Abstract
PURPOSE
To examine the association between total body irradiation (TBI) and subsequent breast cancer in women treated with blood or marrow transplantation (BMT) for hematologic malignancies.
PATIENTS AND METHODS
Participants were drawn from the BMT Survivor Study (BMTSS), a retrospective cohort study that included patients who underwent transplantation between 1974 and 2014 and survived for ≥ 2 years after BMT. Patients with pre-BMT chest radiation or a history of breast cancer were excluded. Participants completed the BMTSS survey, which included details regarding breast cancer diagnosis. Subsequent breast cancer was confirmed by pathology report review or physician notes. Cox proportional hazards models assessed the association between TBI and subsequent breast cancer. Standardized incidence ratios were calculated to determine the excess risk of subsequent breast cancer compared with that in the general population.
RESULTS
A total of 1,464 female BMT survivors (allogeneic: n = 788; autologous: n = 676) participated, with a median follow-up of 9.3 years from BMT. TBI was used in 660 patients (46%). Thirty-seven women developed subsequent breast cancer (allogeneic: n = 19; autologous: n = 18). Multivariable analysis revealed that exposure to TBI was associated with an increased risk of subsequent breast cancer among allogeneic BMT survivors (hazard ratio [HR], 3.7 [95% CI, 1.2 to 11.8]; P = .03) and autologous BMT survivors (HR, 2.6 [95% CI, 1.0 to 6.8]; P = .048). Pre-BMT exposure to alkylating agents was associated with an increased risk of subsequent breast cancer among autologous BMT survivors (HR, 3.3 [95% CI, 1.0 to 9.0]; P = .05). Compared with that in the general population, exposure to TBI at age < 30 years was associated with a 4.4-fold higher risk of subsequent breast cancer in allogeneic BMT survivors and a 4.6-fold higher risk in autologous BMT survivors.
CONCLUSION
The association between TBI and subsequent breast cancer, especially among those exposed at a young age, as well as pre-BMT exposure to alkylating agents, should inform breast cancer screening for early detection.
INTRODUCTION
Steady improvements in survival have resulted in a growing number of blood or marrow transplantation (BMT) survivors; these survivors are uniquely vulnerable to long-term morbidity.1-5 Exposure to high-intensity therapeutic agents, some with carcinogenic potential, results in an excess risk of subsequent malignant neoplasms (SMNs) when compared with the general population.6,7 Among the SMNs, development of radiation-related breast cancer is particularly relevant because of the known benefit of early detection and the ability to tailor screening recommendations to specific populations.8 Although breast cancer developing in childhood cancer survivors treated conventionally with chest radiation is well described,7,9-14 the magnitude of risk of breast cancer among patients treated with total body irradiation (TBI) across all ages remains understudied. We addressed this gap by using the resources offered by the BMT Survivor Study (BMTSS). We also sought to identify the patient and treatment characteristics associated with an increased likelihood of developing subsequent breast cancer.
CONTEXT
Key Objectives
The objectives of this study were to quantify the risk of subsequent breast cancer after exposure to total body irradiation (TBI) conditioning in blood or marrow transplantation (BMT) recipients and to identify subgroups associated with increased risk.
Knowledge Generated
The use of TBI as part of BMT conditioning was associated with increased risk, particularly among women exposed to TBI at age < 30 years. Pre-BMT exposure to alkylating agents was also associated with subsequent breast cancer for women undergoing autologous BMT.
Relevance
The results of this study should be reviewed in the context of current breast cancer screening guidelines. Women who were exposed to TBI at age < 30 years and those who received alkylating agents before BMT should be strongly considered for enhanced breast cancer screening.
PATIENTS AND METHODS
Study Population
The BMTSS is a retrospective cohort study of patients undergoing BMT at 1 of 3 participating institutions (City of Hope [COH], University of Minnesota [UMN], or University of Alabama at Birmingham [UAB]). Eligibility for participation in the BMTSS between 1974 and 2014 included treatment of a life-threatening condition with BMT at any age and survival for ≥ 2 years after undergoing BMT. The BMTSS was approved by institutional review boards at participating sites, and participants provided informed consent. Eligible patients were asked to complete the BMTSS survey, which covered the following: diagnosis of chronic health conditions by a health care provider, relapse of primary cancer or development of SMNs, and age at diagnosis of these conditions; medication use; and sociodemographics (sex, race/ethnicity, education, employment, household income, and health insurance). Loss to follow-up was minimized using searches of public records (LexisNexis Accurint) for updated participant contact information. We restricted our analysis to female participants who were ≥ 18 years of age and alive at study participation; women with a history of breast cancer or thoracic radiation exposure before BMT were excluded.
Clinical and Treatment Information
Information regarding primary cancer diagnosis, transplantation preparative regimens (including dose of TBI), stem-cell source (bone marrow, cord blood, or peripheral blood stem cells), and donor type (autologous, allogeneic) was obtained from institutional databases. Pretransplantation therapeutic exposures (especially alkylating agent and anthracycline exposure) were abstracted from medical records.
All 3 participating institutions delivered TBI in accordance with the American Association for Medical Physics Task Group 29 Report 17.15 UMN preferred a lateral field technique with custom compensators to ensure that the lung received the prescribed dose. COH and UAB used an anteroposterior-posteroanterior beam arrangement, and partial transmission lung blocks (40% to 60% transmission) were routine when ablative doses were prescribed. Supplemental electron fields ensured prescription dose to the marrow containing portions of the chest wall when lung blocks were used. In all cases, the chest wall and breast tissue were intended to receive the prescription dose.
Identification and Confirmation of Breast Cancer
Breast cancers, inclusive of ductal carcinoma in situ, were identified from the completed BMTSS survey, in response to the question, “At any time after your BMT, were you diagnosed with another cancer? [yes/no]”; if yes, then “what was the name of this illness?”; “when was it diagnosed?”; “where was this diagnosed?” Occurrences of breast cancer were confirmed by pathology reports when available or, alternatively, by documentation in the medical records as part of physician notes. Only the first primary breast cancer diagnosed after BMT was included in our analysis.
Analysis of Breast Cancer Incidence in BMTSS Cohort
Breast cancer cumulative incidence was calculated as a function of attained age using the Kaplan-Meier method, given that the cohort included only those alive at study participation. The effect of demographic and treatment variables on the hazard of breast cancer development was assessed using Cox proportional hazards models. Time at risk was calculated from the date of birth to the date of diagnosis of breast cancer or the date of last follow-up, whichever occurred first. Exposure to TBI was treated as a time-dependent covariate.16 Univariable models were assessed first, and variables with P < .1 were selected for inclusion in a multivariable model. Variables examined in the univariable models included age at BMT, race/ethnicity, primary cancer diagnosis for which BMT was performed, stem-cell source, use of TBI (yes/no), pre-BMT exposure to anthracyclines and alkylators, and factors known to be associated with sporadic breast cancer (age at menarche, age at first childbirth, age at menopause, and use of supplemental estrogens). All analyses were stratified by type of BMT (allogeneic or autologous) because of the significant differences in disease characteristics and types of therapeutic exposures.
Comparison with Age-Matched Population
To estimate the risk of breast cancer when compared with that of an age-matched general population, the number of person-years at risk was calculated for the cohort. Annual age-specific breast cancer incidence rates obtained from the SEER program of the National Institutes of Health were used to calculate the expected number of cases.17 The standardized incidence ratio (SIR) was calculated as the ratio of observed to expected cases. The exact 95% CIs were estimated by assuming Poisson distributions for SIRs in which the offset is the number of total person-years and the outcome is the number of breast cancers.18
Sensitivity Analyses
The primary analysis in this study was restricted to patients who were alive at the time of the BMTSS survey. To assess the robustness of the association between TBI and subsequent breast cancer, we repeated the analysis examining the association between TBI and subsequent breast cancer in a combined cohort that also included women who were deceased at the time of the BMTSS survey. The deceased cohort consisted of women who had died before January 1, 2019, but who otherwise met the inclusion criteria for this study and for whom cause of death information was available through linkage of the cohort with the National Death Index (NDI; Fig 1). Any mention of breast cancer on the death record was considered a breast cancer case, regardless of the proximate cause of death. The cumulative incidence of breast cancer occurrence or death in the combined cohort (alive and deceased) was calculated using competing risks methods, treating death as a result of other causes as a competing risk.
FIG 1.
Flowchart of cohort derivation. BMT, blood or marrow transplantation.
RESULTS
A total of 4,270 female patients had undergone BMT at COH, UMN, or UAB between 1974 and 2014, had survived for ≥ 2 years, and were ≥ 18 years of age at consideration for study participation (n = 3,032) or at death (n = 1,238). Among the 3,032 potentially eligible living patients, 180 were deemed ineligible, resulting in 2,852 patients who were alive when approached for BMTSS participation. Of these, 1,587 (55.6%) consented to participate; 123 women who consented were excluded because they had a history of breast cancer before BMT (n = 23), chest radiation before BMT (n = 86), or a mastectomy before BMT (n = 14), yielding a final study cohort of 1,464 women (Fig 1). Nonparticipants were more likely to have undergone autologous BMT (50.4% v 45.4%), were younger at BMT (mean age, 40.3 years v 42.2 years), and were less likely to be non-Hispanic white (48.6% v 64.1%; Appendix Table A1, online only).
The median length of follow-up for the 1,464 living study participants was 9.3 years (range, 2.0-39.9 years) from BMT, and the median age at study participation was 58 years (range, 18-89 years). The most common diagnoses were acute myeloid leukemia or myelodysplastic syndrome (27.5%), non-Hodgkin lymphoma (23.3%), plasma cell dyscrasias (18%), and chronic myelogenous leukemia (10.3%). TBI was used as part of the conditioning regimen for 45.5% of patients. Overall, 53.8% of the study participants received an allogeneic BMT. A comprehensive description of cohort characteristics, stratified by BMT type, is presented in Table 1.
TABLE 1.
Host and Clinical Characteristics of the Patient Cohort
Thirty-seven women developed breast cancer at a median of 9.1 years (range, 0.6-27.2 years) after BMT; 19 in allogeneic BMT recipients and 18 in autologous BMT recipients. The median age at breast cancer diagnosis was 53.9 years (range, 37.6-74.8 years). The estimated 10-year cumulative probability of developing breast cancer was 1.1% (standard deviation [SD], 0.4%) after allogeneic BMT and 2.6% (SD, 0.8%) after autologous BMT (P = .09). Overall, the estimated cumulative probability of developing breast cancer in the entire cohort was 2.7% by age 60 years and 5.2% by age 70 years (Fig 2A). By age 60 years, the cumulative probability of breast cancer approached 3.1% for allogeneic BMT recipients and 2.2% for autologous BMT recipients (Fig 2B).
FIG 2.

(A) Cumulative probability of breast cancer as a function of attained age for the entire cohort. (B) Cumulative probability of breast cancer as a function of attained age and stratified by type of blood or marrow transplantation. Allo, allogeneic; Auto, autologous; CIF, Cumulative Incidence Function.
The cumulative probability of breast cancer for the entire cohort was significantly higher (P = .002) among those exposed to TBI versus those not exposed (Fig 3A: 2.6% v 0.3% by age 50 years, 4.5% v 1.6% by age 60 years, and 8.8% v 3.6% by age 70 years). The cumulative probability of breast cancer among those exposed to TBI versus those not exposed did not achieve statistical significance (P = .1) among autologous BMT recipients (Fig 3B) but was significantly higher in the allogeneic BMT recipients (P = .009; Fig 3C).
FIG 3.

Cumulative probability of breast cancer as a function of attained age, stratified by exposure to total body irradiation (TBI) for (A) the entire cohort, (B) autologous blood or marrow transplantation (BMT) recipients, (C) allogeneic BMT recipients, and (D) women who were exposed to TBI at age younger than 30 years. CIF, Cumulative Incidence Function.
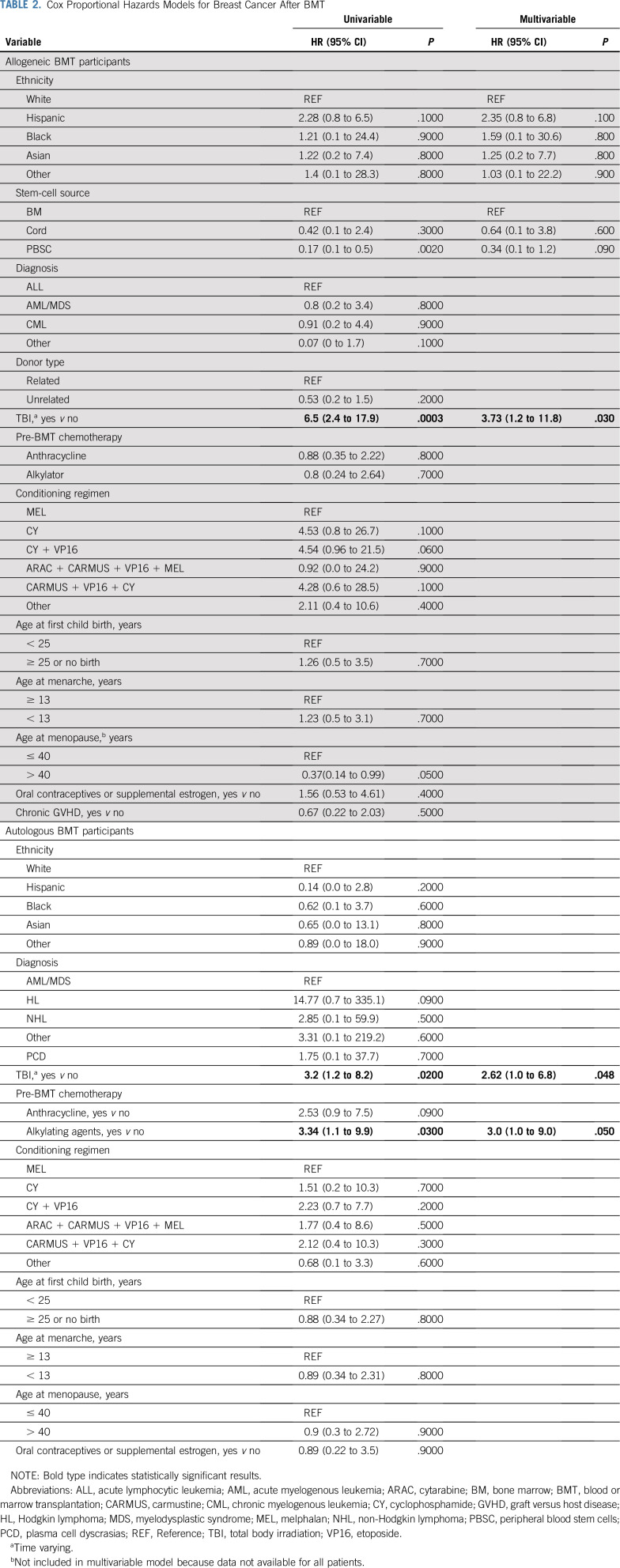
The results of the univariable and multivariable Cox proportional hazards models assessing patient and treatment variables associated with breast cancer risk are presented in Table 2. Exposure to TBI was associated with an increased hazard of breast cancer among both the allogeneic (hazard ratio [HR], 3.73 [95% CI, 1.2 to 11.8]) and autologous (HR, 2.6 [95% CI, 1.0 to 6.8]) BMT recipients. No association between TBI dose and subsequent breast cancer was observed (Appendix Table A2, online only); however, these results should be interpreted with caution because few patients received doses outside of the narrow range of 12-13.2 Gy. Pre-BMT exposure to alkylating agents and anthracyclines was associated with an increased risk of subsequent breast cancer among autologous BMT survivors (alkylators: HR, 3.0 [95% CI, 1.0 to 9.0]; anthracyclines: HR, 2.5 [95% CI, 0.9 to 7.5]), but not among allogeneic BMT recipients (alkylators: HR, 0.8 [95% CI, 0.2 to 2.6]; anthracyclines: HR, 0.9 [95% CI, 0.4 to 2.2]).
TABLE 2.
Cox Proportional Hazards Models for Breast Cancer After BMT
Compared with that of an age-matched general population, the SIR of breast cancer for all study participants was 1.3 (95% CI, 0.9 to 1.7). The SIRs for breast cancer in subgroups of interest, defined by age and exposure status, are presented in Table 3. Patients who received TBI had a 1.5-fold higher risk of developing breast cancer when compared with an age-matched general population (95% CI, 1.0 to 2.3). Conversely, the risk was not elevated for those who did not receive TBI (SIR, 1.0 [95% CI, 0.6 to 1.6]). The largest excess risk of subsequent breast cancer was among BMT recipients who received TBI at age < 30 years (SIR, 4.5 [95% CI, 2 to 8.3]). The excess risk of breast cancer for TBI at age < 30 years was observed for patients who received allogeneic BMT (SIR, 4.4 [95% CI, 1.8 to 9]) as well as autologous BMT (SIR, 4.6 [95% CI, 1.1 to 18.2]). The cumulative probability of developing subsequent breast cancer among those treated with TBI before age 30 years was 1.0% by age 40 years and 13.9% by age 50 years (Fig 3D).
TABLE 3.
SIRS for Breast Cancer
A total of 1,238 women in the study were deceased at the time of the BMTSS survey (after having survived for at least the first 2 years after BMT) but otherwise met the study inclusion criteria and had cause of death information available from the NDI. The median time from BMT to death was 4.6 years, and breast cancer was noted on the death certificate for 18 women. When the cumulative incidence of subsequent breast cancer was calculated for the entire cohort (alive and deceased), exposure to TBI remained associated with higher incidence/mortality because of subsequent breast cancer (P = .02; Appendix Fig A1, online only).
DISCUSSION
We undertook this study to expand our understanding of the association between exposure to TBI and the risk of subsequent breast cancer and to identify subgroups that were at highest risk. Subsequent breast cancer is particularly important to consider among long-term BMT survivors because of the proven efficacy of screening programs and the success of early intervention in other populations.19-21 The 10-year risk of breast cancer among women after allogeneic and autologous BMT was modest. However, those exposed to TBI were at a higher risk of developing breast cancer among both allogeneic and autologous BMT recipients and especially when the exposure occurred at age younger than 30 years. Women who received TBI were at a 1.5-fold higher risk of breast cancer when compared with the general population overall and at a 4.5-fold higher risk when the exposure occurred at age 30 years or younger. We also observed that autologous BMT recipients exposed to alkylating agents and anthracyclines as part of pre-BMT treatment had an increased risk of subsequent breast cancer when compared with women who were not exposed.
The Fred Hutchinson Cancer Research Center (FHCRC) in collaboration with the European BMT (EBMT) Registry described the risk of subsequent breast cancer in allogeneic BMT recipients.22 The 10-year cumulative incidence of breast cancer was 1.0% in the FHCRC cohort and 0.6% in the EBMT cohort and was comparable to our cohort of allogeneic BMT recipients (1.1% at 10 years). Our allogeneic BMT recipients were at a 1.3-fold higher risk of subsequent breast cancer when compared with the general population; these findings are also similar to those observed by the FHCRC/EBMT study, in which the allogeneic BMT recipients were at a 1.4-fold higher risk. The FHCRC/EBMT study found a higher incidence of breast cancer among allogeneic BMT recipients exposed to TBI (17% at 25 years from BMT) when compared with those not exposed (3%). Furthermore, that study found that the use of TBI was associated with a 4-fold higher risk of breast cancer when compared with those not exposed to TBI. The current study confirms the higher risk of breast cancer in allogeneic BMT recipients after TBI-based conditioning in multivariable regression models, in which we found a 3.7-fold higher risk when compared with those not exposed.
However, we extend the findings from the FHCRC/EBMT study to describe the risk of subsequent breast cancer among autologous BMT recipients and also examine the association with pre-BMT chemotherapeutic exposures, as well as other factors associated with increased risk in patients with de novo breast cancer. The cumulative probability of breast cancer was higher among autologous BMT recipients in the BMTSS (2.6% at 10 years after BMT), likely because of the older age of the women who underwent autologous transplantation when compared with allogeneic BMT recipients. The risk of breast cancer among autologous BMT recipients was 2.6-fold higher among those exposed to TBI after adjusting for all known relevant risk factors, and those exposed to TBI at age younger than 30 years were at the highest risk.
To our knowledge, this is the first report of an increased risk of subsequent breast cancer after TBI among female autologous BMT survivors. The observation that exposure to alkylating agents and anthracyclines is associated with an increased risk of subsequent breast cancer has been noted in long-term outcomes studies of childhood cancer survivors23,24; however, to our knowledge, this is the first report of this association among BMT survivors. That an association between pre-BMT alkylating agents/anthracyclines and subsequent breast cancer was observed only within the autologous BMT group is likely because of the higher doses used for the diseases requiring autologous BMT. In this study, we were unable to demonstrate an association between patient characteristics associated with increased risk of de novo breast cancer, such as age at menarche or menopause, and use of supplemental estrogens.
In the current report describing the risk of breast cancer after TBI exposure among allogeneic and autologous BMT recipients, we were intentional in excluding patients who had received chest radiation before BMT to determine an association between TBI and breast cancer. Furthermore, we calculated the risk of subsequent breast cancer by attained age among those exposed to TBI, demonstrating that the risk approached 2.6% by age 50 years, 4.5% by age 60 years, and 8.8% by age 70 years. The risk of breast cancer was particularly high among women exposed to TBI at age < 30 years; these women had a 13.9% risk by age 50 years. These findings provide a framework for screening for breast cancer among TBI-exposed BMT recipients with respect to the age at initiation of screening, and they also provide a rationale for the use of screening strategies similar to those used in other chest-irradiated cancer survivors at an elevated risk of breast cancer.25
Additional strengths of this BMTSS report include the large sample size, combined with detailed information about pre-BMT chemotherapeutic exposures and patient factors that are known to be related to breast cancer, such as age at menarche, childbirth, menopause, and use of exogenous estrogens. Limitations include conducting the primary analysis in participants who were living at the time of the BMTSS survey. However, inclusion of deceased women in the cohort preserved the statistically significant higher risk after exposure to TBI. The BMTSS relies on self-report of conditions diagnosed by a health care provider. We addressed this limitation previously by conducting a study that showed that the accuracy and percentage agreement between self-report and medical records is good.26 Furthermore, we confirmed the breast cancers using pathology reports and clinic notes. Finally, for allogeneic BMT recipients, the magnitude of risk in our study was comparable to that observed in a previous study.22 The possibility of participation bias is always an important consideration, but a number of steps were taken to minimize the potential impact on the study results, and differences in the characteristics of the participants and nonparticipants were noted (Appendix Table A1). We were unable to assess the contribution of underlying familial genetic breast cancer predisposition because this information was not collected as part of the BMTSS survey. We recognize this as an important future direction to better understand breast cancer risk in BMT survivors, particularly because some germline mutations may predispose to both hematologic malignancies and breast cancer.27 We did not observe a relationship between TBI dose and the risk of subsequent breast cancer in this study, but the distribution of TBI doses used for the women included in this study was narrow, and additional research is needed to determine if a dose-response relationship between TBI and subsequent breast cancer exists.
In summary, this study demonstrates an increased risk of breast cancer after TBI exposure among both autologous and allogeneic BMT recipients. This risk is highest among those who are younger than 30 years of age at TBI exposure. Women who received TBI either as part of allogeneic or autologous BMT should therefore be considered for increased screening, particularly if exposed at age younger than 030 years.
APPENDIX
FIG A1.
Cumulative incidence of subsequent breast cancer, stratified by use of total body irradiation, among study cohort and cohort of women deceased at time of Blood or Marrow Transplantation Survivor Study survey. CIF, Cumulative Incidence Function.
TABLE A1.
Characteristics of Study Participants and Nonparticipants

TABLE A2.
TBI Dose and Risk of Subsequent Breast Cancer

PRIOR PRESENTATION
Presented in part at the 2019 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported in part by grants from the National Cancer Institute (R01 CA078938 and U01 CA213140) and the Leukemia Lymphoma Society (R6502-16).
AUTHOR CONTRIBUTIONS
Conception and design: Andrew M. McDonald, Mukta Arora, Smita Bhatia
Financial support: Smita Bhatia
Administrative support: Stephen J. Forman, Mukta Arora, Saro H. Armenian, Smita Bhatia
Provision of study material or patients: Donna Salzman, Saro H. Armenian, Smita Bhatia
Collection and assembly of data: Andrew M. McDonald, Jessica Wu, Lindsey Hageman, Liton Francisco, Michelle Kung, Emily Ness, Kevin Battles, Smita Bhatia
Data analysis and interpretation: Andrew M. McDonald, Yanjun Chen, F. Lennie Wong, Wendy Landier, Donna Salzman, Daniel J. Weisdorf, Stephen J. Forman, Mukta Arora, Saro H. Armenian, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Total Body Irradiation and Risk of Breast Cancer After Blood or Marrow Transplantation: A Blood or Marrow Transplantation Survivor Study Report
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew M. McDonald
Consulting or Advisory Role: Varian Medical Systems
Research Funding: Varian Medical Systems (Inst), Collegium Pharmaceuticals (Inst)
Yanjun Chen
Employment: Edwards Lifesciences
Wendy Landier
Research Funding: Merck Sharp & Dohme (Inst)
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
Stephen J. Forman
Stock and Other Ownership Interests: Mustang Bio, Lixte Biotechnology
Consulting or Advisory Role: Allogene, Lixte, Mustang Bio
Research Funding: Mustang Bio
Patents, Royalties, Other Intellectual Property: Mustang Bio
Mukta Arora
Consulting or Advisory Role: Fate Therapeutics
Research Funding: Syndax (Inst), Kadmon (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderwalde AM, Sun CL, Laddaran L, et al. Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia. 2013;27:1139–1145. doi: 10.1038/leu.2012.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Majhail NS, Tao L, Bredeson C, et al: Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant 19:1498-1501, 2013. [DOI] [PMC free article] [PubMed]
- 5.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139, quiz 3377. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Ramsay NK, Steinbuch M, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87:3633–3639. [PubMed] [Google Scholar]
- 8.Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 9. Henderson TO, Amsterdam A, Bhatia S, et al: Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med 152:444-455; W144-154, 2010. [DOI] [PMC free article] [PubMed]
- 10.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Winter DL, Stiller CA, et al. Risk of breast cancer in female survivors of childhood Hodgkin’s disease in Britain: A population-based study. Int J Cancer. 2007;120:384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmqvist AS, Chen Y, Berano Teh J, et al. Risk of solid subsequent malignant neoplasms after childhood Hodgkin lymphoma-Identification of high-risk populations to guide surveillance: A report from the Late Effects Study Group. Cancer. 2019;125:1373–1383. doi: 10.1002/cncr.31807. [DOI] [PubMed] [Google Scholar]
- 15.https://www.aapm.org/pubs/reports/RPT_17.pdf Van Dyk J, Galvin JM, Glasgow GP, et al: The physical aspects of total and half body photon irradiation: A report of Task Group 29, Radiation Therapy Committee, American Association of Physicists in Medicine. American Institute of Physics,1986. [Google Scholar]
- 16.Hernández L, Terradas M, Camps J, et al. Aging and radiation: Bad companions. Aging Cell. 2015;14:153–161. doi: 10.1111/acel.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. https://wonder.cdc.gov/mortSQL.html Centers for Disease Control and Prevention: Compressed Mortality File: Underlying cause-of-death.
- 18.Sahai H, Khurshid A. Confidence intervals for the mean of a Poisson distribution: A review. Biom J. 1993;35:857–867. [Google Scholar]
- 19.Møller MH, Lousdal ML, Kristiansen IS, et al. Effect of organized mammography screening on breast cancer mortality: A population-based cohort study in Norway. Int J Cancer. 2019;144:697–706. doi: 10.1002/ijc.31832. [DOI] [PubMed] [Google Scholar]
- 20.Sankatsing VDV, van Ravesteyn NT, Heijnsdijk EAM, et al. The effect of population-based mammography screening in Dutch municipalities on breast cancer mortality: 20 years of follow-up. Int J Cancer. 2017;141:671–677. doi: 10.1002/ijc.30754. [DOI] [PubMed] [Google Scholar]
- 21.Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319:154–164. doi: 10.1001/jama.2017.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: A report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111:939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turcotte LM, Liu Q, Yasui Y, et al. Chemotherapy and risk of subsequent malignant neoplasms in the Childhood Cancer Survivor Study Cohort. J Clin Oncol. 2019;37:3310–3319. doi: 10.1200/JCO.19.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veiga LH, Curtis RE, Morton LM, et al. Association of breast cancer risk after childhood cancer with radiation dose to the breast and anthracycline use: A report from the Childhood Cancer Survivor Study. JAMA Pediatr. 2019;173:1171–1179. doi: 10.1001/jamapediatrics.2019.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14:e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louie AD, Robison LL, Bogue M, et al. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 27.Porter CC. Germ line mutations associated with leukemias. Hematology (Am Soc Hematol Educ Program) 2016;2016:302–308. doi: 10.1182/asheducation-2016.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]