Abstract
Parasite monitoring is considered a necessary step for cetacean management and conservation. Between February 2013 and July 2015, 26 dolphins (15 Stenella coeruleoalba, 10 Tursiops truncatus, and one Grampus griseus) stranded along the Tuscan coastline of the protected marine area “Pelagos Sanctuary”, were examined. Organs, tissues, and faecal and blood samples taken from all animals were analysed by parasitological, immunological, and molecular techniques. Twenty-one out of 26 dolphins (80.77%) tested positive for at least one parasite species, and 13/15 (86.7%) S. coeruleoalba, 7/10 (70%) T. truncatus, and the single G. griseus were found positive. Identified parasites included the nematodes Skrjabinalius guevarai (7.69%, 2/26), Halocercus lagenorhynchi (3.85%, 1/26), Halocercus delphini (7.69%, 2/26), Stenurus ovatus (7.69%, 2/26), Crassicauda spp. (7.69%, 2/26); the trematodes Pholeter gastrophilus (26.92%, 7/26), Campula palliata (3.85%, 1/26); the cestodes Phyllobothrium delphini (42.31%, 11/26), Monorygma grimaldii (23.08%, 6/26), Tetrabothrium forsteri (7.69%, 2/26), Strobilocephalus triangularis (7.69%, 2/26), and the acanthocephalan Bolbosoma vasculosum (7.69%, 2/26). Moreover, 6/26 (23%) animals scored positive to Toxoplasma gondii at serology, but PCR confirmed the infection (T. gondii Type II genotype) in a single animal. In examined dolphins, obtained results showed a high prevalence of endoparasites, which included species considered as a cause of severe debilitation or death.
Keywords: parasites, Stenella coeruleoalba, Tursiops truncatus, Grampus griseus, Tuscany (central Italy), Pelagos Sanctuary
1. Introduction
The International Sanctuary for the Protection of Mediterranean Marine Mammals (hereafter Pelagos Sanctuary) is the first International high seas marine protected area worldwide, and it has been added in the list of specially protected areas of Mediterranean interest [1]. The Pelagos Sanctuary encompasses over 87,500 km2 of the north-western Mediterranean Sea. It is bounded to the west by a line extending from the Escampobariou Point (N 43°01′70–E 06°05′90), Giens Peninsula, France to the Falcone Cape (N 40°58′00–E 08°12′00), Sardinia, Italy and to the east by another line extending from the Ferro Cape (N 41°09′18–E 09°31′18), Sardinia, Italy to Fosso Chiarone (N 42°21′24–E 11°31′00), Tuscany, Italy [2,3].
The Sanctuary includes the Ligurian Sea and parts of the Corsican and Tyrrhenian seas, and it is composed by the internal maritime (15% of its extent) and territorial waters (32%) of France, Monaco, and Italy, as well as the adjacent high seas (53%) [2,4]. The Pelagos Sanctuary contains habitat suitable for the breeding and feeding needs of the entire complement of cetacean species regularly found in the Mediterranean Sea [2].
The three most abundant cetaceans in the Pelagos Sanctuary are the fin whale, Balaenoptera physalus (Lacépède, 1804), the striped dolphin, Stenella coeruleoalba (Meyen, 1833), and the bottlenose dolphin, Tursiops truncatus (Montagu, 1821). However, five other species are regular components of the Sanctuary’s cetacean fauna: sperm whale, Physeter macrocephalus; long-finned pilot whale Globicephala melas (Traill, 1809); Risso’s dolphin, Grampus griseus (Cuvier, 1812); common dolphins, Delphinus delphis; and Cuvier’s beaked whale, Ziphius cavirostris (Cuvier 1823) [2,4].
Habitat degradation, interaction with fisheries, and climate change are considered important issues interfering with the conservation of cetaceans worldwide [5,6,7]. Indeed, in the last decades the frequency of cetacean unusual mortality and stranding events has increased worldwide, including in the Mediterranean Sea [8,9,10,11].
Although the role of parasitic diseases as factors in cetacean stranding behaviour is still a topic of current debate, according to some authors, parasites should be included among the potential causes of the cetacean debilitation and death [12]. The damage and mortality of individuals and populations caused by parasitic infections are dependent upon several factors, including the parasite species, its abundance, and the health status of the host [12]. The knowledge of pathological effects and importance of parasites in ecological and evolutionary studies of cetaceans is highlighted, as parasites can influence the behaviour and population size of their hosts and the dynamics of the food chain and community structure [13]. Therefore, information about parasite infections is considered a necessary step towards assessing the impact of parasites on the marine mammal ecology and health and, ultimately, the cetacean population size, and towards the adoption of effective management and conservation plans [14,15].
With the aim to give a contribution to the knowledge of the parasite fauna of stranded cetaceans, this study reports data about parasite species identified in 26 dolphins stranded along the Tuscan coasts of the Pelagos Sanctuary in the period between February 2013 and July 2015.
2. Results and Discussion
Parasites of marine mammals encompass species that may cause serious pathological lesions and have been included among possible factors of cetacean stranding [16,17,18]. Moreover, the evaluation of the effects of parasites on these animals is considered essential for planning cetacean management and conservation measures [19]. Therefore, the knowledge of the cetacean parasitic fauna in a specific geographical area may contribute not only to the acquisition of new information on pathogens of these animals but also to possible tools for parasite control in the area. To date, data about parasite infections of cetaceans stranded along the Tuscan coastline of the “Pelagos Sanctuary” (central Italy) are still scarce [19].
Results obtained in this study showed a high prevalence of parasite infections in dolphins stranded along the coasts of Tuscany in the considered period. Twenty one out of 26 examined dolphins tested positive for parasites, with an overall prevalence of 80.77% (21/26). More specifically, 13/15 (86.67%) striped dolphins, 7/10 (70.00%) bottlenose dolphins, and the single Risso’s dolphin were found positive, but no statistical differences emerged among the different prevalence values found in striped dolphins and bottlenose dolphins (p = 0.390). In Table 1 are summarized data regarding identified parasite species, their prevalence, and the respective 95% confidence intervals observed in examined striped and bottlenose dolphins.
Table 1.
Prevalence and corresponding 95% confidence intervals (95% ICs) of parasites identified in striped dolphins and bottlenose dolphins found stranded between February 2013 and July 2015 along the coastline of Tuscany (Pelagos Sanctuary, central Italy).
| Parasites | Positive | Negative | Prevalence | IC95 % |
|---|---|---|---|---|
| Striped dolphin ( Stenella coeruleoalba , n = 15) | ||||
| Nematodes | ||||
| Halocercus lagenorhynchi | 1 | 14 | 6.67% | 0.00–19.29 |
| Halocercus delphini | 2 | 13 | 13.33% | 0.00–30.54 |
| Crassicauda spp. | 1 | 14 | 6.67% | 0.00–19.29 |
| Trematodes | ||||
| Pholeter gastrophilus | 4 | 11 | 26.67% | 4.29–49.05 |
| Campula palliata | 1 | 14 | 6.67% | 0.00–19.29 |
| Cestodes | ||||
| Phyllobothrium delphini | 11 | 4 | 73.33% | 50.95–95.71 |
| Monorygma grimaldii | 5 | 10 | 33.33% | 9.48–57.19 |
| Tetrabothrium forsteri | 2 | 13 | 13.33% | 0.00–30.54 |
| Strobilocephalus triangularis | 2 | 13 | 13.33% | 0.00–30.54 |
| Protozoa (serology) | ||||
| Toxoplasma gondii | 5 | 10 | 33.33% | 9.48–57.19 |
| Bottlenose dolphin ( Tursiops truncatus, n = 10) | ||||
| Nematodes | ||||
| Skrjabinalius guevarai | 2 | 8 | 20.00% | 0.00–44.79 |
| Stenurus ovatus | 2 | 8 | 20.00% | 0.00–44.79 |
| Crassicauda spp. | 1 | 9 | 10.00% | 0.00–28.59 |
| Trematodes | ||||
| Pholeter gastrophilus | 3 | 7 | 30.00% | 1.60–58.40 |
| Acanthocephalans | ||||
| Bolbosoma vasculosum | 2 | 8 | 20.00% | 0.00–44.79 |
| Protozoa (serology) | ||||
| Toxoplasma gondii | 1 | 9 | 10.00% | 0.00–28.59 |
Overall, all parasite species identified in this study were already reported in previous studies both in dolphins living in the Mediterranean Sea and in other seas [12,20,21,22,23].
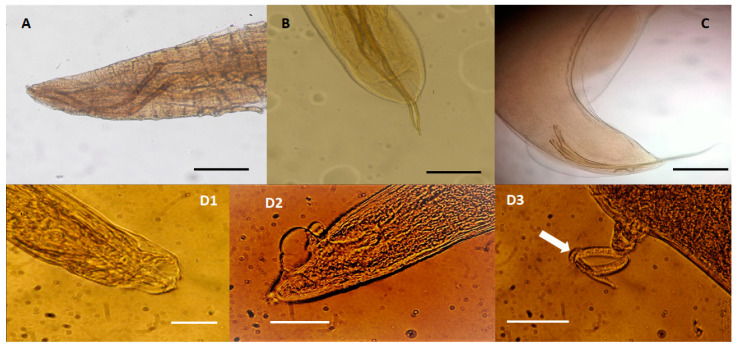
Among nematodes (Table 1, Figure 1), different respiratory species were identified in striped and bottlenose dolphins examined in this study, including Halocercus lagenorhynchi (6.67%, 1/15; Figure 1A) and Halocercus delphini (13.33%, 2/15; Figure 1B) that were identified in striped dolphins (Table 1), while Skrjabinalius guevarai (20.0%, 2/10; Figure 1C) and Stenurus ovatus (20.00%, 2/10; Figure 1D1–D3) were identified in bottlenose dolphins (Table 1). However, while the prevalence of S. guevarai in bottlenose dolphins from this study is similar to that (23.0%) reported by Manfredi et al. [24], the prevalence of S. ovatus is much higher than that (8.6%) reported by Manfredi et al. [24]. H. lagenorhynchi and H. delphini were identified in striped dolphins with a prevalence (respectively, 6.67% and 13.33%) higher than that (6.0%) previously reported in Italy [25]. The pathogenic effects of these nematodes depend on the species involved, the intensity of the infection, and on some factors related to the host. Species infecting the lung parenchyma, such as H. lagenorhynchi and S. guevarai, are a potential cause of death consequent to pneumonia and total or partial occlusion of bronchi and bronchioles and of reduced immersion capacity of infected animals [24,26].
Figure 1.
Nematode species identified in dolphins (15 Stenella coeruleoalba and 10 Tursiops truncatus), stranded along the Tuscan coastline (central Italy) of the “Pelagos Sanctuary” in the period between February 2013 and July 2015. (A) Halocercus lagenorhynchi adult male, measuring about 7 cm in length and 0.38 mm in width, found in the bronchi of a striped dolphin (S. coeruleoalba). Caudal end showing the spicules of about 0.65 mm in length and a copulatory bursa indistinguishable from the cuticle, scale bar 250 µm; (B) Halocercus delphini adult male measuring about 8 cm in length and 0.46 mm in width, found in the bronchi of a striped dolphin (S. coeruleoalba). Caudal end showing the spicules of about 0.73 mm in length, scale bar 250 µm; (C) Skrjabinalius guevarai adult male, measuring 6 cm in length and 0.5 mm in width, found in the bronchi of a bottlenose dolphin (T. truncatus): caudal end showing the spicules (length 0.77–0.80 mm), scale bar 250 µm; (D1–D3) Stenurus ovatus specimens found in the bronchi of a bottlenose dolphin (T. truncatus). (D1) Caudal end of and adult male with a caudal bursa showing two lateral rays (about 0.0465 mm in length and 0.020 mm wide) and a dorsal ray 0.053 mm long and 0.017 mm wide, (scale bar 250 µm); (D2) Caudal end of adult female showing two vulvar lips, one anterior long 0.035 mm and one posterior of about 0.037 mm in length, (scale bar 250 µm). (D3) First stage larva of 0.26 mm in length (arrow, scale bar 250 µm).
In this study, subcutaneous nematodes belonging to the genus Crassicauda (7.67%, 2/26) were detected in a striped dolphin (6.67%, 1/15) and in a bottlenose dolphin (10.00%, 1/10), but their identification at the species level was not possible due to the poor state of parasite conservation. In Italy, the prevalence of Crassicauda spp. nematodes is about 15%, and common, striped, bottlenose, and Risso’s dolphins have been found frequently infected [24,25]. The life cycle of these nematodes is still unknown, but cetaceans act as definitive hosts [27].
Although frequently reported in dolphins [28,29], in this study the detection of anisakid nematodes was not possible, probably due to the lack of availability for parasitological examination of the stomach content of examined dolphins.
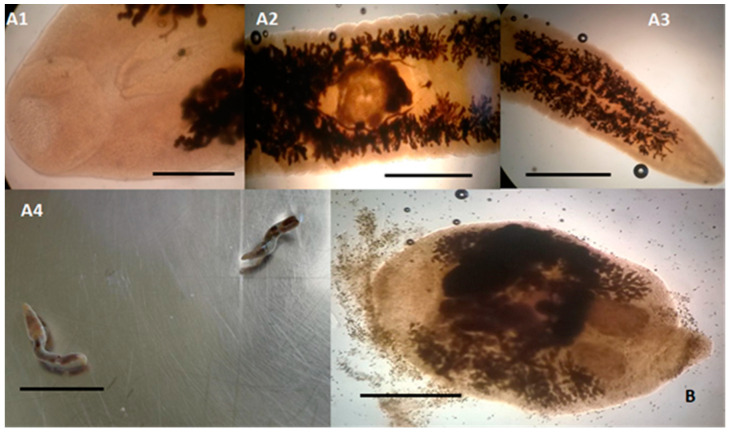
Among flukes (Table 1, Figure 2), the species Campula palliata (Figure 2A1–A4) was identified in 3.85% (1/26) of examined dolphins, precisely a striped dolphin, compared to 12.93% previously observed in Italy [24]. C. palliata infects the liver parenchyma and liver and pancreatic ducts, and it is often responsible for granulomatous hepatitis leading to decreased liver function, liver failure, and secondary bacterial infections [30]. The species Pholeter gastrophilus (Figure 2B) showed an overall prevalence of 26.92% (7/26). However, while the prevalence of this trematode observed in this study in bottlenose dolphins (30.00%, 3/10) was similar to that reported in previous studies in this cetacean species [21,31], in the striped dolphins (26.67%, 4/15) here examined it was lower than that reported in the study of Aznar et al. [22]. The localization site of P. gastrophilus is the gastric submucosa, and it is a common cause of granulomatous gastritis, which hinders the food transit and compromise the stomach motility [32]. How odontocetes, the definitive hosts, acquire P. gastrophilus and C. palliata infection it is not yet known, but it is thought to occur with the ingestion of infected fish and cephalopods, intermediate hosts [11,33].
Figure 2.
Trematodes identified in dolphins (15 Stenella coeruleoalba and 10 Tursiops truncatus), stranded along the Tuscan coastline (central Italy) of the “Pelagos Sanctuary” in the period between February 2013 and July 2015. (A1–A4) Campula palliata adult specimens found in the bile ducts of a striped dolphin (S. coeruleoalba). (A1) Microscopical view of the anterior end showing the oral sucker (scale bar 300 µm); (A2) microscopical view of the middle part of the body showing the ventral sucker (scale bar 1 mm); (A3) microscopical view of the posterior end of the body (scale bar 1 mm); (A4) microscopical view of the entire body of two adult specimens of 12–13 mm in length and 1.7–2.0 mm in width (scale bar 12 mm). (B) Pholeter gastrophilus adult (2.90 mm long and 2.00 mm wide) found in the submucosa of the third gastric compartment of a bottlenose dolphin (T. truncatus) showing a spindle-shaped body, with a cuticle covered with small pointed spines. The uterus, long and folded, is placed marginally and follows the body for almost its entire length. The testicles are ovoid in shape and are placed side by side in the posterior region of the body, while the lobed ovary is placed slightly in front and laterally the testicles, (scale bar 1 mm).
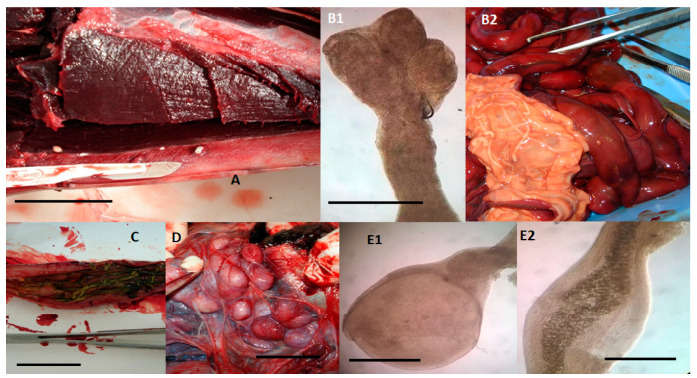
Among cestodes (Table 1, Figure 3), the overall prevalence of the species Phyllobothrium delphini and Monorygma grimaldii was, respectively, 42.30% (11/26) and 23.08% (6/26). However, in striped dolphins, the prevalence of P. delphini (73.33%, 4/15) and M. grimaldii (33.33%, 5/15) was higher than that reported in previous studies [12,24]. The positivity to M. grimaldii was also found in the single examined specimen of Risso’s dolphin. In the life cycle of these parasites, dolphins are second intermediate hosts and contain the second (merocercoid) larval stage, while sharks are the definitive hosts [11,34]. However, it is not yet known how dolphins acquire the infections and which are the first intermediate hosts [11,34]. In dolphins, P. delphini infects the subcutaneous adipose tissue (Figure 3A), while M. grimaldii can be found in the subserosa of the abdominal cavity (Figure 3D). Compared to P. delphini, M. grimaldii is more frequently the cause of suppurative inflammations. Nevertheless, it is assumed that the damage to the subcutaneous adipose tissue caused by a high P. delphini load may have a serious negative impact on swimming abilities of infected dolphins [34].
Figure 3.
Cestodes and Acanthocephalans identified in dolphins (15 Stenella coeruleoalba, 10 Tursiops truncatus, and a Grampus griseus), stranded along the Tuscan coastline (central Italy) of the “Pelagos Sanctuary” in the period between February 2013 and July 2015. (A) Phyllobothrium delphini located in the subcutaneous adipose tissue of the perigenital region of a striped dolphin (S. coeruleoalba), macroscopic view. Merocercoid larvae appear as white oval cystic formations with a diameter of 5–10 mm and containing an invaginated scolex showing four grooves (bothria) and a short neck, scale bar 2 cm. (B1,B2) Tetrabothrium forsteri adults in the intestine of a striped dolphin (S. coeruleoalba), microscopical view of the scolex showing four bothria measuring 0.3–0.69 mm in length and 0.25–0.6 mm in width (B1, scale bar 1 mm), and macroscopic view of the strobila whose length may range from a few millimetres to two metres, while the proglottids are wider than long (B2). (C) Strobilocephalus triangularis adults in the intestine of a striped dolphin (S. coeruleoalba). The size of the strobila varies from a few millimetres to two meters, while the scolexes are 5–6 mm wide and 4–6 mm long with four muscular bothria (scale bar 2.5 cm). (D) Monorygma grimaldii merocercoids in the subserosa of the abdominal cavity of an infected striped dolphin (S. coeruleoalba). Merocercoids appear as white cystic formations with a diameter of 10–20 mm, each containing an invaginated scolex showing four bothria and a very long neck, scale bar 1.5 cm. (E1,E2) Bolbosoma vasculosum female adult specimen about 0.435 mm wide and 0.85 mm long, found in the intestine of a bottlenose dolphin (T. truncatus) showing the bulbar anterior end of the body (E1, scale bar 250 µm) and developed eggs (E2, scale bar 250 µm).
Among cestode species for which dolphins are the definitive hosts (Table 1, Figure 3), the species Tetrabothrium forsteri (7.69%, 2/26; Figure 3B1,B2) and Strobilocephalus triangularis (7.69%, 2/26; Figure 3C) were here identified only in striped dolphins, both with a prevalence of about 13% (2/15). This prevalence is lower than that previously reported [20]. Both species infect the intestine but, while S. triangularis may be responsible for granulomatous nodular lesions, T. forsteri is considered a low-pathogenic parasite [35]. The life cycle of these cestodes is not completely known, but crustaceans are thought to act as first intermediate hosts, while cephalopods and teleost fishes are considered second intermediate or paratenic hosts, containing the plerocercoid larvae, which are infective for dolphins [11].
About acanthocephalans, Bolbosoma vasculosum (7.69%, 2/26; Figure 3E1,E2) was identified in bottlenose dolphins with a prevalence (20.00%, 2/8) lower than that (52.0%) reported by Mateu et al. [20]. These parasites are found in the intestine and are potentially highly pathogenic due to the hooked proboscis, which can cause an intestinal inflammatory reaction with fibrosis [33]. The life cycle of Bolbosoma spp. is thought to involve the pelagic marine zooplankton as intermediate host and different species of fish as transport hosts [36,37]. Juvenile forms (cystacanths) of these acanthocephalans are widely considered to be the infective stage for cetacean definitive hosts [37].
Faecal analysis by the sedimentation technique with formol-ethyl acetate allowed the detection of trematode eggs (P. gastrophilus and C. palliata) in 3/15 striped dolphins (20.0%), while 4/15 striped dolphins faecal samples (26.0%) scored positive for cestode eggs at flotation test. On the contrary, all faecal samples from bottlenose dolphins scored negative.
In recent studies, Giardia duodenalis and Cryptosporidium spp. infections have been recorded in dolphins and included zoonotic genotypes [38,39]. Nevertheless, in this study, as observed in a previous study [40], all dolphins scored negative to Giardia spp. and Cryptosporidium spp. All tests performed on faecal samples (fresh and Lugol, modified Ziehl–Neelsen, and DiffQuik stained faecal smears, immunoassay, and PCR) tested negative for G. duodenalis and Cryptosporidium spp. However, due to the intermittent faecal excretion of these protozoans or to a very small amount of protozoan antigen in the examined samples, false negative results may be possible [41].
T. gondii is a worldwide diffused zoonotic parasite affecting humans and animals, showing an heteroxenous lifecycle involving intermediate hosts, virtually all warm-blooded animals, and felid definitive hosts [42,43]. In dolphins, T. gondii is considered one of the main causes or a main contributing cause of stranding episodes and mortality [18,44,45,46]. In fact, in dolphins, T. gondii infection frequently causes encephalitis, myocarditis, lymphadenitis, abortion, and death [47,48]. Six out of 26 (23.08%) examined dolphins (5 striped dolphins and 1 bottlenose dolphin) tested positive to T. gondii antibodies at serology, but among them only the brain tissue of a single dolphin was positive for Toxoplasma DNA by PCR. In particular, the subject tested PCR positive was a striped dolphin specimen showing a serological positive titre of 1/2560. In previous studies [19,23,48], the seroprevalence of T. gondii in dolphins stranded along the Italian coasts was found to vary from 11% to 93%. Therefore, the serological prevalence observed in this study falls within the seroprevalence range reported in Italy. In some investigations [46,49], T. gondii serological prevalence was found to be higher in the coastal dolphin species, such as the bottlenose dolphin. Nevertheless, in this study the seroprevalence of T. gondii was higher in striped dolphins (33.33%, 5/15), a pelagic species, than in bottlenose dolphins (10.00%, 1/10). Differences between serological and PCR results can be explained by the heterogeneous distribution of T. gondii and parasite absence in the low amount of nervous tissue (few mgs) used for molecular analysis, especially in the case of low infection intensity [50]. It is interesting to note that the only subject found PCR positive in this study showed the highest serological titre. Nevertheless, PCR negativity to T. gondii of nervous tissues from serologically positive dolphins has been previously reported [19,50]. Furthermore, it is also possible that the positivity observed in some animals in this study was due to infections caused by other protozoa antigenically related to T. gondii and reported in dolphins, such as Neospora caninum and Sarcocystis spp. [51,52], or to other false positive results due to the low specificity of the serological test used [53]. However, this serological test is one of the most used and considered valid for the diagnosis of T. gondii [54].
Results of multilocus RFLP-PCR genotyping of T. gondii, using genes described by Su and colleagues [55,56], identified the amplified sample as belonging to Type II genotype, excluding for SAG1 gene where type II and III are indistinguishable (Table 2, Figure S1). Although some atypical genotypes were previously identified in dolphins [57,58], the type II genotype is the most widespread genotype in marine mammals in North America and Europe [57,58].
Table 2.
Genotyping of Toxoplasma gondii isolate from a striped dolphin (Stenella coeruleoalba).
| Genetic Markers | ||||||
|---|---|---|---|---|---|---|
| SAG1 * | 5′ +3′ SAG2 ** | alt SAG2 *** | SAG3 | BTUB | GRA6 | |
| Genotype | II/III | II | II | II | II | II |
How dolphins may acquire T. gondii infection is still controversial, although the ingestion of contaminated water or fish are currently considered the most likely route [44,47,52]. It has been suggested that oocysts poured into the sea with surface waters and sewers containing faeces of the final felid hosts may contaminate the marine environment with oocysts [11]. Oocysts are in fact very resistant in the marine environment, as they can reach infectivity and remain alive and infectious for several months [59]. It is also possible that the discharges of boats may play an important role in pelagic environments, especially if cats are on board [46]. However, it is known that dolphins ingest modest quantities of water, deriving mainly from the diet, and that they feed mainly on fish, cephalopods, and crustaceans that are not intermediate hosts of T. gondii [60]. On the other hand, some recent studies have demonstrated that fish and bivalve molluscs may contain viable T. gondii oocysts and can be involved in the transmission of T. gondii in the marine environment [47,61]. Bivalve molluscs are not usually part of the dolphin diet; however, the actual role of fish in the infection of dolphins has also not yet been clarified.
3. Materials and Methods
3.1. Animals
This study was carried out in the period between February 2013 and July 2015 and involved 26 dolphins found stranded lifeless in an optimal state of preservation on the Tuscan coastline (central Italy) of the Pelagos Sanctuary and promptly submitted for necropsy. Collection and transport of all carcasses were performed according to the EC Regulation 1069/2009. In this period, the following cetaceans were examined: 15 striped dolphins (S. coeruleoalba), 10 bottlenose dolphins (T. truncatus), 1 Risso’s dolphin (G. griseus). Biological data of these animals and geographic localities where they were found are presented in Table 3.
Table 3.
Species, gender, and weight of examined cetaceans found stranded between February 2013 and July 2015 along the coastline of Tuscany (Pelagos Sanctuary, central Italy) and year and geographic localities where animals were found.
| Species | Gender | Weight (kg) | Year of Stranding | Geographic Localities (Geographic Coordinates) |
|---|---|---|---|---|
| Stenella coeruleoalba | M | 49 | 2013 | Tirrenia (Pisa) 43°37′38″ N–10°17′28″ E |
| Stenella coeruleoalba | M | 100 | 2013 | Monte Argentario (Grosseto) 42°26′07″ N–11°07′00″ E |
| Stenella coeruleoalba | M | - | 2013 | Orbetello (Grosseto) 42°26′22″ N–11°12′45″ E |
| Stenella coeruleoalba | F | 45 | 2013 | Follonica (Grosseto) 42°55′08″ N–10°45′41″ E |
| Stenella coeruleoalba | F | 61 | 2013 | Calambrone (Pisa) 43°35′49″ N–10°17′40″ E |
| Stenella coeruleoalba | M | 65 | 2013 | Piombino (Livorno) 42°56′05.37″ N–10°31′19.63″ E |
| Stenella coeruleoalba | F | 25 | 2013 | Bibbona (Livorno) 16′12.64″ N–10°35′54.73″ E |
| Stenella coeruleoalba | M | 50 | 2013 | Livorno 43°32′36″ N–10°19′1″ E |
| Stenella coeruleoalba | M | 6 | 2013 | Livorno 43°32’36” N–10°19′1″ E |
| Stenella coeruleoalba | M | 75 | 2013 | Marina di Grosseto (Grosseto) 42°42′55″ N–10°59′02″ E |
| Stenella coeruleoalba | F | 60 | 2013 | Orbetello (Grosseto) 42°26′22″ N–11°12′45″ E |
| Stenella coeruleoalba | M | 75 | 2014 | Cecina (Livorno) 43°18′42.53″ N–10°31′08.49″ E |
| Stenella coeruleoalba | M | - | 2014 | Piombino (Livorno) 42°56′05.37″ N–10°31′19.63″ E |
| Stenella coeruleoalba | M | 29.5 | 2015 | Portoferraio (Livorno) 42°48′45″ N–10°18′56″ E |
| Stenella coeruleoalba | M | 45 | 2015 | Marina di Massa (Massa) 44°00′33.8″ N–10°06′06.9″ E |
| Tursiops truncatus | M | - | 2013 | Marina di Castagneto Carducci (Livorno) 43°10′39.93″ N–10°32′20.27″ E |
| Tursiops truncatus | * | - | 2013 | Calambrone (Pisa) 43°35′49″N–10°17′40″E |
| Tursiops truncatus | M | 140 | 2013 | Calambrone (Pisa) 43°35′49″N–10°17′40″E |
| Tursiops truncatus | F | 70 | 2013 | Rosignano Marittimo (Livorno) 43°24′32″ N–10°28′26″ E |
| Tursiops truncatus | F | 27 | 2014 | Livorno 43°32′36″ N–10°19′1″ E |
| Tursiops truncatus | M | 300 | 2014 | Marina di Pietrasanta (Lucca) 43°55′35.1″ N–10°11′48.3″ E |
| Tursiops truncatus | M | 30 | 2014 | Tirrenia (Pisa) 43°37′38″ N–10°17′28″ E |
| Tursiops truncatus | M | 140 | 2014 | Rosignano Marittimo (Livorno) 43°24′32″ N–10°28′26″ E |
| Tursiops truncatus | F | 102 | 2015 | Marina di Pisa (Pisa) 43°40′20″ N–10°16′37″ E |
| Tursiops truncatus | F | 43 | 2015 | Livorno 43°32′36″ N–10°19′1″ E |
| Grampus griseus | F | - | 2015 | San Vincenzo (Livorno) 43°06′02.28″ N–10°32′11.96″ E |
-: Data not available; *: sex was not identified.
3.2. Parasitological Examination
All dolphins were necropsied and all organs, including the subcutaneous adipose tissue, lung, liver, stomach, and intestine were examined by using different parasitological techniques aimed to the collection and identification of parasites. However, for most of examined specimens, the stomach content was not fully available for parasitological examination, as it was used for the identification of food composition and feeding habits of dolphins.
After collection, helminths were fixed and preserved in 70% ethanol. When necessary, worms were cleared with lactophenol or glycerine. After clarification, internal and external structures were visualized under an optical microscope and measured by using a micrometric ocular. Parasite identification was based on morphological identification keys available in the literature [11,12,20,23,30,32,35,37,62,63,64,65,66,67].
Moreover, individual faecal samples were collected from all examined dolphins and promptly analysed or stored at 4 °C and examined within 24 h. All samples were analysed macroscopically for macroparasites, such as nematode (sub)adults, proglottids of cestodes, and worm fragments, and then screened microscopically by the flotation test with a low-density solution (33% ZnSO4 solution) and by using the formalin-ethyl acetate centrifugation technique [39,68]. Fresh and Lugol, modified Ziehl–Neelsen, and Diff Quick stained faecal smears were performed for the search of protozoa, especially Giardia spp. and Cryptosporidium spp. [39,68]. In addition, a commercial rapid immunoassay (RIDA QUICK Cryptosporidium/Giardia Combi, R-Biopharm, Darmstadt, Germany) was used on the same faecal samples for the search of G. duodenalis and Cryptosporidium spp. faecal antigens.
PCR analysis was also performed for Giardia spp. and Cryptosporidium spp. on faecal samples as described by Cacciò et al. [69] and Pedraza-Diaz et al. [70], respectively. The extraction of DNA from stool was carried out by using a QIAamp DNA stool mini kit (Qiagen, Milan, Italy), following the instructions of the manufacturer.
During necropsy, blood clots were collected from the heart chamber of each dolphin and centrifuged (at 2500 rpm for 10 min). Obtained serum samples were screened for anti-Toxoplasma gondii antibodies by using a commercial direct agglutination test (Toxo-Screen DAT, BioMérieux, Florence, Italy) accordingly to manufacturer’s instructions. IgM-mediated agglutination was suppressed by using a diluting buffer containing 2-mercaptoethanol. The test was performed in microtitration plates with U-shaped wells. Control and diagnostic sera were diluted from 1:20 to 1:5200. The minimal titre considered as positive was greater or equal to 1:20, as reported for marine mammals [57,71,72,73].
Brain tissue samples were used for molecular analysis aimed to detect T. gondii DNA as described by [74]. The extraction of DNA from brain tissues was carried out by using a DNeasy Blood and Tissue Kit (Qiagen, Milan, Italy), following the manufacturer’s instructions. Positive DNA samples were obtained using BigDye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Life Technologies, Thermo Fisher Scientific, Milan, Italy), purified using BigDye X terminator® Purification Kit (Applied Biosystems, Thermo Fisher Scientific, Milan, Italy), and sequenced with the 310 Genetic Analyser (Applied Biosystems, Thermo Fisher Scientific, Milan, Italy). The samples confirmed as T. gondii positive were genotyped through multilocus PCR-RFLP genotyping by using the genetic markers described by Su and colleagues [55,56].
3.3. Statistical Analyses
The prevalence of parasites and the corresponding 95% confidence intervals (95% CIs) were calculated. Results were statistically analysed in order to evaluate possible differences between data found in the two most represented dolphin species in this study (striped dolphin and bottlenose dolphin). Data were analysed using a χ2 test with the Yates correction or a Fisher test [75], when appropriate. Results were considered significant when the null hypothesis had a probability lower than p < 0.05.
4. Conclusions
Results from this study showed a high prevalence of parasite infections in dolphins stranded along the coasts of Tuscany. For some parasite species, observed prevalence was much higher than that previously reported. The high prevalence of endoparasite infections in the subjects herein examined and the identification of parasite species considered as a cause of severe debilitation or death highlight the importance of parasite monitoring in investigations aimed at evaluating the health status of dolphins in the Pelagos Sanctuary, especially for threatened cetacean species [76]. Moreover, the life cycle of many identified parasite species and, mainly, the routes of infection in dolphins are still poorly understood, and further studies are needed to fill these gaps.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/8/612/s1, Figure S1: Multiplex multilocus nested PCR-RFLP (Mn-PCR-RFLP) analysis of Toxoplasma gondii sample using seven different genetic markers. Nested PCR products from each marker were digested with selected restriction enzymes [56,57], and DNA fragments were separated in agarose.
Author Contributions
Conceptualization, G.T. and S.P.; methodology, G.T., G.F., and S.P.; software and formal analysis, G.F.; investigation, G.T., G.F., A.C., E.R., C.M., and S.P.; resources, G.T. and S.P.; data curation, A.C.; writing—original draft preparation, S.P. and G.F.; writing—review and editing, G.T., G.F., and S.P.; supervision, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Panigada S., Lauriano G., Burt L., Pierantonio N., Donovan G. Monitoring winter and summer abundance of cetaceans in the Pelagos Sanctuary (northwestern Mediterranean Sea) through aerial surveys. PLoS ONE. 2011;6:e22878. doi: 10.1371/journal.pone.0022878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notarbartolo-di-Sciara G., Agardy T., Hyrenbach D., Scovazzi T., Van Klaveren P. The Pelagos Sanctuary for Mediterranean marine mammals. Aquat. Conserv. 2008;18:367–391. doi: 10.1002/aqc.855. [DOI] [Google Scholar]
- 3.Pelagos Sanctuary. [(accessed on 10 June 2020)]; Available online: https://www.sanctuaire-pelagos.org/en/about-us/area-of-application-and-coastal-municipalities.
- 4.Pennino M.G., Arcangeli A., Prado Fonseca V., Campana I., Pierce G.J., Rotta A., Bellido J.M. A spatially explicit risk assessment approach: Cetaceans and marine traffic in the Pelagos Sanctuary (Mediterranean Sea) PLoS ONE. 2017;12:e0179686. doi: 10.1371/journal.pone.0179686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balbuena J.A., Simpkin A. Role of Crassicauda sp. in natural mortality of pantropical spotted dolphins Stenella attenuata: A reassessment. Dis. Aquat. Organ. 2014;108:83–89. doi: 10.3354/dao02694. [DOI] [PubMed] [Google Scholar]
- 6.Lassalle G., Gascuel D., Le Loc’h F., Lobry J., Pierce G.J., Ridoux V., Begona Santos M., Spitz J., Niquil N. An ecosystem approach for the assessment of fisheries impacts on marine top predators: The bay of Biscay case study. ICES J. Mar. Sci. 2012;69:925–938. doi: 10.1093/icesjms/fss049. [DOI] [Google Scholar]
- 7.Lauriano G., Pierantonio N., Donovan G., Panigada S. Abundance and distribution of Tursiops truncatus in the Western Mediterranean Sea: An assessment towards the Marine Strategy Framework Directive requirements. Mar. Environ. Res. 2014;100:86–93. doi: 10.1016/j.marenvres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Mazzariol S., Centelleghe C., Cozzi B., Povinelli M., Marcer F., Ferri N., Di Francesco G., Badagliacca P., Profeta F., Olivieri V., et al. Multidisciplinary studies on a sick-leader syndrome-associated mass stranding of sperm whales (Physeter macrocephalus) along the Adriatic coast of Italy. Sci. Rep. 2018;8:11577. doi: 10.1038/s41598-018-29966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrabar J., Bočina I., Gudan Kurilj A., Đuras M., Mladineo I. Gastric lesions in dolphins stranded along the Eastern Adriatic coast. Dis. Aquat. Organ. 2017;125:125–139. doi: 10.3354/dao03137. [DOI] [PubMed] [Google Scholar]
- 10.Casalone C., Mazzariol S., Pautasso A., Di Guardo G., Di Nocera F., Lucifora G., Ligios C., Franco A., Fichi G., Cocumelli C., et al. Cetacean strandings in Italy: An unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis. Aquat. Organ. 2014;109:81–86. doi: 10.3354/dao02726. [DOI] [PubMed] [Google Scholar]
- 11.Raga J.A., Aznar F.J., Balbuena J.A., Fernandez M. Parasites. In: Perrin W.F., Wursig B., Thewissen H.G.M., editors. Encyclopedia of Marine Mammals. 2nd ed. Academic Press; San Diego, CA, USA: 2008. pp. 821–830. [Google Scholar]
- 12.Oliveira J.B., Morales J.A., González-Barrientos R.C., Hernández-Gamboa J., Hernández-Mora G. Parasites of cetaceans stranded on the Pacific coast of Costa Rica. Vet. Parasitol. 2011;182:319–328. doi: 10.1016/j.vetpar.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho V.L., Bevilaqua C.M., Iñiguez A.M., Mathews-Cascon H., Bezerra Ribeiro F., Bezerra Pessoa L.M., Oliveira de Meirelles A.C., Gomes Borges J.C., Marigo J., Soares L., et al. Metazoan parasites of cetaceans off the northeastern coast of Brazil. Vet. Parasitol. 2010;173:116–122. doi: 10.1016/j.vetpar.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Poulin R., Blasco-Costa I., Randhawa H.S. Integrating parasitology and marine ecology: Seven challenges towards greater synergy. J. Sea Res. 2016;113:3–10. doi: 10.1016/j.seares.2014.10.019. [DOI] [Google Scholar]
- 15.Lehnert K., Randhawa H., Poulin R. Metazoan parasites from odontocetes of New Zealand: New records. Parasitol. Res. 2017;116:2861–2868. doi: 10.1007/s00436-017-5573-0. [DOI] [PubMed] [Google Scholar]
- 16.Guimarães J.P., Febronio A.M., Vergara-Parente J.E., Werneck M.R. Lesions associated with Halocercus brasiliensis Lins de Almeida, 1933 in the lungs of dolphins stranded in the Northeast of Brazil. J. Parasitol. 2015;101:248–251. doi: 10.1645/14-513.1. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Delgado J., Fernández A., Sierra E., Sacchini S., Andrada M., Vela A.I., Quesada-Canales O., Paz Y., Zucca D., Groch K., et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012) PLoS ONE. 2018;13:e0204444. doi: 10.1371/journal.pone.0204444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obusan M.C.M., Villanueva R.M.D., Siringan M.A.T., Rivera W.L., Aragones L.V. Leptospira spp. and Toxoplasma gondii in stranded representatives of wild cetaceans in the Philippines. BMC Vet. Res. 2019;15:372. doi: 10.1186/s12917-019-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pretti C., Mancianti F., Nardoni S., Ariti G., Monni G., Di Bello D., Marsili S., Papini R. Detection of Toxoplasma gondii infection in dolphins stranded along the Tuscan coast, Italy. Rev. Med. Vet. 2010;161:428–431. [Google Scholar]
- 20.Mateu P., Raga J.A., Fernandez M., Aznar F.J. Intestinal helminth fauna of striped dolphins (Stenella coeruleoalba) in the western Mediterranean: No effects of host body length, age and sex. Mar. Mam. Sci. 2014;30:961–977. doi: 10.1111/mms.12096. [DOI] [Google Scholar]
- 21.Quinones R., Giovannini A., Raga J.A., Fernandez M. Intestinal helminth fauna of bottlenose dolphin Tursiops truncatus and common Dolphin Delphinus delphis from the western mediterranean. J. Parasitol. 2013;99:576–579. doi: 10.1645/GE-3165.1. [DOI] [PubMed] [Google Scholar]
- 22.Aznar F.J., Fognani P., Balbuena J.A., Pietrobelli M., Raga J.A. Distribution of Pholeter gastrophilus (Digenea) within the stomach of four odontocete species: The role of the diet and digestive physiology of hosts. Parasitology. 2006;133:369–380. doi: 10.1017/S0031182006000321. [DOI] [PubMed] [Google Scholar]
- 23.Di Guardo G., Agrimi U., Morelli L., Cardeti G., Terracciano G., Kennedy S. Postmortem investigations on cetaceans found stranded on the coasts of Italy between 1990 and 1993. Vet. Rec. 1995;136:439. doi: 10.1136/vr.136.17.439. [DOI] [PubMed] [Google Scholar]
- 24.Manfredi M.T., Gazzonis A.L., Merella P., Garippa G., Musella V. Cetacei dei Mari Italiani: Diffusione, Spiaggiamenti e Problematiche Sanitarie. [(accessed on 19 May 2020)];2012 Available online: http://www.parassitologia.unina.it/wp-content/themes/para/mappe/parassiti16.pdf.
- 25.Mazzariol S., Marrucchella G., Di Guardo G., Podestà M., Olivieri V., Colangelo P., Kennedy S., Castagnaro M., Cozzi B. Post-mortem findings in cetaceans stranded along Italian Adriatic Sea coastline (2000–2006); Proceedings of the International Whaling Commission’s 59th Annual Meeting; Anchorage, AK, USA. 28–31 May 2007. [Google Scholar]
- 26.Measures L.N. Lungworms of marine mammals. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. 2nd ed. Iowa States University Press; Ames, IA, USA: 2001. pp. 279–300. Chapter 10. [Google Scholar]
- 27.Lambertsen R.H. Crassicaudosis: A parasitic disease threatening the health and population recovery of large baleen whales. Rev. Sci. Tec. 1992;11:1131–1141. doi: 10.20506/rst.11.4.656. [DOI] [PubMed] [Google Scholar]
- 28.Blazekovic K., Pleic I.L., Duras M., Gomercic T., Mladineo I. Three Anisakis spp. isolated from toothed whales stranded along the eastern Adriatic Sea coast. Int. J. Parasitol. 2015;5:17–32. doi: 10.1016/j.ijpara.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Van Beurden S.J., IJsseldijk L.L., Cremers H.J.W.M., Gröne A., Verheije M.H., Begeman L. Anisakis spp. induced granulomatous dermatitis in a harbour porpoise Phocoena phocoena and a bottlenose dolphin Tursiops truncates. Dis. Aquat. Org. 2015;112:257–263. doi: 10.3354/dao02818. [DOI] [PubMed] [Google Scholar]
- 30.Dailey M.D. Parasitic disease. In: Dierauf L.A., Gulland F.M.D., editors. CRC Handbook of Marine Mammals Medicine: Health, Disease and Rehabilitation. 2nd ed. CRC Press, Inc.; Boca Raton, FL, USA: 2001. pp. 357–379. [Google Scholar]
- 31.Romero M.A., Fernández M., Dans S.L., García N.A., González R., Crespo E.A. Gastrointestinal parasites of bottlenose dolphins Tursiops truncatus from the extreme Southwestern Atlantic, with notes on diet composition. Dis. Aquat. Org. 2014;108:61–70. doi: 10.3354/dao02700. [DOI] [PubMed] [Google Scholar]
- 32.Jaber J.R., Perez J., Arbelo M., Zafra R., Fernandez A. Pathological and immunohistochemical study of gastrointestinal lesions in dolphins stranded in the Canary Islands. Vet. Rec. 2006;159:410–414. doi: 10.1136/vr.159.13.410. [DOI] [PubMed] [Google Scholar]
- 33.Gibson D.I., Harri E.A., Bray R.A., Jepson P.D., Kuiken T., Baker J.R., Simpson V.R. A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J. Zool. 1998;244:563–574. doi: 10.1111/j.1469-7998.1998.tb00061.x. [DOI] [Google Scholar]
- 34.Aznar F.J., Agusti C., Littlewood D.T.J., Raga J.A., Olson P.D. Insight into the role of cetaceans in the life cycle of the Tetraphyllideans (Platyhelminthes: Cestoda) Int. J. Parasitol. 2007;37:243–255. doi: 10.1016/j.ijpara.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Khalil L.F., Jones A., Bray R.A. Keys to the Cestode Parasites of Vertebrates. Commonwealth Agricultural Bureaux; Wallingford, UK: 1994. p. 768. [Google Scholar]
- 36.Hoberg E.P., Daoust P.Y., McBurney S. Bolbosoma capitatum and Bolbosoma sp. (Acanthocephala) from sperm whales (Physeter macrocephalus) stranded on Prince Edward Island, Canada. Proc. Helminthol. Soc. Wash. 1993;60:205–210. [Google Scholar]
- 37.Costa G., Chubb J.C., Veltkamp C.J. Cystacanths of Bolbosoma vasculosum in the black scabbard fish Aphanopus carbo, oceanic horse mackerel Trachurus picturatus and common dolphin Delphinus delphis from Madeira, Portugal. J. Helminthol. 2000;74:113–120. doi: 10.1017/S0022149X00000159. [DOI] [PubMed] [Google Scholar]
- 38.Reboredo-Fernández A., Ares-Mazás E., Martínez-Cedeira J.A., Romero-Suances R., Cacciò S.M., Gómez-Couso H. Giardia and Cryptosporidium in cetaceans on the European Atlantic coast. Parasitol. Res. 2015;114:693–698. doi: 10.1007/s00436-014-4235-8. [DOI] [PubMed] [Google Scholar]
- 39.Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living Indo-Pacific bottlenose dolphins (Tursiops aduncus) in the Northern Red Sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- 40.Fayer R., Fair P.A., Bossartf G.D., Santin M. Examination of naturally exposed bottlenose dolphins (Tursiops truncatus) for Microsporidia, Cryptosporidium, and Giardia. J. Parasitol. 2008;94:143–147. doi: 10.1645/GE-1262.1. [DOI] [PubMed] [Google Scholar]
- 41.RIDASCREEN Cryptosporidium_Giardia-Combi. [(accessed on 11 June 2020)]; Available online: https://clinical.r-biopharm.com/wp-content/uploads/sites/3/2017/05/C1121-RIDASCREEN-Cryptosporidium_Giardia-Combi_lang-2017-04-20_EN.pdf.
- 42.Aguirre A.A., Longcore T., Barbieri M., Dabritz H., Hill D., Klein P.N., Lepczyk C., Lilly E.L., McLeod R., Milcarsky J., et al. The one health approach to toxoplasmosis: Epidemiology, control, and prevention strategies. Ecohealth. 2019;16:378–390. doi: 10.1007/s10393-019-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubey J.P., Beattie C.P. Toxoplasmosis of Animals and Man. CRC Press; Boca Raton, FL, USA: 1988. pp. 1–220. [Google Scholar]
- 44.Bigal E., Morick D., Scheinin A.P., Salant H., Berkowitz A., King R., Levy Y., Melero M., Sánchez-Vizcaíno J.M., Goffman O., et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus); A first description from the Eastern Mediterranean Sea. Vet. Parasitol. 2018;258:74–78. doi: 10.1016/j.vetpar.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Di Guardo G., Proietto U., Di Francesco C.E., Marsilio F., Zaccaroni A., Scaravelli D., Mignone W., Garibaldi F., Kennedy S., Forster F., et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet. Pathol. 2010;47:245–253. doi: 10.1177/0300985809358036. [DOI] [PubMed] [Google Scholar]
- 46.Van Bressem M.F., Raga J.A., Di Guardo G., Jepson P.D., Duignan P.J., Siebert U., Barrett T., Santos M.C., Moreno I.B., Siciliano S., et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 2009;86:143–157. doi: 10.3354/dao02101. [DOI] [PubMed] [Google Scholar]
- 47.Massie G.N., Ware M.W., Villegas E.N., Black M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Profeta F., Di Francesco C.E., Marsilio F., Mignone W., Di Nocera F., De Carlo E., Lucifora G., Pietroluongo G., Baffoni M., Cocumelli C., et al. Retrospective seroepidemiological investigations against Morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998–2014) Res. Vet. Sci. 2015;101:89–92. doi: 10.1016/j.rvsc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Cabezon O., Resendes A.R., Domingo M., Raga J.A., Agusti C., Alegre F., Mons J.L., Dubey J.P., Almeria S. Seroprevalence of Toxoplasma gondii antibodies in wild dolphins from the Spanish Mediterranean coast. J. Parasitol. 2004;90:643–644. doi: 10.1645/GE-257R. [DOI] [PubMed] [Google Scholar]
- 50.Van de Velde N., Devleesschauwer B., Leopold M., Begeman L., IJsseldijk L., Hiemstra S., IJzer J., Brownlow A., Davison N., Haelters J., et al. Toxoplasma gondii in stranded marine mammals from the North Sea and Eastern Atlantic Ocean: Findings and diagnostic difficulties. Vet. Parasitol. 2016;230:25–32. doi: 10.1016/j.vetpar.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 51.Resendes A.R., Almería S., Dubey J.P., Obon E., Juan-Salles C., Degollada E., Alegre F., Cabezon O., Pont S., Domingo M. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J. Parasitol. 2002;88:1029–1032. doi: 10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Dubey J.P., Zarnke R., Thomas N.J., Wong S.K., Van Bonn W., Briggs M., Davis J.W., Ewing R., Mense M., Kwok O.C.H., et al. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003;116:275–296. doi: 10.1016/S0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 53.Blanchet M.A., Godfroid J., Breines E.M., Heide-Jørgensen M.P., Nielsen N.H., Hasselmeier J., Iversen M., Jensen S.K., Åsbakk K. West Greenland harbour porpoises assayed for antibodies against Toxoplasma gondii: False positives with the direct agglutination method. Dis. Aquat. Organ. 2014;108:181–186. doi: 10.3354/dao02715. [DOI] [PubMed] [Google Scholar]
- 54.Murata K., Mizuta K., Imazu K., Terasawa F., Taki M., Endoh T. The prevalence of Toxoplasma gondii antibodies in wild and captive cetaceans from Japan. J. Parasitol. 2004;90:896–898. doi: 10.1645/GE-197R. [DOI] [PubMed] [Google Scholar]
- 55.Su C., Zhang X., Dubey J.P. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: A high resolution and simple method for identification of parasites. Int. J. Parasitol. 2006;36:841–848. doi: 10.1016/j.ijpara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- 57.Dubey J.P., Fair P.A., Sundar N., Velmurugan G., Kwok O.C., McFee W.E., Majumdar D., Su C. Isolation of Toxoplasma gondii from bottlenose dolphins (Tursiops truncatus) J. Parasitol. 2008;94:821–823. doi: 10.1645/GE-1444.1. [DOI] [PubMed] [Google Scholar]
- 58.Di Guardo G., Di Cesare A., Otranto D., Casalone C., Iulini B., Mignone W., Tittarelli C., Meloni S., Castagna G., Forster F., et al. Genotyping of Toxoplasma gondii isolates in meningo-encephalitis affected striped dolphins (Stenella coeruleoalba) from Italy. Vet. Parasitol. 2011;183:31–36. doi: 10.1016/j.vetpar.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Lindsay D.S., Dubey J.P. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 2009;95:1019–1020. doi: 10.1645/GE-1919.1. [DOI] [PubMed] [Google Scholar]
- 60.Jones J.L., Dubey J.P. Waterborne toxoplasmosis—Recent developments. Exp. Parasitol. 2009;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Marino A.M.F., Giunta R.P., Salvaggio A., Castello A., Alfonzetti T., Barbagallo A., Aparo A., Scalzo F., Reale S., Buffolano W., et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health. 2019;66:826–834. doi: 10.1111/zph.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aguilar-Aguilar R., Delgado-Estrella A., Moreno-Navarrete R.G. New host report for nematodes in a stranded short-snouted spinner dolphin Stenella clymene (Cetacea: Delphinidae) from the Mexican Caribbean coast. Helminthologia. 2010;47:136–138. doi: 10.2478/s11687-010-0020-0. [DOI] [Google Scholar]
- 63.Baylis H.A., Daubney R. A revision of the lungworms of cetaceans. Parasitology. 1925;17:201–216. doi: 10.1017/S0031182000004595. [DOI] [Google Scholar]
- 64.Dawes B. The Trematoda, with Special Reference to British and Other European Forms. Cambridge University Press; Cambridge, UK: 1968. p. 364. [Google Scholar]
- 65.Dailey M.D. Parasites of marine mammals. In: Rohde K., editor. Marine Parasitology. CABI Publishing; Wallingford, UK: 2005. pp. 408–414. [Google Scholar]
- 66.Kuwamura M., Sawamoto O., Yamate J., Aokii M., Ohnishi Y., Kotani T. Pulmonary vascular proliferation and lungworm (Stenurus ovatus) in a bottlenose dolphin (Tursiops truncates) J. Vet. Med. Sci. 2007;69:531–533. doi: 10.1292/jvms.69.531. [DOI] [PubMed] [Google Scholar]
- 67.Flores-Cascante L., Gendron D. Application of McMaster’s technique in live blue whales. Vet. Rec. 2012;171:220. doi: 10.1136/vr.100749. [DOI] [PubMed] [Google Scholar]
- 68.Grilo M.L., Gomes L., Wohlsein P., de Carvalho L.M., Siebert U., Lehnert K. Cryptosporidium species and Giardia species prevalence in marine mammal species present in the North and Baltic Seas. J. Zoo. Wildl. Med. 2018;49:1002–1006. doi: 10.1638/2017-0255.1. [DOI] [PubMed] [Google Scholar]
- 69.Cacciò S.M., De Giacomo M., Pozio E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 2002;32:1023–1030. doi: 10.1016/S0020-7519(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 70.Pedraza-Diaz S., Amar C., Nichols G.L., McLauchin J. Nested polymerase chain reaction for amplication of the Cryptosporidium oocyst wall protein gene. Emerg. Infect. Dis. 2001;7:49–56. doi: 10.3201/eid0701.010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Measures L.N., Dubey J.P., Labelle P., Martineau D. Seroprevalence of Toxoplasma gondii in Canadian Pinnipeds. J. Wild. Dis. 2004;40:294–300. doi: 10.7589/0090-3558-40.2.294. [DOI] [PubMed] [Google Scholar]
- 72.Dubey J.P., Morales N., Sundar G., Velmurugan V., Gonzalez-Barrientos C.R., Hernandez-Mora G., Su C. Isolation and genetic characterization of Toxoplasma gondii from striped dolphins (Stenella coeruleoalba) J. Parasitol. 2007;93:710–711. doi: 10.1645/GE-1120R.1. [DOI] [PubMed] [Google Scholar]
- 73.Dubey J.P., Mergi J., Gehring E., Sundar N., Velmurugan G.V., Kwok O.C.H., Grigg M.E., Su C., Martineau D. Toxoplasmosis in captive dolphins (Tursiops truncatus) and Walrus (Odobenus rosmarus) J. Parasitol. 2009;95:82–85. doi: 10.1645/GE-1764.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alba P., Terracciano G., Franco A., Lorenzetti S., Cocumelli C., Fichi G., Eleni C., Zygmunt M.S., Cloeckaert A., Battisti A. The presence of Brucella ceti ST26 in a Striped Dolphin (Stenella coeruleoalba) with Meningoencephalitis from the Mediterranean Sea. Vet. Microbiol. 2013;164:158–163. doi: 10.1016/j.vetmic.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Glantz S.A. Statistica per Discipline Biomediche. 5th ed. McGraw-Hill Companies Srl.; Milan, Italy: 2003. [Google Scholar]
- 76.The IUCN Red List of Threatened Species. [(accessed on 26 June 2020)]; Available online: https://www.iucnredlist.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.