To the Editor,
Hypoxemic acute respiratory failure (hARF) secondary to COVID-19 presents with heterogeneous features depending on several determinants, such as the extent of intravascular microthrombosis, superinfections, and other complications [1, 2]. The easiest approach for setting positive end-expiratory pressure (PEEP) and inspiratory oxygen fraction (FiO2) is using PEEP/FiO2 tables [3, 4]. However, because the magnitude of lung recruitability is variable, personalizing PEEP would be desirable [1]. Electrical impedance tomography (EIT) offers this opportunity by bedside estimating both alveolar collapse and lung overdistension throughout a decremental PEEP trial [5].
This investigation (Ethics Committee approval: Ref:4853/AO/20-AOP2012) aims to assess the agreement between EIT-based PEEP values and those recommended by the higher and lower PEEP/FiO2 tables [6] in a series of consecutive intubated COVID-19 hARF patients, admitted to intensive care unit at our institution. Written informed consent was obtained from all patients.
We performed 15 decremental PEEP trials through a dedicated device (Pulmovista500, Drӓger-Medical, Germany) and subsequently analyzed pulmonary perfusion distribution [5]. Five patients were evaluated in a prone position. EIT optimal PEEP (PEEPEIT) was defined as the best compromise between lung collapse and overdistension [5]. All patients were deeply sedated without spontaneous breathing efforts and ventilated in volume control mode with lung-protective settings [3]. PEEPEIT was compared with PEEP from higher and lower PEEP/FiO2 tables [6]. Data, expressed as median and interquartile ranges or 95% confidence interval (CI), were analyzed with the Mann–Whitney test for comparisons and Spearman rank test for correlations, considering p values < 0.05 significant. The Bland–Alman analysis was also performed.
Patients had received invasive ventilation for 12.0 (10.0–14.5) days. Patients’ age was 63 (56–78) years, while body mass index (BMI) was 26.2 (25.4–30.9) kg/m2. Pulmonary shunt and dead space, as assessed by EIT [5], were 4% (2–6%) and 27% (23–36%), respectively. d-dimer was increased [759 (591–1208) mcg/L], while procalcitonin blood concentration was nearly normal [0.53 (0.34–0.70) mcg/L]. PEEPEIT was 12 (10–14) cmH2O and was significantly different from PEEP values of both higher [17 (16–20) cmH2O, p < 0.001] and lower [9 (8–10) cmH2O, p = 0.049] PEEP/FiO2 tables. The Bland–Altman analysis showed that PEEPEIT was 6.2 [CI 3.9–8.4] cmH2O smaller and 2.0 [CI 0.1–4.0] cmH2O greater than PEEP levels recommended, respectively, by the higher and lower PEEP/FiO2 tables (Fig. 1). No correlation was found between PEEPEIT and FiO2 (p = 0.789) (Fig. 2). The loss of lung compliance secondary to lung collapse observed with PEEP values from the lower PEEP/FiO2 table [7.0% (3.2–8.7%)] was not significantly greater, compared to that obtained with PEEPEIT [3.0% (2.0–4.7%)] (p = 0.077). Conversely, the loss of lung compliance consequent to lung overdistension was significantly greater with PEEP values from the higher PEEP/FiO2 table [15.5% (11.0–21.5%)] than with PEEPEIT [4.0% (3.0–4.7%)] (p < 0.001).
Fig. 1.
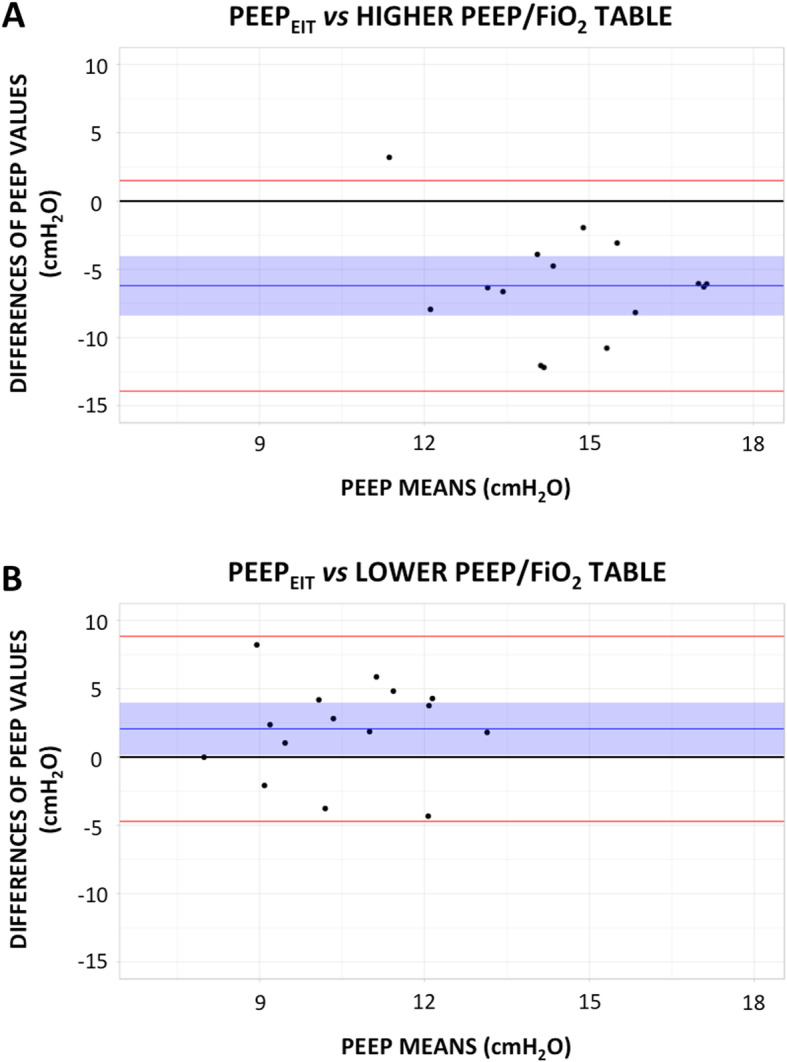
Bland–Altman plot, evaluating the agreement between PEEPEIT and the PEEP values proposed by the higher (a) and lower (b) PEEP/FiO2 tables from the ALVEOLI trial [6]. X-axis: average of paired measurements. Y-axis: difference between paired measurements. The blue line and blue shaded area: bias and 95% confidence interval of the bias between PEEPEIT and the PEEP values suggested by PEEP/FiO2 tables. Red lines: upper and lower limits of agreement between methods
Fig. 2.
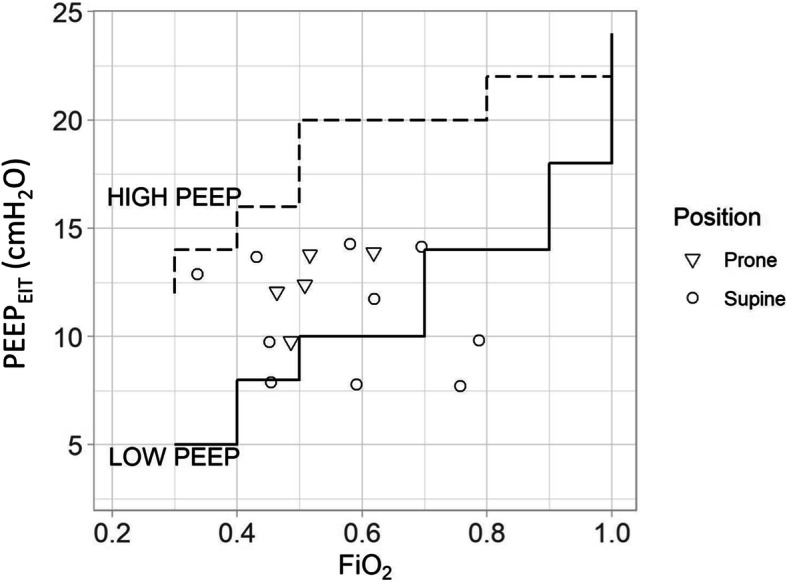
Spearman correlation between PEEPEIT and FiO2 (R = 0.075, p = 0.789). Continuous line: lower PEEP/FiO2 table. Dashed line: higher PEEP/FiO2 table
In contrast to our results, a recent study, utilizing the same EIT device in intubated COVID-19 hARF patients, reported much higher values of PEEPEIT [21 (16–22) cmH2O], closer to those indicated by the higher PEEP/FiO2 table, though without significant correlation [4]. These differences are partly explained by the different criteria for PEEPEIT selection, which in that study was set above the value indicated by the built-in algorithm corresponding to the least lung collapse and overdistension [4]. Also, compared to our study, they enrolled more obese patients, as indicated by the higher BMI [30.0 (27.0–34.0) kg/m2] [4]. Not reported in that study [4], our patients showed increased d-dimer and high fraction of pulmonary dead space, while shunt fraction and procalcitonin were nearly normal, suggesting predominant lung vascular disruption.
In conclusion, we confirm the rationale for individualized PEEP setting in COVID-19 patients intubated for hARF. Whether EIT is the best technique for this purpose and the overall influence of personalizing PEEP on clinical outcome remain to be determined.
Acknowledgements
We feel indebted with all ISTAR3-ICU personnel who made this work possible.
Authors’ contributions
Concept and design: NS, FZ, and PN. Acquisition, analysis, or interpretation of the data: FZ, GA, VG, and NS. Drafting of the manuscript: FZ, VG, NS, and AB. Critical revision of the manuscript for important intellectual content: PN, GA, and AB. Statistical analysis: FZ and NS. Supervision: PN. The authors read and approved the final manuscript.
Funding
None declared.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, PN, upon request.
Ethics approval and consent to participate
The study was approved by the Local Ethical Committee: Comitato Etica per la Ricerca Clinica, Azienda Ospedale Università di Padova, Ref:4853/AO/20-AOP2012. Written informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained for all patients.
Competing interests
PN received royalties from Intersurgical for Helmet Next invention and speaking fees from Philips, Resmed, MSD, and Novartis.
The other authors have no competing interests to declare.
The experimental software for EIT perfusion assessment was kindly provided by Drӓger Medical, Germany, without any financial supports.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicolò Sella and Francesco Zarantonello contributed equally to this work.
References
- 1.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted?. Lancet Respir Med. 2020;8(8):816-21. 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed]
- 2.Boscolo A, Spiezia L, Correale C, et al. Different hypercoagulable profiles in patients with COVID-19 admitted to the internal medicine ward and the intensive care unit [published online ahead of print, 2020 Jul 23]. Thromb Haemost. 2020. 10.1055/s-0040-1714350. [DOI] [PubMed]
- 3.Pasin L, Sella N, Correale C, et al. Regional COVID-19 Network for Coordination of SARS-CoV-2 outbreak in Veneto, Italy [published online ahead of print, 2020 May 15]. J Cardiothorac Vasc Anesth. 2020. 10.1053/j.jvca.2020.05.005. [DOI] [PMC free article] [PubMed]
- 4.van der Zee P, Somhorst P, Endeman H, Gommers D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(2):280–284. doi: 10.1164/rccm.202003-0816LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frerichs I, Amato MB, van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2017;72:83–93. doi: 10.1136/thoraxjnl-2016-208357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. NEJM. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, PN, upon request.


