The DNA and protein complex known as chromatin is subject to posttranslational modifications (PTMs) that regulate cellular functions such that PTM dysregulation can lead to disease, including cancer. One critical PTM is acetylation/deacetylation, which is being investigated as a means to develop targeted cancer therapies. The histone acetyltransferase (HAT) family of proteins performs histone acetylation. In humans, MOF (hMOF), a member of the MYST family of HATs, acetylates histone H4 at lysine 16 (H4K16ac).
KEYWORDS: cancer, DDR, histone acetyltransferase, MOF, stem cell
ABSTRACT
The DNA and protein complex known as chromatin is subject to posttranslational modifications (PTMs) that regulate cellular functions such that PTM dysregulation can lead to disease, including cancer. One critical PTM is acetylation/deacetylation, which is being investigated as a means to develop targeted cancer therapies. The histone acetyltransferase (HAT) family of proteins performs histone acetylation. In humans, MOF (hMOF), a member of the MYST family of HATs, acetylates histone H4 at lysine 16 (H4K16ac). MOF-mediated acetylation plays a critical role in the DNA damage response (DDR) and embryonic stem cell development. Functionally, MOF is found in two distinct complexes: NSL (nonspecific lethal) in humans and MSL (male-specific lethal) in flies. The NSL complex is also able to acetylate additional histone H4 sites. Dysregulation of MOF activity occurs in multiple cancers, including ovarian cancer, medulloblastoma, breast cancer, colorectal cancer, and lung cancer. Bioinformatics analysis of KAT8, the gene encoding hMOF, indicated that it is highly overexpressed in kidney tumors as part of a concerted gene coexpression program that can support high levels of chromosome segregation and cell proliferation. The linkage between MOF and tumor proliferation suggests that there are additional functions of MOF that remain to be discovered.
INTRODUCTION
The nucleosome in eukaryotes is a structural complex of genomic DNA bound to an octameric histone core. It is comprised of two copies each of histones (H2A, H2B, H3, and H4). Histones have numerous sites subject to posttranslational modifications (PTMs), like acetylation, methylation, and phosphorylation, etc. (1), which are utilized to fine-tune gene function. As such, dysregulation of histone modification can give rise to genomic instability, a key player in the development of cancer (2, 3).
MOF was first reported as a part of the dosage compensation complex (DCC) (4) or male-specific lethal (MSL) complex in Drosophila melanogaster, where it plays a key role in equalizing gene expression on the X chromosome between female and male flies (5–7). The MSL complex binds to the male X chromosome and acetylates H4 at lysine 16 (H4K16ac), leading to enhanced transcription (5–7). The loss of H4K16 acetylation due to MOF gene mutations therefore leads to fly death, hence the MSL phenotype and MSL complex name (8). In Drosophila, the MSL complex is a multiprotein assembly comprising the MSL1, MSL2, MSL3, MLE, and MOF proteins as well as two noncoding RNAs (rox1 and rox2). In humans, MSL (hMSL) is a complex that is comprised of MOF, MSL1, MSL2, and MSL3 and has the same substrate specificity as that of the Drosophila MSL complex, with MOF being the integral component of the MSL complex and directly involved in acetylating H4K16 (9).
MOF activity is associated with a wide range of cellular functions, including cell cycle regulation, the DNA damage response (DDR), and stem cell development (8, 10, 11). The human counterpart of Drosophila mof (MOF) shares significant similarity to Drosophila mof at the protein domain level and, like human MOF, contains a chromodomain, a central histone acetyltransferase (HAT) domain, and a C2HC-type zinc finger domain (12–14). The functions of these domains are highly conserved from fruit flies to humans, and hMOF is also central to H4K16 acetylation, even though dosage compensation in humans is mechanistically different (8). These studies all point to a complex spectrum of functions for MOF that, to date, are known to impact the cell cycle, the DDR, and stem cell development. This review addresses the complex yet vital functions of MOF and its link to cell proliferation in relation to cancer development.
MOF as a central histone acetyltransferase in global H4K16 acetylation.
In cells, histone acetylation and deacetylation are controlled by HATs and histone deacetylases (HDACs), which play an important role in the regulation of gene function (9, 12, 15). MOF is a member of the MYST (MOZ, Ybf2, Sas2, and Tip60) family of HATs (8) and is characterized by the presence of a MYST domain, which catalyzes the transfer of an acetyl group from acetyl coenzyme A to a histone lysine (8). A chromatin immunoprecipitation study of the genome-wide H4K16ac distribution in HEK293 cells identified 25,893 DNA regions (average length of 692 nucleotides). It was found that chromosome ends have areas with high H4K16ac levels, while centromeric regions were largely free of H4K14ac, with 82% of H4K16ac modification sites being located within genes. The results of this study imply that the role of H4K16ac in transcription regulation is cell type dependent and is secondary compared to other epigenetic markers like DNA methylation, which may override the histone code in differentiated cells. It appears from this study that MOF-dependent transcriptional regulation could be important only when cells are exposed to certain stimuli, suggesting that MOF may have a critical function in the transcriptional regulation of certain genes in specific cell types (16). It is known that stimuli like DNA damage modulate MOF function and its associated H4K16ac in physiological phenomena like the DDR and oncogenesis (17, 18).
MOF as a regulator of gene expression beyond histones.
MOF is one of the main HATs involved in histone acetylation, but it also acetylates proteins other than histone H4, including transcription factors like p53 and Nrf2 (19, 20). Sykes and coworkers reported that p53 Lys120 modification occurs rapidly after DNA damage and is catalyzed by MOF and Tip60 (19). Mutation of p53 at Lys120 occurs frequently in human cancers and reduces its ability to induce apoptosis by abolishing the transcription of proapoptotic genes like Bax and PUMA (19). The depletion of MOF and Tip60 also inhibits Bax and PUMA transcriptional activation. Those authors postulate that MOF- and Tip60-mediated Lys120 acetylation is a mechanism by which cells shift the balance between p53-induced cell cycle arrest and apoptosis (19). In addition to p53, Chen et al. (20) reported that MOF acetylates Nrf2 in lung cancer cells, where the MOF/KAT8 gene is reportedly overexpressed. MOF acetylates Nrf2 at Lys588 and thereby increases Nrf2 nuclear retention. This helps cells endure the replication stress associated with cancer, suggesting the possibility of targeting MOF as part of precision medicine in the treatment of non-small-cell lung cancer (NSCLC).
The chromatin nucleolar remodeling complex (NoRC) silences rRNA genes (rDNA) by inducing the formation of heterochromatin at rDNA promoters (21). TIP5 is the largest subunit of the NoRC. Histone H2A.X is specifically deposited at rDNA, which is responsible for the recruitment of NoRC to repress rRNA synthesis, thereby impacting embryonic stem cell (ESC) proliferation (21). MOF acetylates TIP5 at Lys633, which is adjacent to the TIP5 RNA binding domain (22). The acetylation of TIP5 at Lys633 is deacetylated by SIRT1 (sirtuin 1), thereby regulating the interaction of the NoRC with promoter-associated RNA and consequently influencing heterochromatin formation, nucleosome positioning, and rDNA silencing. The MOF-induced NoRC facilitates the fine-tuning of cellular function by responding to cellular bioenergetics, and its level fluctuates during S phase (22).
MOF as a regulator of the DNA damage response.
Ataxia telangiectasia mutated (ATM), a serine/threonine kinase and a central protein in the initiation of DNA double-strand break (DSB) repair, is mutated in patients with ataxia telangiectasia (23). Such patients have a progressive neurodegenerative disease that involves cerebellar atrophy, neuromotor dysfunction, and radiosensitivity that contributes to a genetic predisposition to innumerable types of cancer. After the induction of DNA damage, ATM phosphorylates multiple downstream substrates to orchestrate a collective DDR (24). One of the early events in the cellular response to DNA damage is the autophosphorylation of ATM at Ser1981, which activates ATM by converting it from an inactive dimeric form to an active monomeric form, which subsequently initiates the downstream signaling cascade (25–27).
In a seminal 2005 study, Gupta and coworkers (28) reported that MOF interacts with ATM and that the overexpression of wild-type MOF enhances DNA repair of ionizing radiation (IR)-induced damage and that MOF depletion impairs IR-induced DNA repair. Furthermore, the normal IR-induced increase in ATM autophosphorylation was decreased after MOF ablation (28). Additionally, MOF was required for the optimal functioning of both the nonhomologous end joining (NHEJ) and homologous recombination (HR) DDR pathways (29, 30). The role of MOF in the DDR was confirmed by Li et al. (17), who showed that Mof depletion in mouse embryonic fibroblasts reduced the formation of repair foci containing Mdc1, 53BP1, and Brca1. Gupta and coworkers (31) further reported that MOF is phosphorylated at Thr392 by ATM and that mutation of Thr392 abrogated DNA repair in S- and G2-phase but not G1-phase cells (31). MOFT392A mutant overexpression results in a failure of 53BP1 displacement and reduced BRCA1 accumulation at DNA DSBs in S/G2-phase cells. Collectively, these data support a model where MOF modulates 53BP1 function to help regulate DSB repair pathway choices.
MOF is required for oncogenesis and embryogenesis, and MOF overexpression can enhance oncogenesis and tumor relapse (18). The induction of replicative stress in MOF-depleted cells was recently shown to be accompanied by decreased replication fork speed, increased fork stalling, and additional firings at new origins (32). Proliferating cell nuclear antigen (PCNA), a central coordinator of replication protein recruitment (33), was found to interact with MOF, which influenced PCNA ubiquitination and recruitment at DNA damage sites. The depletion of MOF also reduced the DDR by decreasing the recruitment of MRE11, RPA70, and RAD51 to damage sites. MOF depletion also reduced DNA end resection and decreased CHK1 phosphorylation after exposure to replicative stress (32). These results point to a model where MOF is an important component of the cell DNA repair machinery, as it aids in the recruitment of important components of the complex DDR pathway.
Role of MOF in cellular death.
MOF plays an integral role in embryogenesis, as the deletion of the mouse Mof gene results in cell death and early embryonic lethality (18). The corresponding loss of H4K16ac after Mof depletion correlates with reduced proliferation and cell death. Kumar and coworkers generated Purkinje cell-specific Mof-deficient mice using the Cre/loxP recombination system, Mofflox/flox mice, which contained two loxP sites flanking exon 3 of the Mof gene. These mice were crossed with pcp2-Cre transgenic mice [B6.129-Tg(Pcp2-cre)2Mpin/J] expressing Cre recombinase under the control of an L7/Pcp2 promoter (Mofff/Pcp2-Cre− mice), and it was reported that Purkinje cell-specific gene deletion in mice leads to neurological abnormalities resembling those seen in individuals with ataxia telangiectasia: impaired fine motor coordination, balance deficits, and a backward-walking phenotype (34). Gupta and coworkers (35) reported that T-cell-specific Mof deletion (Moff/f/Lck-Cre+ mice) resulted in a smaller thymus, a larger spleen, and a blockage of T-cell differentiation. The mice also displayed reduced body weight and genomic instability in B cells (35), suggesting that aberrant chromatin-modifying factors could contribute to leukemia and lymphoma development. As noted above, MOF acetylates p53 at Lys120 in cells exposed to IR and upregulates the expression of proapoptotic factors like Bax and PUMA, thereby promoting apoptosis.
As in T-cell development, studies in mice with a male germ cell-specific Mof gene deletion have identified a role in cell development (36). In mice, Mof influences meiotic sex chromosome inactivation by facilitating three waves of H2AX phosphorylation expansion in meiotic prophase I (36). The meiotic prophase starts with the leptotene phase, in which condensation of the chromosome occurs, followed by the zygotene phase, where there is synapsis between homologous chromosomes. After the zygotene phase, cells enter the pachytene phase, where crossing over between chromosomes occurs, followed by the diplotene phase and dissolution of the synaptonemal complex. Mof deletion blocked the chromatin-wide expansion of H2AX phosphorylation in most leptotene spermatocytes (36). However, γ-H2AX foci could still be observed, indicating that Mof is not required for the initiation of H2AX phosphorylation at this stage in germ cells. Collectively, these studies suggest that MOF is involved in the expansion, but not initiation, of the first two waves of H2AX phosphorylation. Functionally, MOF loss is associated with massive germ cell loss in the testes, with a higher ratio of leptotene to zygotene spermatocytes and a lower ratio of pachytene spermatocytes (36).
The H4K16ac state has been shown to correlate with autophagy-related cell death (37, 38). Autophagy has evolved as a mechanism to overcome cell stress, but it also contributes to cell death in certain cases (38, 39). The downregulation of hMOF/Kat8/MYST correlates with reduced H4K16ac levels (37), which impacts autophagy by altering autophagy-related gene expression. SIRT1 is a NAD-dependent deacetylase, and its overexpression results in increased basal levels of autophagy. SIRT1 can form a molecular complex with essential components of the autophagy molecular machinery, including the autophagy genes Atg5, Atg7, and Atg8 (40). SIRT1 has many histone targets, but H4K16 is its primary histone target (41, 42); therefore, hMOF works in a manner antagonistic to SIRT1 by inducing H4K16 acetylation. Thus, the balance between SIRT1 deacetylase and MOF acetylation activities in H4K16 acetylation regulates autophagy and the outcome of autophagy in cells.
Role of MOF in embryonic stem cell development.
ESCs are pluripotent cells that can undergo repeated cycles of self-renewal or differentiate into any cell lineage. Since mouse embryos with a deletion of Mof did not develop beyond the expanded blastocyst stage, it was suspected that MOF might play an important role in stem cell development (43). Building upon these observations, Li and coworkers (44) first reported the direct involvement of MOF in regulating the embryonic stem cell core transcription network. By using MOF/KAT8 conditional knockout embryonic stem cell lines, those authors showed that MOF directly regulates core ESC transcription factors such as Nanog, Oct4, and Sox2. Interestingly, the MOF-null phenotype was rescued by the ectopic expression of Nanog (44), indicating that Nanog is the key downstream MOF target in ESCs. Furthermore, 80% of Nanog target genes were found to have MOF binding sites. Yet although MOF and Nanog targets show significant similarity, these proteins do not bind the same sequence (44). Chromatin immunoprecipitation sequencing (ChIP-seq) analysis indicates that MOF binding sites in mammals are enriched at transcription start sites (TSSs) and evenly distributed in downstream coding regions, which is a distinct ESC feature. Ang and coworkers (45) have shown that knocking down the MLL complex Dpy30/Rbbp5 or Wdr5 gene significantly reduced histone H3 lysine 4 trimethylation (H3K4me3) in ESCs. In continuation of this work, it was reported that only Wdr5 knockdown significantly changes the expression of self-renewal genes (i.e., Nanog, Oct4, and Sox2) (44). The depletion of Mof leads to both increased and decreased expression levels of its direct targets. This suggests that Mof functions as a chromatin modulator, regulating the chromatin environment and thereby fine-tuning the transcription machinery as it passes the transcribed region. Mof is required for Wdr5 recruitment to chromatin, and Wdr5 functions as part of the Mof complex, regulating transcription in ESCs (44). Additionally, Mof regulates H3K4me3 at important bivalent domains, including those at promoters of stem cell regulatory genes like Nanog and Sox2, which was further supported by genome-wide analyses (41). Mujoo and coworkers (46) further reported that MOF and its associated H4K16 acetylation, as well as Nanog, were downregulated during the differentiation of induced pluripotent stem cells (iPSCs).
MOF has been established as part of the MSL complex in Drosophila melanogaster, and the MSL complex is involved in the 2-fold upregulation of H4K16ac and gene transcription from the single male X chromosome (47). This function of the MSL complex thus is balancing gene expression from a single male X chromosome to expression from two X chromosomes in females. The NSL (nonspecific lethal) complex, which includes NSL1, NSL3, MCRS2, and MBD-R2, has been found to localize at the promoters of over 4,000 genes. In Drosophila melanogaster, NSL proteins are enriched at gene promoters of constitutively active housekeeping genes, and NSL target promoters are highly enriched in histone modifications like H4K16ac, H3K9ac, H3K4me2, and H3K4me3, which are known to be markers of active chromatin (48). In mouse embryonic stem cells (mESCs), Mof-associated MSL and NSL complexes bind to both specific and common sets of genes (49). By using a combination of ChIP-seq and genetic knockdown experiments, NSL was shown to bind specifically to promoters, while MSL binds to gene bodies: NSL regulates cell growth, and MSL specifically acts in H4K16 acetylation. MSL is essential for regulating key mESC-specific and bivalent developmental genes. In this study, the authors also reported an evident overlap between the genes upon which the NSL and MSL complexes act (49). However, both NSL and MSL also each have their own set of exclusive genes to which they bind: NSL is more specific to housekeeping gene functions, while MSL binding is more specific to embryonic stem cell and neuronal progenitor cell development. Proteomic analysis also found the incorporation of Msl1 and Nsl1 together with Mof in their respective endogenous complexes. Further experiments suggested the potential existence of free Mof that is not part of either the NSL or MSL complex.
Interestingly, both the MSL and NSL complexes act synergistically through distinct pathways for a fail-safe mechanism to repress the X chromosome in ESCs. Both complexes influence transcription by targeting promoters and TSS-distal enhancers. Unlike Drosophila, the MSL complex here is not enriched at the X chromosome. However, it is important for the regulation of the mammalian X chromosome by regulating Tsix, the major repressor of Xist long noncoding RNA (lncRNA). The depletion of MSL leads to reduced Tsix expression, thereby leading to an enhanced accumulation of Xist lncRNA, which is involved in the inactivation of one of two X chromosomes in females during the differentiation of stem cells. At the same time, the NSL complex orchestrates the production of multiple proteins that drive the development of embryonic stem cells as well as the repression of X chromosome inactivation (50). Valerio and coworkers (51) used vav1-cre-induced MOF/KAT8 conditional knockouts to establish that MOF is required for hematopoietic cell maintenance and hematopoietic stem cell (HSC) engraftment during adult hematopoiesis. This work further supports the hypothesis that chromatin remodelers like MOF are crucial for hematopoiesis during various stages of development. The roles of MOF in different aspects of stem cell development are summarized in Table 1.
TABLE 1.
MOF regulation of embryonic stem cell development
| Stem cell type | Molecular target(s) | Biological function(s) | Reference |
|---|---|---|---|
| Mof in mouse embryonic stem cell development | H4K16 acetylation | Maintain normal chromatin structure of early male and female embryos | 43 |
| Regulate expanded blastocyst stage | |||
| Loss leads to peri-implantation lethality | |||
| Nanog, Oct4, Sox2, Wdr5, H3K4me | Function as a chromatin modulator | 44 | |
| Fine-tune the transcription machinery as they pass the transcribed region | |||
| NSL, MSL | NSL binds exclusively at promoters | 49 | |
| MSL binds in gene bodies | |||
| Essential for mouse embryonic stem cell pluripotency and early development | |||
| NSL and MSL complexes act synergistically through distinct pathways for a fail-safe mechanism to repress the X chromosome | |||
| Tsix, Xist lncRNA | Tsix regulates Xist lncRNA, resulting in inactivation of 1 of 2 X chromosomes in females | 50 | |
| Mof in hematopoietic stem cell development | H4K16 acetylation | Maintenance of hematopoietic cell and HSC engraftment during adult hematopoiesis | 51 |
| MOF in iPSCs | H4K16 acetylation, Nanog | Downregulated during differentiation | 46 |
Dysregulation of MOF activity and cancer.
MOF and H4K16 acetylation perform critical functions in the DDR pathway. This observation supports the notion that the overexpression of MOF/KAT8 and its associated H4K16 acetylation could provide a survival advantage to cancer cells, especially since tumor cells overexpressing MOF show an enhanced resolution of DNA damage foci (29). Like many central proteins involved in development and regulation of the DDR pathway, the MOF/KAT8 gene is deregulated in most cancers studied to date. From an analysis of a panel containing normal tissues, cancer cell lines, and primary tumors, Fraga and coworkers found that cancer cells display a loss of monoacetylated and trimethylated forms of histone H4 (52). In a model of multistage carcinogenesis, they showed that these changes appeared early and accumulated during oncogenesis. Subsequent analysis of H4K16 acetylation in breast cancer and medulloblastoma tissues revealed a downregulation of MOF/KAT8 in 40% of cases, while genomic loss occurred in 11% of cases (53). Follow-up studies with different cancers such as renal cell carcinoma (RCC) (54), gastric cancer (55), ovarian cancer (56), and colorectal cancer (57) also indicated reduced expression of MOF/KAT8. Subsequently, Cao et al. (57) reported low expression of MOF/KAT8 in colorectal carcinoma lymph node metastasis and tumor metastasis. However, there was no association between MOF/KAT8 expression and tumor type.
Studies of MOF/KAT8 expression in lung cancers found that the gene is significantly overexpressed in non-small-cell lung cancer (NSCLC), and H4K16 acetylation was also more evident than in normal tissues (58, 59). Thus, MOF evidently promotes oncogenesis by inducing the cell proliferation, migration, and adhesion of NSCLC cell lines and also promotes S-phase entry via the cell cycle progression protein Skp2 (59). Collectively, these data indicate that MOF promotes NSCLC oncogenesis. In a recent study, Valerio and coworkers (60) found that MOF was important for leukemia cell growth by using an RNA interference (RNAi)-based screen to identify druggable modulators of chromatin in leukemia associated with oncogenic rearrangements of the MLL gene. In endometrial carcinoma, MOF is highly expressed and associated with the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt and Ras–Raf–MEK–extracellular signal-regulated kinase (ERK) signaling pathways, thus enhancing proliferation (61). Furthermore, a critical role for hMOF in the vascular invasion of hepatocellular carcinoma (HCC) via transcription activation of key genes involved in this process confirms the role of MOF in HCC progression and suggests a need for targeting hMOF for therapy (62).
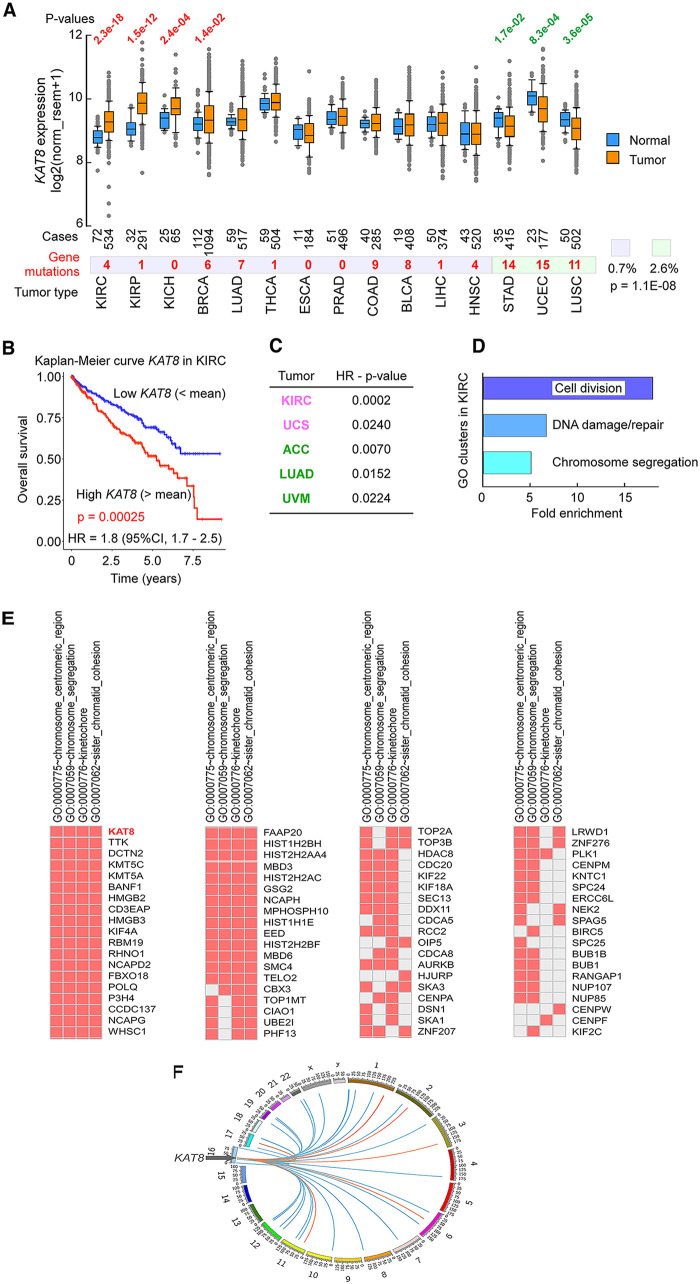
Here, we used our in-house pipeline (63) to conduct an analysis of MOF/KAT8 expression and its relationship to patient survival in cancer from The Cancer Genome Atlas (TCGA). This repository comprises gene expression and clinical data from >11,000 patients with 33 different tumor types and thus provides a robust assessment of the implications of gene dysregulation in cancer. For the 15 tumor types in which matching control samples were also taken from normal tissues of some of the same patients, MOF/KAT8 was significantly overexpressed in the kidney (kidney renal clear cell carcinoma [KIRC], kidney renal papillary cell carcinoma [KIRP], and kidney chromophobe [KICH]) and breast invasive carcinoma (BRCA) and moderately downregulated in stomach adenocarcinoma (STAD), uterine corpus endometrial carcinoma (UCEC), and lung squamous cell carcinoma (LUSC) (Fig. 1A). To address if mutations in the KAT8 gene could impact its expression, we compiled all types of mutations in the KAT8 gene (https://cancer.sanger.ac.uk/cosmic/) and noted that these occurred more commonly in the 3 tumor types with low expression levels (STAD, UCEC, and LUSC) than in the other 12 types (2.6% versus 0.7%; P = 1.1 × 10−8 by Fisher’s exact test) (Fig. 1A). The methylation status of the KAT8 promoter has been profiled in only a few TCGA samples, 8 with probe cg02220965 and 1 with probe cg06205614, but in all cases, there is evidence of hypermethylation. Therefore, it is possible that the high number of KAT8 mutations in tumors where KAT8 is downregulated arises as a secondary effect of gene silencing, perhaps due in part to decreased DNA repair in regions of low transcription (64).
FIG 1.
MOF/KAT8 expression in cancer genomes from TCGA. (A) Box plot of MOF/KAT8 expression in tumors and matched controls with the number of samples profiled (P values from Wilcoxon tests). Red, number of mutations in the KAT8 gene in all samples subjected to mutation analysis (https://cancer.sanger.ac.uk/cosmic/); purple highlighting, high or normal KAT8 mRNA levels in the tumor; green highlighting, low mRNA levels in the tumor (P value from Fisher’s exact test). (B) Kaplan-Meier curves and hazard ratios (HR) from the Cox proportional-hazards regression model in kidney renal clear cell carcinoma (KIRC) patients with high (above the mean) and low (below the mean) MOF/KAT8 mRNA values (P value from a log rank test). CI, confidence interval. (C) P values of hazard ratios for patients with poor outcomes when expressing high (above the mean) (magenta) or low (below the mean) (green) MOF/KAT8 mRNA levels in the tumor. (D) Bar plot of fold enrichments of the 3 main clusters of related gene ontology (GO) terms from GSEA for 20,529 genes in KIRC with significant hazard ratios for poor outcomes when MOF/KAT8 mRNA levels were above the mean in the tumor. (E) List of genes comprising the GO terms of the “chromosome segregation” cluster from panel D. (F) Circos plot of the chromosomal location of genes from panel E significantly (P < 0.01 from a t distribution of linear regression) correlated (blue links) or anticorrelated (orange links) with MOF/KAT8 mRNA levels in KIRC patients. THCA, thyroid carcinoma; ESCA, esophageal carcinoma; PRAD, prostate adenocarcinoma; COAD, colon adenocarcinoma; BLCA, bladder urothelial carcinoma; LIHC, liver hepatocellular carcinoma; HNSC, head and neck squamous cell carcinoma; UCS, uterine carcinosarcoma.
The exceptional overexpression of MOF/KAT8 in KIRC prompted us to examine whether its dysregulation could impact patient survival by analyzing the Kaplan-Meier (KM) estimator and the associated Cox-derived hazard ratios. The KM survival analysis is a nonparametric statistical test used to compare the fractions of patients living for a given period under different conditions, in our case high and low KAT8 expression, whereas the Cox hazard ratio is a measure of the increase or decrease in the hazard that an event (in our case, death) will occur. These tests predicted that KIRC patients with MOF/KAT8 mRNA levels above the mean (in the tumor) would have a shorter life span than those with lower levels (Fig. 1B). This analysis of TCGA data suggests that MOF supports tumor growth and/or metastasis in KIRC. This role may be tumor type specific, as we observed the opposite trend in adrenocortical carcinoma (ACC), lung adenocarcinoma (LUAD), and uveal melanoma (UVM), although here, the hazard ratios were considerably weaker than in KIRC (Fig. 1C).
To aid in the interpretation of how increased MOF/KAT8 expression would impact patient outcomes, we extended the Cox-derived hazard ratios to all 20,529 genes in KIRC. We then looked for sets of genes enriched in specific “gene ontology terms” using gene set enrichment analysis (GSEA), which informs on dysregulated pathways impacting patient survival. At a threshold of a P value of <0.001, there were 2,413 genes that would confer poor survival in patients in which any of these genes were expressed at mRNA levels above the mean. These genes arose predominantly from 3 clusters of pathways involved in regulating the cell cycle and cell proliferation (cluster 1), the response to DNA damage and DNA repair (cluster 2), and chromosome segregation and centrosome assembly (cluster 3) (Fig. 1D). MOF/KAT8 was found in the third cluster, along with 75 other genes, about half of which play a role in the centromeric region, chromosome segregation, kinetochore function, and sister chromatid cohesion (Fig. 1E).
We then performed a comprehensive gene expression correlation analysis (GECA) between MOF/KAT8 and all other genes in the TCGA data set to assess the extent to which its expression would be coordinated with those of other genes in the cluster 3 pathways (Fig. 1D and E). In both tumor and normal tissues, MOF/KAT8 expression tended to correlate with those of cluster 3 genes, although in tumors, the strongest coexpressions were observed with nearby genes on 16p, where MOF/KAT8 resides. In KIRC, 40/75 genes from cluster 3 were significantly coexpressed with MOF/KAT8 (Fig. 1F). This fraction is significant compared to that of all genes exhibiting coexpression with MOF/KAT8 in KIRC (7,601/20,529; P = 0.005 by Fisher’s exact test), confirming the prediction that genes within the MOF/KAT8 pathway(s) are coregulated.
Taken together, these data and the analyses reported here suggest that hMOF plays an important role in regulating histone modification in genes that perform functions in chromosome segregation and, to a broader extent, cell division and proliferation, a function mirroring that observed in Drosophila (65). Overexpression in cancer may reflect increased cell proliferation, which triggers a replication stress response, as suggested by the concomitant activation of DNA damage and repair pathways, ultimately supporting tumor growth at the expense of patient outcomes.
All the above-described studies point to a model whereby the upregulation or downregulation of MOF and the accompanying H4K16 acetylation levels contribute to oncogenesis. However, it has been reported that hMOF suppresses hepatocellular carcinoma growth (66). Therefore, MOF/KAT8 expression has to be tightly regulated in cells since histone acetylation levels represent a dynamic balance between histone acetyltransferases and histone deacetylases, which in turn are regulated by other enzymes. Much more research is required to dissect out how MOF activity is regulated in cells.
Conclusion and future directions.
In this review, we examine the myriad functions of MOF and how it has emerged as a key protein in regulating multiple important cancer-relevant cellular events. Notably, these functions include the DDR, oncogenesis, embryogenesis, and stem cell development, as summarized in Table 2. MOF exists as part of two distinct complexes (MSL and NSL), which regulate H4K16 acetylation, and targets of the NSL complex extend beyond H4K16 acetylation. Research into these multiprotein complexes is still progressing, with new evidence emerging from various studies that will shed light on these targets. Future work will elucidate how these complexes are regulated and how these multiprotein complexes modulate gene function for optimal cellular output. One main MOF research focus is to decipher the upstream regulator(s) of MOF activity and the distinct downstream targets of MOF, as it has been found to act in multiple cellular processes, and the list keeps growing.
TABLE 2.
MOF functional roles in different aspects of the DNA damage response, embryonic stem cell development, cellular death, and H4K16 acetylation
| Biological function | Function(s) of MOF | Reference(s) |
|---|---|---|
| Regulation of gene expression by H4K16ac | Regulation of gene functions by influencing opening of chromatin | 9, 14 |
| MOF is essential for oncogenesis and embryogenesis | 18 | |
| DNA damage response | Interacts with and impacts ATM autophosphorylation at Ser1981 | 28 |
| Required for optimal functioning of NHEJ and HR pathways | 29 | |
| MOF phosphorylation at T392 site by ATM regulates DNA DSB pathway choice | 31 | |
| Reduced MOF levels correlate with reduced replication fork speed | 32 | |
| Cellular death | p53 lysine 120 modification influences activation of proapoptotic genes | 19 |
| MOF depletion leads to downregulation of autophagy | 37 | |
| MOF depletion results in death of Purkinje cells, leading to neurological abnormality | 34 | |
| Stem cell development | Regulation of embryonic stem cell core transcriptional network | 44 |
| MOF is associated with differentiation of iPSCs | 46 | |
| MOF is required for hematopoietic stem cell maintenance | 51 | |
A recent and surprising result is that MOF regulates transcription and respiration in mitochondria (67). Chatterjee and coworkers (67) found that MOF, along with its NSL complex, is present in mitochondria and regulates oxidative phosphorylation (OXPHOS) by influencing the expression of respiratory genes from both nuclear DNA and mitochondrial DNA (mtDNA). MOF, along with members of the NSL complex, KANSL1 and KANSL3, was found in the mitochondria. It was found that MOF regulates the expression of genes involved in OXPHOS in aerobically respiring cells. A reduction in the level of MOF/KANSL1 resulted in a significant downregulation of mtDNA transcription, leading to impaired cellular respiration. This study indicates the importance of MOF enzymatic activity in mitochondrial function (67). Furthermore, MOF binds mitochondrial DNA, and MOF/KAT8 conditional knockout mice developed hypertrophic cardiomyopathy and cardiac failure due to the involvement of MOF in oxidative phosphorylation. Moreover, Mof influences H2AX phosphorylation and spermatogenesis in mice and regulates male meiosis by influencing the expansion of all three waves of H2AX phosphorylation from the leptotene to the pachytene stages (36). Furthermore, Horikoshi and coworkers (68) reported the significance of preexisting levels of H4K16ac by hMOF in gene-rich regions to impact DSB repair by the homologous recombination pathway. These recent results suggest that there is much more to MOF biology and its myriad functions.
We note that MOF is dysregulated in many cancer tissues, including ovarian cancer, medulloblastoma, colorectal cancer, renal cell carcinoma, as well as non-small-cell lung cancer. Intriguingly, MOF/KAT8 levels are both downregulated and upregulated within different types of cancers (Fig. 1). This finding exemplifies the rebalancing of MOF in specific tumors and emphasizes the need for further research to understand the molecular roles of MOF in cancer pathogenesis and their relationship to cancer prognoses. Since MOF is associated with stem cell development and oncogenesis, another important aspect will be whether MOF dysregulation contributes to cancer stem cell maintenance. From the collective literature review and cancer database analyses here, we suggest that MOF dysregulation merits examination as a useful tumor marker and a potential guide for precision cancer therapies.
ACKNOWLEDGMENTS
This work was supported by grants GM109768-06 and RO1 CA129537 (T.K.P.) plus CA092584 and R35 CA220430 (J.A.T.) from the National Institutes of Health. J.A.T. is also supported by Cancer Prevention Research Institute of Texas (CPRIT) grants RP180813 and RP130397 and a Robert A. Welch Chemistry Chair. The research used resources from the Texas Advanced Computing Center (TACC) supported by National Science Foundation grant ACI-1134872.
We declare no competing interests.
REFERENCES
- 1.Hunt CR, Ramnarain D, Horikoshi N, Iyengar P, Pandita RK, Shay JW, Pandita TK. 2013. Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat Res 179:383–392. doi: 10.1667/RR3308.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldmann T, Schneider R. 2013. Targeting histone modifications—epigenetics in cancer. Curr Opin Cell Biol 25:184–189. doi: 10.1016/j.ceb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Kelly TK, Jones PA. 2010. Epigenetics in cancer. Carcinogenesis 31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belote JM, Lucchesi JC. 1980. Male-specific lethal mutations of Drosophila melanogaster. Genetics 96:165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi JC. 1997. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J 16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bone JR, Kuroda MI. 1996. Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila. Genetics 144:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilfiker A, Yang Y, Hayes DH, Beard CA, Manning JE, Lucchesi JC. 1994. Dosage compensation in Drosophila: the X-chromosomal binding of MSL-1 and MLE is dependent on Sxl activity. EMBO J 13:3542–3550. doi: 10.1002/j.1460-2075.1994.tb06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rea S, Xouri G, Akhtar A. 2007. Males absent on the first (MOF): from flies to humans. Oncogene 26:5385–5394. doi: 10.1038/sj.onc.1210607. [DOI] [PubMed] [Google Scholar]
- 9.Pillus L. 2008. MYSTs mark chromatin for chromosomal functions. Curr Opin Cell Biol 20:326–333. doi: 10.1016/j.ceb.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikh BN, Bechtel-Walz W, Lucci J, Karpiuk O, Hild I, Hartleben B, Vornweg J, Helmstadter M, Sahyoun AH, Bhardwaj V, Stehle T, Diehl S, Kretz O, Voss AK, Thomas T, Manke T, Huber TB, Akhtar A. 2016. MOF maintains transcriptional programs regulating cellular stress response. Oncogene 35:2698–2710. doi: 10.1038/onc.2015.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mujoo K, Hunt CR, Horikoshi N, Pandita TK. 2017. A multifaceted role for MOF histone modifying factor in genome maintenance. Mech Ageing Dev 161:177–180. doi: 10.1016/j.mad.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Conrad T, Akhtar A. 2012. Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription. Nat Rev Genet 13:123–134. doi: 10.1038/nrg3124. [DOI] [PubMed] [Google Scholar]
- 14.Morales V, Straub T, Neumann MF, Mengus G, Akhtar A, Becker PB. 2004. Functional integration of the histone acetyltransferase MOF into the dosage compensation complex. EMBO J 23:2258–2268. doi: 10.1038/sj.emboj.7600235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberland M, Montgomery RL, Olson EN. 2009. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horikoshi N, Kumar P, Sharma GG, Chen M, Hunt CR, Westover K, Chowdhury S, Pandita TK. 2013. Genome-wide distribution of histone H4 lysine 16 acetylation sites and their relationship to gene expression. Genome Integr 4:3. doi: 10.1186/2041-9414-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, Min J, Dou Y. 2010. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Mol Cell Biol 30:5335–5347. doi: 10.1128/MCB.00350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK, Ludwig T, Pandita TK. 2008. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol 28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykes SM, Stanek TJ, Frank A, Murphy ME, McMahon SB. 2009. Acetylation of the DNA binding domain regulates transcription-independent apoptosis by p53. J Biol Chem 284:20197–20205. doi: 10.1074/jbc.M109.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Ye X, Tang N, Shen S, Li Z, Niu X, Lu S, Xu L. 2014. The histone acetylranseferase [sic] hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer. Br J Pharmacol 171:3196–3211. doi: 10.1111/bph.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eleuteri B, Aranda S, Ernfors P. 2018. NoRC recruitment by H2A.X deposition at rRNA gene promoter limits embryonic stem cell proliferation. Cell Rep 23:1853–1866. doi: 10.1016/j.celrep.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Schmitz KM, Mayer C, Yuan X, Akhtar A, Grummt I. 2009. Reversible acetylation of the chromatin remodelling complex NoRC is required for non-coding RNA-dependent silencing. Nat Cell Biol 11:1010–1016. doi: 10.1038/ncb1914. [DOI] [PubMed] [Google Scholar]
- 23.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NG, Taylor AM, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 24.Shiloh Y, Ziv Y. 2013. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14:197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 25.Bakkenist CJ, Kastan MB. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 26.Pandita TK. 2003. A multifaceted role for ATM in genome maintenance. Expert Rev Mol Med 5:1–21. doi: 10.1017/S1462399403006318. [DOI] [PubMed] [Google Scholar]
- 27.Pandita TK, Lieberman HB, Lim DS, Dhar S, Zheng W, Taya Y, Kastan MB. 2000. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 19:1386–1391. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK. 2005. Involvement of human MOF in ATM function. Mol Cell Biol 25:5292–5305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ, Cote J, Pandita TK. 2010. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol 30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Tian GW, Tang LR, Li G. 2019. hMOF reduction enhances radiosensitivity through the homologous recombination pathway in non-small-cell lung cancer. Onco Targets Ther 12:3065–3075. doi: 10.2147/OTT.S192568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Hunt CR, Hegde ML, Chakraborty S, Chakraborty S, Udayakumar D, Horikoshi N, Singh M, Ramnarain DB, Hittelman WN, Namjoshi S, Asaithamby A, Hazra TK, Ludwig T, Pandita RK, Tyler JK, Pandita TK. 2014. MOF phosphorylation by ATM regulates 53BP1-mediated double-strand break repair pathway choice. Cell Rep 8:177–189. doi: 10.1016/j.celrep.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh DK, Pandita RK, Singh M, Chakraborty S, Hambarde S, Ramnarain D, Charaka V, Ahmed KM, Hunt CR, Pandita TK. 2018. MOF suppresses replication stress and contributes to resolution of stalled replication forks. Mol Cell Biol 38:e00484-17. doi: 10.1128/MCB.00484-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mailand N, Gibbs-Seymour I, Bekker-Jensen S. 2013. Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol 14:269–282. doi: 10.1038/nrm3562. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Hunt CR, Gupta A, Nannepaga S, Pandita RK, Shay JW, Bachoo R, Ludwig T, Burns DK, Pandita TK. 2011. Purkinje cell-specific males absent on the first (mMof) gene deletion results in an ataxia-telangiectasia-like neurological phenotype and backward walking in mice. Proc Natl Acad Sci U S A 108:3636–3641. doi: 10.1073/pnas.1016524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Hunt CR, Pandita RK, Pae J, Komal K, Singh M, Shay JW, Kumar R, Ariizumi K, Horikoshi N, Hittelman WN, Guha C, Ludwig T, Pandita TK. 2013. T-cell-specific deletion of Mof blocks their differentiation and results in genomic instability in mice. Mutagenesis 28:263–270. doi: 10.1093/mutage/ges080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H, Gao Q, Zheng W, Yin S, Wang L, Zhong L, Ali A, Khan T, Hao Q, Fang H, Sun X, Xu P, Pandita TK, Jiang X, Shi Q. 2018. MOF influences meiotic expansion of H2AX phosphorylation and spermatogenesis in mice. PLoS Genet 14:e1007300. doi: 10.1371/journal.pgen.1007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fullgrabe J, Lynch-Day MA, Heldring N, Li W, Struijk RB, Ma Q, Hermanson O, Rosenfeld MG, Klionsky DJ, Joseph B. 2013. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature 500:468–471. doi: 10.1038/nature12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fullgrabe J, Klionsky DJ, Joseph B. 2013. Histone post-translational modifications regulate autophagy flux and outcome. Autophagy 9:1621–1623. doi: 10.4161/auto.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. 2008. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaquero A, Sternglanz R, Reinberg D. 2007. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene 26:5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- 42.Hajji N, Wallenborg K, Vlachos P, Fullgrabe J, Hermanson O, Joseph B. 2010. Opposing effects of hMOF and SIRT1 on H4K16 acetylation and the sensitivity to the topoisomerase II inhibitor etoposide. Oncogene 29:2192–2204. doi: 10.1038/onc.2009.505. [DOI] [PubMed] [Google Scholar]
- 43.Thomas T, Dixon MP, Kueh AJ, Voss AK. 2008. Mof (MYST1 or KAT8) is essential for progression of embryonic development past the blastocyst stage and required for normal chromatin architecture. Mol Cell Biol 28:5093–5105. doi: 10.1128/MCB.02202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Li L, Pandey R, Byun JS, Gardner K, Qin Z, Dou Y. 2012. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell 11:163–178. doi: 10.1016/j.stem.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, Wang J, Rendl M, Bernstein E, Schaniel C, Lemischka IR. 2011. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mujoo K, Pandita RK, Tiwari A, Charaka V, Chakraborty S, Singh DK, Hambarde S, Hittelman WN, Horikoshi N, Hunt CR, Khanna KK, Kots AY, Butler EB, Murad F, Pandita TK. 2017. Differentiation of human induced pluripotent or embryonic stem cells decreases the DNA damage repair by homologous recombination. Stem Cell Rep 9:1660–1674. doi: 10.1016/j.stemcr.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC. 2005. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol 25:9175–9188. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikh BN, Guhathakurta S, Akhtar A. 2019. The non-specific lethal (NSL) complex at the crossroads of transcriptional control and cellular homeostasis. EMBO Rep 20:e47630. doi: 10.15252/embr.201847630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravens S, Fournier M, Ye T, Stierle M, Dembele D, Chavant V, Tora L. 2014. Mof-associated complexes have overlapping and unique roles in regulating pluripotency in embryonic stem cells and during differentiation. Elife 3:e02104. doi: 10.7554/eLife.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chelmicki T, Dundar F, Turley MJ, Khanam T, Aktas T, Ramirez F, Gendrel AV, Wright PR, Videm P, Backofen R, Heard E, Manke T, Akhtar A. 2014. MOF-associated complexes ensure stem cell identity and Xist repression. Elife 3:e02024. doi: 10.7554/eLife.02024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valerio DG, Xu H, Eisold ME, Woolthuis CM, Pandita TK, Armstrong SA. 2017. Histone acetyltransferase activity of MOF is required for adult but not early fetal hematopoiesis in mice. Blood 129:48–59. doi: 10.1182/blood-2016-05-714568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Perez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. 2005. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 53.Pfister S, Rea S, Taipale M, Mendrzyk F, Straub B, Ittrich C, Thuerigen O, Sinn HP, Akhtar A, Lichter P. 2008. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int J Cancer 122:1207–1213. doi: 10.1002/ijc.23283. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Zhang R, Wu D, Lu Z, Sun W, Cai Y, Wang C, Jin J. 2013. Epigenetic change in kidney tumor: downregulation of histone acetyltransferase MYST1 in human renal cell carcinoma. J Exp Clin Cancer Res 32:8. doi: 10.1186/1756-9966-32-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L, Yang J, Zhao L, Yu X, Wang L, Wang F, Cai Y, Jin J. 2015. Expression of hMOF, but not HDAC4, is responsible for the global histone H4K16 acetylation in gastric carcinoma. Int J Oncol 46:2535–2545. doi: 10.3892/ijo.2015.2956. [DOI] [PubMed] [Google Scholar]
- 56.Cai M, Hu Z, Liu J, Gao J, Tan M, Zhang D, Zhu L, Liu S, Hou R, Lin B. 2015. Expression of hMOF in different ovarian tissues and its effects on ovarian cancer prognosis. Oncol Rep 33:685–692. doi: 10.3892/or.2014.3649. [DOI] [PubMed] [Google Scholar]
- 57.Cao L, Zhu L, Yang J, Su J, Ni J, Du Y, Liu D, Wang Y, Wang F, Jin J, Cai Y. 2014. Correlation of low expression of hMOF with clinicopathological features of colorectal carcinoma, gastric cancer and renal cell carcinoma. Int J Oncol 44:1207–1214. doi: 10.3892/ijo.2014.2266. [DOI] [PubMed] [Google Scholar]
- 58.Song JS, Chun S-M, Lee JY, Kim DK, Kim YH, Jang SJ. 2011. The histone acetyltransferase hMOF is overexpressed in non-small cell lung carcinoma. Korean J Pathol 45:386–396. doi: 10.4132/KoreanJPathol.2011.45.4.386. [DOI] [Google Scholar]
- 59.Zhao L, Wang DL, Liu Y, Chen S, Sun FL. 2013. Histone acetyltransferase hMOF promotes S phase entry and tumorigenesis in lung cancer. Cell Signal 25:1689–1698. doi: 10.1016/j.cellsig.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Valerio DG, Xu H, Chen CW, Hoshii T, Eisold ME, Delaney C, Cusan M, Deshpande AJ, Huang CH, Lujambio A, Zheng YG, Zuber J, Pandita TK, Lowe SW, Armstrong SA. 2017. Histone acetyltransferase activity of MOF is required for MLL-AF9 leukemogenesis. Cancer Res 77:1753–1762. doi: 10.1158/0008-5472.CAN-16-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi Y, Tan M, Zheng M, Jin S, Wang H, Liu J, Wang P, Nie X, Gao L, Lin B. 2020. Estrogen/estrogen receptor promotes the proliferation of endometrial carcinoma cells by enhancing hMOF expression. Jpn J Clin Oncol 50:241–253. doi: 10.1093/jjco/hyz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pote N, Cros J, Laouirem S, Raffenne J, Negrao M, Albuquerque M, Bedossa P, Godinho Ferreira M, Ait Si Ali S, Fior R, Paradis V. 2020. The histone acetyltransferase hMOF promotes vascular invasion in hepatocellular carcinoma. Liver Int 40:956–967. doi: 10.1111/liv.14381. [DOI] [PubMed] [Google Scholar]
- 63.Bacolla A, Ye Z, Ahmed Z, Tainer JA. 2019. Cancer mutational burden is shaped by G4 DNA, replication stress and mitochondrial dysfunction. Prog Biophys Mol Biol 147:47–61. doi: 10.1016/j.pbiomolbio.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Supek F, Lehner B. 2019. Scales and mechanisms of somatic mutation rate variation across the human genome. DNA Repair (Amst) 81:102647. doi: 10.1016/j.dnarep.2019.102647. [DOI] [PubMed] [Google Scholar]
- 65.Pavlova GA, Popova JV, Andreyeva EN, Yarinich LA, Lebedev MO, Razuvaeva AV, Dubatolova TD, Oshchepkova AL, Pellacani C, Somma MP, Pindyurin AV, Gatti M. 2019. RNAi-mediated depletion of the NSL complex subunits leads to abnormal chromosome segregation and defective centrosome duplication in Drosophila mitosis. PLoS Genet 15:e1008371. doi: 10.1371/journal.pgen.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Liu H, Pan H, Yang Y, Huang G, Yang Y, Zhou WP, Pan ZY. 2014. The histone acetyltransferase hMOF suppresses hepatocellular carcinoma growth. Biochem Biophys Res Commun 452:575–580. doi: 10.1016/j.bbrc.2014.08.122. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee A, Seyfferth J, Lucci J, Gilsbach R, Preissl S, Bottinger L, Martensson CU, Panhale A, Stehle T, Kretz O, Sahyoun AH, Avilov S, Eimer S, Hein L, Pfanner N, Becker T, Akhtar A. 2016. MOF acetyl transferase regulates transcription and respiration in mitochondria. Cell 167:722–738.e23. doi: 10.1016/j.cell.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 68.Horikoshi N, Sharma D, Leonard F, Pandita RK, Charaka VK, Hambarde S, Horikoshi NT, Gaur Khaitan P, Chakraborty S, Cote J, Godin B, Hunt CR, Pandita TK. 2019. Pre-existing H4K16ac levels in euchromatin drive DNA repair by homologous recombination in S-phase. Commun Biol 2:253. doi: 10.1038/s42003-019-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]