Abstract
The modification of the microbiome through fecal microbiota transplantation (FMT) is becoming a very promising therapeutic option for inflammatory bowel disease (IBD) patients. Our pilot study aimed to assess the effectiveness of multi-session FMT treatment in active ulcerative colitis (UC) patients. Ten patients with UC were treated with multi-session FMT (200 mL) from healthy donors, via colonoscopy/gastroscopy. Patients were evaluated as follows: at baseline, at week 7, and after 6 months, routine blood tests (including C reactive protein (CRP) and calprotectin) were performed. 16S rRNA gene (V3V4) sequencing was used for metagenomic analysis. The severity of UC was classified based on the Truelove–Witts index. The assessment of microbial diversity showed significant differences between recipients and healthy donors. FMT contributed to long-term, significant clinical and biochemical improvement. Metagenomic analysis revealed an increase in the amount of Lactobacillaceaea, Micrococcaceae, Prevotellaceae, and TM7 phylumsp.oral clone EW055 during FMT, whereas Staphylococcaceae and Bacillaceae declined significantly. A positive increase in the proportion of the genera Bifidobacterium, Lactobacillus, Rothia, Streptococcus, and Veillonella and a decrease in Bacillus, Bacteroides, and Staphylococcus were observed based on the correlation between calprotectin and Bacillus and Staphylococcus; ferritin and Lactobacillus, Veillonella, and Bifidobacterium abundance was indicated. A positive change in the abundance of Firmicutes was observed during FMT and after 6 months. The application of multi-session FMT led to the restoration of recipients’ microbiota and resulted in the remission of patients with active UC.
Keywords: fecal microbiota transplantation (FMT), inflammatory bowel disease (IBD), ulcerative colitis (UC), gut microbiota
1. Introduction
Inflammatory bowel disease (IBD) is a chronic progressive and idiopathic inflammatory disorder of the gastrointestinal tract (GI) tract, which includes ulcerative colitis (UC) and Crohn’s disease (CD). Although the etiology of IBD remains largely unknown, it involves a complex interaction between the genetic, environmental, and aberrant immune responses [1]. In recent times, the involvement of gut microbiota is considered a new probable factor strongly connected with IBD pathogenesis [2]. However, it is still unclear whether this dysbiosis is a primary or secondary event in the relationship with IBD. In this regard, Tomasello et al. [3] reported several mechanisms that are responsible for gut dysbiosis. Among the various etiopathogenic hypotheses, the most important one suggests that change in the saprophytic microbial flora causes mucosal damage. Modifications of intracellular tight junctions cause significant penetration of antigens, which leads to activation of the intestinal lymphatic system (MALT) [4]. Moreover, commensal microbiota plays a crucial role in the regulation of intestinal immune homeostasis. For example, Bacteroides fragilis through the action of the bacterial-derived polysaccharide A (PSA) affects systemic Th1 response [5]. Additionally, intestinal microbiota composition is regulated by specialized ileal epithelial cells. Paneth cells act by secreting granule contents that include antimicrobial peptides (secretory phospholipase A2 and α-defensins), thus any defects in the autophagy pathway lead to cell pathology [6]. So far, previous studies have presented inconsistent and inconclusive results on alterations in the intestinal microbiota in IBD patients. The gut microbial composition of patients with IBD is characterized by lower taxonomic levels of the most frequently assessed beneficial bacteria, such as Faecalibacterium prausnitzii and Roseburia intestinalis [7]. These microbes are responsible for butyric acid production, and as a result, can protect and modulate the intestinal immune response. Additionally, UC patients’ microbiota may harbor adherent/invasive Escherichia coli and Shigella species of the Enterobacteriaceae family [8]. Nevertheless, some changes in microbiota composition are observed in CD patients only, with no further confirmation in UC patients (i.e., in Actinobacteria, Firmicutes, Proteobacteria, Bacteroidetes, and Verrucomicrobia) [9,10,11,12,13], which makes the clinical investigation of microbiota in this group of patients even more complicated. Currently, one of the recent therapeutic options for UC patients, fecal microbiota transplantation (FMT), is applied in the clinical setting with a focus on restoring dysbiosis. FMT is an infusion of stool from a healthy donor into the colon, or its delivery through the upper gastrointestinal tract, to a recipient with a disease believed to be related to an unhealthy gut microbiome [14]. Recent studies [15,16,17,18,19] have proven the efficacy of FMT in the treatment of Clostridium difficile infection, with cure rates up to 90%. From a long-term perspective, it might be possible that FMT will be useful in the maintenance of IBD remission, which is of high importance in this treatment.
2. Materials and Methods
2.1. Study Design and Patients
This was a clinically controlled intervention study conducted at the Department of Gastroenterology of Heliodor Swiecicki Clinical Hospital, at Poznan University of Medical Sciences between January 2018 and December 2019. The study protocol was approved on 10 November 2016 by the Research Ethical Committee of Poznan University of Medical Sciences in Poland (No. 1004/16) and followed the requirements of the Declaration of Helsinki. All patients (donors and recipients of FMT) provided written consent.
Ten patients participated in the study, where multi-session FMT treatment was performed in weeks 1–6. Simultaneously, at the baseline, after the 6th course of FMT administration (week 7), and at the end of the follow-up period (6 months), stool sample analyses were performed to assess the changes in the microbiome and the concentration of fecal calprotectin. Six healthy stool donors were recruited from volunteers who reported to Heliodor Swiecicki Clinical Hospital in Poznan. Finally, 42 fecal samples collected from donors and recipients were used for microbial profiling.
2.2. Characteristics of FMT Recipients
Eligible patients were adults (aged over 18) with moderately to severely active UC (Truelove–Witts Severity Index 2–3), with no previous FMT history. The prevailing body mass index (BMI) of enrolled patients was 22 ± 2.75 kg/m2. UC was diagnosed based on clinical, endoscopic, and histological criteria. Exclusion criteria were as follows: colonic surgery, gastrointestinal infection including parasitic and C. difficile infections, indeterminate colitis, irritable bowel syndrome, food allergy, co-morbid chronic disease, history of cancer, pregnancy, use of antibiotics or probiotics during the 3 months before enrolment. During the study, concomitant treatments using 5-aminosalicylic acid immunomodulators were permitted, as long as the dose was stable for 4 weeks, and oral corticosteroids with the mandatory taper of 4–5 mg per week. However, neither the use of antibiotics nor probiotics was allowed.
2.3. Characteristics of Fecal Donors
Fecal donors were healthy individuals with regular Body Mass Index (BMI: 18.5–24.9 kg/m2), who consumed a diet following the principles of proper nutrition, were unrelated to recipients, and underwent detailed tests to rule out some diseases. A detailed medical history interview, physical examination, body composition analysis, and evaluation according to the following criteria were performed [20,21]. Following the guidelines by Cammarota et al. [22], the exclusion criteria for fecal donors were as follows: the presence of HIV, hepatitis B and C virus, cytomegalovirus, Epstein–Barr virus, toxoplasmosis, syphilis, Clostridium difficile, Yersinia spp., Campylobacter jejuni Shigella spp., Helicobacter pylori, parasites and potential carriers of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), extended-spectrum-β-lactamase (ESBL) producing bacteria, carbapenem-resistant Enterobacteriaceae (CRE), New Delhi metallo-β-lactamase (NDM), Klebsiella pneumoniae carbapenemase (KPC), and OXA-48. An additional exclusion criterion was the use of antibiotic therapy in the last 6 months. Basic biochemical tests (CRP, TSH, creatinine) and complete blood count (CBC) were performed. All samples were stored at −80 °C immediately after collection. Donors’ fecal samples were examined twice in the pre-screening period and 6 weeks later to minimize the risk of disease transmission.
2.4. Fecal Sample Preparation and FMT Intervention
From the collection of feces to the preparation of the transplant, strict procedures were followed to ensure sterile conditions. In the filtration process, the material was deprived of food residues; 180 mL of the mixture was taken successively, poured into disposable sterile sealed bottles, and then 20 mL of sterile glycerol as a cryoprotectant was added to achieve the mixture of feces: saline: glycerol in a ratio of 2:7:1. The bottles were successively frozen at −80 °C to ensure that the value of the material was identical to fresh material. On the day of transplantation, the material was thawed according to the procedure. The bottles with the material were additionally wrapped with aluminum foil to minimize the harmful effects of light and provide the patient with psychological comfort during the procedure. Intestinal microbiota transplantation was performed via colonoscopy or gastroduodenoscopy issues. Participants received loperamide 2 mg orally to reduce intestinal peristalsis and to maintain the suspension for a longer period. Fifty grams of fecal material was diluted with 150–200 mL sterile saline solution (0.9%). Infusion of the fecal suspension was ongoing via the biopsy channel of the flexible endoscope using the biopsy channel cap with extension tubing over the course of 5 min followed by 100 mL of physiological saline. The first dosage of FMT was given during the colonoscopy approach, throughout which 200 mL of a fecal suspension of donor stool was injected into the cecum. Additionally, during colonoscopy, the severity and extent of UC were estimated. Five subsequent sessions of FMT were performed via the upper GI tract route once a week for five consecutive weeks. A volume of 200 mL of fecal suspension was infused into the descending part of the duodenum. No side effects were reported.
2.5. Metagenomic Analysis and Data Processing
2.5.1. Sample Collection and DNA Extraction
Stool samples from donors and recipients (before and 1 month and 6 months after FMT) were immediately stored at −80 °C until genetic material isolation. From an approximately 200 μL feces aliquot, the bacterial DNA was extracted by using a Genomic Mini AX Bacteria+ Kit (A&A Biotechnology, Gdansk, Poland) according to the manufacturer’s protocol.
2.5.2. 16. S rRNA Gene Amplification and Sequencing
16S rRNA gene variable regions V3 and V4 were amplified in a two-step barcoding approach according to the 16S Metagenomic Sequencing Library Preparation protocol (https://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html, Part# 15044223Rev.B, Nov 27, 2013) using Illumina primers with overhang adapters (Forward: 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG3′, Reverse: 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATC3′), indices (Nextera® XT Index Kit 96, Illumina, San Diego, CA, USA), and KAPA HiFi HotStart ReadyMix PCR Kit (KAPA Biosystems, Cape Town, South Africa). Library concentration and quality were assessed on the Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the Agilent 2100 Bioanalyzer System (Agilent, Santa Clara, CA, USA). Subsequently, an 8 pM pooled library was enriched with a 20% PhiX Control v3 (Illumina, San Diego, CA, USA), and paired-end sequencing was performed on the Illumina MiSeq platform using the MiSeq Reagent Kit v3 (600 cycles).
2.6. Biochemical Assessment
In order to monitor the patient’s clinical features, routine diagnostic blood tests were performed including a hemoglobin count (Hb (g/L)), white cell count (WBC (109/L)), red blood count (RBC (1012/L)), platelet count (PLT (109/L)), markers of inflammation such as C reactive protein (CRP (mg/dL)), iron (Fe (ug/dL)), total iron-binding capacity (TIBC (ug/dL)), serum ferritin (ug/L) as an indicator of iron storage, total protein level (TP g/dL), and albumin (g/dL). Additionally, fecal calprotectin as a non-invasive test for the direct evaluation of intestinal inflammation was measured using a commercially available quantitative enzyme-linked immunoassay (PhiCal Calprotectin Elisa Kit, Immunodiagnostic, Bensheim, Germany). Blood samples were taken after 14 h of fasting. Biochemical assessments and metagenomic analysis were performed in a certified laboratory according to standardized procedures and good laboratory practice at the baseline (week 0), after the 6th course of therapeutic intervention, and at the end of the follow-up study.
2.7. Statistical Analysis
Obtained sequencing reads were processed for quality check, demultiplexing, trimming, and alignment using CLC Genomic Workbench 8.5 and CLC Microbial Genomics Module 1.2. (Qiagen Bioinformatics, Aarhus, Denmark). Chimeric sequences were removed and operational taxonomic units (OTUs) were clustered against the SILVA v119 97% 16S rDNA gene database, following Quast et al. [23].
The results are presented as percentage average with 1 standard deviation for normally distributed continuous variables, or median (interquartile range) for non-normally distributed continuous variables as tested by the Shapiro–Wilk test. A p-value of less than 0.05 was considered significant. The associations between clinical parameters and bacteria were tested by the Spearman rank coefficient. For comparison of OTU richness in all studied groups, Kruskal–Wallis (p = 0.0013) and U Mann–Whitney tests were used. Comparisons between two groups were analyzed by the U Mann–Whitney test, and for more than two groups by ANOVA with post hoc Tukey test or Friedman with post hoc Dunn test for normally distributed continuous variables or non-normally distributed variables, respectively. Statistical analysis was performed using Dell Inc. (2016). Dell Statistica (data analysis software system), version 13. software.dell.com.
3. Results
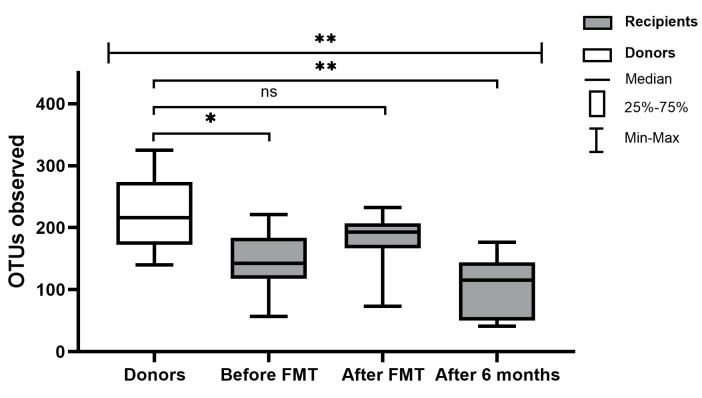
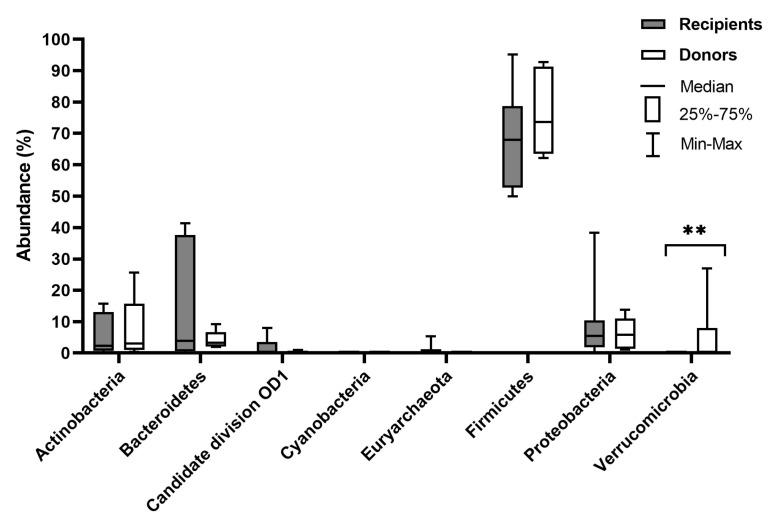
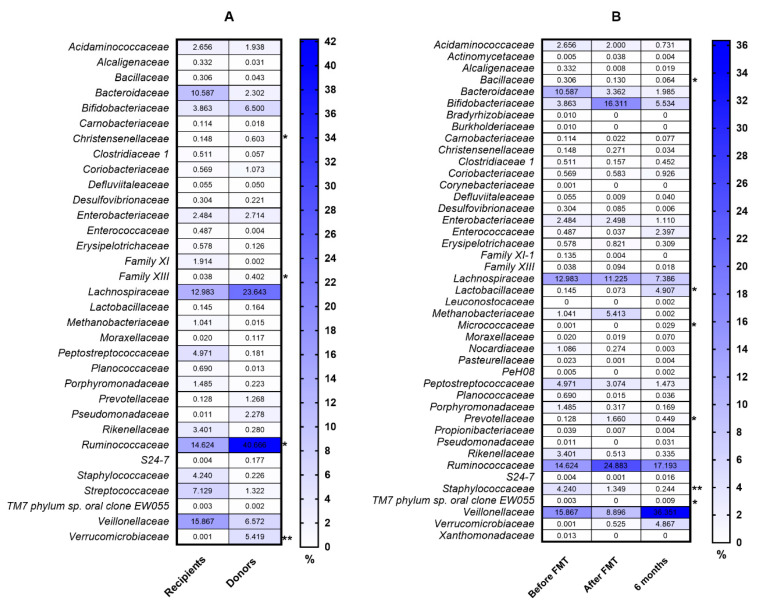
The clinical characteristics of patients are presented in Table 1. The diversity and richness of the fecal microbiota were significantly higher in donors compared with UC patients. At week 7 after FMT, the diversity and richness of the recipients’ fecal microbiota increased but then slightly decreased after 6 months (Figure 1). Assessment of phylum microbial diversity between recipients and donors indicated statistically significant changes in Verrucomicrobiota (Figure 2). Metagenomic analysis also indicated statistical changes in the family diversity of Christensenellaceae, Family XIII, Ruminococcaceae, and Verrucomicrobiaceae (Figure 3A). Within the range of genera, significant changes between donors’ and recipients’ microbiota were observed in the numbers of Akkermansia, Alloprevotella, Christensenella, Coprococcus, Dorea, Faecalibacterium, Incertae Sedis-03, Incertae Sedis-04, Incertae Sedis-06, Ruminococcus, and Subdoligranulum (Figure 4A).
Table 1.
Baseline patient characteristics.
| Analyzed Variable | N = 10 |
|---|---|
| Age (years) | 47.5 ± 18.16 |
| Disease duration (years) | 5.9 (3−10) |
| BMI (kg/m2) | 22 ± 2.75 |
| Male sex | 5 |
| Disease extent | |
| -Proctitis | 0 |
| -Left-sided colitis | 4 |
| -Extensive colitis | 4 |
| -Pancolitis | 2 |
| Medical treatment * | |
| -Oral 5-ASA | 10 |
| -Oral steroids | 4 |
| -Immunomodulators | 4 |
| Nonsmoker | 6 |
* Aminosalicylates: mesalazine, sulfasalazine; corticosteroids: prednisone, methylprednisolone; immunomodulators: azathioprine, 6-mercaptopurine.
Figure 1.
Gut microbial diversity in terms of operational taxonomic unit (OTU) richness in donors and ulcerative colitis (UC) patients in the peri-fecal microbiota transplantation (FMT) period. * p < 0.05, ** p < 0.01, ns: no significance.
Figure 2.
Comparison of microbial composition at the phylum level between recipients and donors. ** p < 0.005.
Figure 3.
Comparison of microbial composition at the family level between recipients and donors (A) and in UC patients in the peri-FMT period (B). * p < 0.05, ** p < 0.01.
Figure 4.
Comparison of microbial composition at the genus level between recipients and donors (A) and in UC patients in the peri-FMT period (B). The numbers in rectangles represent the abundance for each detected bacterial genus in %. Statistical significance: * p < 0.05, ** p < 0.01, *** p < 0.001.
Positive changes in the abundance of the phylum Firmicutes over the course of FMT were indicated in recipient patients, where a statistically significant increase was observed between baseline and the end of the follow-up period (6 months) (Figure 5). Metagenomic analysis indicated a significant increase in the family abundance of Lactobacillaceaea, Micrococcaceae, Prevotellaceae, and TM7 phylum sp. oral clone EW055 during FMT, whereas Bacillaceae and Staphylococcaceae declined significantly (p < 0.05) (Figure 3B). Moreover, positive, significant changes in the genus diversity were shown in the abundance of Anaerococcus, Bifidobacterium, Lactobacillus, Rothia, TM7 phylum sp. oral clone EW055, and Veillonella. In contrast, we observed a decreased richness of Bacillus, Bacteroides, and Staphylococcus after FMT and 6 months later, while Streptococcus decreased after FMT but increased at 6 months post FMT (Figure 4B).
Figure 5.
Comparison of microbial composition at the phylum level between recipients and donors (A) and in UC patients in the peri-FMT period (B). The numbers in rectangles represent the abundance for each detected bacterial phylum in %. Statistical significance: * p < 0.05.
Biochemical results are shown in Table 2. The disease activity was determined based on clinical and biochemical indicators. The comparison of biomarkers such as red blood count (RBC), hematocrit HCT, total iron-binding capacity (TIBC), C reactive protein (CRP), total protein level (TP), and calprotectin over the three time points of analysis (baseline, 7 weeks, and 6 months) showed statistically significant and clinically beneficial changes (p < 0.05). These significant, positive changes in biomarker concentrations were maintained during the follow-up period (6 months after FMT) for all of them. However, directly after FMT (at the 7th week), statistically significant changes were observed only for CRP and TP. Disease activity was classified based on the Truelove and Witts score (p < 0.05), which was also significant in the follow-up period. Additionally, before FMT administration we observed a positive correlation in recipients’ stool between calprotectin level and Bacillus and Staphylococcus genera, and Staphylococcaceae family richness. Furthermore, we noted that low serum ferritin concentration coexisted with decreased abundance of the Lactobacillaceae family, Lactobacillus genera, and Veillonella species, whereas augmented Bifidobacterium in recipients’ stool 6 months after FMT contributed to an increase in the level of serum ferritin (Table 3).
Table 2.
Biochemical results before and after FMT and after 6 months.
| Analyzed Parameter | n | Before FMT | After FMT | After 6 Months | p-Value |
|---|---|---|---|---|---|
| WBC (103/L) | 10 | 22.40 (20–24) | 7.15 (5.2–9.4) | 7.2 (6.2–9.4) | 0.904 b |
| RBC (10⁶/L) | 10 | 3.97 ± 0.62 | 4.11 ± 0.63 | 4.438 ± 0.46 | 0.029 a |
| Before vs. After 0.675 | |||||
| Before vs. After 6mo 0.027 | |||||
| After vs. After 6mo 0.141 | |||||
| HGB (g/dL) | 10 | 11.61 ± 2.38 | 12.18 ± 1.88 | 12.63 ± 1.92 | 0.099 a |
| HCT (%) | HCT | 36 (2.7–39) | 36.65 (33.6–39.5) | 40.35 (35.5–42.7) | 0.049 b |
| Before vs. After 0.281 | |||||
| Before vs. After 6mo 0.057 | |||||
| After vs. After 6mo 1.000 | |||||
| MCV (fL) | 10 | 86.75 (84–88) | 86.35 (84.9–88) | 85.35 (84.9–87) | 0.905 b |
| RDW-CV (fL) | 10 | 12.55 (12.3–13.7) | 13.7 (12.1–16) | 13.85 (12.8–14.5) | 0.283 b |
| PLT (103/L) | 10 | 313.4 ± 128.17 | 344.7 ± 118.83 | 299.1 ± 104.82 | 0.059 a |
| Iron (µg/dL) | 10 | 48.1 ± 24.6 | 51.7 ± 22.92 | 71.1 ± 39.75 | 0.075 a |
| TIBC (µg/dL) | 10 | 243.2 ± 72.81 | 278.6 ± 36.81 | 316.5 ± 45.01 | 0.004 a |
| Before vs. After 0.171 | |||||
| Before vs. After 6mo 0.003 | |||||
| After vs. After 6mo 0.135 | |||||
| CRP (mg/L) | 10 | 9.5 (7.7–82) | 5.2 (3.2–5.7) | 3.4 (2.4–7.4) | 0.0004 b |
| Before vs. After 0.011 | |||||
| Before vs. After 6mo 0.0004 | |||||
| After vs. After 6mo 1.000 | |||||
| Ferritin (ng/mL) | 10 | 33 (23–50) | 35.5 (19–42) | 37 (24–44) | 0.388 b |
| TP (g/dL) | 10 | 6.7 ± 0.45 | 7.18 ± 0.51 | 7.325 ± 0.52 | 0.001 a |
| Before vs. After 0.013 | |||||
| Before vs. After 6mo 0.002 | |||||
| After vs. After 6mo 0.604 | |||||
| ALB (g/dL) | 10 | 3.72 ± 0.65 | 4.06 ± 0.28 | 4.196 ± 0.33 | 0.127 a |
| Calprotectin (µg/g) | 10 | 1500 (969–1590) | 1095 (1000–1280) | 510 (91–800) | 0.002 b |
| Before vs. After 0.221 | |||||
| Before vs. After 6mo 0.001 | |||||
| After vs. After 6mo 0.221 | |||||
| Disease activity (Truelove and Witts Severity Index) | 10 | 3 (3–3) | 2 (2–2) | 1 (1–2) | 0.0001 b |
| Before vs. After 0.016 | |||||
| Before vs. After 6mo 0.0003 | |||||
| After vs. After 6mo 0.791 |
a ANOVA post hoc Tukey, b Friedman post hoc Dunn.
Table 3.
Selected biochemical parameters and bacteria, before and after FMT and after 6 months.
| Phylum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selected Bacteria | Before FMT | After FMT | After 6 Months | ||||||
| CRP | Ferritin | CALPR * | CRP | Ferritin | CALPR * | CRP | Ferritin | CALPR * | |
| Firmicutes | 0.405 (0.297) | 0.947 (0.024) | 0.575 (−0.202) | 0.382 (0.311) | 0.556 (−0.212) | 0.275 (−0.383) | 0.511 (−0.236) | 0.345 (−0.334) | 0.128 (−0.515) |
| Family | |||||||||
| Lactobacillaceae | 0.851 (0.068) | 0.035 (−0.668) | 0.427 (−0.283) | 0.228 (−0.419) | 0.570 (0.205) | 0.903 (0.045) | 0.379 (0.313) | 0.852 (−0.068) | 0.672 (0.153) |
| Staphylococcaceae | 0.336 (0.340) | 0.662 (0.159) | 0.001 (0.861) | 0.353 (0.329) | 0.059 (0.612) | 0.097 (0.553) | 0.211 (0.433) | 0.293 (0.370) | 0.131 (0.511) |
| Genus | |||||||||
| Anaerococcus | 0.793 (−0.096) | 0.756 (0.113) | 0.97 (−0.014) | 0.227 (−0.42) | 0.141 (−0.5) | 0.918 (−0.037) | 0.416 (0.29) | 0.415 (0.291) | 0.416 (0.29) |
| Bacillus | 0.135 (0.506) | 0.424 (0.285) | 0.003 (0.829) | 0.021 (0.713) | 0.947 (−0.024) | 0.973 (0.012) | 0.173 (0.467) | 0.102 (0.547) | 0.65 (0.164) |
| Bacteroides | 0.511 (−0.236) | 0.854 (−0.067) | 0.461 (−0.264) | 0.802 (−0.091) | 0.108 (−0.539) | 0.106 (−0.541) | 0.679 (−0.15) | 0.615 (0.182) | 0.199 (0.444) |
| Bifidobacterium | 0.651 (0.164) | 0.364 (0.322) | 0.599 (0.19) | 0.464 (−0.262) | 0.31 (0.358) | 0.213 (0.432) | 0.365 (0.321) | 0.028 (0.687) | 0.855 (0.067) |
| Lactobacillus | 0.851 (0.068) | 0.035 (−0.668) | 0.428 (−0.283) | 0.228 (−0.419) | 0.57 (−0.205) | 0.903 (0.045) | 0.379 (0.313) | 0.853 (−0.068) | 0.672 (0.153) |
| Staphylococcus | 0.336 (0.34) | 0.662 (0.159) | 0.001 (0.862) | 0.353 (0.329) | 0.06 (0.612) | 0.097 (0.553) | 0.211 (0.433) | 0.293 (0.37) | 0.131 (0.511) |
| Veillonella | 0.551 (0.215) | 0.046 (−0.64) | 0.878 (0.056) | 0.865 (−0.062) | 0.906 (0.043) | 0.598 (−0.191) | 0.385 (−0.309) | 0.097 (−0.553) | 0.385 (−0.309) |
* Calprotectin (CALPR).
4. Discussion
In the current study, the effectiveness of multi-session FMT was proven as a promising therapeutic option for moderately to severely active UC patients. The high percentage of patients (60%) that achieved clinically significant improvement highlights the importance of FMT in long-term microbial diversity modulation in UC patients. No serious adverse effects were observed. Therefore, FMT can be characterized as a safe procedure in the clinical setting.
There are several factors such as delivery route, frequency, dosage, and microbiota resources that may significantly affect the outcome of FMT in UC patients. The most adequate route of administration for FMT remains to be defined. Multiple approaches to FMT including upper-gut delivery (oral capsules of fecal microbiota), mid-gut delivery (naso-intestinal tubes, gastroduodenoscopy, transendoscopic enteral tubing (TET), intestinal stoma, percutaneous endoscopic gastrostomy with jejunal tubes (PEG-J)), or lower-gut delivery (colonoscopy, enema, infusions through colonic stomas and a new delivering technic colonic transendoscopic enteral tubing (TET) or combinations) have been described in several studies [24,25,26,27]. Moayyedi et al. [28] performed FMT via enema, and Rossen et al. [29] used a nasoduodenal tube. The numerous adverse effects noted with the use of the naso-intestinal route convinced us that FMT should be performed using colonoscopy and gastroscopy [30]. The same approach was followed by Goyal et al. [31]. It has to be mentioned that the intensity and duration of FMT may also affect the outcome. In the current study, patients received fecal material once weekly for six consecutive weeks. In another study [32], stool was administered five days per week for eight weeks. Rossen et al. [29] delivered fecal material at weeks 0 and 3. Clinical improvement was observed in cases with higher frequency and longer duration of FMT, thus indicating this approach as superior to rare and short-term interventions, in which no significant differences in clinical and endoscopic remission in UC patients were observed [29]. In general, greater intensity and duration of FMT result in a higher rate of remission in patients with UC. Moreover, the efficacy of FMT in UC patients is influenced by the taxonomic composition of the donor’s intestinal microbiota.
According to the literature published so far, no statistically significant differences were found when fresh stools were used for FMT as compared to frozen-thawed fecal material [33]. However, it has not been established whether single donor vs. multi-donor stool has better efficacy in re-establishing enteric homeostasis. It would seem that multi-donor stools may present with greater microbial diversity. It should be highlighted that microbial diversity also plays a crucial role in preventing the overgrowth of pathogens in UC. Therefore, donors chosen for the study were healthy individuals with a beneficial microbiota composition, without any co-morbidities. The metagenomics analysis indicated statistically significant differences between donors and recipients in the family diversity of Christensenellaceae, Family XIII, Ruminococcaceae, and Verrucomicrobiaceae, which could directly influence changes in specific genus microbial diversity. As shown in the meta-analysis by Mancabelli et al. [34], Christensenellaceae is an indicator of a healthy gut. Indeed, a growing body of evidence supports the consistent reduction of Christensenellaceae in patients with UC [9,35] and Crohn’s disease [9]. Christensenellaceae is a recently described family associated with a lean BMI and short-chain fatty acids (SCFAs) [36,37,38]. A noteworthy phylum is the Verrucomicrobia. It is a widely held view that reduced levels of Verrucomicrobia in the gastrointestinal tract are indicative of gut dysbiosis and are observed in UC patients [39,40,41]. This is consistent with our metagenomic analysis, in which microbiota of healthy donors revealed a relatively higher abundance of Verrucomicrobia. Alam et al. [42] reported in a mouse model that Akkermansia muciniphila (which belongs to the Verrucomicrobia phylum) plays a major role in intestinal wound healing and is considered to be a “probiont” species that contributes towards the repair of mucosal wounds. Furthermore, Akkermansia is associated with the immune response and the induction of interleukin-10 (IL-10) cytokine and SFCA production, supplying energy to goblet cells that produce mucin [43]. Patients with refractory UC, who achieved remission after FMT had a significantly higher relative abundance of Ruminococcaceae including Ruminococcus spp. and Akkermansia muciniphila [38]. This could be due to their ability to process nondigestible carbohydrates and promote the production of SFCAs as well. According to Paramsothy et al. [44], increased abundance of Bacteroides (B. fragilis and B. finegoldii) in donor stool may be associated with observed remission in patients receiving FMT, while changes in Streptococcus may not be related to FMT response. Santoru et al. [10] also showed that the microbial profile is characterized by a significantly higher presence of Verrucomicrobia, Firmicutes, Proteobacteria, and Fusobacteria in the IBD group. Additionally, bacterial taxa such as Coprococcus, Mucispirillum, Odoribacter, Prevotella, and Oscillospira were increased in abundance specifically in the early regenerative mucosa [42].
Six months after FMT, a rise in the abundance of the Micrococcaceae family in the recipients’ stools was observed. Walujkar et al. [45] showed an increase in the abundance of the genus Micrococcus in mucosal-associated microbiota (MAM) of exacerbated UC patients when compared with the remission phase. This was in the line with a study by Kiernan et al. [46] where a lower abundance of Actinobacteria phyla including Mycobacteriaceae, Micrococcaceae, and Streptomycineae was detected in healthy individuals. In the current study, an increase in the Micrococcaceae family may be an indication that microbiota composition is going to change in the pro-inflammatory direction, and from a long-term perspective, FMT will be needed in UC patients as a regular repeated procedure. Chen et al. [47] reported changes in the abundance of Faecalibacterium prausnitzii following FMT, which is of high importance for inflammatory diseases. In the intestine, F. prausnitzii produces butyrate, which can reduce intestinal mucosa inflammation by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor [48]. Another study [49] showed differences in the abundance of bacteria such as Lachnospiraceae, Bacteroidetes, Proteobacteria, and Clostridium clusters IV and XIVa (Firmicutes) in UC patients after FMT. This is in line with the current study, where beneficial changes in the abundance of the phylum Firmicutes over the course of FMT were indicated in recipient UC patients. Dutta et al. [50] also reported, during FMT, a significant rise in Lachnospiraceae, which have anti-inflammatory qualities. Data concerning the Bacteroidetes phylum are not unambiguous. According to our baseline data, a relatively higher abundance of Bacteroides was noted in recipients compared to donors, and it declined after FMT. Some studies [51,52,53] reported a decrease in the abundance of the Bacteroides in Crohn’s disease and UC patients. In contrast, Andoh et al. [54] reported an increased abundance of this phylum in IBD patients.
Analysis of our recipients’ stools indicated a significantly different abundance of Bifidobacterium and Lactobacillus genera, comparing the pre and post-fecal microbiota transplantation samples and at 6 months after FMT. The Lactobacilli and Bifidobacteria are responsible for the process of dietary carbohydrate fermentation in the colon, leading to the production of SCFAs such as butyrate, propionate, and acetate [55,56,57,58]. Due to their potential therapeutic anti-inflammatory components, some strains of Lactobacilli might positively influence the activity of inflammation in IBD patients. According to Nishida et al. [59], an increasing proportion of Bifidobacterium and Lactobacillus in recipients’ stools might contribute to a favorable response to FMT. Nevertheless, some studies [60,61,62] conducted in IBD patients have shown a significant depletion of beneficial Lactobacillus in both UC and CD individuals. At the baseline of our study, an overabundance of Staphylococcus taxa was observed in recipients, and it was consistently depleted after FMT. The mechanism of colonization of Staphylococcus in the human gut is still barely understood. However, some studies suggest an association of Staphylococcus aureus with IBD [63,64].
The Staphylococcus genus was found more frequently in UC patients with coexisting arthritis [65]. Furthermore, we observed an increased abundance of the Prevotellecaeae family in our recipients’ microbiota concomitantly after FMT and 6 months later. A similar observation was presented by Paramsmothy et al. [32], showing that Prevotella was a prominent feature in several patients during and after fecal microbiota transplantation. Several studies [66,67,68] have shown associations between the Mediterranean diet and an increased abundance of Prevotella.
From the clinical point of view, there are also several biomarkers such as C reactive protein (CRP), erythrocyte sedimentation rate (ESR), platelets, and fecal calprotectin that can be used for objective evaluation of disease activity and inflammation in IBD patients [69]. In the current study, the concentration of CRP and calprotectin significantly improved directly after FMT (p < 0.05), and the effect was maintained in the follow-up period of observation. Similarly to our results, Uygun et al. [70] reported that CRP and sedimentation level improved after FMT in both responder and non-responder to this therapy groups of patients. Comparably, Cold et al. [71] in an open-labeled pilot study confirmed that median fecal calprotectin decreased significantly during the FMT treatment period, but increased again in the follow-up. Additionally, we observed a positive correlation between calprotectin level and Bacillus and Staphylococcus richness in recipients’ stools before FMT administration. Calprotectin belongs to the antimicrobial proteins that inhibit the growth of microbial species [72,73]. Damo et al. [74] reported that calprotectin hinders the growth of bacterial pathogens such as Staphylococcus aureus, S. epidermidis, S. lugdunesis, and Enterococcus fecalis, etc. Furthermore, we noticed that low serum ferritin concentration coexisted with decreased abundance of Lactobacillus and Veillonella species, whereas augmented Bifidobacterium in recipients’ stools 6 months after FMT contributed to an increased level of serum ferritin. One third of IBD patients suffer from iron deficiency anemia (IDA) or anemia of chronic disease (ACD). Das et al. [75] showed that gut microbial metabolites may regulate host systemic iron homeostasis via ferritin regulation. Lactobacillus species are the crucial bacteria in ascertaining intestinal iron levels and attenuating host iron absorption.
This study has some limitations. The small number of patients who enrolled in this study means that it should be treated as a pilot in the further evaluation of FMT efficacy in UC patients, where a control group should be introduced to avoid potential bias in the data analysis.
5. Conclusions
FMT seems to be a promising therapy for the management of moderately to severely active UC. Our pilot study demonstrated that six rounds of intensive, weekly FMT administration contributed to clinical (Truelove and Witts score) and biochemical (CRP, calprotectin) improvement not only immediately after FMT, but also persisting up to 6 months of follow-up. Metagenomic analysis revealed significant differences in microbial diversity and richness between recipients and donors. After FMT, we observed a positive increase in the amount of Lactobacillaceaea, Micrococcaceae, Prevotellaceae, and TM7 phylum sp. oral clone EW055, whereas Staphylococcaceae and Bacillaceae declined significantly. Furthermore, we revealed a positive change in the abundance of Firmicutes both during FMT and after 6 months. It seems that the efficacy of this study might be related to the good donor microbial characteristics and the planned scheme and route (one colonoscopy and five rounds of gastroduodenoscopy) of FMT.
Abbreviations
| FMT | Fecal Microbiota Transplantation |
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative Colitis |
| CD | Crohn’s Disease |
| BMI | Body Mass Index |
| MAM | Mucosal-Associated Microbiota |
| CALPR | Calprotectin |
| SCFAs | Short-Chain Fatty Acids |
| CRP | C Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| IDA | Iron Deficiency Anemia |
| ACD | Anemia of Chronic Disease |
Author Contributions
Conceptualization, D.M.-W.; methodology, D.M.-W., H.T., and O.Z.-B.; software, A.S. and Ł.W.; formal analysis, O.Z.-B. and M.S.-Z.; investigation, D.M.-W., M.G., H.T., and A.B.; resources, D.M., and M.G.; data curation, D.M.-W. and M.G.; writing—original draft preparation, D.M.-W. and M.S.-M.; figures, M.S.-Z.; writing—review and editing, D.M.-W. and M.S.-M.; supervision, R.S. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant (number 502-01-222-38-000) for young scientists from Poznan University of Medical Sciences, Poznan, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang Y.-Z., Li Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. WJG. 2014;20:91. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knox N.C., Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiome as a target for IBD treatment: Are we there yet? Curr. Treat. Options Gastroenterol. 2019;17:115–126. doi: 10.1007/s11938-019-00221-w. [DOI] [PubMed] [Google Scholar]
- 3.Tomasello G., Bellavia M., Palumbo V.D., Gioviale M.C., Damiani P., Lo Monte A.I. From gut microflora imbalance to mycobacteria infection: Is there a relationship with chronic intestinal inflammatory diseases? Ann. Ital. Di Chir. 2011;82:3613–3668. [PubMed] [Google Scholar]
- 4.Arseneau K.O., Cominelli F. Leukocytapheresis in ulcerative colitis: A possible alternative to biological therapy? Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver. 2009;41:551. doi: 10.1016/j.dld.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Porter E., Bevins C.L., Ghosh D., Ganz T. The multifaceted Paneth cell. Cellular and molecular life sciences. CMLS. 2002;59:1561–1570. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Z.-H., Zhu C.-X., Quan Y.-S., Yang Z.-Y., Wu S., Luo W.-W., Tan B., Wang X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018;24:5. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha S., Xu B., Wang X., Zhang Y., Wang H., Kong X., Zhu H., Wu K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 2013;75:245–251. doi: 10.1016/j.diagmicrobio.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Imhann F., Vila A.V., Bonder M.J., Fu J., Gevers D., Visschedijk M.C., Spekhorst L.M., Alberts R., Franke L., Van Dullemen H.M. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoru M.L., Piras C., Murgia A., Palmas V., Camboni T., Liggi S., Ibba I., Lai M.A., Orrù S., Blois S. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017;7:11–14. doi: 10.1038/s41598-017-10034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michail S., Durbin M., Turner D., Griffiths A.M., Mack D.R., Hyams J., Leleiko N., Kenche H., Stolfi A., Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 2012;18:1799–1808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Gao X., Ghozlane A., Hu H., Li X., Xiao Y., Li D., Yu G., Zhang T. Characteristics of faecal microbiota in paediatric Crohn’s disease and their dynamic changes during infliximab therapy. J. Crohn’s Colitis. 2018;12:337–346. doi: 10.1093/ecco-jcc/jjx153. [DOI] [PubMed] [Google Scholar]
- 13.Liguori G., Lamas B., Richard M.L., Brandi G., Da Costa G., Hoffmann T.W., Di Simone M.P., Calabrese C., Poggioli G., Langella P. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J. Crohn’s Colitis. 2016;10:296–305. doi: 10.1093/ecco-jcc/jjv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.O., Gluck M. Fecal microbiota transplantation: An update on clinical practice. Clin. Endosc. 2019;52:137. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., De Vos W.M., Visser C.E., Kuijper E.J., Bartelsman J.F., Tijssen J.G. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 16.Quraishi M.N., Widlak M., Bhala N.A., Moore D., Price M., Sharma N., Iqbal T. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017;46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 17.Kellingray L., Le Gall G., Defernez M., Beales I.L., Franslem-Elumogo N., Narbad A. Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J. Infect. 2018;77:107–118. doi: 10.1016/j.jinf.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Kelly C.R., Khoruts A., Staley C., Sadowsky M.J., Abd M., Alani M., Bakow B., Curran P., McKenney J., Tisch A. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: A randomized trial. Ann. Intern. Med. 2016;165:609–616. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui B., Xu F., Zhang F. Methodology, not concept of fecal microbiota transplantation, affects clinical findings. Gastroenterology. 2016;150:285–286. doi: 10.1053/j.gastro.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F., Cui B., He X., Nie Y., Wu K., Fan D., Group F.-s.S. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borody T.J., Paramsothy S., Agrawal G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 2013;15:337. doi: 10.1007/s11894-013-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cammarota G., Ianiro G., Tilg H., Rajilić-Stojanović M., Kump P., Satokari R., Sokol H., Arkkila P., Pintus C., Hart A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchaert C., Strubbe B., Peeters H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastro Enterol. Belg. 2019;82:519–528. [PubMed] [Google Scholar]
- 25.Cui B., Li P., Xu L., Peng Z., Xiang J., He Z., Zhang T., Ji G., Nie Y., Wu K. Step-up fecal microbiota transplantation (FMT) strategy. Gut Microbes. 2016;7:323–328. doi: 10.1080/19490976.2016.1151608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long C., Yu Y., Cui B., Jagessar S.A.R., Zhang J., Ji G., Huang G., Zhang F. A novel quick transendoscopic enteral tubing in mid-gut: Technique and training with video. BMC Gastroenterol. 2018;18:37. doi: 10.1186/s12876-018-0766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni X., Fan S., Zhang Y., Wang Z., Ding L., Li Y., Li J. Coordinated hospital-home fecal microbiota transplantation via percutaneous endoscopic cecostomy for recurrent steroid-dependent ulcerative colitis. Gut Liver. 2016;10:975. doi: 10.5009/gnl15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Rossen N.G., MacDonald J.K., De Vries E.M., D’Haens G.R., De Vos W.M., Zoetendal E.G., Ponsioen C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. WJG. 2015;21:5359. doi: 10.3748/wjg.v21.i17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassam Z., Lee C.H., Yuan Y., Hunt R.H. Fecal Microbiota Transplantation forClostridium difficileInfection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 31.Goyal A., Yeh A., Bush B.R., Firek B.A., Siebold L.M., Rogers M.B., Kufen A.D., Morowitz M.J. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24:410–421. doi: 10.1093/ibd/izx035. [DOI] [PubMed] [Google Scholar]
- 32.Paramsothy S., Nielsen S., Kamm M.A., Deshpande N.P., Faith J.J., Clemente J.C., Paramsothy R., Walsh A.J., Van Den Bogaerde J., Samuel D. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156:1440–1454. doi: 10.1053/j.gastro.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Lee C.H., Steiner T., Petrof E.O., Smieja M., Roscoe D., Nematallah A., Weese J.S., Collins S., Moayyedi P., Crowther M. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 34.Mancabelli L., Milani C., Lugli G.A., Turroni F., Cocconi D., Van Sinderen D., Ventura M. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017;93 doi: 10.1093/femsec/fix153. [DOI] [PubMed] [Google Scholar]
- 35.Rajilić-Stojanović M., Shanahan F., Guarner F., De Vos W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013;19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 36.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kummen M., Holm K., Anmarkrud J.A., Nygård S., Vesterhus M., Høivik M.L., Trøseid M., Marschall H.-U., Schrumpf E., Moum B. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 38.Kump P., Wurm P., Gröchenig H., Wenzl H., Petritsch W., Halwachs B., Wagner M., Stadlbauer V., Eherer A., Hoffmann K. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47:67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan I., Ullah N., Zha L., Bai Y., Khan A., Zhao T., Che T., Zhang C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019;8:126. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigsnæs L.K., Brynskov J., Steenholdt C., Wilcks A., Licht T.R. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef. Microbes. 2012;3:287–297. doi: 10.3920/BM2012.0018. [DOI] [PubMed] [Google Scholar]
- 41.Hirano A., Umeno J., Okamoto Y., Shibata H., Ogura Y., Moriyama T., Torisu T., Fujioka S., Fuyuno Y., Kawarabayasi Y. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018;33:1590–1597. doi: 10.1111/jgh.14129. [DOI] [PubMed] [Google Scholar]
- 42.Alam A., Leoni G., Quiros M., Wu H., Desai C., Nishio H., Jones R.M., Nusrat A., Neish A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol. 2016;1:1–8. doi: 10.1038/nmicrobiol.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naito Y., Uchiyama K., Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018;63:33–35. doi: 10.3164/jcbn.18-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., Van Den Bogaerde J., Samuel D., Leong R.W., Connor S., Ng W., Paramsothy R. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 45.Walujkar S.A., Kumbhare S.V., Marathe N.P., Patangia D.V., Lawate P.S., Bharadwaj R.S., Shouche Y.S. Molecular profiling of mucosal tissue associated microbiota in patients manifesting acute exacerbations and remission stage of ulcerative colitis. World J. Microbiol. Biotechnol. 2018;34:76. doi: 10.1007/s11274-018-2449-0. [DOI] [PubMed] [Google Scholar]
- 46.Kiernan M.G., Coffey J.C., McDermott K., Cotter P.D., Cabrera-Rubio R., Kiely P.A., Dunne C.P. The human mesenteric lymph node microbiome differentiates between Crohn’s disease and ulcerative colitis. J. Crohn’s Colitis. 2019;13:58–66. doi: 10.1093/ecco-jcc/jjy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H.T., Huang H.L., Xu H.M., Luo Q.L., He J., Li Y.Q., Zhou Y.L., Nie Y.Q., Zhou Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020;19:2650–2660. doi: 10.3892/etm.2020.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 49.Fuentes S., Rossen N.G., Van Der Spek M.J., Hartman J.H., Huuskonen L., Korpela K., Salojärvi J., Aalvink S., De Vos W.M., D’Haens G.R. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. Isme J. 2017;11:1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutta S.K., Girotra M., Garg S., Dutta A., Von Rosenvinge E.C., Maddox C., Song Y., Bartlett J.G., Vinayek R., Fricke W.F. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2014;12:1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 51.Scanlan P.D., Shanahan F., O’Mahony C., Marchesi J.R. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn’s disease. J. Clin. Microbiol. 2006;44:3980–3988. doi: 10.1128/JCM.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabeerdoss J., Jayakanthan P., Pugazhendhi S., Ramakrishna B.S. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J. Med Res. 2015;142:23. doi: 10.4103/0971-5916.162091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank D.N., Amand A.L.S., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andoh A., Imaeda H., Aomatsu T., Inatomi O., Bamba S., Sasaki M., Saito Y., Tsujikawa T., Fujiyama Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 2011;46:479–486. doi: 10.1007/s00535-010-0368-4. [DOI] [PubMed] [Google Scholar]
- 55.LeBlanc J.G., Chain F., Martín R., Bermúdez-Humarán L.G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venegas D.P., Marjorie K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddavide R., Rotolo O., Gabriella C.M., Stasi E., Notarnicola M., Miraglia C., Nouvenne A., Meschi T., Luigi De’ Angelis G., Di Mario F., et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio-Med. Atenei Parm. 2018;89:60. doi: 10.23750/abm.v89i9-S.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Png C.W., Lindén S.K., Gilshenan K.S., Zoetendal E.G., McSweeney C.S., Sly L.I., McGuckin M.A., Florin T.H. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 59.Nishida A., Imaeda H., Ohno M., Inatomi O., Bamba S., Sugimoto M., Andoh A. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J. Gastroenterol. 2017;52:476–482. doi: 10.1007/s00535-016-1271-4. [DOI] [PubMed] [Google Scholar]
- 60.Hourigan S., Chen L., Grigoryan Z., Laroche G., Weidner M., Sears C.L., Oliva-Hemker M. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015;42:741–752. doi: 10.1111/apt.13326. [DOI] [PubMed] [Google Scholar]
- 61.Sabino J., Vieira-Silva S., Machiels K., Joossens M., Falony G., Ballet V., Ferrante M., Van Assche G., Van Der Merwe S., Vermeire S. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu J., Wang A., Ansari S., Hershberg R.M., Mckay D.M. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125:1785–1795. doi: 10.1053/j.gastro.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Vesterlund S., Karp M., Salminen S., Ouwehand A.C. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology. 2006;152:1819–1826. doi: 10.1099/mic.0.28522-0. [DOI] [PubMed] [Google Scholar]
- 65.Salem F., Kindt N., Marchesi J.R., Netter P., Lopez A., Kokten T., Danese S., Jouzeau J.-Y., Peyrin-Biroulet L., Moulin D. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United Eur. Gastroenterol. J. 2019;7:1008–1032. doi: 10.1177/2050640619867555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R.K., Chang H.-W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh T.S., Rampelli S., Jeffery I.B., Santoro A., Neto M., Capri M., Giampieri E., Jennings A., Candela M., Turroni S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut. 2020 doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Mantrana I., Selma-Royo M., Alcantara C., Collado M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018;9:890. doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang T., Cui B., Li P., He Z., Long C., Wei L., Peng Z., Ji G., Zhang F. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0158227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uygun A., Ozturk K., Demirci H., Oger C., Avci I.Y., Turker T., Gulsen M. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine. 2017;96 doi: 10.1097/MD.0000000000006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cold F., Browne P.D., Günther S., Halkjaer S., Petersen A., Al-Gibouri Z., Hansen L., Christensen A. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated–an open-label pilot study. Scand. J. Gastroenterol. 2019;54:289–296. doi: 10.1080/00365521.2019.1585939. [DOI] [PubMed] [Google Scholar]
- 72.Zackular J.P., Chazin W.J., Skaar E.P. Nutritional immunity: S100 proteins at the host-pathogen interface. J. Biol. Chem. 2015;290:18991–18998. doi: 10.1074/jbc.R115.645085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez C.A., Skaar E.P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe. 2018;23:737–748. doi: 10.1016/j.chom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damo S.M., Kehl-Fie T.E., Sugitani N., Holt M.E., Rathi S., Murphy W.J., Zhang Y., Betz C., Hench L., Fritz G. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das N.K., Schwartz A.J., Barthel G., Inohara N., Liu Q., Sankar A., Hill D.R., Ma X., Lamberg O., Schnizlein M.K. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020;31:115–130.e116. doi: 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]