Abstract
Shoulder replacement is indicated for the surgical treatment of proximal humeral fractures in elderly patients, when severe comminution and osteoporosis jeopardize the chances of success of any fixation technique. Two different implants are available for this purpose: anatomical hemiarthroplasty (HA) and reverse total shoulder arthroplasty (RTSA). HA for fractures was popularized by Charles Neer in the ‘50s and for several decades remained the only reliable implant for these injuries. However, many authors reported inconsistent results with HA as a consequence of the high rate of tuberosity and rotator cuff failure. In 1987, Paul Grammont designed the first successful RTSA, which was the end result of a long thought process on functional surgery of the shoulder. This implant was initially used to treat cuff tear arthropathy and shoulder pseudoparalysis, but indications have gradually expanded with time. Since RTSA does not rely on a functional cuff for shoulder elevation, it was felt that results in fractures could be improved by this prosthesis. In this study, the salient features of these implants are described to understand the rationale behind both approaches and highlight their pros and cons. Several clinical studies comparing HA vs RTSA for proximal humeral fractures have been published during the last two decades. A literature review is carried out to analyze and compare outcomes of both implants, analyzing clinical results, radiographic findings and complications. The final goal is to provide an overview of the different factors to consider for making a choice between these two prostheses.
Key words: shoulder hemiarthroplasty, reverse shoulder arthroplasty, proximal humeral fractures, osteoporosis, elderly
Introduction
Proximal humerus fractures are the third most common fractures of the appendicular skeleton in patients over 65, with a higher incidence among the female population.1,2 Conservative treatment is a viable option for the vast majority of patients, but some complex fractures require surgery.
The goal of surgical treatment is to preserve shoulder function and maintain previous levels of activity and autonomy. Surgical options include different fixation techniques (percutaneous pinning,3 intramedullary nailing, plating) as well as shoulder arthroplasty. The choice of the procedure should be made considering several local (fracture pattern, quality of bone, status of the rotator cuff) and general (comorbidities, functional demands, compliance to treatment) factors. Unfortunately, there is insufficient evidence to provide straightforward recommendations and the significant variation in clinical practice among orthopaedic surgeons is indicative of a lack of consensus regarding optimal treatment for these fractures.4,5
Shoulder replacement is frequently indicated for the management of proximal humeral fractures in elderly patients, when severe comminution and poor bone quality jeopardize the chances of success of any fixation technique. Two different implants are available for this purpose: anatomical hemiarthroplasty (HA) and reverse total shoulder arthroplasty (RTSA). The first one was pioneered and popularized by Charles Neer in the 1950s, with a monobloc prosthesis specifically designed for fractures.6 Modern RTSA was developed by Paul Grammont in the 1980s to treat arthritic shoulders with severe destruction of the cuff;7 indications of this implant expanded with time, including acute fractures of the proximal humerus.
The salient features of these implants will be described to understand the rationale behind both approaches and highlight their pros and cons. A review of literature will be also carried out to analyze and compare outcomes of HA and RTSA.
Shoulder hemiarthroplasty
The disappointing results achieved with humeral head excision or arthrodesis in the treatment of unimpacted fracturedislocations led Charles Neer (1917-2011) to consider articular replacement as a better solution for achieving pain relief and improved shoulder function in these complex injuries. In 1955, he published the results achieved in a series of twelve patients (mean age, 51 years) using a monobloc Vitallium prosthesis: at an average follow up of 10 months, eleven patients were free from pain, while range of motion (ROM) was described as poor in only two patients.6
During the following years, Neer popularized this technique and many orthopaedic surgeons adopted HA for the surgical treatment of complex fractures and fracture-dislocations of the proximal humerus. However, subsequent clinical experiences reported inconsistent results with regard to ROM and strength, despite the improvement of implants and surgical techniques.8-10
HA in proximal humeral fractures is one of the most difficult procedures to perform in shoulder surgery. In fact, it is not a simple joint replacement, but it should be considered as an “augmented osteosynthesis”, in which anatomical and stable repair of the tuberosities must be achieved around a properly implanted prosthesis. In complex fractures, the loss of anatomic landmarks can make precise prosthetic implantation and reconstruction of the center of rotation challenging. Height, retroversion and size of the humeral head are critical variables to consider, because malpositioning of the prosthesis will invariably compromise tuberosity reconstruction (Figure 1).11
The greatest determinant of postoperative function is the fate of the tuberosities and the rotator cuff. It is well known that malunion, nonunion and/ or resorption of the tuberosities occur frequently after HA, thus hindering functional recovery of the shoulder. Tuberosity reconstruction is particularly difficult if comminution is present, as typically occurs in elderly patients, in whom the rotator cuff is often compromised, too. Tuberosity and rotator cuff failure ultimately results in proximal humeral migration and pseudoparalysis, with shoulder motion just relying on the scapulothoracic joint (Figure 2). Another critical aspect to consider is rehabilitation. HA requires a demanding rehabilitation, with strict cooperation between the surgeon and the physiotherapist, and high patient’s compliance. There are conflicting opinions about the protocol to adopt in the postoperative period: some surgeons prefer to start passive motion immediately, while others believe that an initial immobilization in a brace is preferable. Rehabilitation lasts several months and elderly patients might encounter difficulties in accomplishing this long lasting program.
Reverse total shoulder arthoplasty
Constrained designs for shoulder arthroplasty were explored by several surgeons in the 70s to overcome the inability of anatomic implants to restore function in cuff-deficient shoulders. However, the disappointing results and the high complication rates reported for these prostheses led to their abandonment.
In 1987, Paul Grammont (1940-2013) designed the first successful RTSA, which was the end result of a long thought process on functional surgery of the shoulder.12 The basic biomechanical principle of his Delta prosthesis was the medialization concept: shifting the center of shoulder rotation to the glenoid surface, Grammont was able to overcome the early failure of the glenoid component due to excessive mechanical torque at the bone-implant interface (Figure 3). Moreover, medialization and distalization of the humerus increased both the moment arm and the tension of the deltoid muscle, thus enhancing its function.
Figure 1.
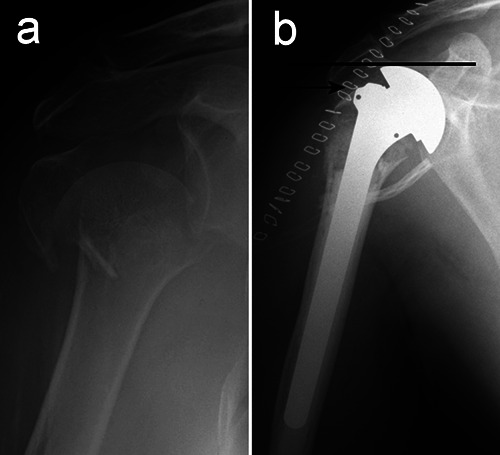
An emblematic case to explain how hemiarthroplasty malpositioning compromises the relationships between the prosthetic head and the tuberosities. a) Preoperative radiogram of a 4-part fracture in a 72-year old lady, treated with hemiarthroplasty. b) Postoperative control showing the excessive height of the prosthetic head (line) Periprosthetic tuberosity reconstruction is correct, but inevitably too low (arrow) in relation to the head.
Figure 2.
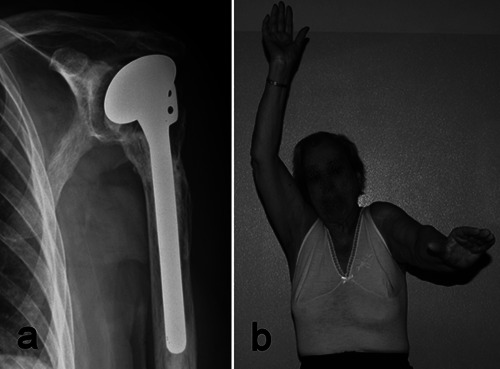
a) Tuberosity resorption and cuff failure with subacromial migration of the prosthetic head four years after hemiarthroplasty in a 77-year old woman. b) Severe limitation (less than 90°) of shoulder elevation, with active motion relying mostly on the scapulo-thoracic joint.
RTSA is a fixed-fulcrum, semiconstrained prosthesis with an inverted geometry of its components. These features allow the arm to be raised overhead even when the rotator cuff is absent. RTSA was initially used to treat cuff tear arthropathy and shoulder pseudoparalysis, but indications have gradually expanded with time. Revision arthroplasties, fracture sequelae, tumors involving the proximal humerus, severe osteoarthritis and acute fractures in elderly patients are now common indications for RTSA. The clinical effectiveness of RTSA is testified by the huge number of implants performed every year worldwide.
RTSA is a fixed-fulcrum, semiconstrained prosthesis with an inverted geometry of its components. These features allow the arm to be raised overhead even when the rotator cuff is absent. RTSA was initially used to treat cuff tear arthropathy and shoulder pseudoparalysis, but indications have gradually expanded with time. Revision arthroplasties, fracture sequelae, tumors involving the proximal humerus, severe osteoarthritis and acute fractures in elderly patients are now common indications for RTSA. The clinical effectiveness of RTSA is testified by the huge number of implants performed every year worldwide.
Much research has been devoted to develop new design solutions thus improving surgical techniques. RTSA is still an evolving field of study and alternative solutions to the original biomechanical principles of Grammont are endlessly proposed in the attempt to improve outcomes and reliability of RTSA.
The spreading use of RTSA for the surgical treatment of acute proximal humerus fractures, particularly in elderly patients, is a direct consequence of the inconsistent results achieved with HA, but it is also conditioned by the easier surgical technique.16-20 RTSA may increase the chances of recovering shoulder function owing to its lesser dependence on tuberosity healing (Figure 5).21-23 In the majority of elderly patients, the loss of shoulder function after HA is common, because of the coexistence of negative prognostic factors, such as tuberosity comminution, rotator cuff degeneration and tears, low compliance and/or lack of logistic support for performing adequate rehabilitation. The anatomo-clinical aspects supporting indication for RTSA in proximal humerus
Figure 3.
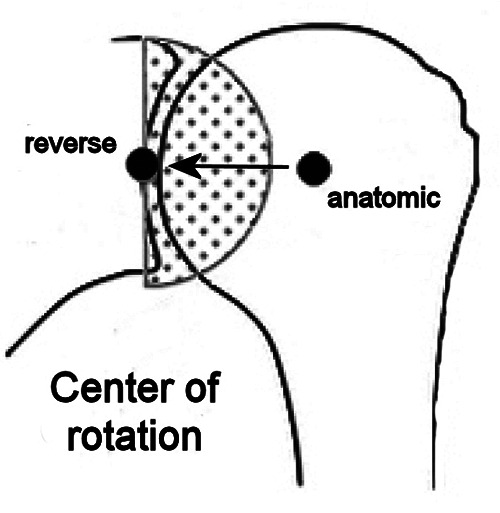
Drawing that illustrates how the center of shoulder rotation shifts medially passing from hemiarthroplasty to reverse total shoulder arthroplasty.
Figure 4.
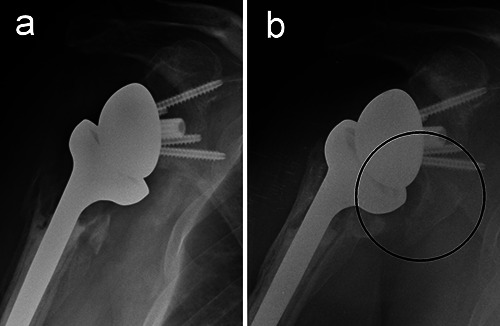
a) Radiogram of a reverse total shoulder arthroplasty one month after surgery in a 73-year old woman. b) Follow up at 6 years: scapular notching and a large inferior osteophyte of the scapular neck are evident (circle).
Table 1.
Variables supporting indication for reverse total shoulder arthroplasty in proximal humerus fractures (when shoulder replacement is required).
| Age | ♀ | > 65-70 |
| ♂ | > 70-75 | |
| Local conditions | - | Large rotator cuff tear |
| - | Comminuted tuberosities | |
| - | Need to replace the glenoid (fractured, concomitant arthritis) | |
| - | Poor blood supply (diabetes, smoking) | |
| General conditions | - | Limited functional demands |
| - | Low compliance for rehabilitation |
Hemiarthroplasty vs reverse total shoulder arthoplasty in proximal humerus fractures
During the last two decades, several clinical studies as well as literature reviews, comparing HA vs RTSA for proximal humeral fractures, were published. Difficulties in this comparison - and generally between all treatment modalities for proximal humeral fractures - are related to different factors: no prospective randomized methodology, bias related to treatment choice, variations in surgical technique, limited follow-up time and variable outcome tools.
Three different aspects should be considered:
clinical results (range of motion, clinical-functional scores);
radiographic results (tuberosity healing, scapular notching, component loosening);
associated morbidity (complications, reoperations).
Clinical results
The initial clinical experiences with RTSA for fractures showed that recovery of active shoulder elevation over 90° in elderly patients could be achieved in the majority of cases and was not strictly dependent from tuberosity healing. For this reason, it was felt that RTSA could be the best option for patients with comminuted tuberosities, rotator cuff tears and severe osteopenia.
Different clinical series, aimed to compare RTSA and HA for fractures in patients older than 70 years, showed that postoperative mean active forward elevation and abduction were better in patients with the reverse prosthesis, while internal and external rotation were superior in patients with HA.16,24-29
Early adopters of RTSA for fractures did not routinely repair the tuberosities, since it was felt that the prosthesis could work without the rotator cuff. The present trend is to reattach at least the greater tuberosity, since the absence of infraspinatus and teres minor invariably leads to loss of external rotation. Moreover, the bulky body of the original Grammont Delta prosthesis was not ideal for periprosthetic reconstruction of the tuberosities: as happened before for anatomic implants, some changes were adopted in new RTSA designs to enhance tuberosity healing.
The clinical and functional results evaluated by different rating scales for the shoulder (Constant, ASES, DASH, SST, etc.) show on average better results of RTSA over HA in elderly patients. It must be highlighted that most of the comparisons among the two implants doesn’t show statistically significant differences. However, results with HA are less homogeneous, tending to split into “very poor” and “very good”, while RTSA outcomes are more predictable, tending to a normal Gaussianlike distribution.
According to the relevant literature on the topic, RTSA is generally more effective than HA in alleviating pain and regaining shoulder strength for arm elevation.30A more favorable clinical outcome leads to higher patient satisfaction, because no pain and enough function allow to achieve selfsufficiency, a critical condition for preserving a good quality of life in the elderly.
Figure 5.
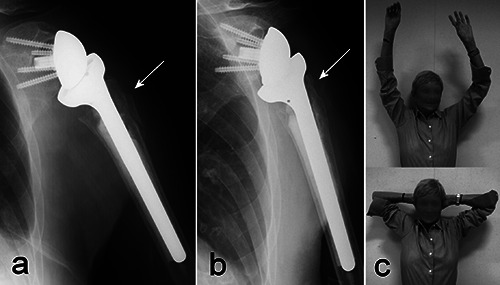
a) Reverse total shoulder arthroplasty implanted for a complex fracture of the proximal humerus in a 76-year old woman. Comminution of the greater tuberosity did not allow an optimal periprosthetic reconstruction (arrow). b) X-rays taken two years after surgery, showing partial resorption of the tuberosity (arrow). c) Shoulder function two years after surgery: active elevation and external rotation are excellent, despite the radiographic findings.
Radiographic results
Much attention has been paid to evaluate tuberosity healing in both HA and RTSA, with the aim to assess the correlation between radiographic findings and clinical results.
There is consensus among authors that favorable outcomes after HA for fractures are strictly dependent from anatomical repair and healing of the tuberosities, a difficult task to accomplish. The chances of a precise reconstruction are critically lowered by fracture comminution; other negative prognostic factors for tuberosity healing in HA are age >75, female gender and three or more comorbidities.31,32
Malunion, nonunion or resorption of the tuberosities result in poor recovery of shoulder function with HA, but not necessarily in shoulder discomfort.10 For this reason, many elderly patients treated with HA for fractures are not willing to undergo further surgery despite an unsatisfactory functional outcome.
The role of tuberosity healing with RTSA is more controversial. Repair of the lesser tuberosity is not essential, since in RTSA the subscapularis is not necessary for shoulder elevation, is not critical for implant stability and might potentially hinder recovery of external rotation. Therefore, the focus is on the greater tuberosity.
Healing of the tuberosity is not influenced by gender and age in RTSA but, similarly to HA, is impaired by comorbidities. Even though some authors did not report any influence on the functional outcome between healed and non-healed tuberosities,33,34 it’s difficult to suppose that active external rotation can be recovered without any connection between the posterior cuff and the humerus.35
Scapular notching is one of the major problems related to RTSA, since repetitive contact between the humeral epiphysis and the scapular neck may result in polyethylene wear debris, chronic inflammation and osteolysis.36 However, its consequences on clinical outcomes are variable and unclear.
The incidence and severity of notching were high with first-generation RTSA, but the scapular notch rate in fracture patients tended to be lower than that reported for cuff-deficient patients.37 The potential progression of scapular notching is worrisome, although the long-term effects of this finding have yet to be elucidated. Severe erosions of the scapular neck causing glenoid component loosening are infrequent, particularly with newer RTSA designs.
Radiolucent lines at the humeral stem - bone interface are more commonly observed in RTSA than in HA. Rates of radiolucencies and loosening of the humeral component ranging from 30% to 60% were reported both in fractures and cuff-deficient shoulders with first-generation RTSA at medium term. In most of the cases, proximal humeral osteolysis was associated with scapular notching and therefore with the inflammatory reaction induced by polyethylene wear debris.
The primary role played by polyethylene particles on humeral bone resorption is confirmed by the observations in anatomical shoulder replacement, in which the incidence and severity of radioluncencies around the humeral component are significantly higher in total arthroplasty (with a polyethylene glenoid component) than in HA.38
Associated morbidity
Complication rates of shoulder replacement for fractures vary considerably according to accuracy in clinical surveillance and are most likely underreported.
In the elderly population, fracture patients have a higher mortality than the agematched non-fracture population, especially after hip fractures. An increased mortality rate in the long term has been found after proximal humeral fractures, too.39 Poor general health in fracture patients contributes to increased mortality rather than just the fracture itself. However, shoulder arthroplasty is a major surgical procedure that can have negative repercussions on frail patients and, consequently, on their survival. The only study comparing conservative treatment versus RTSA in a cohort of fractured patients over 75 years of age reported a lower one-year mortality rate in the surgical group (8,1% vs 10,8%), but the difference was not significant.
The early experiences with RTSA were characterized by a high incidence of complications, that markedly declined over time as a result of surgical expertise and improved implant designs. According to a literature review focusing on shoulder replacement for proximal humerus fractures, the overall complication rates of RTSA and HA are 9,6% and 4,1%, respectively.40 However, these data refer to a population including all age groups. Studies specifically addressed to evaluate outcomes in elderly patients indicate an opposite trend, with a complication rate for HA that is almost twice as high the one for RTSA (20,5% vs. 11.8%).30
The most frequently reported complication for HA is stiffness, followed by neuropathy (brachial plexus, axillary and ulnar nerves), dislocation and infection. On the other hand, complex regional pain syndrome is the most common complication observed after RTSA: it can be hypothesized that inadequate rehabilitation has a primary role in the onset of this condition. Dislocation, infection and aseptic component loosening, in decreasing order of incidence, are other relevant complications of RTSA.30,40
Reoperations (excluding revision and resection arthroplasty) tend to be more frequent for RTSA. The most common reoperation after RTSA is debridement and irrigation for wound infection, while for HA is lysis of adhesions, followed by reduction and internal fixation of migrated tuberosities.40
Tuberosity failure is the main indication for revision surgery in HA for fractures: most of the times the procedure consists in revision to a reverse shoulder. There are few revision options for RTSA: another reverse is the only intervention that can preserve some shoulder function, while conversion to HA or resection arthroplasty are exclusively salvage - and not functional - procedures. RTSA revisions are less frequent than HA revisions, but this figure might be influenced by the surgeons’ attitude in restraining indications to RTSA revisions, that can be very challenging in elderly patients.40
Conclusions
Most of proximal humeral fractures in elderly patients can be successfully treated conservatively. However, complex fractures in selected patients may require surgery, and shoulder replacement is one of the available treatment options.
Acceptable pain relief has been reported with HA by several authors, but functional results are often compromised by displacement, nonunion or resorption of tuberosities. It is well known that anatomical healing of the tuberosities is the main determinant for a successful HA, because it is the prerequisite for rotator cuff recovery. The risk of HA failure is increased in case of fracture comminution, osteopenia, compromised vascularity and/or cuff insufficiency, as typically occurs in old patients.
RTSA is a semiconstrained prosthesis that allows to use the deltoid muscle as a compensation for the deficient rotator cuff: a stable center of rotation is created in the glenoid, allowing active flexion and abduction of the arm. During the last two decades, RTSA has gained popularity in the treatment of proximal humeral fractures in elderly patients. Results with RTSA are also somewhat dependent on tuberosity position and healing, particularly for the recovery of external rotation, but not as critically as in HA.
Literature comparing HA and RTSA results is not univocal: RTSA resulted in better functional outcomes compared with HA in some studies, with no difference seen in others. There are many variables affecting the outcome of shoulder arthroplasty for fractures, and social factors have been considered more predictive than age for the final result.41
There is concern about the long-term survival of RTSA, even though new implant designs have decreased the risk of prosthetic failures caused by scapular notching, polyethylene wear and component loosening. The mechanical drawbacks of RTSA cannot be completely overcome and further studies are needed to understand which alternative solutions to Grammont’s original principles might really improve RTSA reliability over time.
In light of an elevated risk of postoperative complications and limited revision options, surgeons should attempt to identify those patients at greatest risk of poor outcome with conservative methods or HA before proceeding to RTSA.
Operative interventions and rehabilitation after a shoulder fracture are resource consuming: for this reason, an accurate assessment of general health, functional needs and compliance to treatment is mandatory before decision making. RTSA treatment is significantly more expensive than HA treatment,42 and further investigation will have to elucidate whether there are functional or financial benefits in choosing RTSA for the treatment of proximal humerus fractures in the elderly.
Funding Statement
Funding: none.
References
- 1.Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury 2006;37:691-7. [DOI] [PubMed] [Google Scholar]
- 2.Calvo E, Morcillo D, Foruria AM, et al. Nondisplaced proximal humeral fractures: high incidence among outpatient-treated osteoporotic fractures and severe impact on upper extremity function and patient subjective health perception. J Shoulder Elbow Surg 2011;20:795-801. [DOI] [PubMed] [Google Scholar]
- 3.Rosa MA, Maccauro G, Nizegorodcew T, et al. Percutaneous elastic fixation of proximal humeral fractures: operative indications, techniques, results and complications. J Orthop Traumatol 2002; 2:157-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray IR, Amin AK, White TO, Robinson CM. Proximal humeral fractures: current concepts in classification, treatment and outcomes. J Bone Joint Surg Br 2011;93:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Piccioli A, Maccauro G, Rossi B, et al. Surgical treatment of pathologic fractures of humerus. Injury. 2010; 41:1112-6. [DOI] [PubMed] [Google Scholar]
- 6.Neer CS. Articular replacement for the humeral head. J Bone Joint Surg Am 1955;37-A:215-28. [PubMed] [Google Scholar]
- 7.Grammont P, Trouilloud P, Laffay J, Deries X. Study and development of a new shoulder prosthesis. Rhumatologie 1987;39:407-18. [Google Scholar]
- 8.Boons HW, Goosen JH, van Grinsven S, et al. Hemiarthroplasty for humeral fourpart fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop Relat Res 2012;470:3483-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olerud P, Ahrengart L, Ponzer S, et al. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg 2011;20:1025-33. [DOI] [PubMed] [Google Scholar]
- 10.Kontakis G, Koutras C, Tosounidis T, Giannoudis P. Early management of proximal humeral fractures with hemiarthroplasty: a systematic review. J Bone Joint Surg Br 2008;90:1407-13. [DOI] [PubMed] [Google Scholar]
- 11.Boileau P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2002;11:401-12. [DOI] [PubMed] [Google Scholar]
- 12.Baulot E, Sirveaux F, Boileau P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res 2011;469:2425-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong) 2009;17:151-6. [DOI] [PubMed] [Google Scholar]
- 14.Cazeneuve J-F, Cristofari D-J. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res 2011;97:583-9. [DOI] [PubMed] [Google Scholar]
- 15.Wall BT, Mottier F, Walch G. Complications and revision of the reverse prosthesis: A multicenter study of 457 cases. J Shoulder Elbow Surg 2007;16:e55. [Google Scholar]
- 16.Boyle MJ, Youn S-M, Frampton CMA, Ball CM. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg 2013;22:32-7. [DOI] [PubMed] [Google Scholar]
- 17.Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br 2007;89:516-20. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers PN, Slikker W, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg 2014;23:197-204. [DOI] [PubMed] [Google Scholar]
- 19.Lenarz C, Shishani Y, McCrum C, et al. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res 2011;469:3324-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reitman RD, Kerzhner E. Reverse shoulder arthoplasty as treatment for comminuted proximal humeral fractures in elderly patients. Am J Orthop 2011;40:458-61. [PubMed] [Google Scholar]
- 21.Sirveaux F, Navez G, Favard L, et al. Reverse prosthesis for acute proximal humerus fracture, the multicentric study. In: Walch G, Boileau P, Mole D. (eds) Reverse shoulder arthroplasty: clinical results, complications, revisions. Sauramps Medical 2006;73-80. [Google Scholar]
- 22.Klein M, Juschka M, Hinkenjann B, et al. Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma 2008;22: 698-704. [DOI] [PubMed] [Google Scholar]
- 23.Valenti P, Katz D, Kilinc A, et al. Midterm outcome of reverse shoulder prostheses in complex proximal humeral fractures. Acta Orthop Belg 2012;78: 442-9. [PubMed] [Google Scholar]
- 24.Grassi FA, Zorzolo I. Reverse shoulder arthroplasty without subscapularis repair for the treatment of proximal humeral fractures in the elderly. Musculoskelet Surg 2014;98:5-13. [DOI] [PubMed] [Google Scholar]
- 25.Gallinet D, Clappaz P, Garbuio P, et al. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res 2009;95:48-55. [DOI] [PubMed] [Google Scholar]
- 26.Garrigues GE, Johnston PS, Pepe MD, et al. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics 2012;35:e703-8. [DOI] [PubMed] [Google Scholar]
- 27.Baudi P, Campochiaro G, Serafini F, et al. Hemiarthroplasty versus reverse shoulder arthroplasty: comparative study of functional and radiological outcomes in the treatment of acute proximal humerus fracture. Musculoskelet Surg 2014;98:19-25. [DOI] [PubMed] [Google Scholar]
- 28.Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am 2013;95:2050-5. [DOI] [PubMed] [Google Scholar]
- 29.Bonnevialle N, Tournier C, Clavert P, et al. Hemiarthroplasty versus reverse shoulder arthroplasty in 4-part displaced fractures of the proximal humerus: Multicenter retrospective study. Orthop Traumatol Surg Res 2016;102:569-73. [DOI] [PubMed] [Google Scholar]
- 30.Borade A, Familiari F, Choi K, Joseph J. Comparison of reverse total shoulder arthroplasty versus hemiarthroplasty for acute fractures of the proximal humerus: Systematic review. J Postgrad Med 2017;51. [Google Scholar]
- 31.Boileau P, Winter M, Cikes A, et al. Can surgeons predict what makes a good hemiarthroplasty for fracture? J Shoulder Elbow Surg 2013;22:1495-506. [DOI] [PubMed] [Google Scholar]
- 32.Kabir K, Burger C, Fischer P, et al. Health status as an important outcome factor after hemiarthroplasty. J Shoulder Elbow Surg 2009;18:75-82. [DOI] [PubMed] [Google Scholar]
- 33.Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg 2014;23: 1419-26. [DOI] [PubMed] [Google Scholar]
- 34.Reuther F, Petermann M, Stangl R. Reverse Shoulder Arthroplasty in Acute Fractures of the Proximal Humerus: Does Tuberosity Healing Improve Clinical Outcomes? J Orthop Trauma 2019;33:e46-51. [DOI] [PubMed] [Google Scholar]
- 35.Chun Y-M, Kim D-S, Lee D-H, Shin SJ. Reverse shoulder arthroplasty for four-part proximal humerus fracture in elderly patients: can a healed tuberosity improve the functional outcomes? J Shoulder Elbow Surg 2017;26:1216-21. [DOI] [PubMed] [Google Scholar]
- 36.Nyffeler RW, Werner CML, Simmen BR, Gerber C. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br 2004;86:1187-91. [DOI] [PubMed] [Google Scholar]
- 37.Torrens C, Alentorn-Geli E, Mingo F, et al. Reverse shoulder arthroplasty for the treatment of acute complex proximal humeral fractures: Influence of greater tuberosity healing on the functional outcomes. J Orthop Surg (Hong Kong) 2018;26:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Sotelo J, O’Driscoll SW, Torchia ME, et al. Radiographic assessment of cemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg 2001;10:526-31. [DOI] [PubMed] [Google Scholar]
- 39.Olsson C, Petersson C, Nordquist A. Increased mortality after fracture of the surgical neck of the humerus: a casecontrol study of 253 patients with a 12-year follow-up. Acta Orthop Scand 2003;74:714-7. [DOI] [PubMed] [Google Scholar]
- 40.Ferrel JR, Trinh TQ, Fischer RA. Reverse total shoulder arthroplasty versus hemiarthroplasty for proximal humeral fractures: a systematic review. J Orthop Trauma 2015;29:60-8. [DOI] [PubMed] [Google Scholar]
- 41.Clement ND, Duckworth AD, McQueen MM, Court-Brown CM. The outcome of proximal humeral fractures in the elderly: predictors of mortality and function. Bone Joint J 2014;96B:970-7. [DOI] [PubMed] [Google Scholar]
- 42.Solomon JA, Joseph SM, Shishani Y, et al. Cost Analysis of Hemiarthroplasty Versus Reverse Shoulder Arthroplasty for Fractures. Orthopedics 2016;39:230-4. [DOI] [PubMed] [Google Scholar]


