Abstract
We found substantial variation in resistance to the fly-specific pathogen Entomophthora muscae 'Berkeley' (Entomophthoromycota), in 20 lines from the Drosophila melanogaster Genetic Reference Panel (DGRP). Resistance to E. muscae is positively (r = 0.55) correlated with resistance to the broad host range ascomycete entomopathogen Metarhizium anisopliae (Ma549), indicative of generalist (non-specific) defenses. Most of the lines showing above average resistance to Ma549 showed cross-resistance to E. muscae. However, lines that succumbed quickly to Ma549 exhibited the full range of resistance to E. muscae. This suggests fly populations differ in E. muscae-specific resistance mechanisms as well as generic defences effective against both Ma549 and E. muscae. We looked for trade-offs that could account for inter-line variation, but increases (decreases) in disease resistance to E. muscae are not consistently associated with increases (decreases) of resistance to oxidative stress, starvation stress and sleep indices. That these pathogens are dynamic agents of selection on hosts is reflected in this genetic variation for resistance in lines derived from wild populations.
Subject terms: Biological techniques, Pathogenesis
Introduction
Considerable genetic variation in resistance and tolerance to infection can exist within populations1,2. This variation determines the burden of disease, and represents the raw material from which populations can evolve resistance either naturally or artificially (i.e. through selective breeding by humans)3. Insects are no exception to this pattern, and the same population of Drosophila melanogaster can contain resistant and susceptible genotypes to viruses, fungi and bacteria4,5.
Many arthropod pathogenic fungi belong to the phylum Entomophthoromycotina and most of the remainder are distantly related ascomycetes6. We previously demonstrated significant variation in the life-span of 188 Drosophila Genetic Reference Panel (DGRP) lines infected with the ascomycete fungus Metarhizium anisopliae ARSEF 549 (Ma549)5. In addition, we found that resistance to Ma549 was correlated with resistance to the bacterium Pseudomonas aeruginosa (Pa14), and several previously published DGRP phenotypes including oxidative stress sensitivity, starvation stress resistance, hemolymph glucose levels, and sleep indices. As bacteria infect per os and fungi through the cuticle, the cross-resistance to Pa14 and Ma549 is suggestive of general (multipurpose) humoral defense mechanisms that do not involve cuticle or gut immunocompetence. Consistent with this, a genome-wide association study revealed a network of Pa14 and Ma549-resistance genes that are functionally connected through many different aspects of host physiology5. These observations are in line with insertional mutagenesis results: Lu7 reported that 87% of mutated genes in more susceptible Drosophila lines are involved in a broad spectrum of biological functions not connected with canonical immune systems. The large numbers of pleiotropic genes involved in resistance to Ma549 and Pa14 contrasts with the small number of common polymorphisms associated with resistance to viruses4. Interestingly, each viral resistance SNP was associated with resistance to only one virus, which suggests that viral immunity is mediated by a suite of specific factors4.
Most models of hosts and pathogens assume there is a tight relationship of co-evolved interactions between species pairs8. Such hosts and parasites are thought to engage in antagonistic coevolution, where a newly evolved parasite virulence mechanism is negated over time by a newly evolved host immune mechanism and vice versa9. The broad host range of Metarhizium and Beauveria spp. used in several previous studies on Drosophila10–13 suggest that these pathogens have not engaged in a strict coevolutionary arms race with Drosophila14. As study systems, these microbes will not, therefore, tell us about how specialist parasites suppress host immunity, or about any secondary immune mechanisms hosts deploy against specialist parasites15,16.
Given the importance of the Drosophila model system to our understanding of immunity, it is surprising that very little is known about its natural parasites. There may be 5.5 million insect species17, and if every metazoan species has at least one host-specific parasite as some studies suggest18, narrow host range entomopathogenic fungi may exist by the millions as well. However, an ecologically relevant specialist fungal pathogen of Drosophila pathogen that would facilitate understanding of host pathogen evolution and identify specialized immune mechanisms has only recently been identified19. Behavior-manipulating fungal pathogens in the Entomophthora muscae (Entomophthoromycota) species complex are best known for causing epizootic outbreaks in house flies. However, Elya et al.19 identified an epizootic in Californian Drosophila caused by a single strain of E. muscae (E. muscae 'Berkeley'). It remains unclear if E. muscae 'Berkeley' is a distinct lineage (or even species) from those that infect other fly species, and how specific it may be for Drosophila spp. over other dipterans is also unknown19. However, contrary to other fungal infections (e.g., Metarhizium), and consistent with previous E. muscae infections described in house flies20, E. muscae 'Berkeley' invaded the Drosophila’s nervous system and caused a characteristic set of behaviors: on their final day of life, a few hours before sunset, the flies climb upward, extend their proboscides, affixing them to a substrate, then raise their wings, clearing a path for infectious spores to launch from their abdomens19. This robust control of behavior by E. muscae 'Berkeley' indicates a high level of adaptation of the pathogen to the host. However, many aspects of this disease (e.g., the climbing behavior of critically ill hosts), are typical for narrow host range pathogens of arthropods, and probably involve the pathogens taking advantage of sleep behavior in insects, as these behaviors are highly conserved21. Many of the best characterized and most commonly witnessed epizootics are caused by behavior-modifying entomophthoralean species infecting flies, ants, grasshoppers, caterpillars and cicadas22,23.
In this study we bioassayed E. muscae 'Berkeley' (hereafter referred to as E. muscae) against a subset of 20 DGRP lines selected because they represent the genotypes that are the most resistant or susceptible to Ma549 from the DGRP collection. Using this divergent subset, we show that wild-derived populations of Drosophila have substantial differences in susceptibility to E. muscae, and that this variation correlates with resistance to Ma549, and, to a lesser extent, with starvation resistance and sleep indices. However, lines that succumbed quickly to Ma549 covered the whole spectrum of resistance to E. muscae from low to high. This suggests there are additional mechanisms by which disease resistance to E. muscae can be altered, besides those effective against Ma549.
Methods
Divergent DGRP lines
DGRP Freeze 2 lines, originally derived from an out-crossed population in Raleigh, North Carolina, by the Mackay laboratory24 were obtained from the Bloomington Stock center. To characterize natural variation in susceptibility to E. muscae, we used a subset of the 188 DGRP lines deployed in Wang et al.5, comprising the 10 most Ma549 resistant and 10 most Ma549 susceptible DGRP lines. Called the “divergent subset” in Wang et al.5, they represent the most extreme disease phenotypes to Ma549 in the DGRP. Ma549 and Pa14 LT50 data for the divergent subset was previously published in Wang et al.5.
E. muscae exposure of divergent DGRP lines
All flies were reared on cornmeal-based diet (3% weight per volume (w/v) cornmeal, 11% w/v dextrose, 2.3% w/v yeast, 0.64% w/v agar and 0.125% w/v tegosept) at 21 °C on a 12:12 light:dark cycle. For infection, we followed a modified version of the protocol described in Elya et al.19 using E. muscae that has been propagated in Drosophila in vivo since 2015. Briefly, 21 “exposure vials” were prepared, each by embedding six Canton-S Drosophila cadavers freshly killed by E. muscae headfirst into minimal media containing 5% sucrose and 1.5% agar in wide fly vials (Genesee Scientific). For each of the 20 DGRP lines and Canton-S, fifty flies (25 male and 25 female) aged < 5 days post eclosion were transferred to a fresh vial and the plug of the vial was pushed down to confine the flies within 2 cm to improve the likelihood that they would encounter infectious spores. Vials were housed for the first 24 h under high humidity at 21 °C with a 12:12 light:dark cycle, at which point the plug was raised to relieve fly confinement. Flies were housed at 21 °C with ~ 40% humidity for the remainder of the experiment. Each vial was monitored twice daily (once before subjective sunset, once after) for deaths and subsequent sporulation, to confirm death by E. muscae. All experiments were replicated five times, the raw data is provided in Supplemental Table S1.
Data analysis
All statistics were done using R version 3.6.1. To determine the relationship between different phenotypes, we performed Pearson correlations using the package Hmisc. We tested for normality using Shapiro-Wilks test. Comparisons between sexes and Wolbachia infection statuses were done using the non-parametric two-sided Mann–Whitney test.
Results and discussion
To characterize natural variation in pathogen resistance, we quantified susceptibility to E. muscae using the divergent subset of the 10 most and the 10 least Ma549 resistant DGRP lines (selected out of 188 DGRP lines). Age-matched flies from each line were exposed to E. muscae, and survival time was monitored using five replicates (25 flies each), per sex per line. Elya et al.19 report that the Wolbachia-free CantonS Drosophila developed a strong immune response one day after infection with E. muscae but by the third day the fungus had spread throughout the body, and most flies died around four to five days following infection. Unlike Ma549 and Pa14, E. muscae consistently kills hosts at the same zeitgeber time every day (always in the hours leading to subjective sunset), therefore, we used the percentage surviving at five days post-exposure to E. muscae (referred to as a PS5; daily deaths only rarely peak after this time point) as our metric to compare to Ma549 and Pa14. In contrast to E. muscae, DGRP lines die from Ma549 or Pa14 at different rates over a broader range of day’s post-exposure, so post-infection survival for these pathogens is better measured using LT50 values.
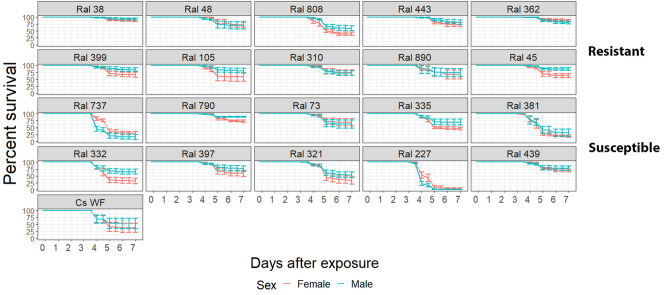
Using the DGRP lines, we show that wild-derived populations of Drosophila have substantial differences in susceptibility with mortalities ranging from 1.6 to 94%, and a mean survival of males (females) of 70% (62%) (Fig. 1). Less than 25% of flies in the most E. muscae-resistant DGRP lines had died seven days post exposure, and most of those that succumbed did so after 4 to 5 days. At the other extreme, almost 100% of RAL 227 flies were dead at five days post exposure. After five days the death rate plateaued off for most lines, and approached that of uninfected flies, suggesting that the survivors had cleared the infection. Thus, variable host susceptibility is illustrated by some DGRP lines dying more than others four to five days post infection with E. muscae.
Figure 1.
Percent survival of DGRP lines tested with E. muscae. Flies of the divergent subset were broken up into two groups, those resistant to Metarhizium anisopliae (top two rows) and those susceptible to M. anisopliae (middle two rows). Canton-S flies (CS WF) (bottom) used previously to establish that E. muscae 'Berkeley' is a Drosophila pathogen19 were used as a positive control. Percentages are an average of five replicates and error bars reflect standard errors.
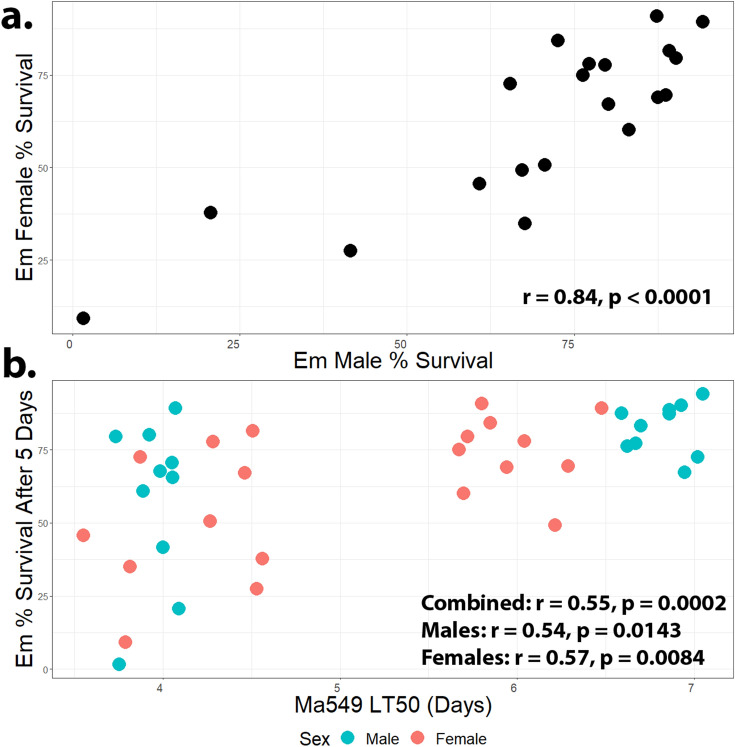
To identify general (multipurpose) defense, the PS5 for males and females exposed to E. muscae was plotted against average LT50 for males (females) infected with Ma549 (Fig. 2b). The data on Ma549 and Pa14 is derived from our earlier publication which used replicates run on different days to randomize environmental variation5. Correlations were moderate but highly significant (r = 0.54, 0.57, p = 0.0143, 0.0084 for males, females respectively), consistent with Drosophila utilizing unspecific generalized defense components against E. muscae and Ma549 (Fig. 2b). We previously reported that LT50 values for Ma549 and P. aeruginosa Pa14 were correlated for both males (r = 0.45, n = 78) and females (r = 0.40, n = 78)5. Correlations between Ma549 and Pa14 in the divergent subset used to assay E. muscae were greater, with r = 0.7 for males (p = 0.0024, n = 16) and r = 0.55 for females (p = 0.0262, n = 16) (Fig S1b). Although correlations were still positive between E. muscae and P. aeruginosa, they were not significant for males (r = 0.09, p = 0.7295, n = 16) or for females (r = 0.39, p = 0.138, n = 16) (Fig S1a). Ma549 is a broad host range generalist insect pathogen, while Pa14 is a human clinical isolate, and so represents a novel association that will have no history of coevolution. Our results suggest that the genetic basis for resistance to a non-coevolved bacterium (Pa14) and an opportunistic broad host range fungus, share more genetic causes than Pa14 and E. muscae.
Figure 2.
Correlation graphs. Positive correlations of (a) % survival of male and female DGRP flies 5 days post-infection with E. muscae, and (b) between flies infected with E. muscae (% survival) or M. anisopliae (Ma549 LT50 values). Ma549 LT50 values were obtained from Wang et al. 20175.
The weak correlations indicate that Drosophila has alleles with pathogen-specific effects to E. muscae. This is consistent with variation in the magnitude and direction of association between Ma549 and E. muscae. We found greater variation in susceptibility to E. muscae among the lines susceptible to Ma549 (male range: 1.6–76.28%, female range: 9.18–84.23%) compared to lines resistant to Ma549 (male range: 77.25–94.2%, female range: 60.13–90.83%) (Fig. 1). Except for RAL 808, the lines resistant to Ma549 were also resistant to E. muscae, while lines that succumbed quickly to Ma549 covered the spectrum of resistance to E. muscae from low to high (Fig. 1). The exception, RAL 808, is the third most resistant line to Ma549, but is moderately susceptible to E. muscae ranking fifteenth out of the twenty lines (PS5 ~ 50%). These results suggest that there are multiple mechanisms by which disease resistance to E. muscae can be altered besides those effective against Ma549. The shared history of E. muscae and D. melanogaster could have resulted in a co-evolutionary process that altered the diversity of resistance genes compared to naïve pairs of hosts and pathogens. Similarly, host–pathogen coevolution increases genetic variation in susceptibility to viruses25. Thus, heritable variation for host resistance was detectable for two natural viruses of D. melanogaster, but not for two non-natural viruses4.
To identify sexual dimorphism, we measured disease resistance separately for males and females infected with E. muscae (Fig. 2a). Cross-sex genetic correlations were high (r = 0.84, p < 0.0001, n = 20), indicating that many of the same variants affect E. muscae resistance in males and females. Females flies died more quickly than males when infected with Ma5495 (p = 0.00039, n = 188). This difference is not significant overall for females of the divergent set (p = 0.37, n = 20), but females group separately from males in the most resistant DGRP lines (Fig. 2b). Females were also slightly more susceptible than males to E. muscae though this fell short of significance (p = 0.2, n = 20). As observed previously for Ma5495, RAL 737 was exceptional, as females of this fly line were more resistant to E. muscae than males (Fig S2).
Wolbachia pipientis is a natural intracellular symbiont of many arthropods, and Wolbachia may confer protection against the fungus Beauveria bassiana in one D. melanogaster line26. Wolbachia status in the DGRP lines was without significant effect on susceptibility to Ma5495. Eleven of the twenty divergent lines were positive for Wolbachia, seven of these eleven were present in the ten most susceptible lines producing no significant effect on the susceptibility to E. muscae for males (p = 0.15, n = 20 or females p = 0.71, n = 20).
Resistance to multiple pathogens should have a selective advantage unless this general defense is traded off against other (pathogen-independent) fitness components27. In the absence of such a trade-off, directional selection would presumably lead to fixation of genotypes showing general resistance. Table S2 shows the divergent subset, and their life cycle parameters and rankings in publicly available data from other publications, including our data for Ma549 and Pa14. Figure S1 presents correlations between the disease resistance phenotypes in our studies and these other traits. The small sample size (n = 20) of the divergent set reduces the discriminatory power of correlation analysis. However, r values for the divergent set and the total population (188 lines) are similar for many phenotypes. For example, correlations between female resistance to Ma549 and paraquat (a source of oxidative stress) are r = 0.46, p = 0.0541 (divergent set, n = 18) and r = 0.31, p < 0.0001, n = 156 (total population), and correlations between female resistance to Ma549 and negative geotaxis are r = 0.29, p = 0.2411 (divergent set, n = 18) and r = 0.2, p < 0.0079, n = 171 (total population). The corresponding values for E. muscae are r = 0.25, p = 0.325, n = 18 (paraquat resistance) and r -0.04, p = 0.861, n = 18 (negative geotaxis).
We previously reported that resistance to Ma549 among 188 DGRP lines was negatively correlated with sleep duration at night in males (r = − 0.32, p < 0.0001, n = 156) and females (r = − 0.28, p = 0.0004, n = 156)5. Conversely, there was a positive association between resistance and the number of sleep bouts in males (r = 0.25, p = 0.0018, n = 156) and females (r = 0.24, p = 0.0028, n = 156)5. Compared to the total population, the resistance of the 20 divergent subset to Ma549 was even more closely associated with the number of nocturnal sleep bouts (males r = 0.67, p = 0.0026, n = 18; females r = 0.67, p = 0.0021, n = 18) and negatively correlated with night sleep duration (males r = − 0.71., p = 0.0009, n = 18; females r = − 0.74, p = 0.0005, n = 18). Hence, compared to the general population, there is a stronger trend for the 10 most resistant DGRP flies to have more sleep bouts than the 10 most susceptible DGRP flies, but these bouts are shorter and total sleep time is less. This trend was retained for E. muscae, but to a lesser degree, and falling short of significance, with the number of nocturnal sleep bouts (males r = 0.29., p = 0.2352, n = 18; females r = 0.35, p = 0.1483, n = 18) and negatively correlated with night sleep duration (males r = − 0.36, p = 0.1402, n = 18; females r = − 0.32, p = 0.1919, n = 18).
Looking at the data on a line-by-line basis shows why the associations are so weak. There are lines with increased levels of resistance to E. muscae and negative geotaxis, oxidative stress or sleep duration, but there are also resistant lines with moderate or low rankings for these indices, suggesting that there are no straightforward associations or trade-offs. Taking starvation stress as an example, as E. muscae colonizes the host’s body it will compete with it for resources19, so it makes intuitive sense that genotypes better able to tolerate starvation would have better tolerance to disease. Resistance to starvation is positively correlated with resistance to E. muscae in both males (r = 0.21, n = 20) and females (r = 0.34, n = 20). Although these values fall short of significance (p > 0.05) (Supplementary Fig S1p), they are higher than the correlations between resistance to Ma549 and starvation in males of (r = 0.17, n = 20) and females (r = − 0.03, n = 20). Resistance to starvation in the total population was only weakly correlated with the resistance of female flies to Ma549 (r = 0.16, p = 0.0335)5, indicating that E. muscae may cause greater nutrient stress to Drosophila than Ma549. However, on a line-by-line basis, DGRP lines RAL 38, RAL 48, RAL 443 and RAL 362 (highly resistant to both Ma549 and E. muscae), ranked 159, 28, 62 and 8 out of 203 DGRP lines for resistance to starvation. RAL 808 (Ma549 resistant, E. muscae susceptible), RAL 439 (Ma549 susceptible, E. muscae resistant) and RAL 227 (susceptible to both Ma549 and E. muscae) ranked 152, 162 and 149, respectively.
Conclusion
Fungal-host interactions include both general broad host range and narrow host range pathogens. E. muscae is a dipteran specialist that naturally causes epizooitic outbreaks in D. melanogaster. Similar to broad host range ascomycete fungi, we identified considerable host genetic variation in resistance to E. muscae infection. However, we found that this variation is unlike ascomycetes, which kills different host genotypes with varying rapidity, but instead reflected considerable differences in the number of flies that died in a narrow window of time four to five days post-exposure. This reflects the unique E. muscae behavioral trait of killing flies in the hours leading to subjective sunset to ultimately maximize fungal dispersal. Despite differences in the co-evolutionary dynamics between D. melanogaster and E. muscae, versus other fungal pathogens such as M. anisopliae, flies showed cross-resistance to both fungi, indicative of generic anti-fungal defences. However, cross-resistance was greater between M. anisopliae and an opportunistic bacterial pathogen, P. aeriginosa, than between M. anisopliae and E. muscae. Also, D. melanogaster lines killed quickly by M. anisopliae Ma549 show greater variation in susceptibility to E. muscae, indicating that Ma549-susceptible individuals vary in evolution or retention of narrow anti-E. muscae mechanisms. Also, with the notable exception of starvation resistance, resistance to Ma549 and Pa14 correlated with non-specific physiological features such as sleep indices, to a greater extent than E. muscae infected flies, consistent with specific defenses being more important. This study demonstrates the continued utility of Drosophila for understanding host-fungus interactions, the clear potential for Drosophila to become a powerful in vivo comparative system to study the diversity of antifungal responses, and supports the utility of E. muscae as a model for studying varied aspects of host–pathogen interactions in the fly.
Supplementary information
Acknowledgements
Jonathan Wang was supported by Hatch project accession no. 1015969 from the USDA National Institute of Food and Agriculture to R.J.S. Carolyn Elya was supported by a HHMI Hanna H. Gray Fellowship. We appreciate Benjamin de Bivort’s helpful comments and suggestions in revising this manuscript.
Author contributions
J.W. Data analysis, Methodology. C. E. Investigation, Methodology, Experimental design, Data curation. R.J.S. Conceptualization, Data analysis, Manuscript preperation. All authors reviewed and edited the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71262-w.
References
- 1.Hill AVS. Evolution, revolution and heresy in the genetics of infectious disease susceptibility. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:840–849. doi: 10.1098/rstb.2011.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kniskern JM, Barrett LG, Bergelson J. Maladaptation in wild populations of the generalist plant pathogen pseudomonas syringae. Evolution (N. Y.) 2011;65:818–830. doi: 10.1111/j.1558-5646.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGonigle JE, et al. Parallel and costly changes to cellular immunity underlie the evolution of parasitoid resistance in three Drosophila species. PLoS Pathog. 2017 doi: 10.1371/journal.ppat.1006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magwire MM, et al. Genome-wide association studies reveal a simple genetic basis of resistance to naturally coevolving viruses in Drosophila melanogaster. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JB, Lu H-L, St. Leger RJ. The genetic basis for variation in resistance to infection in the Drosophila melanogaster genetic reference panel. PLoS Pathog. 2017;13:e1006260. doi: 10.1371/journal.ppat.1006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araújo, J. P. M. M. & Hughes, D. P. Diversity of Entomopathogenic Fungi. Which Groups Conquered the Insect Body? In Advances in Genetics, Vol. 94 (eds Lovett, B. & St. Leger, R. J.) 1–39 (2016). [DOI] [PubMed]
- 7.Lu H-L, Wang JB, Brown MA, Euerle C, St. Leger RJ. Identification of Drosophila mutants affecting defense to an entomopathogenic fungus. Sci. Rep. 2015;5:12350. doi: 10.1038/srep12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett LG, Kniskern JM, Bodenhausen N, Zhang W, Bergelson J. Continua of specificity and virulence in plant host-pathogen interactions: causes and consequences. New Phytol. 2009;183:513–529. doi: 10.1111/j.1469-8137.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 9.Dawkins R, Krebs JR. Arms races between and within species. Proc. R. Soc. Lond. Biol. Sci. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- 10.Gottar M, et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matskevich AA, Quintin J, Ferrandon D. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur. J. Immunol. 2010;40:1244–1254. doi: 10.1002/eji.200940164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxström-Lindquist K, Terenius O, Faye I. Parasite-specific immune response in adult Drosophila melanogaster: a genomic study. EMBO Rep. 2004;5:207–212. doi: 10.1038/sj.embor.7400073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinsley MC, Blanford S, Jiggins FM. Genetic variation in Drosophila melanogaster pathogen susceptibility. Parasitology. 2006;132:767. doi: 10.1017/S0031182006009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawecki TJ. Red queen meets Santa Rosalia: arms races and the evolution of host specialization in organisms with parasitic lifestyles. Am. Nat. 1998;152:635–651. doi: 10.1086/286195. [DOI] [PubMed] [Google Scholar]
- 15.Keebaugh ES, Schlenke TA. Insights from natural host-parasite interactions: the Drosophila model. Dev. Comp. Immunol. 2014;42:111–123. doi: 10.1016/j.dci.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, H.-L. & St. Leger, R. J. Insect immunity to entomopathogenic fungi. in Advances in Genetics (eds Lovett, B. & St. Leger, R. J.) (2016). 10.1016/bs.adgen.2015.11.002 [DOI] [PubMed]
- 17.Stork NE. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018;63:31–45. doi: 10.1146/annurev-ento-020117-043348. [DOI] [PubMed] [Google Scholar]
- 18.Rohde K. Ecology and biogeography, future perspectives: example marine parasites. Geoinform. Geostat. Overv. 2016 doi: 10.4172/2327-4581.1000140. [DOI] [Google Scholar]
- 19.Elya C, et al. Robust manipulation of the behavior of Drosophila melanogaster by a fungal pathogen in the laboratory. Elife. 2018 doi: 10.7554/eLife.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brobyn PJ, Wilding N. Invasive and developmental processes of Entomophthora muscae infecting houseflies (Musca domestica) Trans. Br. Mycol. Soc. 1983;80:1–8. doi: 10.1016/s0007-1536(83)80157-0. [DOI] [Google Scholar]
- 21.Lovett B, St. Leger RJ, de Fine Licht HH. Going gentle into that pathogen-induced goodnight. J. Invertebr. Pathol. 2020 doi: 10.1016/j.jip.2020.107398. [DOI] [PubMed] [Google Scholar]
- 22.Hughes DP, et al. From so simple a beginning. the evolution of behavioral manipulation by fungi. Adv. Genet. 2016 doi: 10.1016/bs.adgen.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK. Bizarre interactions and endgames: entomopathogenic Fungi and Their Arthropod Hosts. Annu. Rev. Entomol. 2006;51:331–357. doi: 10.1146/annurev.ento.51.110104.150941. [DOI] [PubMed] [Google Scholar]
- 24.Mackay TFC, et al. The Drosophila melanogaster genetic reference panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duxbury EM, et al. Host-pathogen coevolution increases genetic variation in susceptibility to infection. Elife. 2019 doi: 10.7554/eLife.46440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panteleev DI, et al. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russian J. Genet. 2007;43:1066–1089. doi: 10.1134/S1022795407090153. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell-Olds T, Bradley D. Genetics of Brassica rapa. 3. Costs of disease resistance to three fungal pathogens. Evolution. 1996;50:1859–1865. doi: 10.1111/j.1558-5646.1996.tb03572.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).