NV, Aartselaar, Belgium). Four different parameters were examined: thickness of the intestinal wall, epithelial fold architecture, number of goblet cells and infiltration of eosinophilic granulocytes. 13 The pictures were analyzed using Image J (NIH, Bethesda, MD). The thickness of the intestinal wall was measured in 12 sections per fish. The means (in μm) were calculated and subdivided depending on their thickening, ranging from 0 up to 2 (0 = no thickening, ≤75 μm; 1 = moderate thickening, 75-95 μm; 2 = severe thickening, ≥95 μm). The architectural appearance of the epithelial folds was rated in 12 sections per animal according to three categories: 0 (normal appearance), 1 (slight disruption) or 2 (severe disruption: increased interfold distance, and disruption of epithelial integrity). The number of goblet cells were counted on 16 sections per fish (1 frame/section: surface area of a frame: 0.13 mm2). The means were calculated and a score was assigned: 0 = ≥15 goblet cells; 1 = between 10 and 15 goblet cells; and 2 = ≤10 goblet cells. On the same frames, the infiltration of eosinophilic granulocytes was counted according to the following scoring system: 0= hardly any eosinophilic granulocytes present; 1 = moderate infiltration; 2 = severe infiltration, characterized by clusters of filtrating cells. The sum of the four scores ranging from 0 to 8, yielded an overall enterocolitis score, with 0 to 2 indicating no inflammation, 3 to 5 moderate inflammation, and 6 to 8 severe inflammation. To determine the acute phase of inflammation, zebrafish were injected the optimal dose of TNBS and euthanized after 3, 6 and 14 h, 1, 3 and 7 days. The intestines were dissected out and processed for paraffin sectioning and staining as described above. Each experimental and control group in this part, contained 5 adult zebrafish.
Neuronal populations
To identify possible shifts in the neuronal population, wholemount intestinal preparations were made at the acute phase of inflammation. Therefore, the intestine was opened along the mesenteric border and pinned out in a Sylgard-lined Petri dish filled with 4% PFA in PBS for 2 h at room temperature. After fixation, the whole mounts were thoroughly washed in PBS, and further processed for optimal immunostaining as previously described.21,22 Briefly, after rinsing in PBS, the whole mounts were incubated for 1 h in PBS containing 0.05% thimerosal. Next, after washing in PBS, they were immersed for 30 minutes in PBS containing 0.1% NaBH3CN and rinsed in PBS. Double immunostainings on the whole mount preparations were carried out at room temperature as previously described.21,22 The primary and secondary antibodies used, are listed in Table 1.
After embedding of the whole mounts, images were taken with a fluorescence microscope equipped with an Olympus DP70 camera. To study the effect of enterocolitis on the enteric neuronal populations, we used for each population 5 animals, while both control groups contained 3 animals. The intestine was arbitrarily separated into three parts, as previously described: PI, MI, and DI. In every part, 10 randomly chosen regions were photographed and the cells were manually counted as previously described.21,22 For quantification, the immunoreactive cell bodies were counted per visual frame (8.2x104 μm2). All statistical tests were performed using IBM SPSS Statistics 20 (IBM, Armonk, NY, USA). The results were analyzed by ANOVA, followed by a Tukey post-hoc test, when appropriate. Significance was assumed at P<0.05. Confocal images were taken with a PerkinElmer UltraVIEW Vox spinning disk confocal system using 488 and 561 nm lasers.
Results
Dose response
Different TNBS concentrations were tested to find the optimal dose for further experiments. Fish receiving doses from 5 up to 160 mM did not die (cut-off 6 h) and did not show severe discomfort. However, using higher TNBS concentrations, we observed that 73% of the fish receiving 320 mM TNBS survived, while only 55% and 25% of the fish, receiving 480 mM and 640 mM TNBS, respectively, survived (Figure 1). The fish injected with 480 mM and 640 mM TNBS suffered from severe pain and further experiments using these doses were excluded from the study. Furthermore, no high pain scores nor death were observed in both control groups.
Scoring the different histological sections of the samples collected 6 h post-induction (hpi) revealed a moderate inflammation for the concentrations 40, 80 and 160 mM, while the injection of 320 mM TNBS resulted in a severe inflammation (Figure 2). Inflammation caused a thickening of the intestinal wall, disruption of the epithelial architecture, a decrease in the number of goblet cells and an increased infiltration of eosinophilic granulocytes (Figure 3). The zebrafish of the control and vehicle groups did not show any sign of inflammation.
Interestingly, after euthanasia during removal of the intestine, we observed that the gallbladder was bright green in the control and vehicle group, while it changed to dark green in the zebrafish injected with 40 mM and 80 mM TNBS, and brownish in the zebrafish injected with 160 mM and 320 mM TNBS.
Table 1.
List of primary and secondary antibodies, and their respective dilutions used for immunohistochemistry.
| Primary antibodies Antigen | Host | Dilution | Source |
|---|---|---|---|
| 5-HT; serotonin | Rabbit | 1/10 000 | Immunostar Inc, Hudson, WI, USA (20080) |
| ChAT; choline acetyltransferase | Goat | 1/100 | Chemicon, Temecula, CA, USA (AB114P) |
| Hu; Neuron-specific protein | Mouse | 1/500 | Molecular probes Inc, OR, USA, (A-21271) |
| nNOS; neuronal nitric oxide synthase | Rabbit | 1/100 | Santa Cruz Biotechnology, Santa Cruz, CA, USA (sc-1025) |
| Secondary antibodies Complex | Dilution | Company | |
| Donkey anti-rabbit Cy3 | 1/800 | Jackson Immunoresearch Laboratories, West Grove, OR, USA | |
| Donkey anti-mouse DL488 | 1/200 | Jackson Immunoresearch Laboratories | |
| Donkey anti-goat Cy3 | 1/800 | Jackson Immunoresearch Laboratories |
Time response
To assess the acute phase of inflammation, the same experiment was repeated. Fish were rectally injected with a dose of 320 mM TNBS dissolved in 25% ethanol, and were sacrificed at 3, 6 and 14 hpi, 1, 3 and 7 days post-induction (dpi). Histological scoring revealed a very quick response to TNBS (Figure 4). By 3 hpi, severe signs of inflammation were observed (score = 6). The peak of inflammation was reached at 6 hpi (score = 8). At 14 hpi, the inflammatory score declined (score = 6). At 1 dpi, only a moderate inflammation was observed (score = 4) and at 3 dpi the inflammatory score decreased to baseline with a complete recovery at 7 dpi.
Neuronal subpopulations
The effect of TNBS-induced colitis on the three enteric neuronal populations was assessed at the peak of inflammation (dose of 320 mM at 6 hpi). In both inflamed and in non-inflamed intestines, the neuronal density increased from the oral to the aboral side of the intestine, but no significant change in neuronal density was observed when both intestinal tissues were compared (Figure 6A). The proportional distribution of nitrergic neurons did not show any changes either between inflamed and non-inflamed conditions (Figure 5 A,B, and 6B) (control: PI 23.6±1.2%; MI 23.3±2.7%; DI 22.3±1.6%; overall 23.07±1.8% // TNBS: PI 24.1±2.1%; MI 22.91±2.1%; DI 24.05±2.8%; overall 23.7±1.9%), while the cholinergic and serotonergic neuronal proportions showed some changes. The proportion of serotonergic neurons in both controls and TNBS-treated fish decreased along the length of the intestine (Figures 5 C,D and 6C). Upon comparison, TNBStreated fish showed increased proportions of serotonergic neurons in each intestinal part (control: PI 23.6±2.5%; MI 18.4±3.0%; DI 11.5±1.9%; overall 17.8±3.1% // TNBS: PI 28.6±2.6%; MI 23.2±1.4%; DI 18.4±1.6%; overall 23.4±1.3%). In the control samples, the overall proportion of cholinergic neurons was 39.3±3.1% and did not show any differences between the different intestinal regions. The TNBS-treated animals however, displayed a decreased proportion of cholinergic neurons in the DI (control: 38.48±4.6% // TNBS: 21.95±2.5%) (Figure 5 E,F and 6D).
Figure 1.
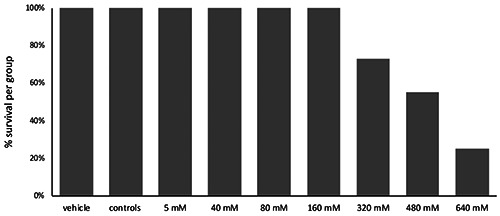
Survival rates per group of fish for the different doses of TNBS administered. Fish receiving doses up to 160 mM survived. Administration of higher doses (from 320 mM to 640 mM) proved inversely proportional to the survival rate.
Figure 2.
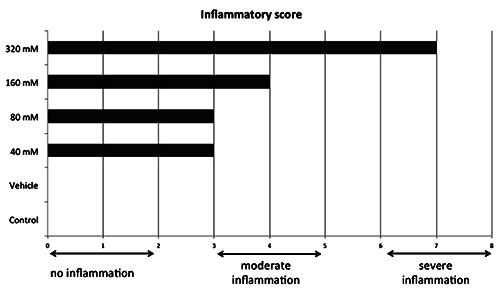
Dose responses for the different experimental groups revealing no inflammation (inflammatory scores 0 to 2) for the control or vehicle group, moderate inflammation (scores 3 to 5) for groups receiving concentrations 40, 80 and 160 mM TNBS, and severe inflammation (score from 6 to 8) for animals injected with 320 mM TNBS.
Discussion
Building on experiments and recommendations concerning chemically induced enterocolitis in zebrafish,7,11-14,25 we optimized a zebrafish model of TNBS-induced enterocolitis after having determined the optimal dose of TNBS (320 mM) and time (6 h) to induce the acute phase of inflammation. This model was used to examine the effect of enterocolitis on the ENS, during the acute phase of inflammation. No effects were observed on the neuronal density in the different parts of the GI wall or on the proportional distribution of the nitrergic enteric neuronal population. However, enterocolitis led to an increase in the proportion of serotonergic neurons over the entire intestine, as well as to a decrease in the proportion of cholinergic neurons in the distal part of the intestine.
A scoring system was used to evaluate the degree of inflammation as previously described in mammalian and zebrafish models for GI inflammation.13,26,27 Our observation of a decreased number of goblet cells at the acute phase of enterocolitis, is in line with studies showing decreased numbers and even depletion of goblet cells in genetic and chemical enterocolitis models in zebrafish,13,28,29 but is not consistent with the results of a previous TNBS studies showing no changes,12,14 or increased numbers of goblet cells.30 In mammals, data on goblet cell depletion are not unequivocal either, with reports of either unchanged or decreased numbers of goblet cells.26,27,31-33 In line with previous work in adult zebrafish, we observed, at the acute phase of enterocolitis, thickening of the intestinal wall, epithelial disruption and infiltration of eosinophils.12,13 These phenomena were also demonstrated in rodent models at the acute phase of TNBS-induced colitis.26,34 IBD studies in man equally reported on thickening of the intestinal wall and a disrupted epithelial architecture.35,36 In man, the number of eosinophils and their in situ activation increased in the acute rather than in the chronic phase of IBD, suggesting a pivotal role for these granulocytes in the observed pathological symptoms.37
Figure 3.

Histological images of the intestine stained with H&E (A, B, C) and Alcian blue/PAS (D,E,F). H&E staining is used to visualize the general morphology (thickness of the intestinal wall and epithelial fold architecture), while PAS staining enables the detection of mucus producing goblet cells and infiltrating eosinophils. These images comprise cases with no inflammation (score 0 to 2) (A,D), moderate inflammation (score 3 to 5) (B,E) and severe inflammation (score 6 to 8) (C,F).
Figure 4.

Time-response curve. The curve depicts the inflammatory scores (from 0 to 8) at different points in time after induction (3 h up to 1 week). The peak of inflammation was reached 6 h after induction.
Data obtained from previous studies in IBD patients and animal models of intestinal inflammation point to a role of enteric neuroplasticity in symptom generation in IBD.1 This is the first study reporting on neuroplasticity in the ENS of the zebrafish, induced by inflammation. A shift in the neurochemical coding of enteric neuronal populations was observed. Among the factors that may have a profound effect on the GI motility and inflammatory responses are an increased proportion of serotonergic neurons over the entire intestine, a decreased proportion of cholinergic neurons in the DI and an unaltered nitrergic population.
The GI tract is the main producer of serotonin (5-HT), i.e., 90-95% of total body 5-HT, in vertebrates. This multifunctional bioamine is mainly produced by enterochromaffin (EC) cells, a major subset of mucosal enteroendocrine cells. A small extent of 5- HT is produced by enteric neurons, which are sparse in the mammalian GI tract. In vertebrates, 5-HT acts through membrane receptors of which seven subfamilies are currently known.38,39 Several studies support the concept of mucosal 5-HT as a proinflammatory mediator in the immune response following GI inflammation, while neuronal 5-HT has neurogenerative and neuroprotective properties.40 One study has reported an increased 5- HT expression in the myenteric plexus of CD patients.15 However, 5-HT-producing EC cells are lacking in the GI epithelium of some teleost species (e.g., goldfish), in which 5-HT is exclusively synthesized by intrinsic enteric neurons.41,42 In the zebrafish, EC cells producing 5-HT are only present in the distal part of the MI and in the DI (own unpublished data). In various animal models of GI inflammation, an increase in EC cell numbers and mucosal 5-HT content as well as a reduced expression of the selective 5-HT reuptake transporter (SERT), as such extending the actions of 5-HT in the GI wall, were observed. In IBD patients, similar changes were observed, although decreased SERT activity was only found in UC patients.40,49 In teleosts, 5-HT causes contraction of the GI tract.42-45 Studies in several species, including zebrafish, have shown that the action of 5-HT on GI smooth muscle is indirect.42-48 In goldfish, neuronal 5-HT regulates GI motility through cholinergic neurons, and also regulates epithelial functions.42,46 However in tilapia, 5-HT acts directly on GI smooth muscle causing relaxation of the GI wall.42 Furthermore, it has been reported in teleosts that the serotonergic neuronal population includes a sensory population. 45 In zebrafish, 18% of the enteric neurons are serotonergic.21 Our results revealed an increased expression of 5-HT due to TNBS-induced colitis in the zebrafish intestine, which is similar to observations in animal models and IBD patients. It is suggested that in the zebrafish intestine, neuronal 5-HT will affect motility, mucosal functions and inflammatory responses during GI inflammation.
In the mammalian GI tract, the neurotransmitter acetylcholine (ACh) is mainly expressed in excitatory motor neurons, but also in diverse interneurons and intestinofugal neurons.3 In the rectum of CD patients, the proportion of cholinergic neurons was not affected, although all nitrergic neurons expressed choline acetyltransferase in comparison with tissue obtained from control persons.50 Furthermore, in UC patients, the proportion of cholinergic neurons remained unaltered in the myenteric plexus of the inflamed colon, although more cholinergic neurons expressed substance P. This remodeling of cholinergic myenteric neurons has been suggested to be part of the neuronal basis underlying the motility disturbances observed in UC patients.51 In rats, TNBS-induced colitis resulted in loss of cholinergic neurons in the myenteric plexus of the inflamed colon, although the proportion of cholinergic neurons was not changed.52 In teleosts, little is known about the cholinergic control of gut motility. In Atlantic cod and rainbow trout, ACh is involved in distension-induced reflex contractions of the stomach. 45 Pharmacological studies in zebrafish embryos and larvae demonstrated that ACh release modulates gut motility.53,54 In vitro studies in goldfish and Atlantic cod showed that ACh induces GI contractions.43,44,47,55,56 Our results did not show any changes in the proportion of cholinergic neurons in the PI or MI of the adult zebrafish; only in the inflamed DI was a decrease detected, pointing to reduced contractility in the DI.
The gasotransmitter nitric oxide is predominantly produced in descending inhibitory motor neurons, but also in descending interneurons in the mammalian GI tract.5 In IBD patients, increased production of NO, due to an overexpression of inducible NOS by intestinal epithelial cells and by an increased proportion of nitrergic enteric neurons, has been reported.15,57-61 However, one study reported similar numbers of nitrergic neurons between controls and CD patients, but this was observed in the submucosal plexus of rectal biopsies.42 The results of TNBS-induced colitis studies in rats are contradictory. No changes in the proportion of nitrergic neurons were observed in some studies, while others reported a decline in the nitrergic enteric population.52,62 Physiological studies in embryos and larvae of zebrafish, and in adult Atlantic cod, revealed that nitric oxide reduces basal tension and contraction amplitude.44,55,56,63 Our results did not reveal any alterations in the nitrergic population, leading us to conclude that the inflammation caused no effect on the inhibitory component of intestinal motility in the zebrafish,
Figure 5.
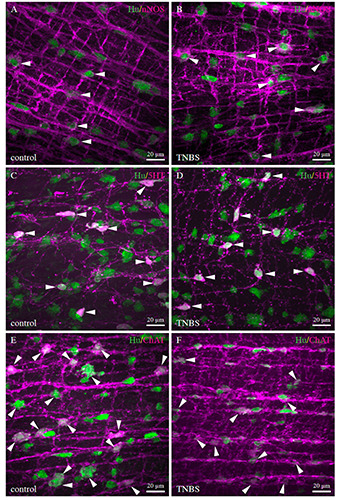
Merged confocal images showing the co-expression of the pan-neuronal marker Hu with the different neurochemical markers of interest for whole mount preparations of the DI for control (A,C,E) and TNBS-treated (B,D,F) zebrafish. Double immunolabelling for nitrergic neurons (A,B) did not reveal any differences between controls and TNBS-treated animals, whereas an increase in the proportion of serotonergic (C-D) and a decrease in the proportion of cholinergic neurons (E,F) was seen in the DI of TNBS-treated animals compared to controls.
Figure 6.
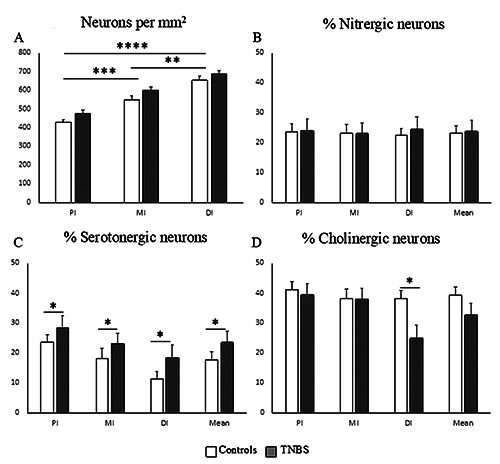
Graphs representing the quantitative results. A) Mean number of neurons (±SEM) in the different intestinal regions per square millimeter. The graph shows increased neuronal numbers toward the DI, but no differences in neuronal numbers between controls and TNBS treated zebrafish. (B-D) Mean percentages of the different neuronal subpopulations (±SEM) for the nitrergic (B), serotonergic (C) and cholinergic neuronal proportions (D) in different intestinal regions, i.e. the proximal (PI), mid (MI) and distal (DI) intestine. Also, the overall mean of the entire intestine is represented. White bars refer to data in non-inflamed (CNTL) and grey bars in inflamed (TNBS) zebrafish intestine. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
In conclusion, the present study described a method of chemically induced colitis in the DI of the adult zebrafish. At the acute phase, the inflammation was accompanied by an imbalance in neuronal content comparable to neuronal changes observed in IBD patients and in experimental mammalian models. Alongside with its versatile properties and the available genetic tools, makes this zebrafish model an excellent resource to unravel mechanisms in IBD pathology and to test new IBD therapies.
Acknowledgements
The authors would like to thank Els Goeman, Martine van Geel and Dominique de Rijck for administrative and technical assistance.
References
- 1.Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation 2010;7:37. doi:10.1186/1742-2094-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014;11:611-27. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 3.Stavely R, Abalo R, Nurgali K. Targeting enteric neurons and plexitis for the management of inflammatory bowel disease. Curr Drug Targets 2020. doi: 10.2174/13894501216662 0051617324. [DOI] [PubMed] [Google Scholar]
- 4.Annese V. Genetics and epigenetics of IBD. Pharmacol Res 2020;159:104892. doi: 10.1016/j.phrs.2020.104892. [DOI] [PubMed] [Google Scholar]
- 5.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012;9:286-94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond) 2016;11:9-15. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol 2016; 64:82-92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Pack M. Modeling intestinal disorders using zebrafish. Methods Cell Biol 2017;138:241-70. doi: 10.1016/bs.mcb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Novoa B, Figueras A. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv Exp Med Biol 2012;946:253-75. doi: 10.1007/978-1-4614-0106-3_15. [DOI] [PubMed] [Google Scholar]
- 10.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol 2004;28:9-28. doi: 10.1016/S0145-305X(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 11.Marjoram L, Bagnat M. Infection, inflammation and healing in zebrafish: intestinal inflammation. Curr Pathobiol Rep 2015;3:147-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiger BM, Gras-Miralles B, Ziogas DC, Karagiannis AKA, Zhen A, Fraenkel P, et al. Intestinal upregulation of melaninconcentrating hormone in TNBS-induced enterocolitis in adult zebrafish. PLoS One 2013;8:e83194. doi: 10.1371/journal. pone.0083194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugman S, Liu K-Y, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA, et al. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 2009;137:1757-67. doi: 10.1053/j.gastro.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 14.Oehlers SH, Flores MV, Hall CJ, Swift S, Crosier KE, Crosier PS. The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis Model Mech 2011;4:832-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut 1997;40:767-74. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil 2008;20:1009-16. doi: 10.1111/j.1365-2982.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 17.Vachon A, Scott FI. The treatment approach to inflammatory bowel disease in 2020. Curr Opin Gastroenterol 2020;36:247-56. [DOI] [PubMed] [Google Scholar]
- 18.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev 2005;122:157-73. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Ng ANY, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 2005;286:114-35. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Pack M, Solnica-Krezel L, Malicki J, Neuhauss SCF, Schier AF, Stemple DL, et al. Mutations affecting development of zebrafish digestive organs. Development 1996;123:321-8. [DOI] [PubMed] [Google Scholar]
- 21.Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans J-P, et al. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J Comp Neurol 2010;518:4419-38. doi: 10.1002%2Fcne.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uyttebroek L, Shepherd IT, Hubens G, Timmermans J-P, van Nassauw L. Expression of neuropeptides and anoctamin 1 in the embryonic and adult zebrafish intestine, revealing neuronal subpopulations and ICC-like cells. Cell Tissue Res 2013;354:355-70. doi: 10.1007/s00441-013-1685-8. [DOI] [PubMed] [Google Scholar]
- 23.Matthews M, Trevarrow B, Matthews J. A virtual tour of the Guide for zebrafish users. Lab Anim (NY) 2002;31:34-40. doi: 10.1038/5000140. [DOI] [PubMed] [Google Scholar]
- 24.Westerfield M. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish, Danio rerio. 4th ed. Eugene: University of Oregon Press; 2007. [Google Scholar]
- 25.Haarder S, Kania PW, Holm TL, von Gersdorff Jorgensen L, Buchmann K. Effect of ES products from Anisakis (Nematoda: Anisakidae) on experimentally induced colitis in adult zebrafish. Parasite Immunol 2017;39:10. doi: 10.1111/pim.12456. [DOI] [PubMed] [Google Scholar]
- 26.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-γ)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol 1999;116:238-45. Available from: doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morampudi V, Bhinder G, Wu X, Dai C, Sham HP, Vallance BA, et al. DNBS/TNBS colitis models: providing insights into inflammatory bowel disease and effects of dietary fat. J Vis Exp 2014;84:e51297. doi: 10.3791/51297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai YR, Lu YF, Lien HW, Huang CJ, Hwang SP. Foxa2 and Hif1ab regulate maturation of intestinal goblet cells by modulating agr2 expression in zebrafish embryos. Biochem J 2016;473:2205-18. doi: 10.1042/bcj20160392. [DOI] [PubMed] [Google Scholar]
- 29.Thakur PC, Davison JM, Stuckenholz C, Lu L, Bahary N. Dysregulated phosphatidylinositol signaling promotes endoplasmic- reticulum-stress-mediated intestinal mucosal injury and inflammation in zebrafish. Dis Model Mech 2014;7:93-106. doi: 10.1242/dmm.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming A, Jankowski J, Goldsmith P. In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. Inflamm Bowel Dis 2010;16:1162-72. doi: 10.1002/ibd.21200. [DOI] [PubMed] [Google Scholar]
- 31.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen T-E, Conklin LS, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009;15:341-52. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linden DR, Chen J-X, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 2003;285:G207-16. Available from: 10.1152/ajpgi.00488.2002 [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer CJ, Sato S, Qiu BS, Keith JC, Evangelista S. Cellular pathology of experimental colitis induced by trinitrobenzenesulphonic acid (TNBS): protective effects of recombinant human interleukin-11. Inflammopharmacology 1997;5:363-81. doi: /10.1007/s10787-997-0033-6. [DOI] [PubMed] [Google Scholar]
- 34.Pontell L, Castelucci P, Bagyanszki M, Jovic T, Thacker M, Nurgali K, et al. Structural changes in the epithelium of the small intestine and immune cell infiltration of enteric ganglia following acute mucosal damage and local inflammation. Virchows Arch 2009;455:55-65. doi: 10.1007/s00428-009-0795-x. [DOI] [PubMed] [Google Scholar]
- 35.Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. Am J Roentgenol 2001;176:1105-16. doi: 10.2214/ajr.176.5.1761105. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, et al. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog 2012;8:e1002593. doi: 10.1371/journal.ppat. 1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeziorska M, Haboubi N, Schofield P, Woolley DE. Distribution and activation of eosinophils in inflammatory bowel disease using an improved immunohistochemical technique. J Pathol 2001;194:484-92. doi: 10.1002/path.904. [DOI] [PubMed] [Google Scholar]
- 38.Erspamer V. Pharmacology of indole-alkylamines. Pharmacol Rev 1954;6:425-87. [PubMed] [Google Scholar]
- 39.Neuhuber W, Worl J. Monoamines in the enteric nervous system. Histochem Cell Biol 2018;150:703-9 doi: 10.1007/s00418-018-1723-4. [DOI] [PubMed] [Google Scholar]
- 40.Shajib MS, Baranov A, Khan WI. Diverse effects of gutderived serotonin in intestinal inflammation. ACS Chem Neurosci 2017;8:920-31. doi: 10.1021/acschemneuro.6b00414. [DOI] [PubMed] [Google Scholar]
- 41.Anderson C, Campbell G. Immunohistochemical study of 5- HT-containing neurons in the teleost intestine: relationship to the presence of enterochromaffin cells. Cell Tissue Res 1988;254:553-9. doi: 10.1007/bf00226505. [DOI] [PubMed] [Google Scholar]
- 42.Kiliaan AJ, Joosten HW, Bakker R, Dekker K, Groot JA. Serotonergic neurons in the intestine of two teleosts, Carassius auratus and Oreochromis mossambicus, and the effect of serotonin on transepithelial ion-selectivity and muscle tension. Neuroscience 1989;31:817-24. doi: 10.1016/0306-4522(89)90444-2. [DOI] [PubMed] [Google Scholar]
- 43.Jensen J, Holmgren S. Neurotransmitters in the intestine of the Atlantic cod, Gadus morhua. Comp Biochem Physiol 1985;82C:81-9. doi: 10.1016/0742-8413(85)90213-0. [DOI] [PubMed] [Google Scholar]
- 44.Karila P, Holmgren S. Enteric reflexes and nitric oxide in the fish intestine. J Exp Biol 1995;198:2405-11. [DOI] [PubMed] [Google Scholar]
- 45.Grans A, Olsson C. Gut motility. Farrell AP, editor. Encyclopedia of fish physiology: from genome to environment, vol. 2. San Diego: Academic Press; 2011. p. 1292-1300. [Google Scholar]
- 46.Velarde E, Delgado MJ, Alonso-Gomez AL. Serotonininduced contraction in isolated intestine from a teleost fish (Carassius auratus): characterization and interactions with melatonin. Neurogastroenterol Motil 2010;22:e364-73. doi: 10.1111/j.1365-2982.2010.01605.x. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Zhang Z, Liang X, Chen Y, Zhang J, Yi H, et al. Study of gastrointestinal tract viability and motility via modulation of serotonin in a zebrafish model by probiotics. Food Funct 2019;10:7416-25. doi: 10.1039/c9fo02129a. [DOI] [PubMed] [Google Scholar]
- 48.James DM, Kozol RO, Kajiwara Y, Wahl AL, Storrs EC, Buxbaum JD, et al. Intestinal dysmotility in a zebrafish (Danio rerio) shank3a;shank3b mutant model of autism. Mol Autism 2019;10:3. doi: 10.1186/s13229-018-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol 2010;161:19-27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, et al. Neurotransmitter coding of enteric neurons in the submucous plexus is changed in non-inflamed rectum of patients with Crohn’s disease. Neurogastroenterol Motil 2001;13:255-64. doi: 10.1046/j.1365-2982.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 51.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 2003;52:84-90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winston JH, Li Q, Sarna SK. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 2013;305:G295-302. doi: 10.1152/ajpgi.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmberg A, Schwerte T, Pelster B, Holmgren S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol 2004;207:4085-94. doi: 10.1242/jeb.01260. [DOI] [PubMed] [Google Scholar]
- 54.Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTXinsensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol 2007;210:1084-91. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- 55.Velarde E, Alonso-Gomez AL, Azpeleta C, Isorna E, De Pedro N, Delgado MJ. Melatonin effects on gut motility are independent of the relaxation mediated by the nitrergic system in the goldfish. Comp Biochem Physiol A 2011;159:367-71. doi: 10.1016/j.cbpa.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 56.Olsson C, Holmgren S. PACAP and nitric oxide inhibit contractions in the proximal intestine of the Atlantic cod, Gadus morhua. J Exp Biol 2000;203:575-83. [DOI] [PubMed] [Google Scholar]
- 57.Avdagić N, Zaćiragić A, Babić N, Hukić M, Šeremet M, Lepara O, et al. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn J Basic Med Sci 2013;13:5-9. doi: 10.17305/bjbms.2013.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn's disease human small intestine. Dig Dis Sci 1999;44:1579-87. doi: 10.1023/a:1026658826010. [DOI] [PubMed] [Google Scholar]
- 59.Geboes K, Mebis K, Rutgeers P, Ectors N, Vantrappen G. Demonstration of nitric oxide positive neurons in Crohn's disease. Gastroenterology 1993;104:A705. [Google Scholar]
- 60.Rachmilewitz D. Increased colonic nitric oxide level in active IBD. Scand J Gastroenterol 2008;43:638. doi: 10.1080/00365520701818045. [DOI] [PubMed] [Google Scholar]
- 61.Reinders CA, Jonkers D, Jansson EA, Stockbrugger RW, Stobberingh EE, Hellstrom PM, et al. Rectal nitric oxide and fecal calprotectin in inflammatory bowel disease. Scand J Gastroenterol 2007;42:1151-7. doi: 10.1080/00365520701320505. [DOI] [PubMed] [Google Scholar]
- 62.Demedts I, Geboes K, Kindt S, Vanden Berghe P, Andrioli A, Janssens J, et al. Neural mechanisms of early postinflammatory dysmotility in rat small intestine. Neurogastroenterol Motil 2006;18:1102-11. doi: 10.1111/j.1365-2982.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 63.Holmberg A, Olsson C, Holmgren S. The effect of endogenous and exogenous nitric oxide on gut motilit in zebrafish Danio rerio embryos and larvae. J Exp Biol 2006;209:2472-9. doi: 10.1242/jeb.02272. [DOI] [PubMed] [Google Scholar]


