Abstract
Purpose of Review
The alarming number of confirmed COVID-19 cases put a strain on the healthcare systems, which had to reallocate human and technical resources to respond to the emergency. Many urologists became integrated into multidisciplinary teams, dealing with this respiratory illness and its unknown management.
It aims to summarize the epidemiological, clinical, diagnostical, and therapeutical characteristics of COVID-19, from a practical perspective, to ease COVID-19 management to non-physician staff.
Recent Findings
We performed a narrative review of the literature regarding COVID-19, updated to May 8th, 2020, at PubMed and COVID resource platforms of the main scientific editorials.
COVID-19, characterized by fever, myalgias, dyspnea, and dry cough, varies widely from asymptomatic infection to death. Arrhythmias and thrombotic events are prevalent.
Lymphopenia and inflammatory reactant elevation on laboratory, as well as bilateral and peripheral ground-glass opacities or consolidations on X-Ray, are usually found in its assessment. Little is known about SARS-CoV-2 immunology.
To date, no therapy has demonstrated efficacy in COVID-19. Of-level or compassionate-use therapies are prescribed in the context of clinical trials. We should become familiar with specific adverse events and pharmacological interactions.
Summary
The COVID-19 pandemic has paralyzed the urological activity, and its long-term consequences are unpredictable.
Despite not being used to deal with respiratory diseases, the urologists become easily qualified to manage COVID-19 by following protocols and being integrated into multidisciplinary teams, helping to overcome the pandemic.
Keywords: COVID-19, Medical management, Review, SARS-CoV-2, Treatment, Urologist
Introduction
Since its first description in December 2019, coronavirus disease 19 (COVID-19), caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has dramatically spread worldwide [1].
The alarming number of confirmed cases overcrowded hospitals, increasing their occupancy to 120% [2]. It was necessary to reallocate human and technical resources [3].
The infection and isolation of many physicians reduced its availability to struggle against COVID-19, so it became essential to recruit surgical staff to assist COVID-19 patients. In this way, some urologists have been integrated into COVID-19 multidisciplinary teams [3].
The remaining urologists continued screening and treating patients, some of whom were asymptomatic COVID-19’s infected patients or whose symptoms were masked under the suspicion of urinary infection. Finally, many patients got infected by SARS-CoV-2 during their hospitalization [4].
Most of the scientific associations, like the European Association of Urology, have considered the importance of urologists’ support in this challenging situation [5]. Some guidelines were drafted concerning the prioritization of the urological activity, helping clinical decision-making [6]. This great effort may avoid a harmful impact on both patients and healthcare urological providers whenever it is possible.
COVID-19’s outbreak and its medium and long-term course uncertainty force physicians to learn from real-life experience and changing emerging evidence [3]. This effort is remarkable in urologists and other surgical staffs, who do not use to deal with respiratory diseases and their medical management.
This document aimed to synthesize the clinical, diagnostical, and therapeutical characteristics of COVID-19. It is made from a practical perspective, adapted to non-physician staff.
Material and Methods
The manuscript is based on the limited scientific data regarding SARS-Cov-2, updated to May 8th, 2020, and the authors’ personal-experience managing COVID-19 at their institutions.
The authors reviewed the literature concerning COVID-19 at PudMed, but also at COVID-19 resource platforms published by editorials like Elsevier, JAMA network, Springer Nature, BioMed Central, The BMJ, Cochrane Library, Wiley. The results were clustered in epidemiology, clinical intercourse, assessment, and treatment of COVID-19.
The initial drafted version was discussed and agreed upon by all authors via a telematics conference. On May 9th, 2020, the final version was approved.
This document reflects an updated and practical perspective of the current evidence adapted to the urology staff.
Results
Epidemiology
With 3,759,967 confirmed cases on May 7th, including 259,474 deaths over 215 counties [7], several systematic reviews have been published about SARS-CoV-2’s epidemiology [8, 9, 10••].
SARS-CoV-2 has high transmission efficiency, with a basic reproduction range (R0) estimated between 2 and 3 in most studies [9, 10••].
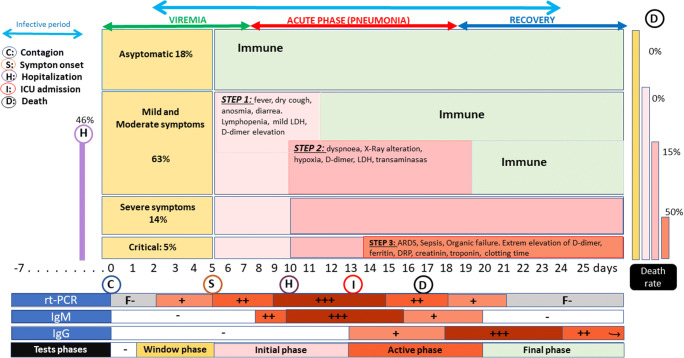
The incubation period was estimated to be 4–6 days, ranging from 2 to 11 days after exposure in most cases [9]. In a practical sense, 14 days are considered its upper limit [10••]. The transmission was estimated to start 1–3 days before symptoms onset [8] (Fig. 1).
Fig. 1.
Diagram of COVID-1 clinical course spectrum and immunological response [11•, 12–14]
The risk of transmission from patients with SARS-CoV-2 infection varies by the kind and duration of exposure, use of preventive measures, and individual factors [8, 9, 15].
Infected symptomatic or asymptomatic patients are the source of infection, being respiratory droplets and direct contact with an infected person/surface the most frequent vehicles. It also occurs by long-time exposure to high-virus concentration respiratory aerosols [10••]. SARS-CoV-2 has been isolated on stool samples, but the feco-oral transmission is not significant [8]. The SARS-CoV-2 yield in urine has not been demonstrated [16]. Not vertical transmission has been demonstrated, although impaired effects have been described in newborns from infected pregnants [9].
Exposure to higher virus concentrations and re-expositions are related to a worse prognosis.
The duration of virus transmissibility is uncertain and seems to be related to the severity of illness. Seven days after the clinical onset, the risk of transmission decreases in mild-symptomatic patients, but it may be extended over 24 days in severe cases [11•, 15].
Clinical Course and Complications
The clinical spectrum of SARS-CoV-2 infection varies widely, including asymptomatic infection, mild upper respiratory tract illness, severe viral pneumonia with respiratory failure, and even death [9, 11•] (Fig. 1).
Fever, fatigue, dry cough, anorexia, myalgias, dyspnea, diarrhea, anosmia, and dysgeusia have been described commonly in COVID-19 since the first Chinese reports [8, 11•, 12].
Hospitalization rates increased with age, ranging between 2.5 in those aged 18–49 years and 12.2 or 17.2 in those aged 65–74 or ≥ 85 years, respectively [17].
Some patients may progress from mild-symptomatic to severe disease (oxygen saturation < 93%, shortness of breath, PaO2/FiO2 < 300 mmHg). Dyspnea development was commonly reported after a median of 5 days since symptoms onset, and admission occurred after a median of 7 days [12]. Acute respiratory distress syndrome (ARDS) is a significant complication in patients with severe disease and can manifest shortly after the onset of dyspnea, being age over 65 years, diabetes mellitus, and hypertension risk factors. Septic shock, thrombotic events (TE), and multiorganic failure are also common complications [11•, 12].
Complimentary Assessment
COVID-19 should be considered in every patient with fever and typical symptoms. All of them should be screened about contact with positive or suspicious cases or residence/travel to CIVID-19’s community transmission areas in the previous 14 days. SARS-Cov-2 assessment should be performed by nasopharyngeal (NP) swap real-time reverse transcription-polymerase chain reaction (rtPCR).
On admission, hemogram, coagulation profile (including D-Dimer), hepatic and renal profiles, and acute reactants (including LDH, ferritin, C-reactive protein, creatine kinase, and procalcitonin) should be performed when possible. Also, arterial gasometry, thoracic X-ray, ECG, and SOFA score should be assessed [11•].
Microbiological studies (HIV, HBV, and HCV serologies, blood culture, pneumococci, and legionella urinary antigens), myocardial enzymes and interleukin-6 determinations may be recommended.
Laboratory Results
Lymphopenia is consistently present at 40% of patients [11•, 18••]. An abnormal coagulation function is commonly found in cases of severe COVID-19 pneumonia [11•, 19]. Elevated inflammatory markers, such as D-dimer, C-reactive protein, LDH, fibrinogen, are commonly present. Other common laboratory findings are shown in Table 1.
Table 1.
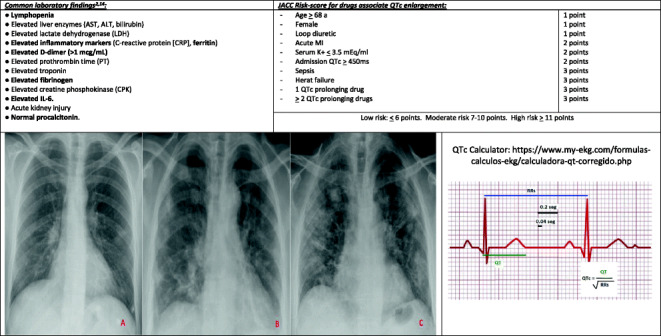
Critical/fatal illness should be suspected of older patients and high SOFA score punctuations. Exuberant inflammatory response findings, D-dimer over 1 μg/mL, and lymphopenia are mortality markers [11•, 12].
According to the literature, we monitored laboratory results every 48–72 h [12].
Electrocardiogram
QTc calculation based on the admission ECG and QTc prolongation score should be performed before the start of COVID-19’s treatment (Table 1) [20–22, 23••]. We should control QTc at least 2–3 times a week, while on treatment with QTc-prolongator drugs.
Radiology
COVID-19 shows non-specific imaging features. Bilateral lung involvement is found in 75% of X-rays. It is characterized by multiple peripheral ground-glass opacities with a subpleural distribution (Table 1; image A) and subsegmental consolidations (Table 1; image B) [12, 24]. In more severe cases, extensive subpleural and peripheral multifocal areas of consolidation, predominantly in lower lobes (Table 1; image C) [24]. Lung involvement increases throughout the illness course, with a peak in severity at 10 to 12 days after symptom onset. No pleural effusions or cavitations have been reported.
The American College of Radiology recommends not using chest CT for its screening or diagnosis [8, 24].
Immunology
The rt PCR is the preferred SARS-CoV-2’s diagnostic test. Viral load is demonstrated in the respiratory tract within 5–6 days of the onset of symptoms [13] (Fig. 1). The pick of viral load is correlated with COVID-19 severity. An NP swap is preferred over oropharyngeal one, due to sensitivity differences (63% vs. 32%) [13]. At the ICU, bronchoalveolar lavage can be performed.
Little evidence exists about the serological course in COVID-19’s patients. Preliminary evidence suggests that some of these antibodies protect, but this, as well as how long any protective effect lasts, must be definitively established [8]. Uncertain correlation between their plasmatic level and clinical severity exists.
After 10 days from symptom onset, most patients increase IgG or IgM level. Seroconversion occurs after 7 days of clinic onset in 50% of them, and in 100% after 14 days [16].
Plasmatic IgM/IgG determination will be useful to study immunological status in healthcare workers [14]. (Fig. 1).
Treatment
To date, no therapy has demonstrated efficacy in COVID-19. Current management consists of supportive care and oxygen support if necessary [10••, 12, 25]. Also, many patients have received off-level or compassionate-use therapies in the context of ethically approved clinical trials or the Monitored Emergency Use of Unregistered Interventions Framework (MEURI) [23••].
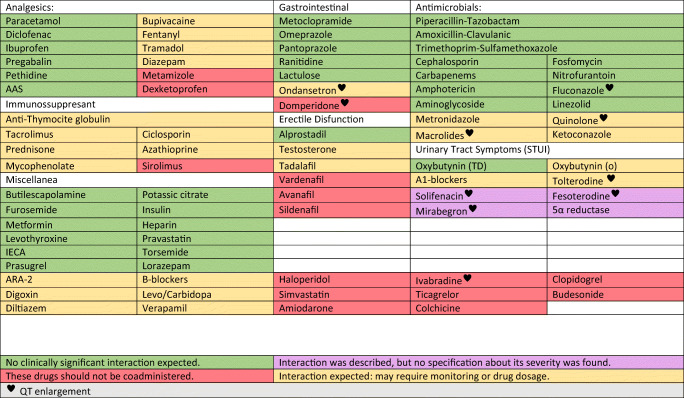
Two main aspects should be considered: we may be unfamiliar to specific adverse events that are potentiated with polimedication, and concomitant medication should be reviewed due to multiple pharmacological interactions (Fig. 2).
Fig. 2.
Interactions between urological drugs and hydroxychloroquine or lopinavir/ritonavir [26]
Table 2 describes the clinical features of the main drugs proposed in COVID-19’s management. Evidence that supports their indication is exposed below.
Table 2.
| Agent | Target | Dose | Contraindications | Toxicities | Adjustments | Recommendations |
|---|---|---|---|---|---|---|
| HIDROXYCHLORQUINE |
- Immunomodulator. - Blockade of viral entry. |
- 400 mg twice daily × 1 day, then 200 mg twice daily × 4 days. |
- Myasthenia gravis. - Retinopathy. - Prolonged QT . - IMAOs and quinolones. - Digoxin, antidiabetic, and antiepileptic’s levels modifications. . QTc prolonger drug interactions. |
- Common: gastrointestinal intolerance. - Severe: QTc prolongation ♥ , hypoglycemia, hematological alterations, neuropsychiatric and CNS effects, and retinopathy. |
- Not-recommended kidney or hepatic adjustment. - May be used in pregnancy if the benefit outweighs the risk. - Cumulative effect: plasmatic half-life: 32 days. |
- ECG monitorization. - Hemogram monitorization. |
| LOPINAVIR/RITONAVIR |
- Inhibition of 3-chymotrypsin-like protease. - Type-1 aspartate protease inhibitor. |
- 400 mg/100 mg twice daily for up to 14 days. |
- Polyurethane feeding-tube contraindicates oral solution. - HIV infection. - Severe hepatic insufficiency. - No-modifiable dugs interaction. |
- Common: gastrointestinal intolerance. - Severe: pancreatitis, hepatopathy, QT prolongation. ♥ - 14% of treatment interruptions: gastrointestinal AEs. |
- Not-recommended kidney or hepatic adjustment. - May be used in pregnancy unless oral solution. |
- Hepatic enzymes monitorization. - PHARMACOLOGICAL INTERACTIONS. |
| AZITROMIZIN | - Antibacterial: inhibition of protein synthesis. |
- 500 mg daily × 3. - 500 mg × 1 + 250 mg daily × 4. |
- Macrolide allergy. - Prolonged QT. - Hepatopathy. |
- QT prolongation, torsade de pointes, tachyarrhythmia. ♥ - Hepatopathy. - Gastrointestinal symptoms. |
- Not-recommended kidney adjustment. |
- ECG monitorization. - Hepatic enzymes monitorization. |
| TOCILIZUMAB | - IL-6 inhibition/reduction. | - 400 mg iv or 8 mg/kg in 1 or 2 doses sparsed 8–12 h. |
- Pregnancy. - AST/ALT > 5 times the upper limit. - Neutropenia < 500 cells. - Thrombocytopenia > 50,000 cells. - Transplanted patients or anti-rejection drugs or immunoregulatory drugs - To be included in other C. Trials. |
Mild: HTA, headache, respiratory infection, TBC reactivation Severe: anaphylaxis, hepatopathy, hematological reactions, intestinal perforation. |
- Not-probed in severe kidney/hepatic impairment. |
- IL-6 levels over 20–40 pc/ml. - Alternatively, D-Dimer over 1500 ng/ml. |
| REMDESIVIR | - RNA polymerase inhibitor. | - 200 mg × 1iv + 100 mg/day × 9. |
- Creatinine clearance < 30 ml/min. - AST/ALT > 5 times the upper limit. - Multiorganic failure. - Concomitant antivirals treatment. |
- Mild: hepatic or renal failure, gastrointestinal intolerance, hypotension. - Severe (23%): multiorganic failure, acute kidney injury, hepatic failure, sepsis. - Discontinuation (12%) |
- Not-recommended kidney or hepatic adjustment. - No probed in severe kidney/hepatic impairment. |
- Compassivity uses if saturation < 94%. - Hepatic enzymes and renal function monitorization. |
| METHYLPREDNISOLONE |
- Immunomodulation. - Anti-inflammatory. - Antifibrotic. |
- 0.5–1 mg/kg/day up to 7 day. - 250 mg/day up to 3 day. |
- Concomitant infection. - Gastrointestinal ulcer. |
Severe: hyperglycemia, psychosis, and avascular necrosis. |
- Dese de-escalation in chronic treatment. - The patient should meet ARDS criteria |
- VERY LIMITED EVIDENCE. - Glycemic control. |
Chloroquine/Hydroxychloroquine
Chloroquine is a well-known and economic antimalarial drug.
An in vitro study demonstrated that chloroquine was highly effective in reducing viral replication [21].
Results from 100 chloroquine-treated patients resulted in improved radiologic findings, enhanced viral clearance, and reduced disease progression. A study based on 36 hydroxychloroquine-treated patients reported 70% of virologic clearance at 6th day, measured by nasopharyngeal swabs [23••].
A harmful increase in cardiac conduction alterations is described in combination therapy with QTc-prolonger drugs.
Lopinavir/Ritonavir
The current evidence suggests a limited role for lopinavir/ritonavir in COVID-19 [22]. A 199 patient-based study (99 receiving lopinavir/ritonavir) was published [20]. No differences were found against the standard of care group in terms of mortality, virus clearance, or time to hospital discharge. Only in the intention to treat analysis, a 1-day difference in the median time to clinical improvement was found (15 days vs. 16 days).
It should start at the early peak of viral replication (initial 7–10 days) to be effective [23••].
We should perform a careful review of concomitant medication due to frequent pharmacological interactions and potential AEs (https://www.covid19-druginteractions.org/).
Azithromycin
Azithromycin has in vitro activity against Zika and Ebola viruses and is commonly prescribed to prevent severe respiratory tract infections when administrated to patients suffering from viral infection [22].
A 100% 6-day virus clearance was described when hydroxychloroquine is combined with azithromycin, but a harmful increase in arrhythmias is described [23••].
Corticosteroids
Low evidence exists about its indication [22]. In a recent series of 201 patients who developed ARDS, methylprednisolone was associated with a decreased mortality risk (46% vs. 62%) [1].
Corticosteroids have an immunomodulatory effect, decreasing inflammatory response that may lead to ARDS and lung fibrosis. Nevertheless, they may increase the risk of secondary infections and delay viral clearance [23••].
Corticosteroids are recommended in the treatment of septic shock, exacerbation of chronic obstructive respiratory disease and these COVID-19’s patients with respiratory deterioration and quick radiological progression associated with sings of cytokine storm (cytopenia, maintained fever, an increase of inflammatory reactants: D-dimer > 1000 ng/mL, ferritin > 1000 ng/mL, fibrinogen > 100 ng/mL, IL-6 > 40 pg/mL) [6, 23••].
In this context, they should be used at low-moderate doses (0.5–1 mg/kg/day up to 7 days) [6].
Tocilizumab
It is a humanized monoclonal antibody against the IL-6 receptor. It decreases the damage caused by the cytokine storm and the amplified immune response in the lung and other organs [23••].
A first report based on 21 patients [17 (85.7%) received 1 dose] showed that the symptoms, hypoxemia, and CT opacity changes were improved immediately after the treatment with tocilizumab. Of them, 75% lowered their oxygen intake (1 patient was taken off the ventilator on the first day after tocilizumab). A total of 90.5% have been discharged within 2 weeks after tocilizumab initiation. Lymphocytes’ percentage normalization in 52.6% and CRP normalization in 84.5% of patients were observed on the fifth day. CT scans’ lesions were absorbed in 19 patients (90.5%) and a little improvement in the others. No AEs were reported [27].
It can be used in patients with extensive bilateral lung opacities or in severe or critical patients who have an elevated level of IL-6 (> 40 pg/mL) [28].
Remdesivir
It is a nucleoside analog prodrug that inhibits viral RNA polymerases. Previously reported at the Ebola disease, it has demonstrated in vitro activity against SARS-Cov-2 [23••].
Despite the FDA approval for its urgent prescription in confirmed patients with oxygen saturations under 94%, clinical trial results are controversial.
The results from 53 patients (30 receiving mechanical ventilation and 4 treated with ECMO) at 18-day median follow-up reported 68% (36 patients) of oxygen-support class improvements (including 17/30 (57%) extubations) and 47% (25/53) of patient discharges. Seven (13%) patients died (6 in those receiving invasive ventilation) [25].
Results from 237 patients, 158 assigned to remdesivir, showed no differences in time to clinical improvement, 28-day mortality, oxygen support, hospitalization, or viral load. Also, described 66% of AEs and 12% of discontinuation [26].
Thromboprophylaxis
Several reports suggest a prothrombotic status with an increased trend of TE [11•].
TE, D-dimer’s elevation, prothrombin time lengthened, and thrombocytopenia are predictors for 28-day mortality and worse prognosis. Moreover, in patients with D-dimer > 6-fold of the standard upper limit or SIC score ≥ 4, the treatment with heparin decreases the 28-day mortality rate [29]. Heparin has anti-inflammatory properties and a protective effect over endothelium and microcirculation [30].
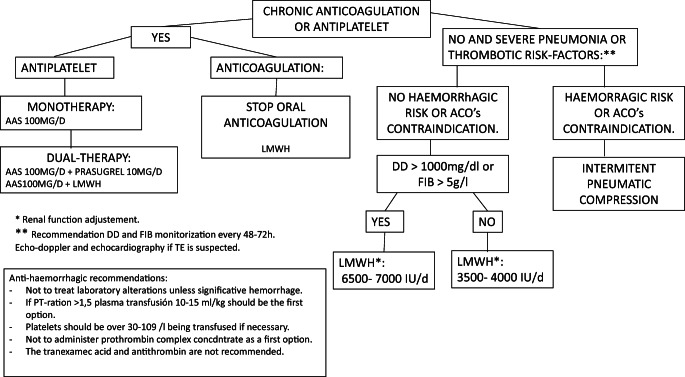
The COVID-19’s treatment interacts with antiplatelets and anticoagulants. The recommendations concerning the management of the coagulation emitted by The Spanish Association for Anesthesiology, Resuscitation, And Pain Therapy (SEDAR) are shown in Fig. 3 [30].
Fig. 3.
Proposal for the management of thrombotic and hemorrhagic events [30]
Pulmonary thromboembolism should be suspected in case of sudden respiratory deterioration associated with ARDS and low arterial pressure [11•].
Discharge Criteria
Post-COVID-19 Experience Learnings
COVID-19 is one of the most shocking worldwide events in the last centuries. No reliable prevision on its personal or communitarian impact can be done.
The consequences of respiratory fibrosis and psychiatric and mobility harm after prolonged isolated hospitalization are unknown. The population has to adapt to looses that are so far unpredictable.
Our hospital was quickly crowed by COVID-19 patients, having to increase its inpatient capacity, especially in ICUs. It reached 120% [2] of its occupancy (more than 1000 beds with 950 of them occupied by COVID-19 patients). From March 13th to April 19th, 2020, 3024 Covid-19-confirmed patients were admitted, 272 of them hospitalized at the ICUs. A total of 487 of them dead and 2004 were discharged.
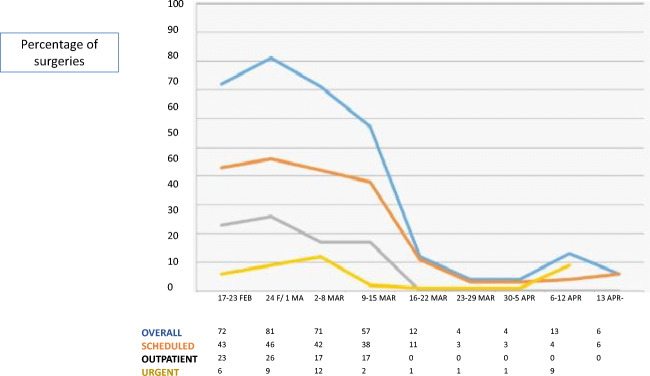
The urology department implemented urgent adaptive measures. Scheduled inpatient clinics were screened and resolved by telephone. The surgical activity radically decreased, non-urgent, or non-priority surgeries were canceled (Fig. 4). Elective admissions were minimized.
Fig. 4.
Evolution of surgical scheduling during the COVID-19 pandemic
It may be appropriate to consider whether the telematic model can be integrated as part of the daily practice.
We highlight the collaborative and engaging character of urologists. Three residents and nine consultants joined multidisciplinary teams (three of them got infected), led by internal medicine, infectologists and pneumologists. We have evaluated the patients’ medical status and complimentary assessment, modified treatment prescriptions, and supported them emotionally; we have also informed their families and other bureaucratic tasks. The remaining ones attended the scheduled activity.
Despite not having received specific training, as urologists, we did not find difficulties in wearing the personal protective equipments or adapting to protocols. The tremendous collaboration into multidisciplinary teams allowed proper clinical management. It is essential to find emotional support and relief since COVID-19’s wards are stressful and crowed. Moreover, feelings of fear of contagion and threat to loved ones are prevalent, and it can lead to emotional isolation that must be avoided.
Conclusions
We are effectively improving how to properly manage COVID, thanks to the experience shared by extraordinarily engaged and unselfish healthcare professionals. Its collaborative work in multidisciplinary teams allows overcoming the COVID-19 pandemic.
Compliance with Ethical Standards
Conflict of Interest
Dr. López-Fando Lavalle reports personal fees from Astellas Pharma SA, personal fees from Neomedic, personal fees from Boston Scientific, personal fees from Wellspect, personal fees from Coloplast, outside the submitted work. Dr. Moreno-Guillén reports grants and personal fees from Gilead Sciences, grants and personal fees from ViiV Healthcare, personal fees from Janssen Cilag, grants and personal fees from MSD, outside the submitted work. The rest of the authors have nothing to disclose.
Human and Animal Rights and Informed Consent
Not applicable. This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Kidney Diseases
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Wu C, Chen X, Cai Y, Xia J’a, Zhou X, Xu S, Huang H, Zhang L, Zhou X, du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hevia V, Lorca J, Hevia M, et al. Pandemia COVID-19: impacto y reacción rápida de la Urología. Actas Urol Esp. 2020. 10.1016/j.acuro.2020.04.006. [DOI] [PMC free article] [PubMed]
- 3.Naspro RPL. Urology in the time of corona. Nat Rev Urol. 2020. [DOI] [PMC free article] [PubMed]
- 4.Lei S, Jiang F, Wating S, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin Med. 2020. [DOI] [PMC free article] [PubMed]
- 5.Ribal MJ, Cornford P, Briganti A, Knoll T, Gravas S, Babjuk M, Harding C, Breda A, Bex A, GORRG Group. Rassweiler JJ, Gözen AS, Pini G, Liatsikos E, Giannarini G, Mottrie A, Subramaniam R, Sofikitis N, Rocco BMC, Xie LP, Witjes JA, Mottet N, Ljungberg B, Rouprêt M, Laguna MP, Salonia A, Bonkat G, Blok BFM, Türk C, Radmayr C, Kitrey ND, Engeler DS, Lumen N, Hakenberg OW, Watkin N, Hamid R, Olsburgh J, Darraugh J, Shepherd R, Smith EJ, Chapple CR, Stenzl A, van Poppel H, Wirth M, Sønksen J, N'Dow J, EAU Section Offices and the EAU Guidelines Panels EAU Guidelines Office Rapid Reaction Group: an organisation-wide collaborative effort to adapt the EAU guidelines recommendations to the COVID-19 era. Eur Urol. 2020;78(1):21–28. doi: 10.1016/j.eururo.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. WHO’s Organ. 2020:1–21.
- 7.WHO. Coronavirus disease 2019 (COVID-19) Situation Report - 87. 2020;(16 April). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200416-sitrep-87-covid-19.pdf?sfvrsn=9523115a_2.
- 8.Mcintosh AK. Coronavirus disease 2019 ( COVID-19): Epidemiology, virology, clinical features, diagnosis, and prevention. UpToDate. 2020;15th April.
- 9.Park M, Cook A, Lim JT, et al. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhikari SP, Meng S, Wu Y-j, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease ( COVID-19 ) during the early outbreak period: a scoping review. J Autoinmun. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Ronghui D, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y-W, Schmidt JE, Persing DH, CWS The laboratory diagnosis of COVID-19 infection: current issues and challenges. JMC. 2020;58(e00512):12–20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López J, Mitjá O, et al. Propuesta de intervenciones de salud pública para el control de la infección SARS-CoV-2 en la comunidad. Control epidemiológico COVID-19. 2020. https://964f74da-ef80-4d30-819c-e4bc9748a9a1.filesusr.com/ugd/e7cd86_ff6a3149457f4b6c96a4f603ea9a4a02.pdf.
- 15.Liu Y, Yan LM, Wan L, Xiang TX, le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020. [DOI] [PubMed]
- 17.Grag S, Kim L, Witaker M, et al. Hospitalization rates and characteristics of patients hospitalized with CDC. Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•• Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rrivera, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020. 10.1016/j.tmaid.2020.101623. This paper is a complete assessment review. [DOI] [PMC free article] [PubMed]
- 19.Tang N, Li D, Xiong Wang ZS. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57(2020):279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infect Dis Soc Am. 2020. 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed]
- 23.•• James M, Sanders ML, Monogue TZ, Jodlowski JBC. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) a review. JAMA. 2020. 10.1001/jama.2020.6019. This paper summarizes the results of the clinical trials involving the different drugs proposed for COVID'19 management. [DOI] [PubMed]
- 24.Soheil Kooraki MH, Lee Myers AG. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol. 2020;17(4):447–451. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Studemeister A, Redinski J, Ahmed S, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang D, Guanhua D, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Mingfeng H, Tiantian L, et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binqing F, Xu X, Wei H, et al. Why tocilizumab could be an effective treatment for severe COVID - 19 ? J Transl Med. 2020;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang N, Bai H, Chen X, Gong J, Dengju Li ZS. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/JTH.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llau JV, Ferrandis R, Sierra P, et al. Propuesta de recomendaciones de manejo de fármacos anticoagulantes y antiagregantes en los pacientes graves con infección por COVID-19. SEDAR. 2020. https://www.sedar.es/images/site/NOTICIAS/coronavirus/RECOMENDACIONES_hemostasia-COVID-final.pdf. [DOI] [PMC free article] [PubMed]