Abstract
In the present study, the antimicrobial effect of Cannabis sativa Futura 75 was evaluated both in vitro against foodborne bacterial pathogens, and on food against naturally occurring microbial groups of minced meat stored for 8 days at 4°C. Ethanol extraction was performed on the grind of the inflorescence. After extraction, ethanol was completely evaporated and substituted by water. Serial dilutions of the extract, the grind and cannabidiol 99% were added to Nutrient Agar and spotted with Listeria monocytogenes, Salmonella Typhimurium, Escherichia coli and Staphylococcus spp. Regarding the evaluation on food, 50 mL of extract, characterised by CBD at concentration of 322,70 μg/mL, were added to 2.5 kg of minced beef meat. Meat was divided into aliquots and stored for 8 days at 4°C. At 0, 1, 2, 3, 4, 7, and 8 days, aerobic bacteria, enterobacteria, coliforms and E. coli were enumerated. All tested products were efficient against Gram +. In particular, extract corresponding to CBD concentration of 0.017 and 0.3 mg/mL were effective against L. monocytogenes and Staphylococcus spp. respectively. After 8 days of storage at 4°C, treated minced meat showed a bright red colour in comparison to a brownish control meat. Moreover, Enterobacteriaceae and coliforms were significantly reduced of 2.3 log CFU/g and 1.6 log CFU/g respectively in treated meat in comparison to the control. Although preliminary, the present study suggests the antimicrobial properties of the extract of Cannabis sativa both in vitro and in minced meat.
Key words: Cannabis sativa, antimicrobial effect, foodborne pathogenes pathogens, process hygiene, bovine minced meat
Introduction
In Europe, Cannabis sativa L. varieties can be legally cultivated if it is registered in the EU Plant variety database of agricultural plant species and its tetrahydrocannabinol (THC) content does not exceed 0.2 % (w/w) hereafter addressed as industrial hemp (EC, 2019a). These plants find different applications in pharmacotherapy (Bonini et al., 2018), agronomy (Das et al., 2017), food industry (Radočaj et al., 2014; Zając et al., 2018), cosmetic (Nuutinen, 2018), sustainable building (Arizzi et al., 2019), animal production (Khan et al., 2010; Neijat et al., 2016; Vispute et al., 2019), broiler meat and chicken egg production (Goldberg et al., 2012; Jing et al., 2017). In Italy the cultivation of industrial hemp is specifically regulated by law N° 242 of 2016, which specifically promotes the use of this plant in research, cosmetics, food industry, biomaterials, sustainable building.
As food or food ingredient, industrial hemp is regulated at European level as novel food and needs pre-market authorisation before commercialisation (EC, 2019b; Regulation EU 2015/2283). However, some products such as hemp seed, seed flour and oil have a long history of consumption and cannot be considered as novel. For them, the use is regulated at national level. With the exception of Romania, where hemp seed and oil can be used without any limit, in other countries such as The Netherlands, Germany, Danmark and Italy, the maximum limits of THC in hemp seed, oil, flour and other foods and drinks is specifically regulated. In particular for Italy, the 30th of October 2018, the Ministry of Health submitted to the European Commission a draft regulation, not yet approved, including the following maximum limits of THC: hemp oil – 5 ppm; hemp seeds and seed flour – 2 ppm; dietary supplement including hemp derivatives: 2 ppm (Italian Ministry of Health, 2018). According to this draft regulation, for all the other hemp derivatives, for example hemp inflorescence, food business operators shall provide evidences on the THC concentration in foodstuffs to competent authorities of official control taking into account specific concentration or dilution factors linked to the food processing (Commission Regulation (EC) No 1881/2006).
Industrial hemp inflorescence, especially resin secreted from the trichomes of female plants, are rich in phytocannabinoids. In C. sativa, cannabinoids are biosynthesized and accumulated as cannabinoid acids, and subsequently decarboxylated into their neutral forms (Bonini et al., 2018). At present around 100 molecules have been characterised with Cannabidiol (CBD) as the one of the most represented in industrial hemp. Properties such as anti-inflammatory, immunomodulation, anti-arthritic have been attributed to CBD (Bonini et al., 2018). Along with these properties, CBD has been described as an antimicrobial agent since the 70ies when CBD was shown to be effective against Gram + bacteria, although not against Gram - (Van Klingeren and Ham, 1976). More recently, MICs of 0.5-1 μg/mL were registered for CBD against different strains of multiresistant and methicillin resistant Staphylococcus aureus (MRSA) (Appendino et al., 2008). Antimicrobial activity of CBD against S. aureus was observed also by other authors (Burstein et al., 2015). Along phytocannabinoids, also terpenoids are found in C. sativa. More than 200 terpenoids have been characterised with limonene, β-myrcene, α- and β-pinene as the most common. Terpenoids act synergistically with phytocannabinoids in the defense strategy against predators (Bonini et al., 2018; Russo et al., 2011). Antimicrobial properties were specifically observed for α- and β-pinene against both Gram + and Gram – bacteria as well as fungi namely, Pseudomonas aeruginosa, Escherichia coli, MRSA and Candida albicans, (da Silva et al., 2012; Dai et al., 2013; Leite et al., 2007).
Due to the presence of different antimicrobial compounds acting synergistically, the antimicrobial activity of Cannabis sativa L. might be potentially higher than those of the single molecules (Russo et al., 2011; Blasco-Benito et al., 2018; Andre et al., 2016). Different types of extracts of seeds or female inflorescence, and resin of industrial hemp have been tested against bacteria, fungi and moulds. For a comprehensive and detailed review please refer to Tandon et al. (2017). With particular reference to bacteria, although with broad diverse results related to plant varieties, extraction methods and parts of the plant tested, industrial hemp extracts showed to be effective against microbial pathogens such as Escherichia coli, Pseudomonas aeruginosa and Staphyloccocus aureus. Among all varieties tested, Futura 75 might be addressed as a promising antimicrobial. Futura 75 variety showed superior antimicrobial properties in comparison to other varieties. In particular, higher MIC values were registered against Enterococcus faecium and Clostridium botulinum (Futura MIC values of 1.55 and 1.76 %v/v respectively) (Nissen et al., 2010). More recently, the water flower extract of Cannabis sativa L. variety Futura 75 was described as effective also against E. coli, P. aeruginosa and S. aureus (Ferrante et al., 2019). Unfortunately, all information on antimicrobial activity of Cannabis sativa were collected from experiments performed in vitro against food-unrelated microbial pathogens. Since the great application of industrial hemp as food ingredient for nutraceutical purposes, knowledges are missing on food safety and hygiene and in particular on the antimicrobial properties of this plant against foodborne-pathogens in vitro as well as against bacteria related to the hygiene of food processing (Commission Regulation (EC) No 2073/2005).
The research question of the present study was to investigate the antimicrobial activity of an extract of the female fluorescence of Cannabis sativa L. variety Futura 75 in vitro against E. coli, Salmonella Typhimurium, Listeria monocytogenes and Staphylococcus spp. and against microbial populations occurring in minced beef stored for 8 days at 4°C.
Materials and Methods
Cannabis sativa L. variety Futura 75
Cannabis sativa L. variety Futura 75 was cultivated without the use of fertilizers, biocidal chemicals or irrigation (ESSENZESATIVESALENTINE, Sogliano Cavour, Italy). Plants were harvested on the first hours of the day light when the amount of resin is maximised. After natural dry out, inflorescences were manually trimmed and grinded.
Preparation of Ethanol extract
The ethanol extract was prepared as previously described with few modifications (Romano e Hazencamp, 2013). Briefly, in each of 9 flasks, 100 mL of ethanol (96 % v/v) (Carlo Erba Reagents, Cornaredo, Italy) were mixed with 5 g of the grind. All flasks were placed on a shaking platform at 120 rpm for 20 min. The content of each of the 9 flasks was filtered by filter paper (Whatman™ Grade 113 qualitative filter paper, diameter 90 mm, pore size 30 μm, SigmaAldrich, Milan, Italy) and combined. In order to avoid any antimicrobial misleading effect of ethanol, the ethanol content of the filtrate was eliminated by evaporation (Rotavapor R-300, Buchi, Cornaredo, Italy), and the residue suspended in 90 mL of physiological solution (0.9% NaCl).
Bacterial cultures and growth conditions
The antimicrobial effect of the plant was tested in vitro against: two strains of Salmonella Typhimurium (ST208 and ST63); two strains of Escherichia coli (EC ATCC 25922 and EC135), two strains of Listeria monocytogenes (LM1 and LM2) and one strain of Staphylococcus spp. (strain S661). For both E. coli and S. Typhimurium, one completely susceptible (ST208 and EC ATCC 25922) and one multiresistant strain (ST63, EC135) were included. Except E. coliATCC 25922, all other strains were from food origin. All strains were kept at -80°C in Brain Heart Infusion (BHI, Thermo Scientific, Milan, Italy), with the addition of 20% glycerol. Upon use, strains were inoculated in BHI and incubated for 24 hours at 37°C.
In vitro evaluation of Cannabis sativa L. susceptibility against foodborne pathogens
In order to evaluate whether the potential antimicrobial effect of industrial hemp was solely due to its CBD content or by additional components, the antimicrobial susceptibilities of different concentrations of the grind, the extract and pure CBD (crystals, purity 99%, ECO HEMP TRADING LTD, Villanova Del Ghebbo, Italy), all with comparable CBD content, were evaluated following a previously reported protocol (Duarte et al., 2016). In particular, the concentrations to be tested were selected based on the following assumptions: 1) 50 mg of pure CBD in 150 mL of Nutrient Agar (NA) was previously suggested as an efficient concentration against Staphylococcus aureus (Duarte et al., 2016) and 2) concentration of CBD in the autoclaved extract was 1316,63 μg/mL (Table 1). Based on these assumptions 4.6 mL of the extract or 60 mg of CBD was added to 100 mL of NA (Thermo Scientific, Milan, Italy) corresponding to a final concentration of CBD in the autoclaved NA of 0.6 mg/mL. Along this concentration, additional 1:2 dilutions were tested in the range 0.017–0.6 mg/mL. The supplements were added to the medium before autoclaving since the sterilisation temperature of 121°C enhances the decarboxylation of the inactive acid form to the active neutral form of cannabinoids. Bacteria suspensions of each food-borne pathogen were prepared in physiological solution and adjusted to a concentration of 1.5 x 108 CFU/mL (corresponding to an OD of 0.08 – 0.1 at 625 nm). On supplemented NA plates, 5 μl of each suspension were spotted, and NA plates incubated for 24-48 hours at 37°C. Inoculated and not supplemented NA plates were included as positive controls.
Table 1.
Phytocannabinoids profile of the grind, autoclaved and not-autoclaved extracts of Cannabis sativa L variety Futura 75.
| Sample | CBDA | CBGA | CBG | CBD | THCV | CBN | Δ9-THC | Δ8-THC | CBC | THCA |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Grind (Cannabis Sativa L.) | 5.40 | 0.10 | 0.03 | 1.59 | nd | 0.01 | 0.11 | nd | 0.07 | 0.14 |
| Dev.std. | 0.15 | 0.02 | 0.01 | 0.05 | - | 0.00 | 0.00 | - | 0.00 | 0.01 |
| CV% | 2.86 | 15.01 | 17.72 | 3.39 | - | 7.88 | 2.63 | - | 5.76 | 9.49 |
| Sample | CBDA | CBGA | CBG | CBD | THCV | CBN | Δ 9-THC | Δ Δ8-THC | CBC | THCA |
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | |
| Autoclaved extract | 89.96 | nd | 29.19 | 1316.63 | nd | 7.34 | 23.76 | nd | 38.99 | nd |
| Dev.std. | 1.62 | - | 0.95 | 13.64 | - | 0.58 | 1.04 | - | 0.45 | - |
| CV% | 1.80 | - | 3.26 | 1.04 | - | 7.89 | 4.39 | - | 1.15 | - |
| Not autoclaved extract | 882.25 | 15.24 | 9.36 | 322.70 | nd | 2.28 | 15.04 | nd | 10.45 | 8.36 |
| Dev.std. | 20.48 | 1.30 | 1.10 | 14.80 | - | 0.09 | 0.62 | - | 0.05 | 0.21 |
| CV% | 2.32 | 8.55 | 11.71 | 4.59 | - | 3.86 | 4.14 | - | 0.43 | 2.54 |
Antimicrobial effect of Cannabis sativa L. against microbial populations of minced beef
In order to test the antimicrobial effect of industrial hemp on microbial indicators of food processing hygiene, 5 kg of minced meat beef was purchased at retail after one day from its production. The meat sample was divided in two aliquots of 2.5 kg each. One aliquot act as control, the second was additioned with 50 mL of the extract. Since the concentration of CBD in the not autoclaved extract was 322,7 μg/mL (Table 1) the final concentration of CBD in minced meat was 6.45 μg/g. In order to mimic domestic storage conditions, aliquots of 80 g each were packed and stored at 4°C along with hemp-free aliquots used as controls. At 0, 1, 2, 3, 4, 7 and 8 days of storage three hemp additioned aliquots and three hemp– free aliquots were tested for, Enterobacteriaceae, coliforms, aerobic colony count and E. coli following standard procedures (International Organisation for Standardisation (ISO), 2001; ISO, 2003; ISO, 2004; ISO, 2006).
Phytocannabinoids detection and quantification
Phytocannabinoids were detected and quantified in the grind as well as in two autoclaved and not autoclaved aliquots of the extract following a previously reported protocol with some minor modifications (Mandrioli et al., 2019). The autoclaved and not-autoclaved aliquots were tested in order to evaluate the exact cannabinoid composition in the in vitro susceptibility and the food experiments respectively. An aliquot of the sample (grind), about 50 mg, was added to 10 mL of methanol-chloroform 9:1 (v/v) extraction solvent. After 10 min on agitation set at 350 oscillations per minute, the sample was ultrasonicated for 10 minutes. After centrifugation for 5 minutes at 1620 xg, the supernatant was collected. The extraction was repeated twice and the two fractions were collected in a 25 mL volumetric flask and brought to volume with methanol/ chloroform (9:1, v/v). The solution was filtered with a 45 μm nylon filter. One mL of this solution was dried under weak nitrogen flow. Dried material was resuspended in 1 mL of acetonitrile, 5.0 μl of which was injected into an RP-HPLCDAD liquid chromatography system. For the extract (autoclaved and not autoclaved),1 mL was transferred and directly dissolved into a 10 mL flask and brought to volume with acetonitrile. For the HPLC analysis, UV detection was used at 230 nm, gradient elution was used at flow rate of 1.5 mL/min according to the following procedure: eluent mixture A water + 0.1% phosphoric acid, B acetonitrile + 0.1% phosphoric acid; gradient elution: 75% of B up to 0.7 min. 85% of B to 2 min. 100% of B to 3.0-3.5 min and 100% of B to 3.6-5.0 min. Data were acquired using Chemstation software for LC3D (Rev.A.08.03 Agilent Technologies, USA). The quantification of analytes was carried out using the external standard method, through the construction of calibration curves prepared with standard reference compounds of chromatographic purity. Standard references of CBDA, CBGA, CBG, CBD, THCV, CBN, Δ9-THC, Δ8-THC, CBC, and THCA were diluted wit acetonitrile in a concentration range between 0.1-100 μg/mL. Eight dilutions of each cannabinoid was used to build the calibration curve. The standard solutions were stored away from light at a temperature of −20 ◦C. The equations of the calibration curves were as follows:
Figure 1.

Minced beef with extract (ETA) and without (CONTR) after 8 days of storage at 4°C.
Table 2.
Susceptibility to CBD and Cannabis sativa of S. Typhimurium (strains ST208, ST63), E. coli (strains EC ATCC 25922, EC135), L. monocytogenes (LM1, LM2) and Staphylococcus spp. (strain S661).
| CBD Concentration (mg/mL) | ST208 | ST63 | EC ATCC | EC135 | LM1 | LM2 | S661 |
|---|---|---|---|---|---|---|---|
| Extract | |||||||
| 0.6 | + | + | + | + | - | - | - |
| 0.3 | + | + | + | + | - | - | - |
| 0.15 | + | + | + | + | - | - | + |
| 0.07 | + | + | + | + | - | - | + |
| 0.035 | + | + | + | + | - | - | + |
| 0.017 | + | + | + | + | - | - | + |
| CBD | |||||||
| 0.6 | + | + | + | + | - | - | - |
| 0.3 | + | + | + | + | - | - | - |
| 0.15 | + | + | + | + | - | - | + |
| 0.07 | + | + | + | + | - | - | + |
| 0.035 | + | + | + | + | - | - | + |
| 0.017 | + | + | + | + | - | - | + |
| Negative control | |||||||
| 0 | + | + | + | + | + | + | + |
| CBD, y=6.5473x-1.6742 (r2=0.9999); CBG, y=5.9001x-0.4766 (r2=0.9964); Δ9-THC, y=6.3627x-0.2229 (r2=0.9962); CBN, y=14.792x-0.6310 (r2=0.9990); CBC, y=15.619x-0.788 (r2=0.9989); CBDA, y=10.018x+1.1843 (r2=0.9981); CBGA, y=9.5259x-1.1675 (r2=0.9959); THCA, y=9.154x-0.9992 (r2=0.9954); Δ8-THC, y=4.8513x+1.0499(r2=0.9979); THCV, y=4.9225x+1.883 (r2=0.9987). |
Results
Semiquantitative evaluation of CBD and THC
In Table 1, the concentrations of CBDA, CBD, Δ9-THC and THCA in the grind as well as in the autoclaved and not autoclaved extract are reported.
The grind of Cannabis sativa L., included in the present study, was characterised by 5,40% of CBDA and 0.11 % of Δ9-THC confirming previously reported data (Palmieri et al., 2019) (Table 1). Other cannabinoids, identified at low percentages, were CBGA (0.10%), CBN (0.01 %) and CBC (0.07%). THCV and Δ8- THC was not detected. Not surprisingly, in the extract a significant shift of cannabinoids from their acid form to their neutral form was observed comparing the autoclaved vs the not autoclaved extract. In particular, in the latter CBDA and CBD were 882.25 μg/mL and 322.70 μg/mL respectively. In the autoclaved extract CBDA was significantly reduced to 89.96 μg/mL, whereas CBD increased up to 1316.63 μg/mL. Moreover, in the autoclaved form, the overall concentration of CBGA+CBG, CBN and CBC increased in comparison to the not autoclaved extract.
In vitro evaluation of Cannabis sativa susceptibility against foodborne pathogens
The antimicrobial properties of Cannabis sativa L. variety Futura 75 were investigated in vitro in comparison to pure CBD against S. Typhimurium, E. coli, L. monocytogenes and Staphylococcus spp. (Table 2). At the tested concentrations, hemp as well as pure CBD were effective in inhibiting Gram positive bacteria but not Gram negative ones (ST208, ST63, EC ATCC and EC135). In particular, all tested concentrations were effective against L. monocytogenes (LM1 and LM2) and 0.3 mg/mL was effective against Staphylococcus spp (S661). This result confirmed previous published data (Duarte et al., 2016).
Figure 2.

Enumeration of enterobacteriaceae in minced meat with extract (treated) and without (control) during storage at 4°C for 8 days.
Figure 3.

Enumeration of coliforms in minced meat with extract (treated) and without (control) during storage at 4°C for 8 days.
Antimicrobial effect of Cannabis sativa on microbial populations of minced beef
The antimicrobial effect of hemp was evaluated on microbial populations naturally occurring on minced beef and representing indicators of food hygiene. For this purpose, hemp extract was added to minced beef which was stored at 4°C for 8 days. The final concentration of CBD in the meat was 6.45 μg/g two order of magnitude lower than the one tested in the in vitro experiment. Surprisingly, after the storage period, visual assessment revealed that hemp extract impact on the colour of minced meat which appeared light red in comparison to dark brownish red colour of the control (minced meat without the extract) (Figure 1). Although red colour after storage is not an indicator of hygiene per se, this observation might suggest an effect of the extract in inhibiting spoilage bacteria such as those belonging to the strict aerobic Pseudomonas genus and/or an antioxidant effect of cannabinoids. Specific reductions were registered on Enterobacteriaceae and coliform enumerations. Both microbial groups showed a significant reduction in minced beef with hemp extract in comparison to control already after 4 days and reaching 2.3 and 1.6 log10 CFU/g reduction respectively after 8 days of storage at 4°C (Figures 2 and 3).
Aerobic colony count showed a slowing down of the growth rate in treated minced beef samples in comparison to control ones at day 4. Subsequently, similar increases up to 2.4-3.0 log10 CFU/g were reached at day 8 in treated and control samples respectively (Figure 4).
E. coli was under the detection limit until day 3. From day 4 to day 8 E.coli increased of 2.4 log10 and 2.9 log10 CFU/g in treated and control samples respectively without significant differences (Figure 5).
Discussion
Cannabis sativa variety 75 was investigated as antimicrobial against foodborne pathogens as well as against microbial populations of minced beef. Results in vitro confirmed previously reported data on inhibitory properties of this plant against Staphylococcus aureus (Appendino et al., 2008; Duarte et al., 2016). Both L. monocytogenes tested strains (LM1 and LM2) were susceptible to the whole range of CBD and plant concentrations tested. In literature, data are available on oil extract efficacy against this microorganism. Nevertheless controversial results are reported with moderate (Minimum Inhibitory Concentration (MIC) > 2048 μg/mL) to high efficacy (MIC 2-32 μg/mL) (Iseppi et al., 2019; Marini et al., 2018). In contrast, S. Typhimurium and E. coli strains were resistant to both CBD and hemp extract. Efficacy against Gram negative bacteria and in particular E. coli is controversial since both susceptibility and resistance to hemp were described (Ferrante et al., 2019; Tardon et al., 2017). In particular, oil, aqueous and ethanol extracts were described as inactive or slightly active against E. coli, whereas high activity was attributed to methanol and petroleum hemp extracts (Tardon et al., 2017). In the present study, results confirmed the lack of efficacy of ethanol extract against E. coli and they extended the same observation to S. Typhimurium, which was never tested before to the best of author’s knowledge. Moreover, the lack of activity was observed independently from the antibiotic susceptibility profile of selected strains. E. coli and S. Typhimurium, multiresistant as well as antibiotic susceptible strains were equally resistant to Cannabis sativa and CBD at the tested concentrations.
Industrial hemp seeds and seed oil have been recently applied as functional food or functional food ingredient thanks to their significant contribution in polyunsaturated fatty acids, optimum Omega-6 and Omega- 3 ratio, fibres and amino acids (Apostol et al. 2015; Frassinetti et al., 2017; Radočaj et al., 2014). However, to the best of author’s knowledges, the effect of hemp extract as natural food preservative was never investigated before. In particular, in the present study the antimicrobial effect of hemp extract was investigated against microbial indicators of food hygiene of minced meat. After one week of storage at 4°C, a significant reduction was achieved for Enterobacteriaceae and coliforms respectively, along with a delay on the growth of aerobic colony count. Moreover, a striking lighter red colour of the minced meat was observed in comparison to the control, suggesting: i) hemp antioxidant properties as previously described (Bonini et al., 2018); ii) reduction of strict aerobic spoilage bacteria of the Pseudomonas genus.
Figure 4.
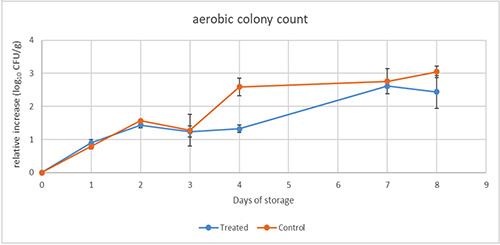
Aerobic colony count in minced meat with extract (treated) and without (control) during storage at 4°C for 8 days.
Figure 5.

Escherichia coli enumeration in minced meat with extract (treated) and without (control) during storage at 4°C for 8 days.
Interestingly, the hemp extract showed antimicrobial properties both after heat treatment due to autoclaving in the in vitro experiment and without heat treatment in the food experiment. In particular, in the food experiment a final CBD concentration of 6.45 μg/g was enough to significantly impact on the growth of tested bacterial groups. As demonstrated by chemical results heat treatment allows the decarboxylation of cannabinoids compounds which shifted from an inactive acid form to an active decarboxylated form. In particular 90% of CBDA and 100 % of THCA and CBGA shifted to their decarboxylated forms CBD, THC and CBG respectively. Additionally, heat treatment might be responsible of the loss of other compounds such as volatile terpenes. Further analyses should be performed in order to detect and quantify the exact profile of terpenoid compounds characterizing the hemp extract as well as the antioxidant activity of cannabinoids in minced meat and their inhibitory effect on spoilage bacteria such as Pseudomonas.
Conclusions
The ethanol extract of industrial hemp (Cannabis sativa L. variety Futura 75) showed in vitro antimicrobial properties against foodborne pathogens such as L. monocytogenes and Staphylococcus spp. but not against S. Typhimurium and E. coli. These results along with the antimicrobial properties observed for hemp extract when mixed to minced beef, suggest that hemp extract might have promising applications as natural food preservative which deserves further investigations especially on the specific molecules as well as their mechanisms of action linked to antimicrobial activity.
References
- Amezquita-Lopez BA, Soto-Beltran M, Lee BG, Yabao JC, Quinones B, 2018. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli, J Microbiol Immunol Infect 51:525-34. [DOI] [PubMed] [Google Scholar]
- Andre CM, Hausman JF, Guerriero G, 2016. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol L, Popa M, Mustatea G, 2015. Cannabis sativa L partially skimmed flour as source of bio-compounds in the bakery industry. Romanian Biotechnological Letters 20:10835-44. [Google Scholar]
- Arizzi A, Brummer M, Martin-Sanchez I, Cultrone G, Viles H., 2015. The influence of the type of lime on the hygric behaviour and bio-receptivity of hemp lime composites used for rendering applications in sustainable new construction and repair works. PLoS One 10:e0125520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M, Mellmann A, Bletz S, Zhang W, Kock R, Kossow A, Prager R, Fruth A, Orth-Holler D, Marejkova M, Morabito S, Caprioli A, Pierard D, Smith G, Jenkins C, Čurova K, Karch H, 2013. Enterohemorrhagic Escherichia coli O26:H11/H−: A New Virulent Clone Emerges in Europe. Clin Infect Dis 56:1373–81. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Zhang W, Mellmann A, Karch H, 2007. Enterohaemorrhagic Escherichia coli O26:H11/H-: a human pathogen in emergence. Berl Munch Tierarztl Wochenschr 120:279-87. [PubMed] [Google Scholar]
- Blasco-Benito S, Seijo-Vila M, Caro- Villalobos M, Tundidor I, Andradas C, Garcia-Taboada E, Wade J, Smith S, Guzman M, Perez-Gomez E, Gordon M, Sanchez C, 2018. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol 157:285-93. [DOI] [PubMed] [Google Scholar]
- Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, Mastinu A, 2018. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227:300-15. [DOI] [PubMed] [Google Scholar]
- Braun V, 2001. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291:67-79. [DOI] [PubMed] [Google Scholar]
- Buchrieser C, Prentice M, Carniel E, 1998. The 102-Kilobase Unstable Region of Yersinia pestis Comprises a High- Pathogenicity Island Linked to a Pigmentation Segment Which Undergoes Internal Rearrangement. J Bacteriol 180:2321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugarel M, Beutin L, Scheutz F, Loukiadis E, Fach P, 2011. Identification of genetic markers for differentiation of Shiga toxin-producing, enteropathogenic, and avirulent strains of Escherichia coli O26. Appl Environ Microbiol 77:2275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugarel M, Beutin L, Fach P, 2010. Lowdensity macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): a new approach for molecular risk assessment of STEC isolates. Appl Environ Microbiol 76:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S., 2015. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 23:1377-85. [DOI] [PubMed] [Google Scholar]
- Chen L, Zheng D, Liu B, Yang J, Jin Q, 2016. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res 44:D694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirila F, Tabaran A, Fit N, Nadas G, Mihaiu M, Tabaran F, Cătoi C, Reget OL, Dan SD, 2017. Concerning Increase in Antimicrobial Resistance in Shiga Toxin-Producing Escherichia coli Isolated from Young Animals during 1980-2016. Microbes Environ 32:252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364:5-24. [Google Scholar]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338:1-26. [Google Scholar]
- Commission Regulation (EU) No 209/2013 of 11 March 2013 amending Regulation (EC) No 2073/2005 as regards microbiological criteria for sprouts and the sampling rules for poultry carcases and fresh poultry meat Text with EEA relevance. OJ L 68:19–23. [Google Scholar]
- Council Regulation (EC) No 73/2009 of 19 January 2009 stablishing common rules for direct support schemes for farmers under the common agricultural policy and establishing certain support schemes for farmers amending Regulations (EC). OJ L 30:1-132. [Google Scholar]
- da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS, 2012. Biological activities of α- pinene and β-pinene enantiomers. Molecules 17:6305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Zhu L, Yang L, Qiu J, 2013. Chemical composition, antioxidant and antimicrobial activities of essential oil from Wedelia prostrata. EXCLI J. 12:479–90. [PMC free article] [PubMed] [Google Scholar]
- Das L, Liu E, Saeed A, Williams DW, Hu H, Li C, Ray AE, Shi J, 2017. Industrial hemp as a potential bioenergy crop in comparison with kenaf, switchgrass and biomass sorghum. Bioresour Technol 244:641-9. [DOI] [PubMed] [Google Scholar]
- Day M, Doumith M, Jenkins C, Dallman TJ, Hopkins KL, Elson R, Godbole G, Woodford N, 2017. Antimicrobial resistance in Shiga toxinproducing Escherichia coli serogroups O157 and O26 isolated from human cases of diarrhoeal disease in England. J Antimicrob Chemother 72:145–52. [DOI] [PubMed] [Google Scholar]
- Delannoy S, Beutin L, Fach P, 2013. Towards a molecular definition of enterohemorrhagic Escherichia coli (EHEC): detection of genes located on O island 57 as markers to distinguish EHEC from closely related enteropathogenic E. coli strains. J Clin Microbiol 51:1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy S, Mariani-Kurkdjian P, Bonacorsi S, Liguori S, Fach P, 2015. Characteristics of emerging humanpathogenic Escherichia coli O26:H11 strains isolated in France between 2010 and 2013 and carrying the stx2d gene only. J Clin Microbiol 53:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douellou T, Delannoy S, Ganet S, Fach P, Loukiadis E, Montel MC, Sergentet-Thevenot D, 2017. Molecular characterization of O157:H7, O26:H11 and O103:H2 Shiga toxin-producing Escherichia coli isolated from dairy products. Int J Food Microbiol 253:59-65. [DOI] [PubMed] [Google Scholar]
- Duarte P, 2016. Determination of the Antibiotic Properties of Cannabidiol. J Gen Pract (Los Angeles) 4:266. [Google Scholar]
- EFSA and ECDC, 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA Journal 15:5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (EC), 2019a. EU Plant variety database. http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm Accessed: July 14th, 2019. [Google Scholar]
- European Commission (EC), 2019b. EU Novel food catalogue. http://ec.europa.eu/food/safety/novel_food/catalogue/search/public/?event=home&seqfce=72&ascii=C Accessed: July 14th, 2019. [Google Scholar]
- Ferrante C, Recinella L, Ronci M, Menghini L, Brunetti L, Chiavaroli A, Leone S, Di Iorio L, Carradori S, Tirillini B, Angelini P, Covino S, Venanzoni R, Orlando G, 2019. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem Toxicol 125:452-61. [DOI] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H, 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573. [DOI] [PubMed] [Google Scholar]
- Garcia V, Garcia-Menino I, Mora A., Flament-Simon SC, Diaz-Jimenez D., Blanco JE, Alonso MP, Blanco J, 2018. Co-occurrence of mcr-1, mcr-4 and mcr- 5 genes in multidrug-resistant ST10 Enterotoxigenic and Shiga toxinproducing Escherichia coli in Spain (2006-2017). Int J Antimicrobial agents 52:104-8. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Gakhar N, Ryland D, Aliani M, Gibson RA, House JD, 2012. Fatty acid profile and sensory characteristics of table eggs from laying hens fed hempseed and hempseed oil. J Food Sci 77:S153-60. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Escalona N, Toro M, Rump LV, Cao G, Nagaraja TG, Meng J, 2016. Virulence Gene Profiles and Clonal Relationships of Escherichia coli O26:H11 Isolates from Feedlot Cattle as Determined by Whole-Genome Sequencing. Appl Environ Microbiol 82:3900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko J, Westerlund-Wikstrom B, 2013. The role of the bacterial flagellum in adhesion and virulence. Biology 2:1242-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Dallman TJ, Shabaan S, Hanson M, Allison L, 2018. Validation of wholegenome sequencing for identification and characterization of shiga toxinproducing escherichia coli to produce standardized data To enable data sharing. J Clin Microbiol 56:e01388-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseppi R, Brighenti V, Licata M, Lambertini A, Sabia C, Messi P, Pellati F, Benvenuti S, 2019. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 24:e2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO, 2001. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of β-glucuronidasepositive Escherichia coli — Part 2: Colony-count technique at 44°C using 5- bromo-4-chloro-3-indolyl β-D-glucuronide. ISO Standard No. 16649-2. [Google Scholar]
- ISO, 2003. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of microorganisms — Colony-count technique at 30°C. ISO Standard No. 4833. [Google Scholar]
- ISO, 2004. Microbiology of food and animal feeding stuffs Horizontal methods for the detection and enumeration of Enterobacteriaceae Part 2: Colony-count method. ISO Standard No. 21528-2. [Google Scholar]
- ISO, 2006. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of coliforms — Colonycount technique. ISO Standard No. 4832. [Google Scholar]
- Italian Ministry of Health, 2018. Regulation on tetrahydrocannabinol (THC) limits in foodstuffs. Draft. [Google Scholar]
- JEMRA, 2016. Joint FAO/WHO Core Expert Group Meeting on VTEC/STEC. [Google Scholar]
- Jing M, Zhao S, House JD, 2017. Performance and tissue fatty acid profile of broiler chickens and laying hens fed hemp oil and HempOmegaTM. Poult Sci 96:1809-19. [DOI] [PubMed] [Google Scholar]
- Khan RU, Durrani FR, Chand N, Anwar H, 2010. Influence of feed supplementation with Cannabis sativa on quality of broilers carcass. Pakistan Vet J 30:34-8. [Google Scholar]
- Koh EI, Robinson AE, Bandara N, Rogers BE, Henderson JP, 2017. Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat Chem Biol 13:1016-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D, Kelly J, 1995. Use of heme and hemoglobin by Escherichia coli O157 and other Shiga-like-toxin-producing E. coli serogroups. Infect Immun 63:700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law No 242/2016 Provisions for the promotion of hemp cultivation and the related agro-industrial sector. Official Journal of the Italian Republic, 30 December. 2016, General Series No 304. [Google Scholar]
- Leite AM, Lima EO, de Souza EL, Diniz MFM, Trajano VN, Medeiros IA, 2007. Inhibitory effect of beta-pinene, alphapinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Revista Brasileira de Ciencias Farmaceuticas 43:121-6. [Google Scholar]
- Lorenz SC, Son I, Maounounen-Laasri A, Lin A, Fischer M, Kase JA, 2013. Prevalence of hemolysin genes and comparison of ehxA subtype patterns in Shiga toxin-producing Escherichia coli (STEC) and non-STEC strains from clinical, food, and animal sources. Appl Environ Microbiol 79:6301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini E, Magi G, Ferretti G, Bacchetti T, Giuliani A, Pugnaloni A, Rippo MR, Facinelli B, 2018. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. essential oil. Front Cell Infect Microbiol 8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijat M, Suh M, Neufeld J, House JD, 2016. Increasing levels of dietary hempseed products leads to differential responses in the fatty acid profiles of egg yolk, liver and plasma of laying hens. Lipids 51:615-33. [DOI] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ, 2015. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol Biol Evol 32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, Biavati B, Monti A, 2010. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81:413-9. [DOI] [PubMed] [Google Scholar]
- Nuutinen T, 2018. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur J Med Chem 157:198-228. [DOI] [PubMed] [Google Scholar]
- Palmieri S, Mascini M, Ricci A, Fanti F, Ottaviani C, Lo Sterzo C, Sergi M, 2019. Identification of Cannabis sativa L. (hemp) Retailers by Means of Multivariate Analysis of Cannabinoids. Molecules 24: e3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelludat C, Rakin A, Jacobi CA, Schubert S, Heesemann J, 1998. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J Bacteriol 180:538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radočaj O, Dimić E, Tsao R, 2014. Effects of hemp (Cannabis sativa L.) seed oil press-cake and decaffeinated green tea leaves (Camellia sinensis) on functional characteristics of gluten-free crackers. J Food Sci 79:C318-25. [DOI] [PubMed] [Google Scholar]
- Rakin A, Schneider L, Podladchikova O, 2012. Hunger for iron: the alternative siderophore iron scavenging systems in highly virulent Yersinia. Frontiers Cell Infect Microbiol 2:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation (EU) 2015/2283 of the European Parliament and the Council of 25 November 2015 on novel foods amending Regulation (EU) No 1169/2011 of the European Parliament and of the council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 OJ L327, 11.12.21015, 1-22. [Google Scholar]
- Romano LL, Hazekamp A, 2013. Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids 7:1-11. [Google Scholar]
- Russo EB, 2011. Taming THC: potential cannabis synergy and phytocannabinoidterpenoid entourage effects. Br J Pharmacol 163:1344-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AG, Payne SM, 1997. Haem irontransport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 23:825-3. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Mancusi R, Delle Donne G, Bacci C, Bassi L., Bonardi S, 2014. Detection of Shiga toxin (Stx)-producing Escherichia coli (STEC) in bovine dairy herds in Northern Italy. Int J Food Microbiol 184:45-49. [DOI] [PubMed] [Google Scholar]
- Um MM, Brugere H, Kerouredan M, Oswald E, Bibbal D, 2018. Antimicrobial Resistance Profiles of Enterohemorrhagic and Enteropathogenic Escherichia coli of Serotypes O157:H7, O26:H11, O103:H2, O111:H8, O145:H28 Compared to Escherichia coli Isolated from the Same Adult Cattle. Microb Drug Resist 24:852-9. [DOI] [PubMed] [Google Scholar]
- Usein CR, Ciontea AS, Militaru CM, Condei M, Dinu S, Oprea M, Cristea D, Michelacci V, Scavia G, Zota LC, Zaharia A, Morabito S, 2017. Molecular characterisation of human Shiga toxinproducing Escherichia coli O26 strains: results of an outbreak investigation, Romania, February to August 2016. Euro Surveill 22:17-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Klingeren B, Ham T, 1976. Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek. 42:9-12. [DOI] [PubMed] [Google Scholar]
- Vispute MM, Sharma D, Mandal AB, Rokade JJ, Tyagi PK, Yadav AS, 2019. Effect of dietary supplementation of hemp (Cannabis sativa) and dill seed (Anethum graveolens) on performance, serum biochemicals and gut health of broiler chickens. J Anim Physiol Anim Nutr (Berl) 103:525-33. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang S, Liu Z, Liu P, Shi Z, Wei J, Shao D, Li B, Ma Z, 2014. Molecular characterization of Enterohemorrhagic E. coli O157 isolated from animal Ffecal and food samples in Eastern China. Sci World J 2014:946394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley JN, Flores KA, Yang X, Chase JA, Cao G, Tang S, Meng J, Atwill ER, 2017. Prevalence and Genomic Characterization of Escherichia coli O157:H7 in Cow-Calf Herds throughout California. Appl Environ Microbiol 83:e00734-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff EE, Duncan D, Torres AG, Mills M, Maase K, Payne SM, 1998. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol 28:1139-52. [DOI] [PubMed] [Google Scholar]
- Zając M, Świątek R, 2018. The effect of hemp seed and linseed addition on the quality of liver pates. Acta Sci Pol Technol Aliment 17:169-76. [DOI] [PubMed] [Google Scholar]
- Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agerso Y, Lund O, Larsen MV, Aarestrup FM, 2013. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother 68:771-7. [DOI] [PubMed] [Google Scholar]


