Abstract
Streptococcus mutans is the principal biofilm forming oral pathogen associated with dental caries. Studies have shown that Candida albicans, a commensal oral fungus is capable of forming pathogenic mixed-species biofilms with S. mutans. The treatment of bacterial and fungal infections using conventional antimicrobial agents has become challenging due to the antimicrobial resistance of the biofilm mode of growth. The present study aimed to evaluate the efficacy of secretory components of Lactobacillus plantarum 108, a potentially promising probiotic strain, against S. mutans and C. albicans single and mixed-species biofilms. L. plantarum 108 supernatant inhibited S. mutans and C. albicans single-species biofilms as shown by XTT reduction assay, crystal violet assay, and colony forming units counting. The probiotic supernatant significantly inhibited the S. mutans and C. albicans mixed-species biofilm formation. The pre-formed mixed-species biofilms were also successfully reduced. Confocal microscopy showed poorly developed biofilm architecture in the probiotic supernatant treated biofilms. Moreover, the expression of S. mutans genes associated with glucosyltransferase activity and C. albicans hyphal specific genes (HWP1, ALS1 and ALS3) were down-regulated in the presence of the probiotic supernatant. Altogether, the data demonstrated the capacity of L. plantarum 108 supernatant to inhibit the S. mutans and C. albicans mixed-species biofilms. Herein, we provide a new insight on the potential of probiotic-based strategies to prevent bacterial-fungal mixed-species biofilms associated with dental caries.
Keywords: Streptococcus mutans, Lactobacillus plantarum, glucosyltransferase, dental caries, probiotics
1. Introduction
Early childhood caries (ECC), a major oral health problem worldwide is an aggressive form of dental caries that affects children under six years of age. According to World Health Organization, the prevalence of ECC is reported to be 17% among two-year old children, 48% among four-year old children and 70% among six-year old children [1]. ECC is related to prolonged intake of dietary sugars that basically comprises of sucrose [2]. Intake of sugary beverage through nipple bottles is very common in children. This increases the adverse effect of sucrose and limits buffering saliva to reach tooth surface [3,4]. Pathogenic bacteria form a biofilm on dental tissues and produce sugar driven acids. This dissolves the mineral structure of the teeth irreversibly, thereby causing dental caries [5]. If left untreated, ECC may lead to cavitation and subsequent pulpal infection causing severe pain which requires expensive treatment [6].
Streptococcus mutans, an acidogenic bacterium forming a virulent plaque biofilm on the tooth surface is considered a key pathogen associated with ECC [7,8]. S. mutans is able to rapidly utilize fermentable dietary sucrose and synthesize extracellular glucans through several exoenzymes, such as glucosyltransferases (Gtfs). This extracellular glucan enhances the bacterial adhesion to the tooth surface, as well as aid in bacterial co-aggregation leading to the development of highly virulent mixed-species biofilms in the oral cavity [9]. These complex biofilms facilitate the development of an acidogenic microenvironment, subsequently demineralising the tooth enamel [10].
Previous studies have demonstrated that S. mutans forms mixed-species biofilms with the human fungal pathogen Candida albicans in dental plaque [11,12]. S. mutans derived Gtfs bind firmly to the cell surface of C. albicans [13]. Adhesion between the foregoing microorganisms has been shown to be significantly enhanced in the presence of sucrose [14,15,16]. Gtfs bound to the C. albicans cell surface produce large amounts of glucans in the presence of sucrose. These glucans in turn increase the binding sites for S. mutans [13,17] resulting in a highly pathogenic mixed-species biofilm [15]. We demonstrated that S. mutans derived GtfB is able to augment the accumulation of C. albicans in mixed-species biofilms and enhance C. albicans growth by cross-feeding of glucose and fructose by sucrose breakdown [18,19]. Moreover, S. mutans was able to up-regulate the expression of C. albicans hypha associated genes such as HWP1, ALS1 and ALS3 which can be attributed to an increase in virulence of the organism in mixed-species biofilms. On the other hand, C. albicans also enhanced the development of S. mutans microcolonies in mixed-species biofilms [20]. Taken together, S. mutans and C. albicans demonstrate a symbiotic relationship in the mixed-species biofilm with complex inter-species interactions. Thus, a complex microenvironment such as the dental plaque displays resistance to most antimicrobials [21,22].
Use of probiotics as a strategy for controlling dental plaque biofilms has recently gained significant interest [23,24]. According to the 2001 definition by the World Health Organization, probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [25]. The oral cavity is a rich and diverse ecosystem inhabited by both bacteria and fungi, collectively occupying and co-existing within various niches as biofilm communities [26]. In the ecological perspective, dental caries is a result of dysbiosis of the oral flora and probiotics can be used for controlling microbial changes in the oral environment. However, when administration of live microorganisms may not be an ideal therapeutic option under certain conditions, application of their secretory components that demonstrate the ability to support the growth of healthy bacterial species and inhibit pathogenic species can be a promising strategy. As a result, focus has shifted towards testing the antimicrobial activity of secretory components from probiotic bacteria [27]. Probiotic bacteria are known to produce various antimicrobial compounds that can efficiently inhibit bacterial adhesion and disrupt biofilm formation [28]. Studies have demonstrated the promising anti-biofilm activity of probiotic-based strategies against S. mutans and C. albicans single-species biofilms [24,29,30,31]. The probiotic activity of Lactobacillus species such as Lactobacillus salivarius and Lactobacillus rhamnosus strains were evaluated against a S. mutans-C. albicans dual-species biofilms and a multi-species biofilm model, respectively [32,33]. The present study aimed to evaluate the efficacy of the secretory supernatant of a promising probiotic strain, Lactobacillus plantarum 108 (Lp108) against S. mutans and C. albicans mixed-species biofilms.
2. Results
2.1. Effect of Lactobacillus plantarum 108 Supernatant on Planktonic Streptococcus mutans and Candida albicans
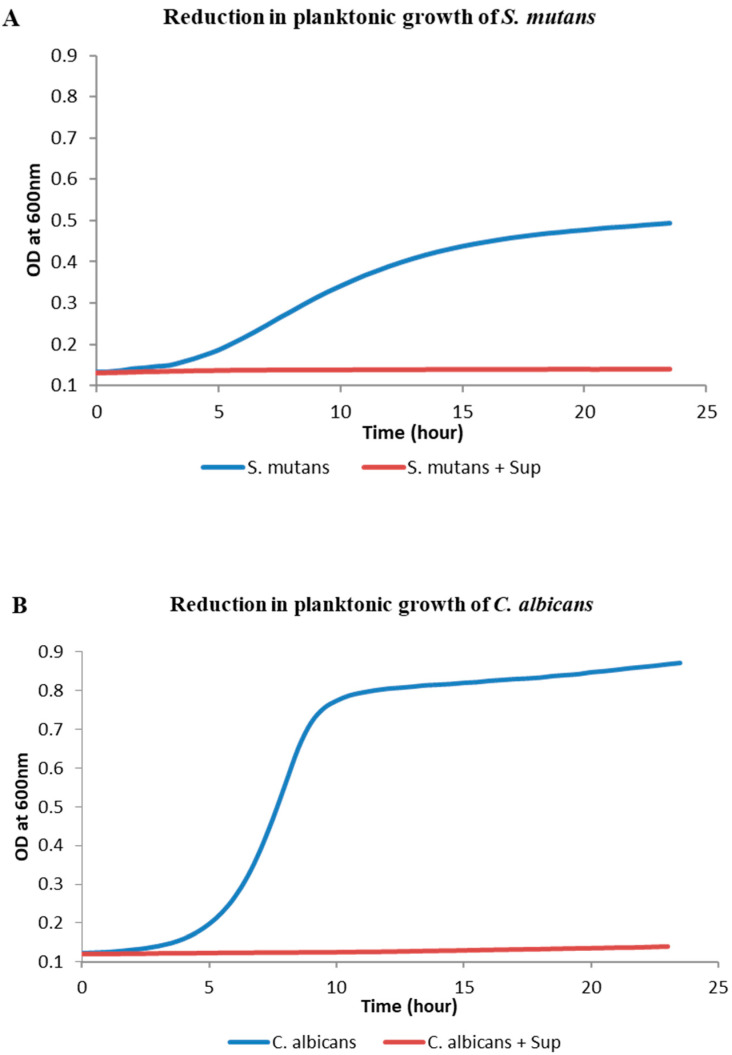
Growth kinetic assay showed that Lp108 supernatant has a significant inhibition on the planktonic mode of growth of both S. mutans and C. albicans compared to the control group without supernatant. In control groups, S. mutans and C. albicans cells showed an exponential growth around five hours after incubation, whereas much lesser growth was detected in the group treated with supernatant (Figure 1).
Figure 1.
Lactobacillus plantarum 108 supernatant (Sup) inhibited the planktonic growth of S. mutans and C. albicans. S. mutans (A) and C. albicans (B) growth evaluated using optical density measurements demonstrated a significant inhibition of growth when supplemented with the supernatant.
2.2. Effect of Lactobacillus Plantarum 108 Supernatant On Streptococcus Mutans Biofilm Formation
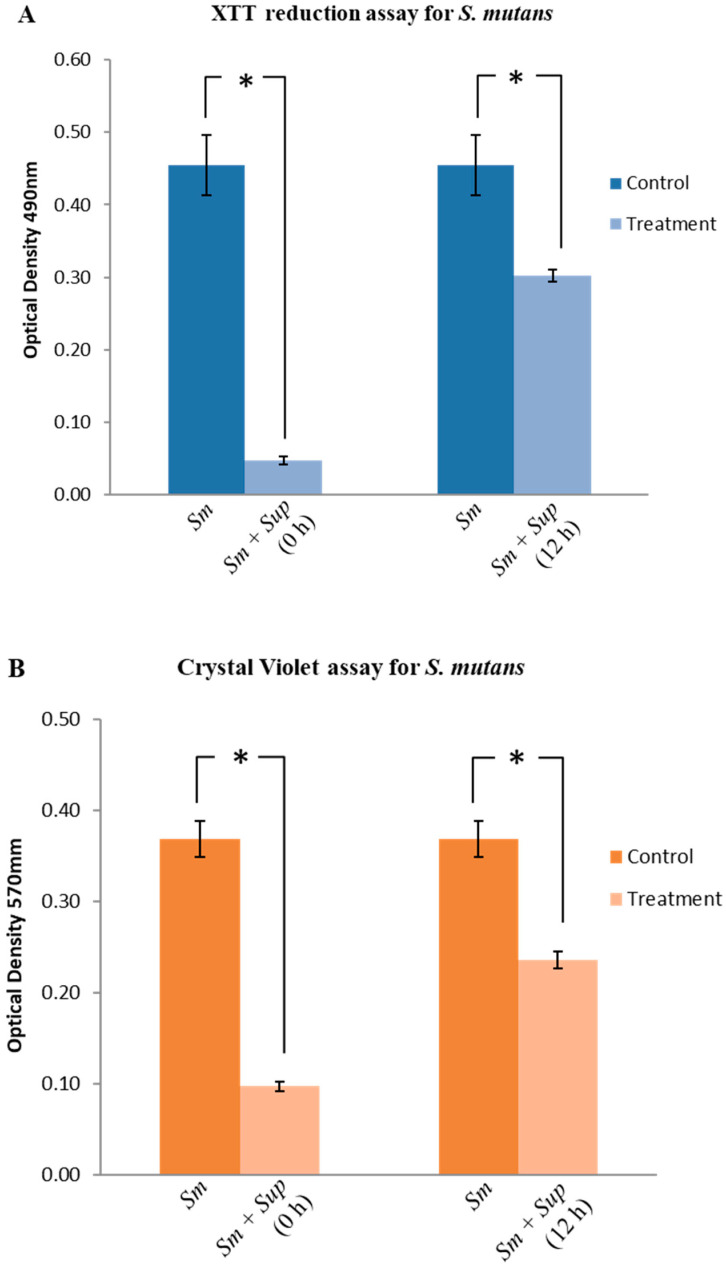
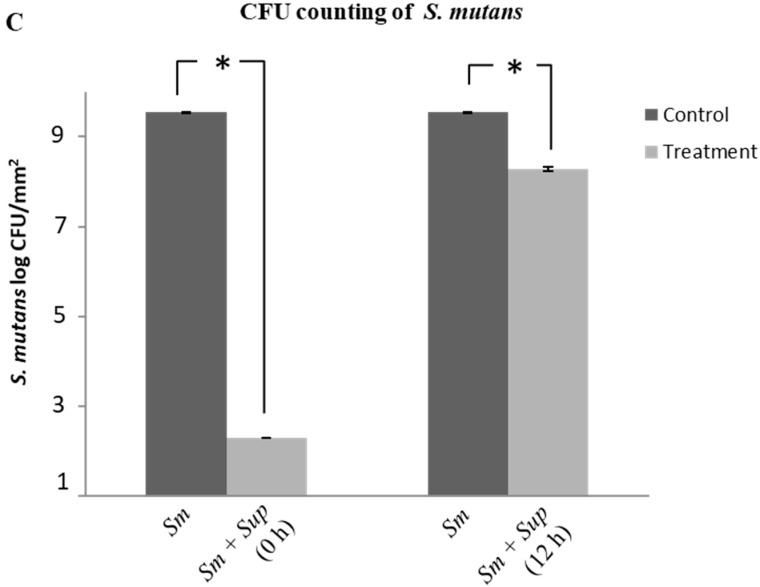
S. mutans was cultured in the presence and absence of the probiotic supernatant and biofilm formation was quantified using Tetrazolium salt 2,3 - bis (2 - methoxy - 4 – nitro - 5- sulfophenyl) -5- [(phenylamino) carbonyl]-2H- tetrazolium hydroxide (XTT) reduction assay [34], Crystal Violet (CV) assay [35], and Colony forming Unit (CFU) counting. When the supernatant was supplemented at the beginning of incubation (0 h), XTT assay showed a significant reduction (89%) in the activity of S. mutans in the presence of Lp108 supernatant compared to the control (Figure 2A; p < 0.05). Similarly, CV assay demonstrated that total biofilm biomass was reduced by 73% when treated with probiotic supernatant (Figure 2B; p <0.05). CFU counting demonstrated 99% reduction in cell numbers in the test group compared to the untreated control (Figure 2C; p < 0.05).
Figure 2.
Lactobacillus plantarum 108 supernatant (Sup) inhibited S. mutans (Sm) biofilm formation and demonstrated activity against preformed biofilms. Lp108 supernatant was introduced at two time points; 0 h for the preventive assay and at 12 h into a preformed biofilm for the therapeutic assay. Biofilms were quantified using (A) XTT reduction assay, (B) Crystal Violet assay and (C) Colony forming unit counting (CFU). Data are presented as mean ± SD and statistical significance indicated by the asterisk (*) was evaluated with respect to the control group without the Lp108 supernatant treatment (p < 0.05).
Activity of probiotic supernatant on pre-formed S. mutans biofilms was also evaluated. An inhibition of S. mutans biofilms by 33%, 36% and 94% was observed with XTT assay, CV assay and CFU assay respectively, following treatment with probiotic supernatant after 12 h of initial incubation (Figure 2; p < 0.05). These results suggest that the Lp108 supernatant was not only able to inhibit the initial colonization of S. mutans biofilm, but also eradicates the preformed biofilms.
2.3. Effect of Lactobacillus Plantarum 108 Supernatant on Candida Albicans Biofilm Formation
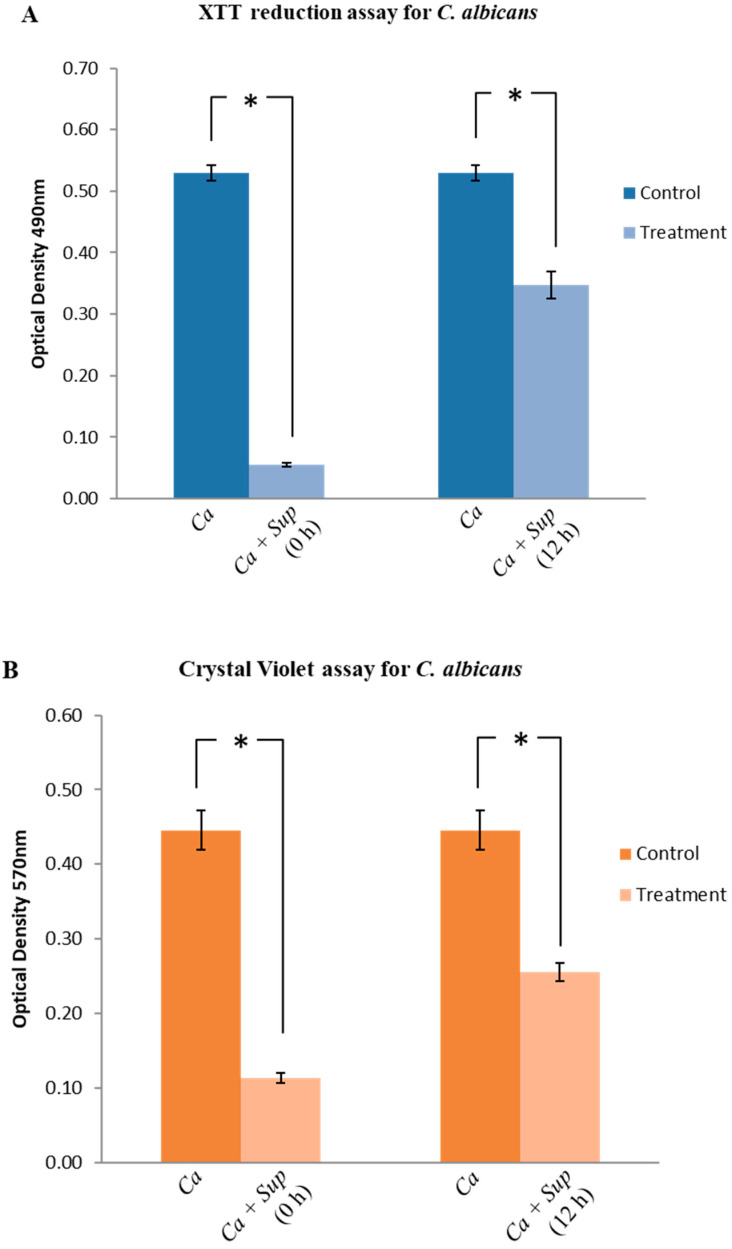
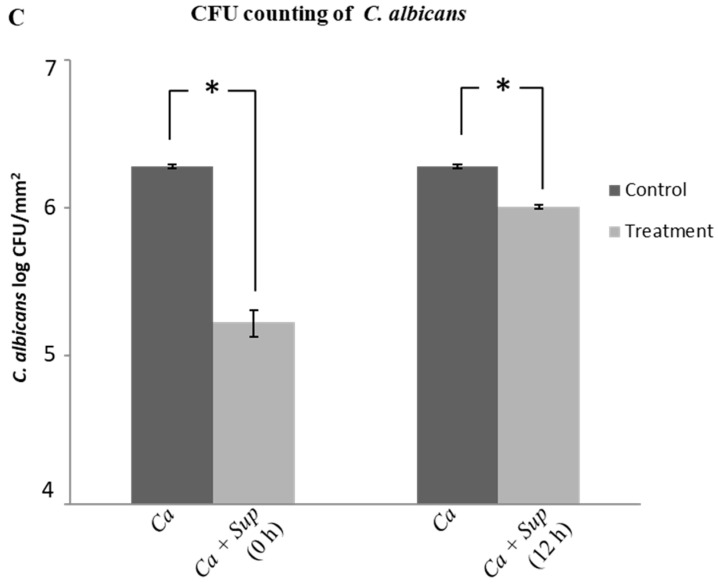
There was a significant inhibition of C. albicans biofilm formation when treated with Lp108 supernatant i.e., 90%, 80% and 91% inhibition in XTT assay, CV assay, and CFU counting, respectively (Figure 3; p < 0.05). Probiotic supernatant also effectively eradicated the preformed C. albicans biofilms. The XTT assay, CV assay and CFU counting demonstrated a reduction by 34%, 42% and 46 % respectively (Figure 3; p < 0.05).
Figure 3.
Lactobacillus plantarum 108 supernatant (Sup) inhibited C. albicans (Ca) biofilm formation and demonstrated therapeutic activity against preformed biofilms. (A) XTT reduction assay, (B) Crystal Violet assay, and (C) Colony forming unit counting (CFU) were used for the quantification of biofilms treated with the supernatant at 0 h for the preventive assay and at 12 h for the therapeutic assay. Data are presented as mean + SD and statistical significance (*) was evaluated with respect to the untreated group (p < 0.05).
2.4. Effect of Lactobacillus plantarum 108 Supernatant on Streptococcus mutans and Candida albicans Mixed-Species Biofilms
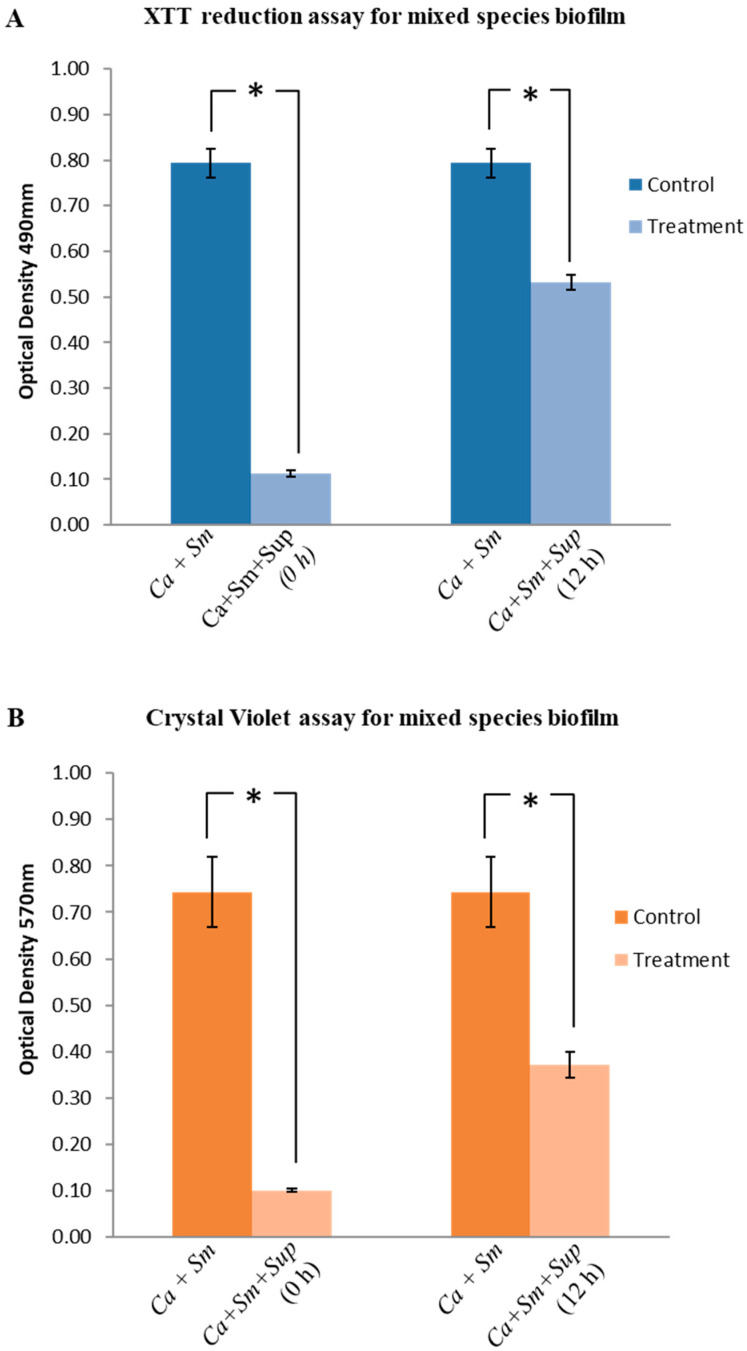
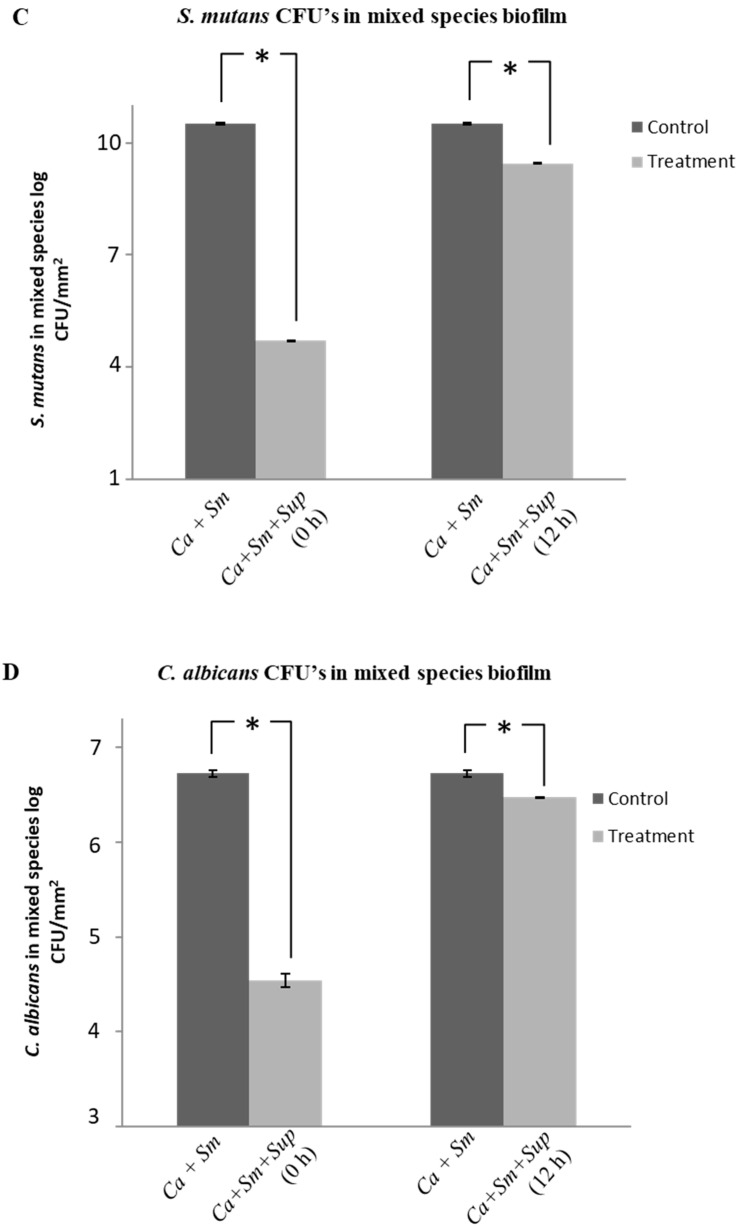
Interestingly, a significant inhibition was observed in mixed-species biofilm formation when the Lp108 supernatant was supplemented at the beginning of the incubation. The XTT reduction assay showed 85% reduction in the mixed-species biofilm (Figure 4A; p < 0.05) whereas CV assay also showed 86% reduction (Figure 4B; p < 0.05) in total biofilm biomass of the treated mixed-species biofilms. The CFU counting method showed a significant reduction in both S. mutans and C. albicans cells in the probiotic-treated biofilm samples compared to the control without supernatant. The probiotic supernatant was able to reduce the S. mutans and C. albicans cells by 99.99% and 99.34% respectively in the mixed-species biofilm (Figure 4C,D; p < 0.05).
Figure 4.
Lactobacillus plantarum 108 supernatant (Sup) inhibited S. mutans (Sm) and C. albicans (Ca) mixed species biofilms. The supernatant was introduced into the mixed-species biofilm at 0 h (preventive assay) and at 12 h (therapeutic assay). Biofilms were quantified using (A) XTT reduction assay, (B) Crystal Violet assay and Colony forming unit counting (CFU) for (C) S. mutans, and (D) C. albicans. Data are presented as mean ± SD and statistical significance (*) was evaluated with respect to the untreated group (p < 0.05).
Further, Lp108 supernatant was also observed to eradicate the preformed S. mutans and C. albicans mixed-species biofilms. The XTT assay, and CV assay showed a reduction in the preformed mixed-species biofilms by 33% and 50% respectively (Figure 4A,B; p < 0.05). In the presence of probiotic supernatant, cultivable S. mutans and C. albicans cells were reduced by 91.50% (Figure 4C; p < 0.05) and 43.68% (Figure 4D) respectively. Foregoing data demonstrate that Lp108 supernatant was effective against S. mutans and C. albicans mixed-species biofilms.
2.5. Structural Analysis of Biofilms by Confocal Laser Scanning Microscopy
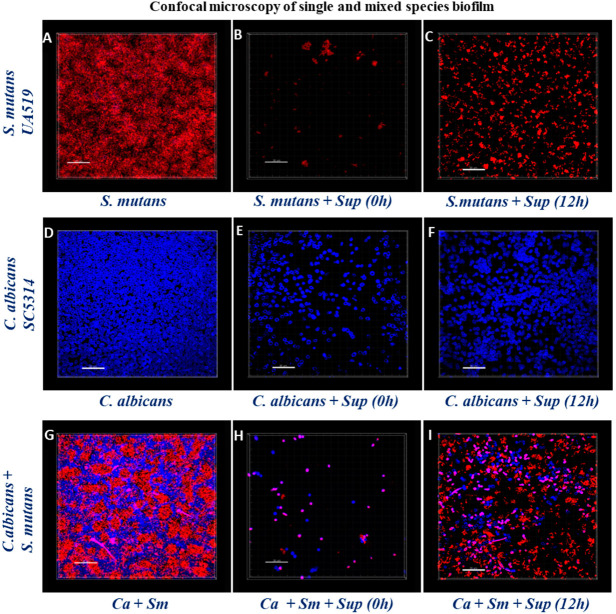
Confocal Laser Scanning Microscopy (CLSM) was used to further validate the efficacy of Lp108 supernatant against S. mutans and C. albicans single and mixed-species biofilms. CLSM images of the control groups showed a dense accumulation of S. mutans (Figure 5A) and C. albicans (Figure 5D) cells in the single-species biofilm showing the typical biofilm architectures. Closely aggregated S. mutans and C. albicans yeast cells clustering together with intermittent hyphal distribution was observed in the S. mutans-C. albicans mixed-species biofilm control (Figure 5G). On the contrary, supplementation of probiotic supernatant significantly inhibited the biofilm formation of S. mutans and C. albicans single and mixed-species biofilms (Figure 5B,E,H). However, preformed biofilms treated with the Lp108 supernatant comparatively had more cells than the biofilms of the inhibitory experiment assay (Figure 5C,F,I). CLSM observations were consistent with the data obtained from the biofilm quantification assays.
Figure 5.
CLSM examination of S. mutans (Sm) and C. albicans (Ca) single and mixed-species biofilm formation in the presence of Lactobacillus plantarum 108 supernatant (Sup). The biofilms were fixed and stained with calcofluor white for C. albicans (in blue) and propidium iodide for S. mutans (in red). Images A–C shows S. mutans single-species biofilms (A) control group, (B) treated with supernatant at 0 h, (C) preformed biofilm treated with the supernatant at 12 h. Images D–F shows C. albicans single-species biofilms (D) control group, (E) treated with supernatant at 0h, (F) preformed biofilm treated with the supernatant at 12 h. Images G–I shows S. mutans and C. albicans mixed-species biofilms (G) Control group, (H) treated with supernatant at 0 h, (I) preformed biofilm treated with the supernatant at 12 h.
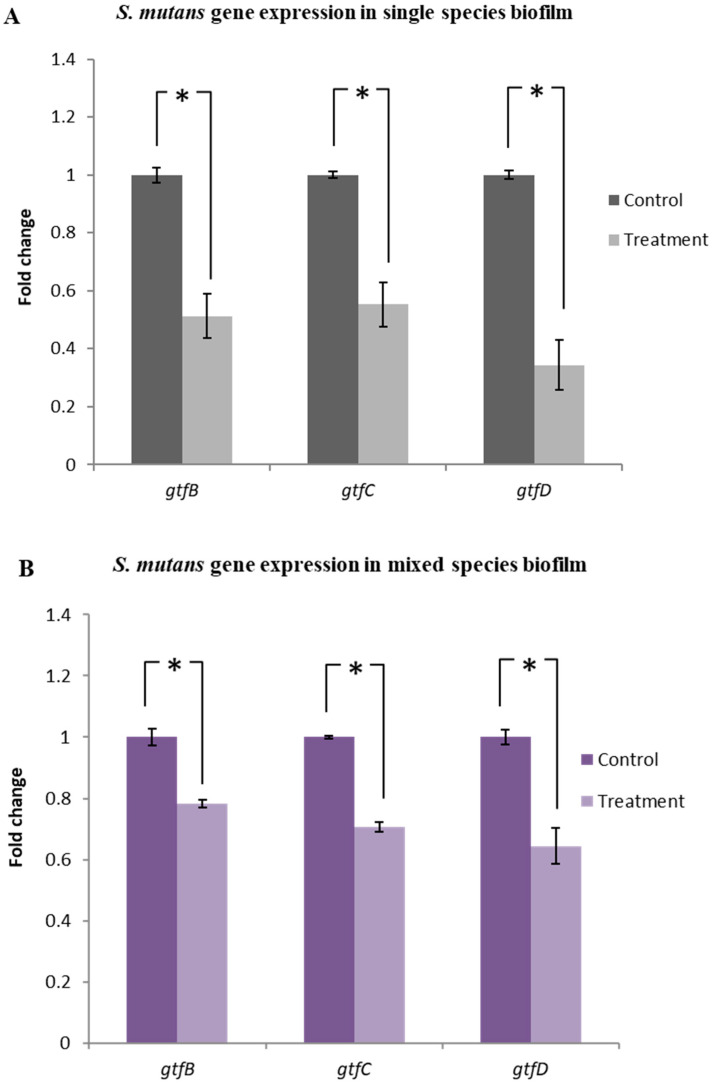
2.6. Lactobacillus plantarum 108 Supernatant Down-Regulated the Gtf Gene Expression in Streptococcus mutans Single and Mixed-Species Biofilms
qRT-PCR analysis of the single-species biofilm demonstrated that the Lp108 supernatant significantly down-regulated the expression of genes associated with Gtf activity of S. mutans. Expression of gtfB, gtfC, and gtfD was downregulated by 48.8%, 44.7%, and 65.7% respectively as compared to the untreated control groups (Figure 6A; p < 0.05). Mixed-species biofilms also showed downregulation in the expression of gtfB, gtfC, and gtfD by 21.8%, 29.3%, and 35.6%, respectively as compared to their corresponding untreated control group (Figure 6B; p < 0.05). Relative fold change compared to control and p values are summarized in Supplementary Table S1.
Figure 6.
Lactobacillus plantarum 108 supernatant down-regulated the gtf gene expression in Streptococcus mutans single and mixed-species biofilms. Quantitative PCR was performed to evaluate the expression of gtfB, gtfC, and gtfD genes in (A) single and (B) mixed species biofilms treated with Lp108 supernatant. Target genes in the sample were normalized with the S. mutans housekeeping gene 16sRNA. The graphs show the relative expression fold change of treated group with respect to the control group. Data represent the mean ± SD of three independent experiments and statistical significance (*) with respect to the control group (p < 0.05).
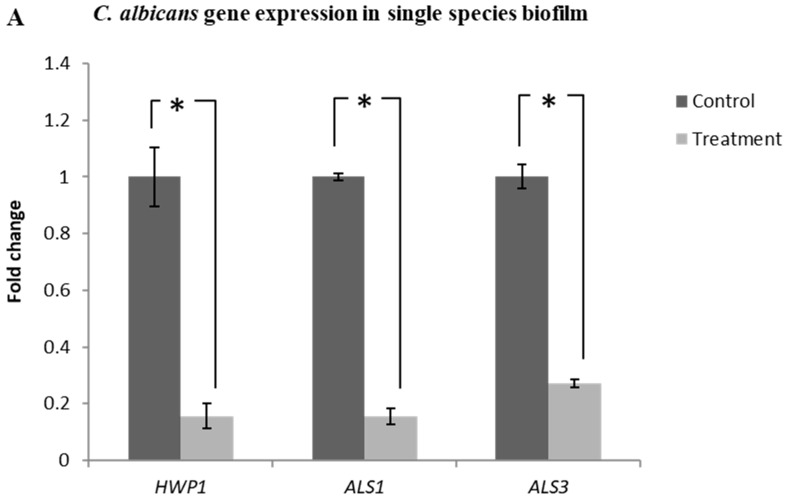
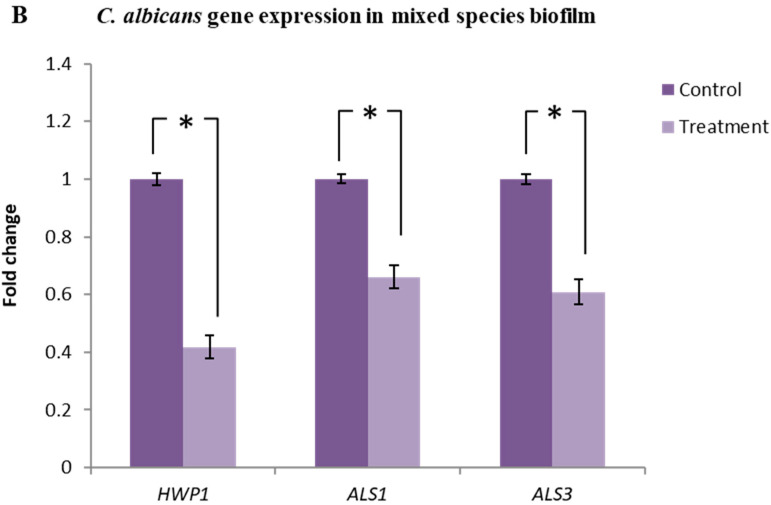
2.7. Lactobacillus plantarum 108 Supernatant Down-Regulated the Expression of HWP1, ALS1 and ALS3 Genes in Candida albicans Single and Mixed-Species Biofilms
The expression of HWP1, ALS1 and ALS3 genes in the single-species C. albicans biofilm treated with Lp108 supernatant was significantly down-regulated with respect to the untreated control groups. HWP1, ALS1 and ALS3 genes were down-regulated by 84.3%, 84.4% and 72.9% respectively (Figure 7A; p < 0.05).
Figure 7.
Lactobacillus plantarum 108 supernatant down-regulated the expression of HWP1, ALS1 and ALS3 genes in Candida albicans single and mixed-species biofilms. Quantitative PCR helped to evaluate the expression of HWP1, ALS1 and ALS3 genes in C. albicans (A) single and (B) mixed species biofilms treated with Lp108 supernatant. Target genes in samples were normalized with the C. albicans housekeeping gene PMA1.The graphs show the relative expression fold change of treated group with respect to the control group. In the presence of supernatant, expression of hyphal growth associated genes were significantly down-regulated in both single and mixed-species biofilms. Data represent the mean ± SD of three independent experiments and statistical significance (*) with respect to control group (p < 0.05).
Down-regulation of hyphal growth associated genes HWP1 (58.3%), ALS1 (33.9%) and ALS3 (39.1%) were also observed in the mixed-species biofilm group treated with probiotic supernatant (Figure 7B; p < 0.05). Relative fold change compared to control and p-values are summarized in supplementary Table S2. Gene expression results were found to be consistent with the inhibitory activity of probiotic supernatant shown by biofilm quantification assays and confocal imaging.
3. Discussion
Probiotic based strategies have shown promising results in inhibiting S. mutans and C. albicans biofilms. Herein we observed that cell-free supernatant from a new probiotic L. plantarum strain (Lp108) inhibits not only the initial colonization but also the preformed S. mutans and C. albicans single as well as mixed-species biofilms. Lp108 supernatant was most effective in inhibiting the early phase of biofilm formation and had comparatively reduced activity against the pre-formed biofilms. Numerous in-vitro studies have shown that different probiotic strains inhibit S. mutans [24,31,36,37] and C. albicans [29,38,39,40] single-species biofilm formation. Clinical studies have also shown the inhibitory capacity of various probiotics against S. mutans [41,42] and C. albicans [43,44,45] in patients suffering from oral health problems.
The antimicrobial effect of the probiotic supernatant could be attributed to the presence of a mixture of antimicrobial peptides and other antimicrobial compounds in the secretome of L. plantarum 108. Previous studies have attempted to purify secretory components from other L. plantarum strains. A secreted low molecular weight compound designated as plantaricin was isolated from a L. plantarum LR/14 strain and was found to have strong bactericidal characteristics, heat stability and tolerance to acids [46]. Subsequently, plantaricin was found to be proteinaceous in nature and exerted its antifungal activity through leakage of intra-cellular contents leading to cell death [47]. Hence, it is likely that the L. plantarum supernatant used in the present study may also possess molecules with similar properties; however other mechanisms of action cannot be ruled out. For example, the nature of the interaction of L. plantarum supernatant with biofilms might be physiochemical. It can be assumed that secretory component of L. plantarum supernatant might have modified the surface energies of the microorganisms and inhibited complex biofilm formation by preventing the microbial coaggregation [48].
Furthermore, as shown by CLSM images, Lp108 supernatant may also have molecules that inhibit the adhesion of S. mutans and C. albicans to solid surfaces. This can be attributed to the biosurfactants and exometabolites in the supernatant that account for reduction in the hydrophobicity of surface substratum by interfering with microbial adhesion and desorption processes [49]. Similar findings have been reported in other studies wherein biosurfactants reduce the microbial adhesion to solid surfaces [48,50,51,52].
Production of glucans from S. mutans is regarded as a crucial virulence factor in the pathogenesis of dental caries [9,53]. Interestingly, our findings revealed that Lp108 supernatant significantly down-regulated the expression of all three gtf genes i.e., gtfB, gtfC, and gtfD in S. mutans single and mixed-species biofilms. Regulatory mechanisms of genes encoding Gtf enzymes in S. mutans is complex and has not been fully elucidated. However, it can be assumed that the active components in the probiotic interfere with the Gtf enzymes production at the gene expression level. As a result, it reduces the S. mutans attachment and biofilm formation. There are also other studies that demonstrate the ability of probiotic strains to down regulate gtf genes in S. mutans [31,54,55,56].
ALS3 and ALS1 genes belong to the ALS family of adhesins which are generally over expressed during in vitro adhesion of C. albicans to the epithelial cells [57,58]. The HWP1 is known to encode the C. albicans protein which is responsible for the maintenance of cell wall integrity, hyphal development and intracellular signaling [57]. HWP1 and ALS3 mutants of C. albicans are defective in biofilm formation [59,60]. Recently, we demonstrated that S. mutans derived GtfB is able to up-regulate the expression of these genes of C. albicans in mixed-species biofilms [18]. Interestingly, in this study, we found that probiotic supernatant significantly down regulated the expression of HWP1, ALS1 and ALS3 of C. albicans in single and mixed-species biofilms. Our results corroborated previous studies that reported down regulation of these genes after treatment of C. albicans with probiotic strains [29,61]. Foregoing findings explain the inhibitory effect of Lp108 supernatant on S. mutans and C. albicans single and mixed-species biofilms.
In conclusion, the results of the present study indicate that Lp108 supernatant was able to inhibit the S. mutans and C. albicans single and mixed-species biofilms. Furthermore, structural analysis through confocal imaging provided evidence for the ability of secretory components of L. plantarum 108 to inhibit the development of biofilm architecture. Finally, through gene expression analysis we were able to demonstrate a significant down-regulation of the genes responsible for glucosyltransferase activity of S. mutans and also that of genes involved in yeast-hypha transition in C. albicans. However, further studies are required to decipher the exact antimicrobial compound/s of this probiotic supernatant that inhibits S. mutans and C. albicans. If proven feasible, probiotic-based anti-biofilm strategies will be highly useful to treat bacterial-fungal mixed-species biofilm infections, including ECC.
4. Materials and Methods
4.1. Microbial Strains and Culture Conditions
L. plantarum 108 strain was identified in a study which screened potential probiotic isolates from human oral cavities at the Tsurumi University School of Dental Medicine [37]. S. mutans UA159, originally isolated from a child with active caries and the fungal strain C. albicans SC5314, a clinic strain originally isolated from a patient with generalized Candida infection were revived from the archival collection stored at -80 °C in the School of Dentistry, National University of Singapore. Standard cell suspensions were prepared in ultra-filtered tryptone yeast extract medium (UFTYE) with 1% (w/v) glucose at pH 7 and pH 5.5 for S. mutans and C. albicans respectively, according to a previously established protocol [18]. Overnight cultures were centrifuged (4000× g, 10 min, 4 °C) and washed twice in phosphate-buffered saline (PBS). Subsequently optical densities (OD) of the S. mutans (OD 0.300) and C. albicans (OD 0.375) cell suspensions were adjusted at a wave length of 520 nm, using a spectrophotometer (UV-1700 Shimadzu, Kyoto, Japan).
4.2. Preparation of Lactobacillus plantarum 108 Supernatant
L. plantarum 108 cells were grown for 18 h in de Man, Rogosa and Sharpe (MRS) broth, prepared by adding 27.5 g of MRS (Sigma-Aldrich) to 500 mL of distilled water and the broth was sterilized by autoclaving. This overnight culture was centrifuged (4000× g, 4 °C, 10 min), washed twice and resuspended in PBS and the optical density of the standard cell suspension was adjusted (OD 0.5 at 600 nm) using a spectrophotometer (UV-1700 Shimadzu, Kyoto, Japan). For supernatant preparation, this standard cell suspension was diluted 1:100 in MRS broth and further incubated for 24 h at 37 °C. The bacterial culture was subjected to centrifugation (4000× g, 4 °C, 10 min), and subsequently the supernatant was filtered through a 0.22 µm pore size membrane (Surfactant-free cellulose acetate, Ministart syringe filter, Sartorius, Singapore). This standardized supernatant was used in all the experiments [37].
4.3. Antimicrobial Activity against Planktonic Streptococcous mutans and Candida albicans
Planktonic cells of S. mutans (2 × 106 CFU/mL) and C. albicans (1 × 106 CFU/mL) were incubated with UFTYE broth containing 2% (w/v) sucrose [15], in 96-well microtiter plates in the presence and absence of Lp108 supernatant. Subsequently, the plate was incubated for 24 h at 37 °C and the optical density was measured at a wavelength of 600 nm using a spectrophotometer (UV-1700 Shimadzu, Kyoto, Japan). The growth was measured at 30 min intervals for 24 h.
4.4. In Vitro Biofilm Formation and Treatment of Biofilms with Lactobacillus plantarum 108 Supernatant
For S. mutans and C. albicans single-species biofilm formation, a cell suspension containing a cell concentration of 1 × 106 CFU/mL yeast cells or 2 × 106 CFU/mL bacterial cells were inoculated in UFTYE broth containing 2% (w/v) sucrose. For mixed-species biofilm formation, equal volumes of 1 × 106 CFU/mL yeast cells and 2 × 106 CFU/mL bacterial cells with UFTYE broth containing 2% (w/v) sucrose were pipetted into each well of a microtiter plate according to a previously established protocol [18].
Two assays were conducted to examine the anti-biofilm activity of cell-free L. plantarum 108 supernatant. Firstly, Lp108 supernatant was introduced into the wells at the beginning (0 h) along with bacterial or fungal cell suspensions to evaluate the preventive ability of the supernatant against biofilms. For the therapeutic assay, respective S. mutans and C. albicans biofilms were formed for 12 h and subsequently the probiotic supernatant was added to these preformed biofilms. Similarly, for mixed-species biofilms probiotic supernatant was added at two different time points (0 h and 12 h).
4.5. Quantification of Biofilms
Biofilms were quantified using three different techniques, namely XTT reduction assay, crystal violet (CV) assay and colony forming units (CFU) counting. XTT assay quantifies the metabolic activity of the biofilms, CV assay estimates the total biomass of biofilms including extracellular matrix, whereas CFU assay indicates the number of cells in the biofilm.
4.5.1. XTT Reduction Assay
Biofilm formation was evaluated using tetrazolium salt XTT reduction assay as previously described [21]. In brief, after removing the culture media, biofilms were washed once with PBS to remove non-adherent cells. Subsequently, 200 μL of XTT solution (4 μM menadione and 0.2 mg/mL XTT in PBS) was added to each well and incubated in the dark for 20 min at 37 °C. Colorimetric changes were measured by using a plate reader at 490 nm (Multiskan™ GO, Thermo Scientific, Singapore).
4.5.2. Crystal Violet Assay
Crystal Violet (CV) assay was performed according to a previously published protocol with minor modifications [35]. In brief, biofilms were washed with PBS once, air dried and fixed in 2% formalin. Subsequently, biofilms were stained with 1% (w/v) of CV for 5 min. After washing, the plates were air dried and an aliquot of 200 μL of 95% ethanol was added and incubated for 15 min. Optical density of 95% ethanol was measured at 570 nm (Plate reader, Multiskan™ GO, Thermo Scientific, Singapore).
4.5.3. Colony-Forming Units Counting
A 10-fold dilution series of the cell suspensions from both single and mixed-species biofilms was prepared in PBS. Different dilutions of C. albicans and S. mutans were spread on glucose minimal medium (GMM) agar (6.79 g/l yeast nitrogen base without amino acids; Difco, 2% glucose and 1% agar; Sigma-Aldrich) and brain-heart infusion (BHI; Difco) agar plates, respectively. For mixed species, GMM and BHI plates were supplemented with 8 μg/mL gentamicin sulfate salt to prevent bacterial growth and 8 μg/mL of amphotericin B to prevent fungal growth. The BHI plates were incubated at 37 °C for 24 h and the GMM plates were incubated at 30 °C for 48 h. The bacterial and fungal colonies were counted and the corresponding log CFU values were calculated.
4.6. Confocal Laser Scanning Microscopy
Single and mixed-species biofilms were formed on 8-well chamber slides (Nunc, Thermo Scientific, Lab-Tek™, Singapore) and the test samples were treated with Lp108 supernatant. For confocal laser scanning microscopy (CLSM), biofilms were stained according to a previously described protocol [18]. In brief, biofilms were fixed with 4% (v/v) paraformaldehyde and stained with 200 μL of propidium iodide (Invitrogen, Thermo Fisher Scientific, Singapore) for S. mutans and 0.001% (w/v) calcofluor white (Sigma-Aldrich, Singapore) for C. albicans. The plates were incubated for 20 min in dark and thereafter washed with PBS. Biofilms were visualized using an Olympus-Fluoview FV1000 TIRF confocal microscope. Z-sections were obtained from representative microscopic fields of three biological replicates. The single and mixed-species biofilm architecture was analysed using Imaris software.
4.7. Gene Expression Analysis by qRT-PCR
The expression of genes associated with S. mutans Gtf enzyme activity such as gtfB, gtfC and gtfD [9] as well as the hyphal growth associated genes in C. albicans such as HWP1, ALS1 and ALS3 were evaluated using quantitative real-time PCR (qRT-PCR) [62]. Biofilm samples were prepared as described above. After incubation, cells from control and probiotic supernatant treated biofilms were harvested and the cell pellets were collected by centrifugation (10,000× g for 10 min). TRIzol reagent (Invitrogen Ambion, Singapore) was used to extract the total RNA from cell pellets according to a previously published protocol [63]. NanoDrop ND 1000 spectrophotometer (Thermo Scientific, Singapore) was used to determine the concentration, purity and quality of the isolated RNA by measuring the absorbance ratio at 260/280 nm and 260/230 nm. RNA was reverse transcribed into cDNA using the M-MLV Reverse Transcriptase system (Promega, Singapore). A list of primers used for amplifying the target and housekeeping genes used are given in the Table 1 and Table 2. Diluted cDNA, gene specific forward and reverse primers and SYBR Green (KAPA SYBR FAST qPCR Kit, Kapa Biosystems, Wilmington, MA, USA) were mixed in reaction mixture and qRT-PCR was performed using the Step One PlusTM Real-Time PCR system (Thermo Fisher Scientific, Singapore). This was done under adequate thermo cycling conditions (Holding stage 95 °C for 3min, cycling stage 95 °C for 3 s, 60 °C for 1 min and Melting curve stage 95 °C for 1 s, 60 °C for 1 min and 95 °C for 15 s) for 40 cycles. The resultant CT values of the target genes of interest were normalized to the CT values of the respective housekeeping gene (16sRNA for S. mutans and PMA1 for C. albicans). Results were analyzed using the relative expression method to calculate the fold changes [64].
Table 1.
Streptococcus mutans forward (F) and reverse (R) primers used for qRT-PCR [31].
| Primer Sequence Used for Streptococcus mutans | ||
|---|---|---|
| Gene | Primer Sequence (5’-3’) | |
| 16sRNA | F: | CCT ACG GGA GGC AGC AGT AG |
| R: | CAA CAG AGC TTT ACG ATC CGA AA | |
| gtfB | F: | AGC AAT GCA GCC AAT CTA CAA AT |
| R: | ACG AAC TTT GCC GTT ATT GTC A | |
| gtfC | F: | GGT TTA ACG TCA AAA TTA GCT GTA TTA GC |
| R: | CTC AAC CAA CCG CCA CTG TT | |
| gtfD | F: | ACA GCA GAC AGC AGC CAA GA |
| R: | ACT GGG TTT GCT GCG TTT G | |
Table 2.
Candida albicans forward (F) and reverse (R) primers used for qRT-PCR [65].
| Primer Sequence Used for Candida albicans | ||
|---|---|---|
| Gene | Primer Sequence (5’-3’) | |
| PMA1 | F: | TTGAAGATGACCACCCAATCC |
| R: | GAAACCTCTGGAAGCAAATTCG | |
| HWP1 | F: | GCTCAACTTATTGCTATCGCTTATTACA |
| R: | GACCGTCTACCTGTGGGACAGT | |
| ALS1 | F: | GAC TAG TGA ACC AAC AAA TAC CAG A |
| R: | CCA GAA GAA ACA GCA GGT GA | |
| ALS3 | F: | AATGGTCCTTATGAATCACCATCTACTA |
| R: | GAGTTTTCATCCATACTTGATTTCACAT | |
4.8. Statistical Analysis
All experiments were carried out in triplicate and on three different occasions. The obtained data was expressed as mean values with the corresponding standard deviations (SD). For pair wise comparison, Student’s t test or Mann-Whitney U test was performed on all data sets to compare treated groups with respect to control groups and p < 0.05 was considered statistically significant (*). SPSS software version 20.0 was used for the statistical analysis.
5. Conclusions
The present study demonstrated the ability of L. plantarum 108 supernatant to inhibit S. mutans and C. albicans single and mixed-species biofilm formation
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/8/478/s1, Table S1: Streptococcus mutans gtf gene expression after incubation with Lactobacillus plantarum supernatant; Table S2: Candida albicans hyphal gene expression after incubation with Lactobacillus plantarum supernatant.
Author Contributions
Research conceptualization, N.S. and C.J.S. Experiment design, N.S., K.E. and C.J.S. Data acquisition, N.S. Data analysis, N.S., K.E. and C.J.S. Data interpretation, N.S., K.E., C.J.S., L.Y.A.C. and T.O. Manuscript drafting, N.S. and C.J.S. Critical review and manuscript revision, N.V., K.E., C.J.S., L.Y.A.C. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by research grant NUHSRO/2014/079/STB/B2B FY14/06 and NMRC/CIRG/1455/2016 to CJS.
Conflicts of Interest
The authors declare that they have no conflict of interest concerning this article.
References
- 1.Rodriguez G., Ruiz B., Faleiros S., Vistoso A., Marro M.L., Sanchez J., Urzua I., Cabello R. Probiotic compared with standard milk for high-caries children: A cluster randomized trial. J. Dent. Res. 2016;95:402–407. doi: 10.1177/0022034515623935. [DOI] [PubMed] [Google Scholar]
- 2.Hallett K.B., O’Rourke P.K. Early childhood caries and infant feeding practice. Community Dent. Health. 2002;19:237–242. [PubMed] [Google Scholar]
- 3.Bowen W.H., Lawrence R.A. Comparison of the cariogenicity of cola, honey, cow milk, human milk, and sucrose. Pediatrics. 2005;116:921–926. doi: 10.1542/peds.2004-2462. [DOI] [PubMed] [Google Scholar]
- 4.Chestnutt I.G., Murdoch C., Robson K.F. Parents and carers’ choice of drinks for infants and toddlers, in areas of social and economic disadvantage. Community Dent. Health. 2003;20:139–145. [PubMed] [Google Scholar]
- 5.Nigel B.P., Domenick T.Z., Phil D.M., Kim E., Jane A.W., Francisco R.-G., Junji T., Svante T., Georgios T., Amid I. Dental caries. Nat. Rev. Dis. Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz R.J., Koo H., McDermott M.P., Whelehan M.T., Ragusa P., Kopycka-Kedzierawski D.T., Karp J.M., Billings R. Adjunctive chemotherapeutic suppression of Mutans Streptococci in the setting of severe early childhood caries: An exploratory study. J. Public Health Dent. 2009;69:163–167. doi: 10.1111/j.1752-7325.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi N., Nyvad B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 8.Socransky S.S., Haffajee A.D. Dental biofilms: Difficult therapeutic targets. Periodontology. 2000;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries. Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo H., Falsetta M.L., Klein M.I. The Exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013;92:1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Carvalho F.G., Silva D.S., Hebling J., Spolidorio L.C., Spolidorio D.M. Presence of Mutans Streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral. Biol. 2006;51:1024–1028. doi: 10.1016/j.archoralbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Raja M., Hannan A., Ali K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010;44:272–276. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 13.Gregoire S., Xiao J., Silva B.B., Gonzalez I., Agidi P.S., Klein M.I., Ambatipudi K.S., Rosalen P.L., Bauserman R., Waugh R.E., et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011;77:6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira-Cenci T., Deng D.M., Kraneveld E.A., Manders E.M., Cury A.A., Cate J.M., Crielaard W. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral. Biol. 2008;53:755–764. doi: 10.1016/j.archoralbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Falsetta M.L., Klein M.I., Colonne P.M., Scott-Anne K., Gregoire S., Pai C.H., Gonzalez-Begne M., Watson G., Krysan D.J., Bowen W.H., et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms In Vivo. Infect. Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metwalli K.H., Khan S.A., Krom B.P., Jabra-Rizk M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013;9:e1003616. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branting C., Sund M.L., Linder L.E. The influence of Streptococcus mutans on adhesion of Candida albicans to acrylic surfaces in vitro. Archs. Oral. Biol. 1989;34:347–353. doi: 10.1016/0003-9969(89)90108-8. [DOI] [PubMed] [Google Scholar]
- 18.Ellepola K., Liu Y., Cao T., Koo H., Seneviratne C.J. Bacterial GtfB Augments Candida albicans Accumulation in Cross-Kingdom Biofilms. J. Dent. Res. 2017;96:1129–1135. doi: 10.1177/0022034517714414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellepola K., Truong T., Liu Y., Lin Q., Lim T.K., Lee Y.M., Cao T., Koo H., Seneviratne C.J. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-Candida albicans mixed-species biofilms. Infect. Immun. 2019;87:e00339-19. doi: 10.1128/IAI.00339-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Sengupta A., Niepa T., Lee B., Weljie A., Freitas-Blanco V., Murata R., Stebe K., Lee D., Koo H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017;7:41332. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seneviratne C.J., Jin L., Samaranayake L.P. Biofilm lifestyle of Candida: A mini review. Oral. Dis. 2008;14:582–590. doi: 10.1111/j.1601-0825.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 22.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug. Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 23.Soderling E.M., Marttinen A.M., Haukioja A.L. Probiotic lactobacilli interfere with Streptococcus mutans biofilm formation In Vitro. Curr. Microbiol. 2011;62:618–622. doi: 10.1007/s00284-010-9752-9. [DOI] [PubMed] [Google Scholar]
- 24.Saha S., Tomaro-Duchesneau C., Rodes L., Malhotra M., Tabrizian M., Prakash S. Investigation of probiotic bacteria as dental caries and periodontal disease biotherapeutics. Benef. Microbes. 2014;5:447–460. doi: 10.3920/BM2014.0011. [DOI] [PubMed] [Google Scholar]
- 25.Ohshima T., Kojima Y., Seneviratne C.J., Maeda N. Therapeutic application of synbiotics, a fusion of probiotics and prebiotics, and biogenics as a new concept for oral Candida Infections: A Mini Review. Front. Microbiol. 2016;7:10. doi: 10.3389/fmicb.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell L.E., Millhouse E., Sherry L., Kean R., Malcolm J., Nile C.J., Ramage G. Polymicrobial Candida biofilms: Friends and foe in the oral cavity. FEMS Yeast Res. 2015:15. doi: 10.1093/femsyr/fov077. [DOI] [PubMed] [Google Scholar]
- 27.Bandara H.M., Matsubara V.H., Samaranayake L.P. Future therapies targeted towards eliminating Candida biofilms and associated infections. Expert. Rev. Anti. Infect. Ther. 2017;15:299–318. doi: 10.1080/14787210.2017.1268530. [DOI] [PubMed] [Google Scholar]
- 28.Ohshima T., Kojima Y., Seneviratne C.J., Maeda N. Microbial Biofilms Omics Biology, Antimicrobials and Clinical Implications. CRC Press; Boca Raton, FL, USA: 2017. Synbiotics, a fusion of probiotics and prebiotics, and biogenics against oral biofilm associated diseases; pp. 237–260. [Google Scholar]
- 29.James K.M., MacDonald K.W., Chanyi R.M., Cadieux P.A., Burton J.P. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J. Med. Microbiol. 2016;65:328–336. doi: 10.1099/jmm.0.000226. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara V.H., Bandara H.M., Ishikawa K.H., Mayer M.P., Samaranayake L.P. The role of probiotic bacteria in managing periodontal disease: A systematic review. Expert. Rev. Anti. Infect. Ther. 2016;14:643–655. doi: 10.1080/14787210.2016.1194198. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.H., Kim Y.J. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch. Microbiol. 2014;196:601–609. doi: 10.1007/s00203-014-0998-7. [DOI] [PubMed] [Google Scholar]
- 32.Krzyściak W., Kościelniak D., Papież M., Vyhouskaya P., Zagórska-Świeży K., Kołodziej I., Bystrowska B., Jurczak A. Effect of a Lactobacillus Salivarius Probiotic on a Double-Species Streptococcus Mutans and Candida Albicans Caries Biofilm. Nutrients. 2017;9:1242. doi: 10.3390/nu9111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Q., Stamatova I., Kainulainen V., Korpela R., Meurman J.H. Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol. 2016;16:149. doi: 10.1186/s12866-016-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seneviratne C.J., Zeng G., Truong T., Sze S., Wong W., Samaranayake L., Chan F.Y., Wang Y.-M., Wang H., Gao J., et al. New “haploid biofilm model” unravels IRA2 as a novel regulator of Candida albicans biofilm formation. Sci. Rep. 2015;5:12433. doi: 10.1038/srep12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Toole G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011;47:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang M.S., Oh J.S., Lee H.C., Lim H.S., Lee S.W., Yang K.H., Choi N.K., Kim S.M. Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J. Microbiol. 2011;49:193–199. doi: 10.1007/s12275-011-0252-9. [DOI] [PubMed] [Google Scholar]
- 37.Kojima Y., Ohshima T., Seneviratne C.J., Maeda N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral Biosci. 2016;58:27–32. doi: 10.1016/j.job.2015.08.004. [DOI] [Google Scholar]
- 38.Matsubara V.H., Wang Y., Bandara H.M., Mayer M.P., Samaranayake L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016;100:6415–6426. doi: 10.1007/s00253-016-7527-3. [DOI] [PubMed] [Google Scholar]
- 39.Kheradmand E., Rafii F., Yazdi M.H., Sepahi A.A. The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans. DARU. 2014;22:48. doi: 10.1186/2008-2231-22-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasslof P., Hedberg M., Twetman S., Stecksén-Blicks C. Growth inhibition of oral Mutans Streptococci and Candida by commercial probiotic Lactobacilli - an in vitro study. BMC. Oral. Health. 2010;10:18. doi: 10.1186/1472-6831-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashwin D., Ke V., Taranath M., Ramagoni N.K., Nara A., Sarpangala M. Effect of probiotic containing ice-cream on salivary Mutans Streptococci (SMS) levels in children of 6-12 years of age: A randomized controlled double blind study with six-months follow up. J. Clin. Diagn. Res. 2015;9:ZC06–ZC09. doi: 10.7860/JCDR/2015/10942.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toiviainen A., Jalasvuori H., Lahti E., Gursoy U., Salminen S., Fontana M., Flannagan S., Eckert G., Kokaras A., Paster B., et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary Mutans Streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral. Investig. 2015;19:77–83. doi: 10.1007/s00784-014-1221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraft-Bodi E., Jorgensen M.R., Keller M.K., Kragelund C., Twetman S. Effect of probiotic bacteria on oral Candida in frail elderly. J. Dent. Res. 2015;94:181S–186S. doi: 10.1177/0022034515595950. [DOI] [PubMed] [Google Scholar]
- 44.Sutula J., Coulthwaite L.A., Thomas L.V., Verran J. The effect of a commercial probiotic drink containing Lactobacillus casei strain Shirota on oral health in healthy dentate people. Microb. Ecol. Health Dis. 2013;24:21003. doi: 10.3402/mehd.v24i0.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendonca F.H., Santos S.S., Faria I.D., Goncalves-e-Silva C.R., Jorge A.O., Leão M.V. Effects of probiotic bacteria on Candida presence and IgA pnti-Candida in the oral oavity of elderly. Braz. Dental. J. 2012;23:534–538. doi: 10.1590/S0103-64402012000500011. [DOI] [PubMed] [Google Scholar]
- 46.Tiwari S.K., Srivastava S. Purification and characterization of plantaricin LR14: A novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl. Microbiol. Biotechnol. 2008;79:759–767. doi: 10.1007/s00253-008-1482-6. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A., Srivastava S. Anti-Candida activity of spent culture filtrate of Lactobacillus plantarum strain LR/14. J. Mycol. Med. 2013;24:25–34. doi: 10.1016/j.mycmed.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Ceresa C., Tessarolo F., Caola I., Nollo G., Cavallo M., Rinaldi M., Fracchia L. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus-derived biosurfactant. J. Appl. Microbiol. 2015;118:1116–1125. doi: 10.1111/jam.12760. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues L., Banat I.M., Teixeira J., Oliveira R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006;57:609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 50.Saravanakumari P., Mani K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 2010;101:8851–8854. doi: 10.1016/j.biortech.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 51.Fracchia L., Cavallo M., Allegrone G., Martinotti M.G. A Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers. Appl. Microbiol. Biotechnol. 2010;2:827–837. [Google Scholar]
- 52.Kuyukina M.S., Ivshina I.B., Korshunova I.O., Stukova G.I., Krivoruchko A.V. Diverse effects of a biosurfactant from Rhodococcus ruber IEGM 231 on the adhesion of resting and growing bacteria to polystyrene. AMB Express. 2016;6:14. doi: 10.1186/s13568-016-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattos-Graner R.O., Smith D.J., King W.F., Mayer M. Water-insoluble glucan synthesis by Mutans Streptococcal strains correlates with caries incidence in 12- to 30-month-old children. J. Dent. Res. 2000;79:1371–1377. doi: 10.1177/00220345000790060401. [DOI] [PubMed] [Google Scholar]
- 54.Salehi R., Savabi O., Kazemi M., Kamali S., Salehi A.R., Eslami G., Tahmourespour A. Effects of Lactobacillus reuteri-derived biosurfactant on the gene expression profile of essential adhesion genes (gtfB, gtfC and ftf) of Streptococcus mutans. Adv. Biomed. Res. 2014;3:169. doi: 10.4103/2277-9175.139134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savabi O., Kazemi M., Kamali S., Salehi A.R., Eslami G., Tahmourespour A., Salehi R. Effects of biosurfactant produced by Lactobacillus casei on gtfB, gtfC, and ftf gene expression level in S. mutans by real-time RT-PCR. Adv. Biomed. Res. 2014;3:231. doi: 10.4103/2277-9175.145729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tahmourespour A., Salehi R., Kermanshahi R.K., Eslami G. The anti-biofouling effect of Lactobacillus fermentum-derived biosurfactant against Streptococcus mutans. Biofouling. 2011;27:385–392. doi: 10.1080/08927014.2011.575458. [DOI] [PubMed] [Google Scholar]
- 57.Sudbery P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 58.Murciano C., Moyes D.L., Runglall M., Tobouti P., Islam A., Hoyer L.L., Naglik J.R. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS ONE. 2012;7:e33362. doi: 10.1371/journal.pone.0033362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nobile C.J., Nett J.E., Andes D.R., Mitchell A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell. 2006;5:1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nobile C.J., Andes D.R., Nett J.E., Smith F.J., Yue F., Phan Q.T., Edwards J.E., Filler S.G., Mitchell A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandara H.M., Cheung B.P., Watt R.M., Jin L.J., Samaranayake L.P. Secretory products of Escherichia coli biofilm modulate Candida biofilm formation and hyphal development. J. Investig. Clin. Dent. 2013;4:186–199. doi: 10.1111/jicd.12048. [DOI] [PubMed] [Google Scholar]
- 62.Finkel J.S., Mitchell A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011;9:109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rio D.C., Ares M., Hannon G.J., Nilsen T.W. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harb. Protoc. 2010;2010:5439. doi: 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 64.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 65.Theberge S., Semlali A., Alamri A., Leung K.P., Rouabhia M.C. albicans growth, transition, biofilm formation and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013;13:246. doi: 10.1186/1471-2180-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.