Abstract
Climate change is expanding the global at-risk population for vector-borne diseases (VBDs). The World Health Organization (WHO) health emergency and disaster risk management (health-EDRM) framework emphasises the importance of primary prevention of biological hazards and its value in protecting against VBDs. The framework encourages stakeholder coordination and information sharing, though there is still a need to reinforce prevention and recovery within disaster management. This keyword-search based narrative literature review searched databases PubMed, Google Scholar, Embase and Medline between January 2000 and May 2020, and identified 134 publications. In total, 10 health-EDRM primary prevention measures are summarised at three levels (personal, environmental and household). Enabling factor, limiting factors, co-benefits and strength of evidence were identified. Current studies on primary prevention measures for VBDs focus on health risk-reduction, with minimal evaluation of actual disease reduction. Although prevention against mosquito-borne diseases, notably malaria, has been well-studied, research on other vectors and VBDs remains limited. Other gaps included the limited evidence pertaining to prevention in resource-poor settings and the efficacy of alternatives, discrepancies amongst agencies’ recommendations, and limited studies on the impact of technological advancements and habitat change on VBD prevalence. Health-EDRM primary prevention measures for VBDs require high-priority research to facilitate multifaceted, multi-sectoral, coordinated responses that will enable effective risk mitigation.
Keywords: health-EDRM, primary prevention, vector-borne disease, biological hazards, climate change, narrative review
1. Introduction
Vector-borne diseases (VBDs) are viral, parasitic and bacterial illnesses transmitted to humans through vectors such as mosquitoes, sand flies and ticks. Common VBDs affecting human health include malaria, yellow fever, dengue, Zika, chikungunya, Lyme disease, tick-borne encephalitis, leishmaniasis and African trypanosomiasis [1]. The complacency towards and reduced emphasis on vector control [2] and the redirection of health resources, together with population growth, urbanisation and globalization, have contributed to the increased frequency of VBD outbreaks in tropical areas of the world in the past decade [2]. With the impact of climate change on ecological and human living environment, the burden of VBDs has expanded from tropical and subtropical areas to temperate regions, placing 80% of the world’s population at risk [3]. This shift in the human vulnerability profile has been attributed to rising temperatures, which favour the migration and geographical expansion of disease vectors [4]. Furthermore, altered precipitation patterns favour larval breeding and have accelerated VBD spread [5]. Contact patterns between humans and pathogens, vectors or hosts may also be altered by climate change in an unpredictable manner [4]. Increased occurrences of natural hazards, such as floods and cyclones, pose a further risk of VBD outbreaks [4]. Geographical areas that were previously unaffected are now facing growing risks [6,7], but are often underequipped in disaster prevention, preparedness and response capacities.
The World Health Organization (WHO) estimates that VBDs currently account for over 17% of the global burden of infectious diseases [1]. As indicated in the Global Burden of Disease Study [8], VBDs have substantial disability weights [9] and can be detrimental to the socioeconomic development of communities. Malaria is a disease which accounts for more than 50% of total deaths caused by VBD [10], and high-risk countries have on average a gross domestic product per capita growth that is over five times lower than countries not affected by the disease [11]. The economic burden of VBDs stems from increased household expenditure on disease prevention and management, lost income from minimised productivity due to sickness or care for the ill [3], damages to crops and livestock by disease vectors [2], and other impacting factors. The United Nations Sustainable Development Goals (SDG) emphasise good health and well-being (SDG 3) [12]. Collaborative initiatives and investments prioritising prevention and treatment research by international bodies in recent decades, such as efforts by the Global Fund [13], have contributed to the alleviation of the global disease burden induced by VBDs [10].
The WHO health-emergency and disaster risk management (health-EDRM) framework was developed in 2018 as an integrated approach for the utilisation and management of resources in addressing current and emerging risks to public health, with the aim of promoting joint action and coherence in implementing other global strategies such as the International Health Regulations (2005), the Sendai Framework for Disaster Risk Reduction 2015–2030, the Paris Agreement on Climate Change, and the Sustainable Development Goals 2015–2030 [14]. Overall, the framework guides the structured analysis and management of health risks brought on by emergencies and disasters, focusing on risk mitigation through hazard and vulnerability reduction, preparedness, response, and recovery measures [14,15]. Health-EDRM emphasises the significance of community involvement to mitigating and counteracting the potential negative impacts of hazardous events such as VBD outbreaks, which are considered biological hazards [14].
The concept of prioritising health in disaster risk management policies was already recognised in the Sendai Framework for Disaster Risk Reduction 2015–2030 [16]. Health actors at all levels have engaged with each other and the WHO in the implementation and monitoring of disaster risk reduction. WHO offices at the regional level, and country governments, have incorporated disaster risk management policies in the health sector, which is an important step in contextualising actions for implementation [17]. The Sendai Framework has been crucial in highlighting health as a core dimension of disaster risk management, and has paved the way for the establishment of the WHO Health-EDRM Research Network, strengthening research and knowledge-sharing globally, allowing for the enhancement of evidence-based policies and practices [17]. There is a crucial need for multi-sectoral, coordinated approaches between the countries’ governments, health systems and other stakeholders, especially in the area of recording and reporting against the framework [17]. Additionally, systems need to reinforce the recognition of prevention and recovery within disaster management [17].
The health-EDRM framework outlines a hierarchisation of health risk prevention into primary, secondary and tertiary prevention [14,18]. Primary prevention mitigates against the onset of disease through health promotion targeted at behavioural modification and health risk reduction. Secondary prevention involves inhibiting disease progression through strategies such as screening and early detection. Tertiary prevention focuses on treatment and rehabilitation in order to minimise disabilities and complications [18,19]. Taking into consideration financial, clinical and infrastructural costs, primary prevention can effectively alleviate the burden of VBDs in a community, if necessary through measures that address a wide spectrum of VBDs, such as targeting diseases transmittable through multiple vectors [20] or focusing on vectors that are capable of transmitting multiple diseases [1]. Primary prevention measures often offer the most cost-effective outcomes and enhance health protection through increased community resilience against diseases where treatment is unavailable or access to healthcare is complicated. Secondary and tertiary prevention measures require significant human resources and health infrastructural support, and may therefore be costly, with higher programmatic risks, causing further economic stress on impacted communities.
There is a large amount of available evidence and research concerning clinical treatment approaches to some VBDs, such as Malaria. However, other VBDs, such as dengue, chikungunya, tick-borne encephalitis, Japanese encephalitis, yellow fever and leishmaniasis, lack standardised or straightforward treatments, and rely primarily on therapeutic interventions built on symptom management [21]. There are ongoing clinical trials in these areas, such as vaccine development for Zika and chikungunya, research into rapid malaria tests, as well as drug trials for chikungunya [22].
This narrative literature review examines published evidence on health-EDRM primary prevention measures for VBD risk mitigation, maps the contextual effectiveness or limitations of each preventive measure, and aims to identify areas of research that need be strengthened in order to develop effective strategies for VBD prevention. The strength of the available scientific evidence is evaluated for each of the prevention measures. Based on the health-EDRM framework, which emphasises the context-based determination of intervention efficacy, analysis of enabling and limiting factors is also included for each measure [14].
2. Materials and Methods
A keyword search-based narrative literature review was conducted using the databases PubMed, Google Scholar, Embase, Medline and ScienceDirect. The search was conducted in May 2020 and included English language-based international peer-reviewed articles, online reports, electronic books and press releases, as well as grey literature by institutions such as the WHO, the United Nations, the Global Fund, the United Nations Children’s Fund, the International Energy Agency, the World Bank, the United States Centres for Disease Control and Prevention, the U.S. Food and Drug Administration, and the Hong Kong Centre for Health Protection, published between January 2000 and May 2020. The snowballing search methodology was also applied. Specific keywords and phrases used can be found in Appendix A. The emergence, primary prevention, associated risk factors and management of VBDs were reviewed in order to generate 10 core primary prevention measures for discussion.
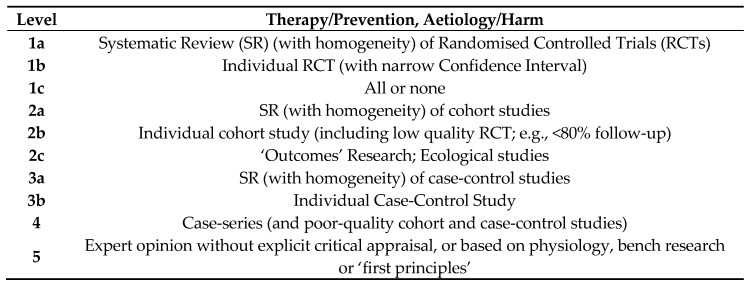
With reference to the Oxford Centre for Evidence-Based Medicine (OCEBM) 2009 Levels of Evidence (Figure 1) criteria, the identified papers were categorised into their respective levels according to strength of evidence based on the study design and methodology [23]. Reviewed literature that could not be categorised using the OCEBM Levels of Evidence was classified as ‘Others’, which includes, but is not limited to, news articles or releases, books, textbooks, position papers, guidelines, case reports and organisational reports.
Figure 1.
The Oxford Centre for Evidence-Based Medicine (OCEBM) 2009 Levels of Evidence (adapted from www.cebm.net) [23].
3. Results
The search identified 134 relevant publications, all of which were included in the results analysis.
Using the identified research, 10 core bottom-up primary prevention measures were proposed and discussed based on the health-EDRM framework. Five personal protection practices (wear protective clothing when outdoors, avoid heading outdoors to vector-prone areas and during peak biting conditions, apply insect repellent, sleep under bed nets, receive prophylactic vaccinations and chemoprophylaxis), three environmental management practices (use insect-killing traps, manage stagnant water appropriately, manage waste appropriately), and two customary household practices (minimise household entry points, cover exposed foodstuffs) were included. Table 1 and Table 2 (personal), Table 3 (environmental) and Table 4 (customary household) highlight relevant health risk, desired behavioural change, potential co-benefits, enabling and limiting factors, alternatives, and strength of evidence available in published literature with regard to these primary prevention measures. Table 5 categorises all 134 reviewed publications according to the OCEBM Levels of Evidence [23]. Of note, a number of the reviewed articles report an assessment of more than one primary prevention measure. The review results indicate that approximately 60% of the studied literature relate to personal protection, 24% to environmental management, and merely 16% focus on customary household practices. Measures such as outdoor avoidance, sleeping under bed nets and receiving prophylactic vaccinations and chemoprophylaxis are amongst the most commonly reported studies. Details on the precise breakdown of each reviewed reference can be found in Table S1.
Table 1.
Personal Protection Practices as Health Emergency and Disaster Risk Management (Health-EDRM) Primary Prevention Approaches against Vector-borne Diseases (VBDs) (Part 1).
| Parametres | Wear Protective Clothing When Outdoors | Avoid Heading Outdoors to Vector-Prone Areas and During Peak Biting Conditions | |
|---|---|---|---|
| Vector-Prone Areas | Peak Biting Conditions | ||
| Risk |
|
|
|
| Behavioural Change |
|
||
| Co-benefit(s) |
|
||
| Enabling Factor(s) |
|
|
|
| Limiting Factor(s) and/or Alternative(s) |
|
|
|
|
|
||
| |||
| Strength of Evidence |
|
|
|
Table 2.
Personal Protection Practices as Health-EDRM Primary Prevention Approaches against VBDs (Part 2).
| Parametre | Apply Insect Repellent | Sleep Under Bed Nets | Receive Prophylactic Vaccinations and Chemoprophylaxis |
|---|---|---|---|
| Risk |
|
||
| Behavioural Change |
|
|
|
| Co-benefit(s) |
|
|
|
| Enabling Factor(s) |
|
|
|
| Limiting Factor(s) and/or Alternative(s) |
|
|
|
| Strength of Evidence |
|
|
|
Table 3.
Environmental Management Practices as Health-EDRM Primary Prevention Approaches against VBDs.
| Parametre | Use Insect-Killing Traps | Manage Stagnant Water Appropriately | Manage Waste Appropriately |
|---|---|---|---|
| Risk |
|
|
|
| Behavioural Change |
|
|
|
| Co-benefit(s) |
|
|
|
| Enabling Factor(s) |
|
|
|
| Limiting Factor(s) and/or Alternative(s) |
|
|
|
| Strength of Evidence |
|
|
|
Table 4.
Customary Household Practices as Health-EDRM Primary Prevention Approaches against VBDs.
| Parametre | Minimise Household Entry Points | Cover Exposed Foodstuffs | |
|---|---|---|---|
| Wall Cracks | Door and Window Openings | ||
| Risk |
|
|
|
|
|
||
| Behavioural Change |
|
||
|
|||
| Co-benefit(s) |
|
||
|
|
||
| Enabling Factor(s) |
|
|
|
| Limiting Factor(s) and/or Alternative(s) |
|
|
|
|
|
||
| Strength of Evidence |
|
|
|
Table 5.
Overview of Health-EDRM Primary Prevention Approaches against VBDs in the Reviewed Articles, Categorised by the Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence. (Please see Table S1 for details.).
| Category | Intervention | Number of Reviewed Articles under Each Category in the OCEBM Levels of Evidence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 4 | 5 | Others * | Total | ||
| Personal Protection Practices | Wear Protective Clothing When Outdoors | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 4 | 3 | 14 |
| Avoid Heading Outdoors to Vector-Prone Areas and During Peak Biting Conditions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 16 | 4 | 23 | |
| Apply Insect Repellent | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 9 | 5 | 17 | |
| Sleep Under Bed Nets | 2 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 5 | 7 | 3 | 22 | |
| Receive Prophylactic Vaccinations and Chemoprophylaxis | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 8 | 6 | 20 | |
| Environmental Management Practices | Use Insect-Killing Traps | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 5 | 14 |
| Manage Stagnant Water Appropriately | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 11 | 1 | 15 | |
| Manage Waste Appropriately | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | 2 | 9 | |
| Customary Household Practices | Minimise Household Entry Points | 1 | 3 | 0 | 1 | 1 | 0 | 0 | 1 | 6 | 3 | 2 | 18 |
| Cover Exposed Foodstuffs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 7 | |
| Total | 4 | 9 | 0 | 3 | 7 | 0 | 2 | 3 | 27 | 72 | 32 | 159 ** | |
* ‘Others’ includes but is not limited to news articles or releases, books, textbooks, position papers, guidelines, case reports and organisational reports.** Of the 134 publications reviewed, some included findings on more than one primary prevention measure, and are counted more than once in Table 5.
4. Discussion
VBDs are classified as biological hazards under the WHO health-EDRM framework [14] and their associated health risks should be managed according to the disaster management cycle (prevention, mitigation, preparedness, response and recovery), which encompasses both top-down and bottom-up interventions [157,158]. Top-down interventions require well-driven bottom-up initiatives to achieve effective primary prevention and to modify community health risk reduction-related measures [159]. Both the WHO health-EDRM framework [14] and the WHO global vector control response 2017–2030 framework [3] emphasise community engagement and mobilisation in enhancing protection against VBDs. The scientific effectiveness and feasibility of the community-level implementation of the 10 proposed primary prevention measures in this review can each be influenced by distinctive external factors, particularly with regards to access to financial or material resources.
Health promotion enables people to have more control over the improvement of their health outcomes, and is done through enhancing health literacy, encouraging behavioural change, and developing supportive policies [160]. There are numerous models which explore behavioural change as a result of education-based health promotion, one of which is the ‘knowledge, attitudes, practices model’, which prompts behavioural changes through knowledge enhancement [160]. In the case of vaccinations and chemoprophylaxis, it is critical for health interventions to enhance individual knowledge and awareness on why and how to receive prophylaxis as a primary prevention mechanism against VBDs, particularly in addressing misconceptions which underestimate the danger of VBDs [81]. Behaviour can be changed through addressing attitudes, such as misunderstandings [81], perception of social norms, cultural traditions and religious beliefs, for example in the case of ultra-orthodox Jewish communities who do not practice vaccination [81,82]. Finally, the behavioural change theory should consider how to promote practice. The viability and efficacy of the practice itself is favoured or limited by a variety of factors; policies will have to address barriers to accessing, and augmenting motivation in, the community [159].
The enabling and limiting factors that impact the effective uptake of primary prevention measures are closely interlinked. This review identified a number of determinants of success, including adequate resources, risk awareness, and well-coordinated supportive systems. A number of primary prevention measures rely on the availability and affordability of material resources, such as insect repellents, protective clothing, UV lamps, household building materials and bed nets (which additionally require space and equipment to set up [73]). Resource-deprived communities, which are at a higher risk of facing vulnerability, may lack the necessary material or financial resources. Materials must be accompanied by knowledge of their appropriate use. Inadequate information can lead to the improper maintenance of vector-prevention commodities, subsequently compromising their efficacy. For example, damaged bed nets with holes and improper bed net usage have been shown to lead to outcomes worse than no usage at all [64,65,66]. Some measures may also be affected by other health conditions, such as allergic reactions to insect repellent active ingredients [76], while others may be limited by cultural concerns, as demonstrated in the case of vaccination hesitancy in certain religious communities [81,82]. The feasibility of certain measures, such as the avoidance of outdoors, is dependent on an individual’s personal, professional and socioeconomic situation. Avoidance of going outdoors into vector-prone areas and during peak biting conditions can be impractical, such as in farming populations that need to spend long periods outdoors, and in tropical areas where the climate is ‘peak-biting’—hot and humid—all year long [50]. Similarly, there may be cases where access to a fully enclosed shelter or household improvements are not feasible, such as for those who are homeless or living in temporary shelters. Beyond resource access, proper education and personal circumstances, some primary prevention measures rely heavily on infrastructural and systemic support. Ensuring community access to vaccinations and chemoprophylaxis requires functioning health systems able to provide the necessary services, including an adequate supply of vaccines or medicine, trained health workers for administration and education, and an established clinic (fixed or mobile) from where the vaccine or drug can be distributed. Health system infrastructure is a critical enabling factor lacking in many rural or resource-poor contexts [84]. The environmental management of vectors also requires a robust and coordinated top-down waste management system [109,117], with multi-sectoral collaboration [161] between the health, environmental and civil engineering sectors, as well as other local and national-level authorities. Authorities should ensure the sufficiency of waste collection points such as waste bins [123], which can affect proper waste disposal, and the supply of electricity [118], which can affect the use of insect-killing traps, particularly in developing contexts [116]. Therefore, the success or failure of a community’s uptake of primary prevention measures is shaped by the availability of material resources and information, supportive health and civil infrastructure, policy formulation, geographical climate, individual or professional flexibilities, and social contexts. Nonetheless, it should always be noted that each measure offers its contribution towards VBD prevention, and the measures serve as an alternative to one another. When one measure cannot be carried out, the practice of other measures is not necessarily impeded.
In comparing the strength of evidence of the reviewed literature (Table 5, please see Table S1 for details), the largest proportion (45%) fell into Level 5 classification, which covers a wide range of study designs and methodologies, such as entomological studies, observational exploratory studies, experimental studies, modelling studies, qualitative studies, and expert opinions. 20% of the reviewed literature was categorised into ‘Others’, which includes but is not limited to news releases, reports by international organisations like the WHO, and textbooks. Level 4 publications, such as cross-sectional mixed method studies, behavioural surveys, household surveys, questionnaires, interventional studies and case series studies contributed a relatively large portion (17%), with many addressing the knowledge, perceptions, acceptance and opinions of populations with regards to VBD-prevention measures. Regarding individual primary prevention measures, evidence is most lacking at all levels with regard to the practices of covering exposed foodstuffs (4%) and proper waste management (6%). The literature relevant to sleeping under bed nets and minimising household entry points was significantly stronger in study design. There is published evidence on the risk reduction relating to wearing protective clothing and the management of stagnant water; however, while a multitude of studies emphasised the impact of primary prevention measures on VBD health risk reduction, a limited number of studies focused on the impact of the measure itself on disease prevention efficacy or outcome. For instance, many studies demonstrate the potential VBD-related health risks of exposed foodstuffs [136,137,138,139] and household entry points [140,141]; however, there are limited studies that demonstrate the effectiveness of covering food or household crack-repairing on disease incidence reduction within a community [156]. Similarly, for solid waste management, while evidence on the health risks [134,135] associated with improper solid waste accumulation is available, there is a lack of in-depth comparative studies between different waste management system models and their strengths and weaknesses.
The methodology used for this review is limited in that it does not include non-English-based literature, non-electronically-accessible literature, grey literature outside of those areas deliberately searched, any publications before 2000, or any publication not identified due to incompatibility with the keywords used for the literature search. Notably, publications documenting experiences from low-resource VBD-endemic settings that are not readily accessible via mainstream databases or online platforms may not have been included in this review.
Certain areas were found to be lacking in the updated evidence. On the efficacy of light-coloured clothing, while the WHO provides recommendations for protective wear against VBDs [21], the search generated no clear evidence, that had been updated within the past two decades, to support the rationale behind vector landing preferences on darker surfaces, and vice versa. Recommendations concerning the appropriate concentration of DEET in insect repellent are often inconsistent across international organisations and governments. More extensive research is needed to better establish the correlation between DEET concentration, repellent strength and duration of efficacy. In addition, while there are various observational studies on the correlation between modern technological advancements, such as air conditioning, and decreased disease vector bites [162,163,164,165], there is limited updated scientific evidence available on the precise impacts of such advancements on changes to vector habitat. Addressing these research gaps will facilitate better-grounded and more evidence-based institutional guidelines.
The best available evidence is always evolving, requiring the continuous updating of guidelines and recommendations. The ongoing research on VBD prophylactic strategies is very active, as well as that on the development of insecticide resistance regarding insecticide-treated bed nets [166,167] and insect repellents [168]. In light of the many different designs, parameters, sample sizes and investigation methods used, it is often difficult to evaluate and compare related studies, thus resulting in a lack of standardisation in guidelines. For instance, a variety of attraction and killing mechanisms, as well as door and window screen designs [141], are used in different studies to evaluate insect-killing trap and household modification efficacies. Efforts to achieve increased consistency in the methodology of published research are crucial to making comparative analyses between studies on different VBD-prevention commodities possible [169,170,171,172].
Three areas are particularly lacking in the published evidence. Firstly, there has been minimal research done on available alternatives to the proposed practices. Taking the case of insect repellents, numerous studies are available to prove the efficacy [59,60,61,85] and explore the potential safety concerns [86,87,88] of DEET. However, the strength of research supporting the repellence of natural alternatives like plant oils is variable [74]. For instance, limited and conflicting findings on citronella efficacy were identified [74,85], and potential health hazards, like dermatitis under high-concentration neem-oil use, are indicated, with less stringent safety testing conducted compared to DEET [74]. Secondly, limited research is available on other disease vectors such as sand flies and ticks. A bulk of the literature identified in this analysis focuses on mosquitoes—the discussions on common vector breeding grounds [52,106,107,108] and the efficacy of insect-killing traps seldom involve other disease vectors [128]. There is a need for research into effective methods to better understand the breeding habitat ecology of sand flies in immature stages, which will facilitate the development of targeted control strategies such as source reduction, which are not yet possible as sand fly larvae can be difficult to detect, in contrast to other vectors such as mosquitoes [173,174,175]. Similarly, in the case of insect-killing traps, only limited studies demonstrate their potential in targeting sand flies in addition to mosquitoes [129], and evidence on tick elimination by the traps is lacking entirely. Thirdly, research on the spectrum of VBDs is disproportionately distributed; studies are oftentimes skewed towards more prevalent VBDs, such as malaria. While consideration is given to other VBDs such as Zika or tick-borne encephalitis, this literature review occasionally extrapolates the primary prevention measures proposed for the more extensively-researched diseases so as to apply them to other VBDs as well—for example, the determination of the time of day with peak biting conditions was based on Plasmodium-infected (malaria) mosquitoes being active from dusk to dawn [29,30,31]. Further research on these three areas is necessary in order to develop comprehensive and informed guidelines or policies that can be implemented in varying contexts to mitigate against the risk and alleviate the disease burden of VBDs.
This review has identified major research gaps in the current published literature relating to health-EDRM primary prevention measures for VBDs (Table 6). Strengthening the available evidence in these areas will create a scientific basis on which governments, policy-makers and community stakeholders can develop effective, targeted and achievable strategies for protecting at-risk populations against VBDs. Aspects of the WHO health-EDRM framework can be applied to address these research gaps. Increasing capacities for information and knowledge management can support collection, analysis and dissemination across multiple sectors, allowing for the comparative evaluation of available evidence, as well as the development of consistent guidelines and recommendations [14]. This is particularly important for any research undertaken in resource-poor contexts, which will provide necessary evidence towards developing effective and targeted VBD prevention measures in such contexts. The framework highlights the need for more multifaceted and multisectoral approaches, the lessons of which will lead to the further development of evidence-based strategies [14].
Table 6.
Major Research Gaps in Current Published Literature Relating to Health-EDRM Primary Prevention Measures for VBDs.
| Research Gaps | |
|---|---|
| 1 | Current studies on health-EDRM primary prevention measures for VBDs mostly focus on health risk reduction practices, yet efficacy evaluation on actual disease reduction is lacking. |
| 2 | Available literature is mostly classified as cross-sectional studies. Evidence on efficacy of the prevention measure based on randomised controlled studies or extensive cohort studies is limited. |
| 3 | Comparative evaluations for variations of certain primary prevention measures, such as efficacy of different insect-killing mechanisms or household modification materials, are limited. |
| 4 | Research outcomes are skewed towards certain vectors (e.g., mosquitoes). Research evidence on other vectors such as sand flies or ticks is limited. |
| 5 | Research outcomes are skewed towards certain VBDs (e.g., malaria). Research evidence on other VBDs such as Zika, chikungunya, or tick-borne encephalitis is limited. |
| 6 | Research and evidence on available alternatives to the proposed practices (e.g., using natural substitutes as opposed to chemical-based insect repellents) is limited. |
| 7 | Updated research on evidence relating technological advancements and the rapid change of ecological and human living environments to behavioural practices against VBDs is limited. |
| 8 | Consistency in recommendations from research papers, policies, and frontline international agencies (e.g., as in DEET concentration recommendations) is lacking. |
| 9 | Literature highlighting the effectiveness of multi-faceted, multi-sectoral and coordinated responses in enabling effective risk mitigation for population-level protection is lacking. |
All 10 primary prevention measures require sustainable, continuous implementation and maintenance in order to be truly effective in preventing VBDs. Primary prevention measures focusing on stagnant water, waste management and the covering of exposed foodstuffs offer the long-term co-benefit of mitigating risks arising from other biological hazards under the health-EDRM framework [14], such as water-borne and food-borne diseases [139]. Practising continuous primary prevention is particularly necessary as long as certain VBDs do not have standardised effective treatment options, and if vector-elimination is not feasible. Some preventive measures face more complex challenges in practise without adequate health or governance infrastructure. Others are more easily implemented, but are nonetheless reliant on materials such as insect repellents or bed nets, which can be an obstacle in resource-poor settings where the population is already facing vulnerability to impoverishment or disease. It is crucial for policymakers to ensure that systems are able to identify and assess needs, and provide the necessary support for the sustainable and fair distribution of resources. Empowering bottom-up initiatives requires well-coordinated top-down policies [83] that effectively disseminate resources and information, especially in resource-deprived, rural, or health-illiterate populations. A strong, accessible health system is key to providing materials and education to the at-risk population. Centralised, coordinated and well-regulated infrastructure, such as a uniform waste management system [176], can significantly enhance the efficacy of primary prevention practices.
Climate change and its associated consequences, such as changing weather patterns and increased disaster occurrences [18], have shifted the epidemiological patterns of VBDs, as well as the volume and spread of the at-risk population, thus affecting the development policies and strategies for mitigating the VBD burden on health systems. Rising temperatures and unpredictable precipitation patterns, for example, lengthen peak-biting periods and further complicate the capacity for outdoor avoidance, especially in tropical areas which are sultry throughout the year. The increased incidence of hydro-meteorological hazards such as floods and cyclones brings about more extreme rainfall, as well as increased humidity and water accumulation [18], and impact stagnant water management, thus possibly facilitating further larval habitat development for disease vectors [18]. Insect vectors cannot regulate their internal temperatures and are very sensitive to changes, which has caused them to invade new areas in order to adapt [177]. This puts previously unexposed populations at risk, who may lack protective immunity or the experience, resources or services necessary to mitigate the prevalence of disease [6]. The WHO health-EDRM framework stresses the importance of strengthening health systems, with an increased emphasis on climate change adaptation [14], to reducing health risks associated with hazardous events, including VBD outbreaks. It is important for governing bodies to consider the associated challenges of climate change during policy formulation, with the inclusion of climate change scenarios in disaster risk assessments [18]. Considering the limitation of the predicted impact of climate change on VBD transmission, governing bodies should enhance individual capacities and community resilience in cases of sudden VBD surges [178]. For instance, early warning systems should be in place to communicate the health risks associated with seasonal VBD outbreaks to vulnerable populations in advance [18]. As such, primary prevention measures that emphasise the broader aspects of environmental management, resource distribution and public education must not be overlooked. Public education, to encourage early symptom identification and subsequent health-seeking behaviours, can serve as a steppingstone in propagating secondary and tertiary VBD intervention amongst vulnerable populations.
In light of the growing burden of VBDs and emerging public health threats, a progressive primary prevention model is key to disaster risk reduction, as encompassed in the four priorities set out in the Sendai Framework for Disaster Risk Reduction (risk understanding, governance, preparedness and resilience) [16]. In terms of disaster risk understanding, a thorough examination of the enabling and limiting circumstances is required in at-risk populations, including local disease prevention capacity, specific VBD characteristics, and risk drivers such as climate change [16,18]. Disaster governance should be strengthened through stakeholder involvement and multi-sectorial collaboration, as well as through adopting a well-coordinated top-down approach to empowering bottom-up community initiatives in a sustainable manner. Resilience enhancement should be driven by global investments in innovation and research, for instance the development of better prophylactic strategies and better vector-prevention commodity designs for utilisation against VBDs. Finally, disaster preparedness can be reinforced through raised awareness, secured healthcare accessibility and health-seeking behaviour encouragement, so as to better equip vulnerable populations facing future VBD outbreaks.
5. Conclusions
This narrative study identified 10 health-EDRM primary prevention measures against VBDs. Resource availability, risk awareness and systemic support were identified as the core enabling factors for the success of these measures. Resources, health and civil infrastructure, policy formulation, geographical climate and socioeconomic factors were the core sources of limitations, which necessitate the need to consider alternatives. Evidence supporting the effectiveness of alternative preventive measures is lacking, in particular with regards to prevention in resource-poor settings. Similarly, evidence related to preventive measures focusses heavily on mosquitoes, whereas research on effective prevention against diseases transmitted by other vectors such as sand flies and ticks is lacking. At a global level, the necessity of VBD prevention increases with the growing impact of climate change and globalisation.
Health risks associated with VBDs will remain an ongoing biological hazard to communities, and thus sustainability of practice is crucial. As recommended by the WHO health-EDRM framework, in addition to the health sector, the successful adoption of primary prevention measures against VBDs requires a multi-faceted, multi-sectoral and coordinated response, encompassing sectors such as meteorology for hazard prediction, education for health awareness and promotion, and the environmental and civil engineering sectors for waste collection and water management.
In conclusion, this review has shown that evidence of the effectiveness and management of primary prevention practices is focused on a narrow spectrum of VBDs and vector types. In order to fill research gaps, the scope of VBD research should be broadened, and standardised protocols should be adopted so as to better prepare communities for disaster risk mitigation and to build the capacities of populations that are vulnerable with regards to health-EDRM practices.
Supplementary Materials
The following is available online at https://www.mdpi.com/1660-4601/17/16/5981/s1, Table S1: Relevant Intervention(s), Study Design, Relevant Key Finding(s) and/or Conclusion of Each Reviewed Article Referenced (n = 134).
Appendix A. Keywords Used for Literature Search
‘bed nets’, ‘blue-light irradiation’, ‘bottom-up approach’, ‘breeding sites’, ‘carbon dioxide’, ‘cement’, ‘chemoprophylaxis’, ‘chikungunya’, ‘climate change’, ‘clothes moth larvae’, ‘clothes wear and tear’, ‘cockroaches’, ‘crack repair’, ‘dengue’, ‘diethyltoluamide (DEET) ’, ‘disease burden’, ‘door screening’, ‘doors and windows burglary’, ‘electricity access’, ‘fall injury water’, ‘floods’, ‘food decay’, ‘food fermentation’, ‘food mould and fungi’, ‘food-borne pathogens’, ‘forests’, ‘health hazards’, ‘health-EDRM’, ‘heat stroke’, ‘heat-seeking ability’, ‘heavy rain’, ‘household waste management’, ‘housing improvements’, ‘humidity’, ‘immunisation’, ‘infectious disease’, ‘insect repellents’, ‘insect traps’, ‘insecticide-treated nets’, ‘Japanese encephalitis’, ‘larval habitats’, ‘larvicides’, ‘lime’, ‘living environment’, ‘long clothing’, ‘long-lasting insecticide-treated nets’, ‘malaria’, ‘mosquito larvae’, ‘mosquito traps’, ‘mosquitoes’, ‘mould development water’, ‘mud’, ‘natural repellents’, ‘octenol’, ‘pesticide’, ‘primary prevention’, ‘protective behaviour’, ‘protective clothing’, ‘rodents’, ‘rubber plantations’, ‘sand flies’, ‘solid waste management’, ‘sticky traps’, ‘sunburns’, ‘temperature’, ‘tick-borne diseases’, ‘tick-borne encephalitis’, ‘ticks’, ‘top-down approach’, ‘tropical climates’, ‘ultraviolet irradiation’, ‘vaccination’, ‘vaccine complacency’, ‘vaccine hesitancy’, ‘VBDs’, ‘vector attraction’, ‘vector biting’, ‘vector contamination’, ‘vector exposure risk’, ‘vector human movement’, ‘vector landing preference’, ‘vector light clothing’, ‘vector net’, ‘vector traps’, ‘vectors’, ‘wall cracks’, ‘waste management’, ‘waste mismanagement’, ‘water storage’, ‘water supply’, ‘West Nile virus’, ‘window screening’, ‘yellow fever’, ‘Zika’.
Author Contributions
Conceptualization, E.Y.Y.C. and C.D.; methodology, T.S.T.S. and T.S.S.; formal analysis, T.S.T.S; T.S.S.; writing—original draft preparation, E.Y.Y.C.; T.S.T.S.; T.S.S.; C.D.; writing—review and editing, Z.H.; S.L.; K.K.C.H.; S.L.A.T.; K.O.K.; P.-H.C.; R.K.; R.S.; supervision, E.Y.Y.C.; funding acquisition, E.Y.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CCOUC-University of Oxford research fund (2019–2023).
Conflicts of Interest
The authors declare no conflict of interest. Professor Emily Ying Yang Chan serves as the Co-Chair of the Health-EDRM Global Research Network and Ryoma Kayano serves as the Secretary of the Health-EDRM Global Research Network.
References
- 1.Vector-Borne Diseases. [(accessed on 31 May 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
- 2.Lemon S.M., Sparling P.F., Hamburg M.A., Relman D.A., Choffnes E.R., Mack A. Vector-Borne Diseases: Understanding the Environmental, Human Health, and Ecological Connections. The National Academies Press; Washington, DC, USA: 2008. pp. 1–27. [PubMed] [Google Scholar]
- 3.WHO . Global Vector Control Response 2017–2030. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 4.Wu X., Lu Y., Zhou S., Chen L., Xu B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016;86:14–23. doi: 10.1016/j.envint.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Hoshen M.B., Morse A.P. A weather-driven model of malaria transmission. Malar. J. 2004;3 doi: 10.1186/1475-2875-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caminade C., McIntyre K.M., Jones A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019;1436:157–173. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouque F., Reeder J.C. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect. Dis. Poverty. 2019;8 doi: 10.1186/s40249-019-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathers C. International Encyclopedia of Public Health. Academic Press; Cambridge, MA, USA: 2016. Global Burden of Disease. [Google Scholar]
- 9.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . World Malaria Report 2019. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 11.Mccarthy D., Wolf H., Wu Y. Malaria and Growth. The World Bank; Washington, DC, USA: 2000. pp. 2–26. [DOI] [Google Scholar]
- 12.United Nations . The Sustainable Development Goals Report 2019. United Nations; New York, NY, USA: 2019. [Google Scholar]
- 13.The Global Fund: Malaria. [(accessed on 31 May 2020)]; Available online: https://www.theglobalfund.org/en/malaria/
- 14.WHO . Health Emergency and Disaster Risk Management: Overview. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 15.World Health Organisation . Emergency Risk Management for Health—Overview. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 16.World Health Organization . Sendai Framework for Disaster Risk Reduction 2015–2030. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 17.Wright N., Fagan L., Lapitan J.M., Kayano R., Abrahams J., Huda Q., Murray V. Health Emergency and Disaster Risk Management: Five Years into Implementation of the Sendai Framework. Int. J. Disaster Risk Sci. 2020;11:206–217. doi: 10.1007/s13753-020-00274-x. [DOI] [Google Scholar]
- 18.Chan E.Y.Y., Shaw R. Public Health and Disasters: Health Emergency and Disaster Risk Management in Asia. Springer; Berlin/Heidelberg, Germany: 2020. [Google Scholar]
- 19.Boslaugh S. Encyclopedia of Epidemiology. SAGE Publications, Inc.; Thousand Oaks, CA, USA: 2008. Prevention: Primary, Secondary, and Tertiary; pp. 839–840. [Google Scholar]
- 20.Alison M. Global Health Impacts of Vector-Borne Diseases. The National Academies Press; Washington, DC, USA: 2016. Global Health Impacts of Vector-Borne Diseases; pp. 1–59. [PubMed] [Google Scholar]
- 21.World Health Organization . A Global Brief on Vector-Borne Diseases. World Health Organization; Geneva, Switzerland: 2014. p. 9. [Google Scholar]
- 22.World Health Organisation . International Clinical Trials Registry Platform. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 23.OCEBM Levels of Evidence Working Group “The Oxford 2009 Levels of Evidence”. Oxford Center for Evidence-Based Medicine. [(accessed on 10 August 2020)]; Available online: https://www.cebm.net/index.aspx?o=5653.
- 24.Achee N.L., Youngblood L., Bangs M.J., Lavery J.V., James S. Considerations for the use of human participants in vector biology research: A tool for investigators and regulators. Vector-Borne Zoonotic Dis. 2015;15:89–102. doi: 10.1089/vbz.2014.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saavedra M.P., Conn J.E., Alava F., Carrasco-Escobar G., Prussing C., Bickersmith S.A., Sangama J.L., Fernandez-Miñope C., Guzman M., Tong C., et al. Higher risk of malaria transmission outdoors than indoors by Nyssorhynchus darlingi in riverine communities in the Peruvian Amazon. Parasites Vectors. 2019;12 doi: 10.1186/s13071-019-3619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakkhara P., Chongsuvivatwong V., Thammapalo S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2013;107:789–796. doi: 10.1093/trstmh/trt083. [DOI] [PubMed] [Google Scholar]
- 27.Wallace J.W., Nicholson W.L., Perniciaro J.L., Vaughn M.F., Funkhouser S., Juliano J.J., Lee S., Kakumanu M.L., Ponnusamy L., Apperson C.S., et al. Incident Tick-Borne Infections in a Cohort of North Carolina Outdoor Workers. Vector-Borne Zoonotic Dis. 2016;16:302–308. doi: 10.1089/vbz.2015.1887. [DOI] [PubMed] [Google Scholar]
- 28.Tangena J.A.A., Thammavong P., Lindsay S.W., Brey P.T. Risk of exposure to potential vector mosquitoes for rural workers in Northern Lao PDR. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndoen E., Wild C., Dale P., Sipe N., Dale M. Dusk to dawn activity patterns of anopheline mosquitoes in West Timor and Java, Indonesia. Southeast Asian J. Trop. Med. Public Health. 2011;42:550–561. [PubMed] [Google Scholar]
- 30.Van Bortel W., Trung H.D., Hoi L.X., Van Ham N., Van Chut N., Luu N.D., Roelants P., Denis L., Speybroeck N., D’Alessandro U., et al. Malaria transmission and vector behaviour in a forested malaria focus in central Vietnam and the implications for vector control. Malar. J. 2010;9 doi: 10.1186/1475-2875-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeb M., Elliott S.J., Gibson B., Fearon M., Nosal R., Drebot M., D’Cuhna C., Harrington D., Smith S., George P., et al. Protective behavior and West Nile virus risk. Emerg. Infect. Dis. 2005;11:1433–1436. doi: 10.3201/eid1109.041184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013 doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokarevich N., Tronin A., Gnativ B., Revich B., Blinova O., Evengard B. Impact of air temperature variation on the ixodid ticks habitat and tick-borne encephalitis incidence in the Russian Arctic: The case of the Komi Republic. Int. J. Circumpolar Health. 2017;76 doi: 10.1080/22423982.2017.1298882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhury F.R., Ibrahim Q.S.U., Shafiqul Bari M., Jahangir Alam M.M., Dunachie S.J., Rodriguez-Morales A.J., Ismail Patwary M. The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS ONE. 2018;15:e0199579. doi: 10.1371/journal.pone.0199579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vázquez M., Muehlenbein C., Cartter M., Hayes E.B., Ertel S., Shapiro E.D. Effectiveness of personal protective measures to prevent lyme disease. Emerg. Infect. Dis. 2008;14:210–216. doi: 10.3201/eid1402.070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnard D.R. Global Collaboration for Development of Pesticides for Public Health (GCDPP) Repellents and Toxicants for Personal Protection. Volume 46. World Health Organization; Geneva, Switzerland: 2000. pp. 408–418. [Google Scholar]
- 37.Donohoe H., Pennington-Gray L., Omodior O. Lyme disease: Current issues, implications, and recommendations for tourism management. Tour. Manag. 2015;46:408–418. doi: 10.1016/j.tourman.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linos E., Keiser E., Fu T., Colditz G., Chen S., Tang J.Y. Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness. Cancer Causes Control. 2011 doi: 10.1007/s10552-011-9780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szykitka W. Big Book of Self-Reliant Living: Advice and Information on just about Everything You Need to Know to Live on Planet Earth. The Lyons Press; Guilford, CT, USA: 2010. p. 65. [Google Scholar]
- 40.Harahap R. Sumatran Tigers Seen on Plantation in Riau. [(accessed on 31 May 2020)]; Available online: https://www.thejakartapost.com/news/2019/02/28/sumatran-tigers-seen-on-plantation-in-riau.html.
- 41.Takahata C., Nielsen S.E., Takii A., Izumiyama S. Habitat selection of a large carnivore along human-wildlife boundaries in a highly modified landscape. PLoS ONE. 2014;9:e0086181. doi: 10.1371/journal.pone.0086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu S., Wang A., Bian G., He T., Yi B., Lu B., Li X., Xu G. Relationship between weather factors and heat stroke in Ningbo city. Chin. J. Endem. 2016;37:1131–1136. doi: 10.3760/cma.j.issn.0254-6450.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Kenny G.P., Wilson T.E., Flouris A.D., Fujii N. Handbook of Clinical Neurology. Elsevier; Amsterdam, The Netherlands: 2018. Heat exhaustion; pp. 505–529. [DOI] [PubMed] [Google Scholar]
- 44.Zeng J., Zhang X., Yang J., Bao J., Xiang H., Dear K., Liu Q., Lin S., Lawrence W.R., Lin A., et al. Humidity may modify the relationship between temperature and cardiovascular mortality in Zhejiang province, China. Int. J. Environ. Res. Public Health. 2017;14:1383. doi: 10.3390/ijerph14111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin L.W., Lin H.Y., Hsu C.Y., Rau H.H., Chen P.L. Effect of weather and time on trauma events determined using emergency medical service registry data. Injury. 2015;46:1814–1820. doi: 10.1016/j.injury.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Crawshaw A.F., Maung T.M., Shafique M., Sint N., Nicholas S., Li M.S., Roca-Feltrer A., Hii J. Acceptability of insecticide-treated clothing for malaria prevention among migrant rubber tappers in Myanmar: A cluster-randomized non-inferiority crossover trial. Malar. J. 2017;16 doi: 10.1186/s12936-017-1737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiu L., Floris I. Susceptibility of environmentally friendly sheep wool insulation panels to the common clothes moth tineola bisselliella in laboratory assays. Insects. 2019;10:379. doi: 10.3390/insects10110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao C., Kuklane K., Östergren P.O., Kjellstrom T. Occupational heat stress assessment and protective strategies in the context of climate change. Int. J. Biometeorol. 2018;62:359–371. doi: 10.1007/s00484-017-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unger A., Riley L.W. Slum health: From understanding to action. PLoS Med. 2007;4:1561–1566. doi: 10.1371/journal.pmed.0040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobel A.H. Tropical Weather. Nat. Educ. Knowl. 2012;3:2. [Google Scholar]
- 51.Stjernberg L., Berglund J. Detecting ticks on light versus dark clothing. Scand. J. Infect. Dis. 2009;37:361–364. doi: 10.1080/00365540410021216. [DOI] [PubMed] [Google Scholar]
- 52.Dejenie T., Yohannes M., Assmelash T. Characterization of Mosquito Breeding Sites in and in the Vicinity of Tigray Microdams. Ethiop. J. Health Sci. 2011;21:57–66. doi: 10.4314/ejhs.v21i1.69045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumodan P.K. Species diversity of mosquito breeding in rubber plantations of Kerala, India. J. Am. Mosq. Control Assoc. 2012;28:114–115. doi: 10.2987/11-6208R.1. [DOI] [PubMed] [Google Scholar]
- 54.Mackensie J.S., Lindsay M.D., Broom A.K. Effect of climate and weather on the transmission of Ross River and Murray Valley encephalitis viruses. Microbiol. Aust. 2000;21:40. [Google Scholar]
- 55.Reinhold J.M., Lazzari C.R., Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects. 2018;9:158. doi: 10.3390/insects9040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eldridge B.F., Edman J.D., Moncayo A.C. Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods. J. Med. Entomol. 2000;116:15086–15095. doi: 10.1603/0022-2585-38.5.768. [DOI] [Google Scholar]
- 57.Sherrard-Smith E., Skarp J.E., Beale A.D., Fornadel C., Norris L.C., Moore S.J., Mihreteab S., Charlwood J.D., Bhatt S., Winskill P., et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. USA. 2019 doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutherst R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004;17:136–167. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leal W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014;6:93–98. doi: 10.1016/j.cois.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.CDC, Centers for Disease Control and Prevention . Fight the Bite for Protection from Malaria Guidelines for DEET Insect Repellent Use. CDC; Atlanta, GA, USA: 2005. p. 1. [Google Scholar]
- 61.Staub D., Debrunner M., Amsler L., Steffen R. Effectiveness of a repellent containing DEET and EBAAP for preventing tick bites. Wilderness Environ. Med. 2002;13:12–20. doi: 10.1580/1080-6032(2002)013[0012:EOARCD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 62.Onyett H., Bortolussi R., Bridger N.A., Finlay J.C., Martin S., McDonald J.C., Robinson J.L., Salvadori M.I., Vanderkooi O.G., Allen U.D., et al. Preventing mosquito and tick bites: A Canadian update. Paediatr. Child Health. 2014;19:326–328. doi: 10.1093/pch/19.6.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tips for Using Insect Repellents. [(accessed on 31 May 2020)]; Available online: https://www.chp.gov.hk/en/features/38927.html.
- 64.Msellemu D., Shemdoe A., Makungu C., Mlacha Y., Kannady K., Dongus S., Killeen G.F., Dillip A. The underlying reasons for very high levels of bed net use, and higher malaria infection prevalence among bed net users than non-users in the Tanzanian city of Dar es Salaam: A qualitative study. Malar. J. 2017;16 doi: 10.1186/s12936-017-2067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ochomo E.O., Bayoh N.M., Walker E.D., Abongo B.O., Ombok M.O., Ouma C., Githeko A.K., Vulule J., Yan G., Gimnig J.E. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malar. J. 2013;12 doi: 10.1186/1475-2875-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah M.P., Steinhardt L.C., Mwandama D., Mzilahowa T., Gimnig J.E., Bauleni A., Wong J., Wiegand R., Mathanga D.P., Lindblade K.A. The effectiveness of older insecticide-treated bed nets (ITNs) to prevent malaria infection in an area of moderate pyrethroid resistance: Results from a cohort study in Malawi. Malar. J. 2020;19 doi: 10.1186/s12936-020-3106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Insecticide-Treated Bed Nets. [(accessed on 31 May 2020)]; Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html.
- 68.Jayanti P., Acharya I.A. A Study on Efficacy of LLINS As Compared To In-Use ITNs Amongst Troops in a Malaria Endemic Area. J. Trop. Dis. 2015;3 doi: 10.4172/2329-891x.1000175. [DOI] [Google Scholar]
- 69.Clem A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McBride W.J.H. Chemoprophylaxis of tropical infectious diseases. Pharmaceuticals. 2010;3:1561–1575. doi: 10.3390/ph3051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson A.L., Dhiman R.C., Kitron U., Scott T.W., van den Berg H., Lindsay S.W. Benefit of Insecticide-Treated Nets, Curtains and Screening on Vector Borne Diseases, Excluding Malaria: A Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J.W., Liao Y.M., Liu H., Nie R.H., Havumaki J. Use of bed nets and factors that influence bed net use among jinuo ethnic minority in southern China. PLoS ONE. 2014;9:e0103780. doi: 10.1371/journal.pone.0103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das M.L., Singh S.P., Vanlerberghe V., Rijai S., Rai M., Karki P., Sundar S., Boelaert M. Population preference of net texture prior to bed net trial in Kala-Azar-endemic areas. PLoS Negl. Trop. Dis. 2007;1 doi: 10.1371/journal.pntd.0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maia M.F., Moore S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011;10 doi: 10.1186/1475-2875-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batish D.R., Singh H.P., Kohli R.K., Kaur S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008;256:2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 76.McHenry M., Lacuesta G. Severe allergic reaction to diethyltoluamide (DEET) containing insect repellent. Allergy, Asthma Clin. Immunol. 2014;10 doi: 10.1186/1710-1492-10-S2-A30. [DOI] [Google Scholar]
- 77.Nelson K.E., Williams C.M. Infectious Disease Epidemiology: Theory and Practice. Jones & Bartlett Publishers; Burlington, MA, USA: 2008. pp. 1014–1015. [Google Scholar]
- 78.Von Seidlein L., Ikonomidis K., Bruun R., Jawara M., Pinder M., Knols B.G.J., Knudsen J.B. Airflow attenuation and bed net utilization: Observations from Africa and Asia. Malar. J. 2012;11 doi: 10.1186/1475-2875-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ntonifor N.H., Veyufambom S. Assessing the effective use of mosquito nets in the prevention of malaria in some parts of Mezam division, Northwest Region Cameroon. Malar. J. 2016;15 doi: 10.1186/s12936-016-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulford J., Hetzel M.W., Bryant M., Siba P.M., Mueller I. Reported reasons for not using a mosquito net when one is available: A review of the published literature. Malar. J. 2011;10 doi: 10.1186/1475-2875-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pugliese-Garcia M., Heyerdahl L.W., Mwamba C., Nkwemu S., Chilengi R., Demolis R., Guillermet E., Sharma A. Factors influencing vaccine acceptance and hesitancy in three informal settlements in Lusaka, Zambia. Vaccine. 2018;36:5617–5624. doi: 10.1016/j.vaccine.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muhsen K., Abed El-Hai R., Amit-Aharon A., Nehama H., Gondia M., Davidovitch N., Goren S., Cohen D. Risk factors of underutilization of childhood immunizations in ultraorthodox Jewish communities in Israel despite high access to health care services. Vaccine. 2012;30:2109–2115. doi: 10.1016/j.vaccine.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 83.The Lancet Infectious Diseases Malaria vaccination: A major milestone. Lancet Infect. Dis. 2019;19:559. doi: 10.1016/S1473-3099(19)30222-1. [DOI] [PubMed] [Google Scholar]
- 84.Malande O.O., Munube D., Afaayo R.N., Annet K., Bodo B., Bakainaga A., Ayebare E., Njunwamukama S., Mworozi E.A., Musyoki A.M. Barriers to effective uptake and provision of immunization in a rural district in Uganda. PLoS ONE. 2019;14:e0212270. doi: 10.1371/journal.pone.0212270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez S.D., Drake L.L., Price D.P., Hammond J.I., Hansen I.A., Liu N. The efficacy of some commercially available insect repellents for Aedes aegypti (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae) J. Insect Sci. 2015;15 doi: 10.1093/jisesa/iev125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Legeay S., Clere N., Hilairet G., Do Q.T., Bernard P., Quignard J.F., Apaire-Marchais V., Lapied B., Faure S. The insect repellent N,N-diethyl-m-Toluamide (DEET) induces angiogenesis via allosteric modulation of the M3 muscarinic receptor in endothelial cells. Sci. Rep. 2016;6 doi: 10.1038/srep28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swale D.R., Bloomquist J.R. Is DEET a dangerous neurotoxicant? Pest Manag. Sci. 2019;75 doi: 10.1002/ps.5476. [DOI] [PubMed] [Google Scholar]
- 88.Koren G., Matsui D., Bailey B. DEET-based insect repellents: Safety implications for children and pregnant and lactating women. Can. Med. Assoc. J. 2003;169:209–212. [PMC free article] [PubMed] [Google Scholar]
- 89.Lenhart A., Orelus N., Maskill R., Alexander N., Streit T., McCall P.J. Insecticide-treated bednets to control dengue vectors: Preliminary evidence from a controlled trial in Haiti. Trop. Med. Int. Heal. 2008;13:56–57. doi: 10.1111/j.1365-3156.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 90.Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., Battle K.E., Moyes C.L., Henry A., Eckhoff P.A., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hawley W.A., Phillips-Howard P.A., Ter Kuile F.O., Terlouw D.J., Vulule J.M., Ombok M., Nahlen B.L., Gimnig J.E., Kariuki S.K., Kolczak M.S., et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am. J. Trop. Med. Hyg. 2003;68:121–127. doi: 10.4269/ajtmh.2003.68.121. [DOI] [PubMed] [Google Scholar]
- 92.Killeen G.F., Smith T.A., Ferguson H.M., Mshinda H., Abdulla S., Lengeler C., Kachur S.P. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4:1246–1258. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hauser G., Thiévent K., Koella J.C. The ability of Anopheles gambiae mosquitoes to bite through a permethrin-treated net and the consequences for their fitness. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-44679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization Vaccines and vaccination against yellow fever WHO Position Paper—June Note de synthèse: Position de l’ OMS sur les vaccins et la vaccination contre la fièvre jaune, juin 2013. Relevé Épidémiologique Hebdomadaire. 2013;88:269–284. [PubMed] [Google Scholar]
- 95.Gotuzzo E., Yactayo S., Córdova E. Review article: Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013;89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.WHO Publication Vaccines against tick-borne encephalitis: WHO position paper—Recommendations. Vaccine. 2011;86:241–256. doi: 10.1016/j.vaccine.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Bogovic P. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases. 2015 doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.World Health Organization Japanese Encephalitis Vaccines: WHO position paper, February 2015—Recommendations. Vaccine. 2016;90:69–88. doi: 10.1016/j.vaccine.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 99.Hegde N.R., Gore M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccines Immunother. 2017;13:1320–1337. doi: 10.1080/21645515.2017.1285472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malaria Vaccine Pilot Launched in Malawi. [(accessed on 31 May 2020)]; Available online: https://www.who.int/news-room/detail/23-04-2019-malaria-vaccine-pilot-launched-in-malawi.
- 101.Jover J.A., Leon L., Pato E., Loza E., Rosales Z., Matias M.A., Mendez-Fernandez R., Díaz-Valle D., Benitez-del-Castillo J.M., Abasolo L. Long-term use of antimalarial drugs in rheumatic diseases. Clin. Exp. Rheumatol. 2012;30:380–387. [PubMed] [Google Scholar]
- 102.Schwartz E. Prophylaxis of Malaria. Mediterr. J. Hematol. Infect. Dis. 2012;4 doi: 10.4084/mjhid.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L.H., Wilson M.E., Schlagenhauf P. Prevention of malaria in long-term travelers. J. Am. Med. Assoc. 2006;296:2234–2244. doi: 10.1001/jama.296.18.2234. [DOI] [PubMed] [Google Scholar]
- 104.First FDA-Approved Vaccine for the Prevention of Dengue Diseases in Endemic Regions. [(accessed on 31 May 2020)]; Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-dengue-disease-endemic-regions#:~:text=The_U.S._Food_and_Drug,who_live_in_endemic_areas.
- 105.Da Silveira L.T.C., Tura B., Santos M. Systematic review of dengue vaccine efficacy. BMC Infect. Dis. 2019;19 doi: 10.1186/s12879-019-4369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du S., Liu Y., Liu J., Zhao J., Champagne C., Tong L., Zhang R., Zhang F., Qin C.F., Ma P., et al. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monteiro V.V.S., Navegantes-Lima K.C., De Lemos A.B., Da Silva G.L., De Souza Gomes R., Reis J.F., Junior L.C.R., Da Silva O.S., Romão P.R.T., Monteiro M.C. Aedes-chikungunya virus interaction: Key role of vector midguts microbiota and its saliva in the host infection. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soleimani-Ahmadi M., Vatandoost H., Zare M. Characterization of larval habitats for anopheline mosquitoes in a malarious area under elimination program in the southeast of Iran. Asian Pac. J. Trop. Biomed. 2014;4:73–80. doi: 10.12980/APJTB.4.2014C899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziraba A.K., Haregu T.N., Mberu B. A review and framework for understanding the potential impact of poor solid waste management on health in developing countries. Arch. Public Health. 2016;74 doi: 10.1186/s13690-016-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hori M., Shibuya K., Sato M., Saito Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 2014;4 doi: 10.1038/srep07383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puri A., Kumar M., Johal E. Solid-waste management in Jalandhar city and its impact on community health. Indian J. Occup. Environ. Med. 2008;12:76–81. doi: 10.4103/0019-5278.43265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Achudume A.C., Olawale J.T. Microbial pathogens of public health significance in waste dumps and common sites. J. Environ. Biol. 2007;28:151–154. [PubMed] [Google Scholar]
- 113.Bell J.L., Collins J.W., Wolf L., Gronqvist R., Chiou S., Chang W.R., Sorock G., Courtney T., Lombardi D., Evanoff B. Evaluation of a comprehensive slip, trip and fall prevention programme for hospital employees. Ergonomics. 2009;51:1905–1925. doi: 10.1080/00140130802248092. [DOI] [PubMed] [Google Scholar]
- 114.Weinhold B. A spreading concern: Inhalational health effects of mold. Environ. Health Perspect. 2007;115:300–305. doi: 10.1289/ehp.115-a300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mattiello A., Chiodini P., Bianco E., Forgione N., Flammia I., Gallo C., Pizzuti R., Panico S. Health effects associated with the disposal of solid waste in landfills and incinerators in populations living in surrounding areas: A systematic review. Int. J. Public Health. 2013;58:725–735. doi: 10.1007/s00038-013-0496-8. [DOI] [PubMed] [Google Scholar]
- 116.Ferronato N., Torretta V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health. 2019;16:1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abeyewickreme W., Wickremasinghe A.R., Karunatilake K., Sommerfeld J., Axel K. Community mobilization and household level waste management for dengue vector control in Gampaha district of Sri Lanka; an intervention study. Pathog. Glob. Health. 2012;106:479–487. doi: 10.1179/2047773212Y.0000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.SDG7: Data and Projections. [(accessed on 31 May 2020)]; Available online: https://www.iea.org/reports/sdg7-data-and-projections/access-to-electricity.
- 119.Ritchie S.A., Cortis G., Paton C., Townsend M., Shroyer D., Zborowski P., Hall-Mendelin S., Van Den Hurk A.F. A Simple Non-Powered Passive Trap for the Collection of Mosquitoes for Arbovirus Surveillance. J. Med. Entomol. 2013;50:185–194. doi: 10.1603/ME12112. [DOI] [PubMed] [Google Scholar]
- 120.Lu Y., Bei Y., Zhang J. Are Yellow Sticky Traps an Effective Method for Control of Sweetpotato Whitefly, Bemisia tabaci, in the Greenhouse or Field? J. Insect Sci. 2012;12 doi: 10.1673/031.012.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.García-Betancourt T., Higuera-Mendieta D.R., González-Uribe C., Cortés S., Quintero J. Understanding water storage practices of urban residents of an endemic dengue area in Colombia: Perceptions, rationale and socio-demographic characteristics. PLoS ONE. 2015;10:e0129054. doi: 10.1371/journal.pone.0129054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dambach P., Jorge M.M., Traoré I., Phalkey R., Sawadogo H., Zabré P., Kagoné M., Sié A., Sauerborn R., Becker N., et al. A qualitative study of community perception and acceptance of biological larviciding for malaria mosquito control in rural Burkina Faso. BMC Public Health. 2018;18 doi: 10.1186/s12889-018-5299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yukalang N., Clarke B., Ross K. Barriers to effective municipal solid waste management in a rapidly urbanizing area in Thailand. Int. J. Environ. Res. Public Health. 2017;14:1013. doi: 10.3390/ijerph14091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shockley Cruz M., Lindner R., Cruz M.S., Lindner R. Ph.D. Thesis. University of Georgia Department of Entomology; Athens, GA, USA: 2011. Insect Vision: Ultraviolet, Color, and LED Light. [Google Scholar]
- 125.Van Loon J.J.A., Smallegange R.C., Bukovinszkiné-Kiss G., Jacobs F., De Rijk M., Mukabana W.R., Verhulst N.O., Menger D.J., Takken W. Mosquito Attraction: Crucial Role of Carbon Dioxide in Formulation of a Five-Component Blend of Human-Derived Volatiles. J. Chem. Ecol. 2015;41:567–573. doi: 10.1007/s10886-015-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Hara J.E., UsUpensky I., Bostanian N.J., Capinera J.L., Chapman R., Barfield C.S., Swisher M.E., Barfield C.S., Heppner J., Fitzgerald T.D., et al. Encyclopedia of Entomology. Springer Science & Business Media; Berlin, Germany: 2008. Traps for Capturing Insects; pp. 3675–4007. [Google Scholar]
- 127.Zhou Y.H., Zhang Z.W., Fu Y.F., Zhang G.C., Yuan S. Carbon dioxide, odorants, heat and visible cues affect wild mosquito landing in open spaces. Front. Behav. Neurosci. 2018;12 doi: 10.3389/fnbeh.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorenzi O.D., Major C., Acevedo V., Perez-Padilla J., Rivera A., Biggerstaff B.J., Munoz-Jordan J., Waterman S., Barrera R., Sharp T.M. Reduced incidence of Chikungunya virus infection in communities with ongoing aedes aegypti mosquito trap intervention studies—Salinas and Guayama, Puerto Rico, November 2015–february 2016. Morb. Mortal. Wkly. Rep. 2016;65:479–480. doi: 10.15585/mmwr.mm6518e3. [DOI] [PubMed] [Google Scholar]
- 129.Junnila A., Kline D.L., Müller G.C. Comparative efficacy of small commercial traps for the capture of adult Phlebotomus papatasi. J. Vector Ecol. 2011;36:172–178. doi: 10.1111/j.1948-7134.2011.00128.x. [DOI] [PubMed] [Google Scholar]
- 130.Sliney D.H., Gilbert D.W., Lyon T. Ultraviolet safety assessments of insect light traps. J. Occup. Environ. Hyg. 2016;13:413–424. doi: 10.1080/15459624.2015.1125489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Urban J.E., Broce A. Killing of flies in electrocuting insect traps releases bacteria and viruses. Curr. Microbiol. 2000;41:267–270. doi: 10.1007/s002840010132. [DOI] [PubMed] [Google Scholar]
- 132.Getachew D., Tekie H., Gebre-Michael T., Balkew M., Mesfin A. Breeding sites of aedes aegypti: Potential dengue vectors in dire Dawa, east Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015;2015 doi: 10.1155/2015/706276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.UNICEF . Djibouti Humanitarian Situation Report No. 2 Flood Response. UNICEF; New York, NY, USA: 2019. pp. 1–5. [Google Scholar]
- 134.Krystosik A., Njoroge G., Odhiambo L., Forsyth J.E., Mutuku F., LaBeaud A.D. Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Front. Public Health. 2020;7 doi: 10.3389/fpubh.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Banerjee S., Aditya G., Saha G.K. Household wastes as larval habitats of dengue vectors: Comparison between urban and rural areas of Kolkata, India. PLoS ONE. 2015;10:e0138082. doi: 10.1371/journal.pone.0138082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Becher P.G., Hagman A., Verschut V., Chakraborty A., Rozpędowska E., Lebreton S., Bengtsson M., Flick G., Witzgall P., Piškur J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018;8:2962–2974. doi: 10.1002/ece3.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Billeter J.C., Wolfner M.F. Chemical Cues that Guide Female Reproduction in Drosophila melanogaster. J. Chem. Ecol. 2018;44:750–769. doi: 10.1007/s10886-018-0947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boadi K.O., Kuitunen M. Environmental and health impacts of household solid waste handling and disposal practices in Third World cities: The case of the Accra Metropolitan Area, Ghana. J. Environ. Health. 2005;68:32–36. [PubMed] [Google Scholar]
- 139.Barreiro C., Albano H., Silva J., Teixeira P. Role of Flies as Vectors of Foodborne Pathogens in Rural Areas. ISRN Microbiol. 2013;2013 doi: 10.1155/2013/718780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zamora D.M.B., Hernández M.M., Torres N., Zúniga C., Sosa W., De Abrego V., Escobar M.C.M. Information to act: Household characteristics are predictors of domestic infestation with the Chagas vector Triatoma dimidiata in central America. Am. J. Trop. Med. Hyg. 2015;93 doi: 10.4269/ajtmh.14-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jawara M., Jatta E., Bell D., Burkot T.R., Bradley J., Hunt V., Kandeh B., Jones C., Manjang A.M., Pinder M., et al. New prototype screened doors and windows for excluding mosquitoes from houses: A pilot study in rural Gambia. Am. J. Trop. Med. Hyg. 2018;99:1475–1484. doi: 10.4269/ajtmh.18-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Che-Mendoza A., Medina-Barreiro A., Koyoc-Cardeña E., Uc-Puc V., Contreras-Perera Y., Herrera-Bojórquez J., Dzul-Manzanilla F., Correa-Morales F., Ranson H., Lenhart A., et al. House screening with insecticide-treated netting provides sustained reductions in domestic populations of Aedes aegypti in Merida, Mexico. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tusting L.S., Ippolito M.M., Willey B.A., Kleinschmidt I., Dorsey G., Gosling R.D., Lindsay S.W. The evidence for improving housing to reduce malaria: A systematic review and meta-analysis. Malar. J. 2015;14 doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Massebo F., Lindtjørn B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: A randomized trial. Malar. J. 2013;12 doi: 10.1186/1475-2875-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Musoke D., Karani G., Ssempebwa J.C., Musoke M.B. Integrated approach to malaria prevention at household level in rural communities in Uganda: Experiences from a pilot project. Malar. J. 2018;18:1144–1156. doi: 10.1186/1475-2875-12-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matsui E.C. Management of rodent exposure and allergy in the pediatric population. Curr. Allergy Asthma Rep. 2013;13 doi: 10.1007/s11882-013-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hopkins A.S., Whitetail-Eagle J., Corneli A.L., Person B., Ettestad P.J., DiMenna M., Norstog J., Creswell J., Khan A.S., Olson J.G., et al. Experimental evaluation of rodent exclusion methods to reduce hantavirus transmission to residents in a Native American community in New Mexico. Vector Borne Zoonotic Dis. 2002;2:61–68. doi: 10.1089/153036602321131850. [DOI] [PubMed] [Google Scholar]
- 148.Jones C.H., Benítez-Valladares D., Guillermo-May G., Dzul-Manzanilla F., Che-Mendoza A., Barrera-Pérez M., Selem-Salas C., Chablé-Santos J., Sommerfeld J., Kroeger A., et al. Use and acceptance of long lasting insecticidal net screens for dengue prevention in Acapulco, Guerrero, Mexico. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bonner P.C., Schmidt W.P., Belmain S.R., Oshin B., Baglole D., Borchert M. Poor housing quality increases risk of rodent infestation and lassa fever in refugee camps of sierra leone. Am. J. Trop. Med. Hyg. 2007;77:169–175. doi: 10.4269/ajtmh.2007.77.169. [DOI] [PubMed] [Google Scholar]
- 150.Safan M.A., Etman Z.A., Konswa A. Evaluation of polyurethane resin injection for concrete leak repair. Case Stud. Constr. Mater. 2019;11 doi: 10.1016/j.cscm.2019.e00307. [DOI] [Google Scholar]
- 151.Tseloni A., Farrell G., Thompson R., Evans E., Tilley N. Domestic burglary drop and the security hypothesis. Crime Sci. 2017;6 doi: 10.1186/s40163-017-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carter A.D. Ph.D. Thesis. Georgia State University; Atlanta, GA, USA: 2014. Are Housing Improvements an Effective Supplemental Vector Control Strategy to Reduce Malaria Transmission? A Systematic Review. [Google Scholar]
- 153.Bublitz D.A.C., Poché R.M., Garlapati R. Measures to control Phlebotomus argentipes and visceral leishmaniasis in India. J. Arthropod. Borne. Dis. 2016;10:113–126. [PMC free article] [PubMed] [Google Scholar]
- 154.Kaindoa E.W., Finda M., Kiplagat J., Mkandawile G., Nyoni A., Coetzee M., Okumu F.O. Housing gaps, mosquitoes and public viewpoints: A mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar. J. 2018;17 doi: 10.1186/s12936-018-2450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ogoma S.B., Kannady K., Sikulu M., Chaki P.P., Govella N.J., Mukabana W.R., Killeen G.F. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar. J. 2009;8 doi: 10.1186/1475-2875-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ranjan A., Sur D., Singh V.P., Siddique N.A., Manna B., Lal C.S., Sinha P.K., Kishore K., Bhattacharya S.K. Risk factors for Indian kala-azar. Am. J. Trop. Med. Hyg. 2005;73:74–78. doi: 10.4269/ajtmh.2005.73.74. [DOI] [PubMed] [Google Scholar]
- 157.Chan E.Y.Y., Shaw R. Public Health Humanitarian Responses to Natural Disasters. Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 158.Ryan J. Environmental Health in Emergencies and Disasters: A Practical Guide. Emerg. Med. J. 2002;22:610. doi: 10.1136/emj.2003.011981. [DOI] [Google Scholar]
- 159.Laaser U., Dorey S., Nurse J. A plea for global health action bottom-up. Front. Public Health. 2016;4 doi: 10.3389/fpubh.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hou S.I. Health Education: Theoretical Concepts, Effective Strategies and Core Competencies. Volume 15. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 161.Naranjo D.P., Qualls W.A., Jurado H., Perez J.C., De Xue R., Gomez E., Beier J.C. Vector control programs in Saint Johns County, Florida and Guayas, Ecuador: Successes and barriers to integrated vector management. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Demanou M., Pouillot R., Grandadam M., Boisier P., Kamgang B., Hervé J.P., Rogier C., Rousset D., Paupy C. Evidence of Dengue Virus Transmission and Factors Associated with the Presence of Anti-Dengue Virus Antibodies in Humans in Three Major Towns in Cameroon. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poinsignon A., Boulanger D., Binetruy F., Elguero E., Darriet F., Gallian P., De Lamballerie X., Charrel R.N., Remoue F. Risk factors of exposure to Aedes albopictus bites in mainland France using an immunological biomarker. Epidemiol. Infect. 2019;147 doi: 10.1017/S0950268819001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reiter P., Lathrop S., Bunning M., Biggerstaff B., Singer D., Tiwari T., Baber L., Amador M., Thirion J., Hayes J., et al. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bloch D., Roth N.M., Caraballo E.V., Muñoz-Jordan J., Hunsperger E., Rivera A., Pérez-Padilla J., Rivera Garcia B., Sharp T.M. Use of Household Cluster Investigations to Identify Factors Associated with Chikungunya Virus Infection and Frequency of Case Reporting in Puerto Rico. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mathanga D.P., Mwandama D.A., Bauleni A., Chisaka J., Shah M.P., Landman K.Z., Lindblade K.A., Steinhardt L.C. The effectiveness of long-lasting, insecticide-treated nets in a setting of pyrethroid resistance: A case-control study among febrile children 6 to 59 months of age in Machinga District, Malawi. Malar. J. 2015;14 doi: 10.1186/s12936-015-0961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pryce J., Richardson M., Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 2019;11 doi: 10.1002/14651858.CD000363.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deletre E., Martin T., Duménil C., Chandre F. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasites Vectors. 2019;12 doi: 10.1186/s13071-019-3343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.WHO . Handbook for Integrated Vector Management. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 170.Wilson A.L., Boelaert M., Kleinschmidt I., Pinder M., Scott T.W., Tusting L.S., Lindsay S.W. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31:380–390. doi: 10.1016/j.pt.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 171.Corrin T., Waddell L., Greig J., Young I., Hierlihy C., Mascarenhas M. Risk perceptions, attitudes, and knowledge of chikungunya among the public and health professionals: A systematic review. Trop. Med. Health. 2017;45 doi: 10.1186/s41182-017-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Degroote S., Zinszer K., Ridde V. Interventions for vector-borne diseases focused on housing and hygiene in urban areas: A scoping review. Infect. Dis. Poverty. 2018;7 doi: 10.1186/s40249-018-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Giantsis I.A., Chaskopoulou A. Broadening the tools for studying sand fly breeding habitats: A novel molecular approach for the detection of phlebotomine larval DNA in soil substrates. Acta Trop. 2019;190:123–128. doi: 10.1016/j.actatropica.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 174.Moncaz A., Faiman R., Kirstein O., Warburg A. Breeding sites of Phlebotomus sergenti, the sand fly vector of cutaneous leishmaniasis in the Judean desert. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vivero R.J., Torres-Gutierrez C., Bejarano E.E., Peña H.C., Estrada L.G., Florez F., Ortega E., Aparicio Y., Muskus C.E. Study on natural breeding sites of sand flies (Diptera: Phlebotominae) in areas of Leishmania transmission in Colombia. Parasites Vectors. 2015;8 doi: 10.1186/s13071-015-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.World Health Organization . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]
- 177.Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020;1 doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Campbell-Lendrum D., Manga L., Bagayoko M., Sommerfeld J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2013.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.