Abstract
PURPOSE
The oligometastatic paradigm hypothesizes that patients with a limited number of metastases may achieve long-term disease control, or even cure, if all sites of disease can be ablated. However, long-term randomized data that test this paradigm are lacking.
METHODS
We enrolled patients with a controlled primary malignancy and 1-5 metastatic lesions, with all metastases amenable to stereotactic ablative radiotherapy (SABR). We stratified by the number of metastases (1-3 v 4-5) and randomized in a 1:2 ratio between palliative standard-of-care (SOC) treatments (arm 1) and SOC plus SABR (arm 2). We used a randomized phase II screening design with a primary end point of overall survival (OS), using an α of .20 (wherein P < .20 indicates a positive trial). Secondary end points included progression-free survival (PFS), toxicity, and quality of life (QOL). Herein, we present long-term outcomes from the trial.
RESULTS
Between 2012 and 2016, 99 patients were randomly assigned at 10 centers internationally. The most common primary tumor types were breast (n = 18), lung (n = 18), colorectal (n = 18), and prostate (n = 16). Median follow-up was 51 months. The 5-year OS rate was 17.7% in arm 1 (95% CI, 6% to 34%) versus 42.3% in arm 2 (95% CI, 28% to 56%; stratified log-rank P = .006). The 5-year PFS rate was not reached in arm 1 (3.2%; 95% CI, 0% to 14% at 4 years with last patient censored) and 17.3% in arm 2 (95% CI, 8% to 30%; P = .001). There were no new grade 2-5 adverse events and no differences in QOL between arms.
CONCLUSION
With extended follow-up, the impact of SABR on OS was larger in magnitude than in the initial analysis and durable over time. There were no new safety signals, and SABR had no detrimental impact on QOL.
INTRODUCTION
It has been hypothesized for nearly a century that patients with a small burden of metastatic disease can benefit from ablation of all metastases,1 with some achieving long-term disease control or even cure. Although surgery was historically the primary modality used to ablate metastases,2 newer and less-invasive modalities are now available, including stereotactic ablative radiotherapy (SABR).3 Although the clinical use of surgery and SABR has increased rapidly in recent decades,3,4 randomized data that prove the existence of the oligometastatic state have been lacking.5
CONTEXT
Key Objective
To determine the impact of stereotactic ablative radiotherapy (SABR) on overall survival (OS) in patients with a controlled primary tumor and 1-5 oligometastases.
Knowledge Generated
In this long-term report from an international randomized phase II trial, patients who received SABR demonstrated a 22-month improvement in median OS compared with patients who received a standard-of-care approach alone, corresponding to an absolute survival benefit of 25% at 5 years. There were no new safety signals detected.
Relevance
These data add to the growing evidence base that suggests that SABR can improve long-term outcomes in patients with a limited burden of metastatic disease. These results may influence treatment decisions while awaiting the results of phase III trials.
Preclinical and translational studies provide evidence in support of the oligometastatic hypothesis.6 The development of metastases requires a series of key steps known as the invasion-metastasis cascade.7 The cascade includes steps that lead to tumor cell intravasation into the circulatory system, with subsequent hematogeneous dissemination and extravasation, followed by survival and colonization in a distant organ. The large majority of cells that reach a distant organ either die or enter dormancy, whereas only a small percentage proliferate to develop into metastases.6,7 Phylogenetic analyses of primary tumors and metastases using next-generation sequencing have allowed for the creation of timelines of metastasis development.8-10 In some patients, a solitary metastasis can be present for years as a single site of disease but then subsequently seed further widespread metastases thereafter.8-10 Metastases can also reseed the primary tumor.11
The biologic evidence is supported by numerous single-arm studies that tested ablative therapies in patients with oligometastases.5 Such studies have often demonstrated better-than-expected long-term survivals for a population of patients with metastatic disease. However, conclusions from single-arm studies are limited by a lack of a control arm, which leads to uncertainties about whether long-term survivals reported are due to the ablative therapies themselves or merely to the selection of fit patients with slow-growing indolent disease.5 This debate has resulted in substantial international variation in the patterns of practice in treating patients with oligometastases.12
There are now supportive data from randomized phase II studies that have tested the impact of ablative therapies on overall survival (OS)13,14 or on surrogate end points such as progression-free survival (PFS).13-18 One of these, Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastases (SABR-COMET), assessed the impact of SABR on OS in patients with a controlled primary tumor and 1-5 metastatic lesions. The initial report of SABR-COMET demonstrated a 13-month improvement in median OS, the primary end point, after a median follow-up of 28 months.14 However, because of the larger-than-expected number of patients who achieved 5-year survival, the SABR-COMET protocol was modified to extend follow-up beyond 5 years to capture long-term outcomes. Herein, we report the extended outcomes of the trial > 40 months after completion of accrual.
METHODS
Study Design
SABR-COMET was an open-label phase II randomized international study that enrolled patients from 10 centers. Appropriate regulatory approval, including ethics approval, was obtained in all jurisdictions. The trial was registered before activation. Because the trial details and statistical analyses have been published in detail,14,19 a short synopsis is provided here.
Participants
The main inclusion requirements were age ≥ 18 years, Eastern Cooperative Oncology Group performance score 0-1, and a life expectancy ≥ 6 months, and patients were required to have 1-5 metastases and a controlled primary tumor. All metastatic lesions had to be eligible for SABR in accordance with protocol-specified dose constraints. The main exclusion criteria included serious medical comorbidities that prohibited radiotherapy, prior radiotherapy to a site that required treatment, malignant pleural effusion, tumors in proximity to the spinal cord (within 3 mm), and brain metastasis that required surgical decompression. All patients provided written informed consent.
Randomization and Masking
Patients were randomly assigned using a computer-generated randomization list with permuted blocks of 9 after stratification by the number of metastases (1-3 v 4-5). There was no blinding of patients or physicians.
Procedures
In the control arm, standard palliative radiotherapy was delivered with the goal of alleviating symptoms or preventing complications, with recommended doses ranging from 8 Gy in 1 fraction to 30 Gy in 10 fractions. In the SABR arm, patients received SABR to all sites of metastatic disease. A full table of allowable SABR doses is provided in the protocol (Data Supplement, online only). Patients in the SABR arm who subsequently developed new metastases were eligible for additional SABR, if feasible. In both arms, palliative standard-of-care systemic therapy was recommended as indicated, using a pragmatic approach wherein the choice of systemic agents was at the discretion of the medical oncologist. Any further palliative systemic therapy or palliative radiation therapy after progression were at the discretion of the treating physicians.
Patients were seen in follow-up every 3 months after random assignment in years 1-2 and every 6 months until year 5, with regular imaging as outlined in the protocol. The trial was amended in October 2016 to continue annual visits until year 10.
Outcomes
The primary end point was OS, and secondary end points were quality of life (QOL), as assessed with the Functional Assessment of Cancer Therapy: General (FACT-G); toxicity, on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4); progression-free survival (PFS); lesional control (LC) rate; and the number of cycles of further chemotherapy/systemic therapy. This latter end point was not easily ascertainable because of patients receiving palliative systemic therapy at other centers and was therefore reported as a binary variable (ie, further systemic therapy received: yes v no) in the primary analysis and again here. An additional post hoc end point was analyzed here: time to development of new metastases, which was defined as time from random assignment to development of new metastatic lesions, treating death as a result of any cause as a competing event. To address the possible imbalance that arises from the distribution of patients with prostate cancer between the 2 arms, a post hoc sensitivity analysis was performed to examine the impact of SABR on OS after excluding all patients with prostate cancer.
Statistical Methods
SABR-COMET used a randomized phase II screening design14 with a 2-sided α of .20 and a power of 80%. In this approach, the α is set higher than the .05 level used in phase III trials, with the recognition that even if the phase II trial is positive (ie, if P for the primary end point is < .20), such a positive result is not usually considered definitive proof without a subsequent phase III trial. However, a finding with P < .005 in a phase II screening trial may be considered definitive.20
All analyses were based on the intention-to-treat principle. OS and PFS were calculated using Kaplan-Meier estimates and differences were compared using stratified log-rank tests (adjusting for stratification). Hazard ratios (HRs) were calculated using Cox proportional hazards regression adjusted for stratification. QOL was measured using FACT-G scores, with differences between groups over time compared using linear mixed modeling (with time and treatment arms as fixed effects and patient number as random effect). Differences in rates of grade ≥ 2 toxicity and in receipt of systemic therapy were compared using the χ2 test or Fisher’s exact test as appropriate. Time to development of new metastases was estimated using cumulative incidence functions, with death considered a competing event, and differences were compared using the stratified Gray’s test (adjusting for stratification). Statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC) with 2-sided statistical testing at the .05 significance level.
The trial closed in August 2016, and after 1 year of follow-up and time to resolve data queries, the data set was locked for the previously published primary analyses on January 18, 2018. Data collection continued thereafter, and the data set was locked for this long-term analysis on January 30, 2020. The first author (D.A.P.) and statistician (A.W.) had full access to the data, vouch for the integrity of the data and the adherence to the study protocol, and are responsible for the decision to submit the report for publication.
Role of the Funding Source
The funding bodies had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Data Sharing Statement
The trial protocol did not include a data sharing plan, and therefore data from the trial will not be shared publicly as sharing was not included in the ethics approvals.
RESULTS
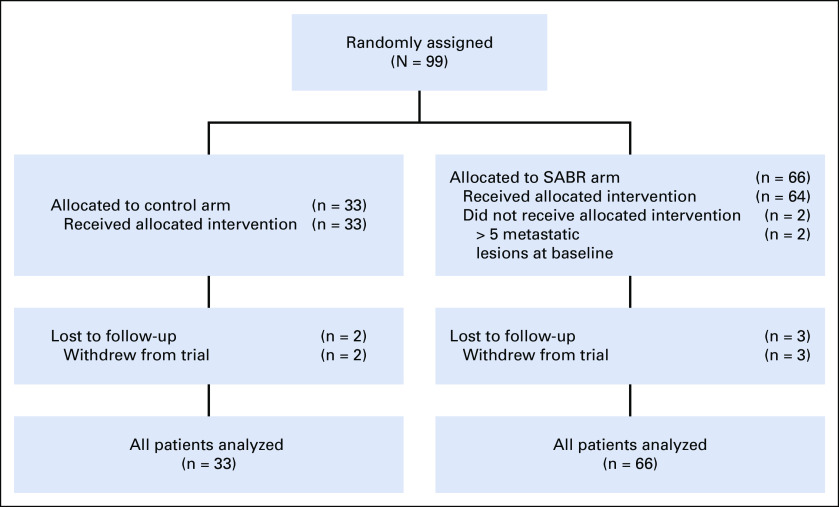
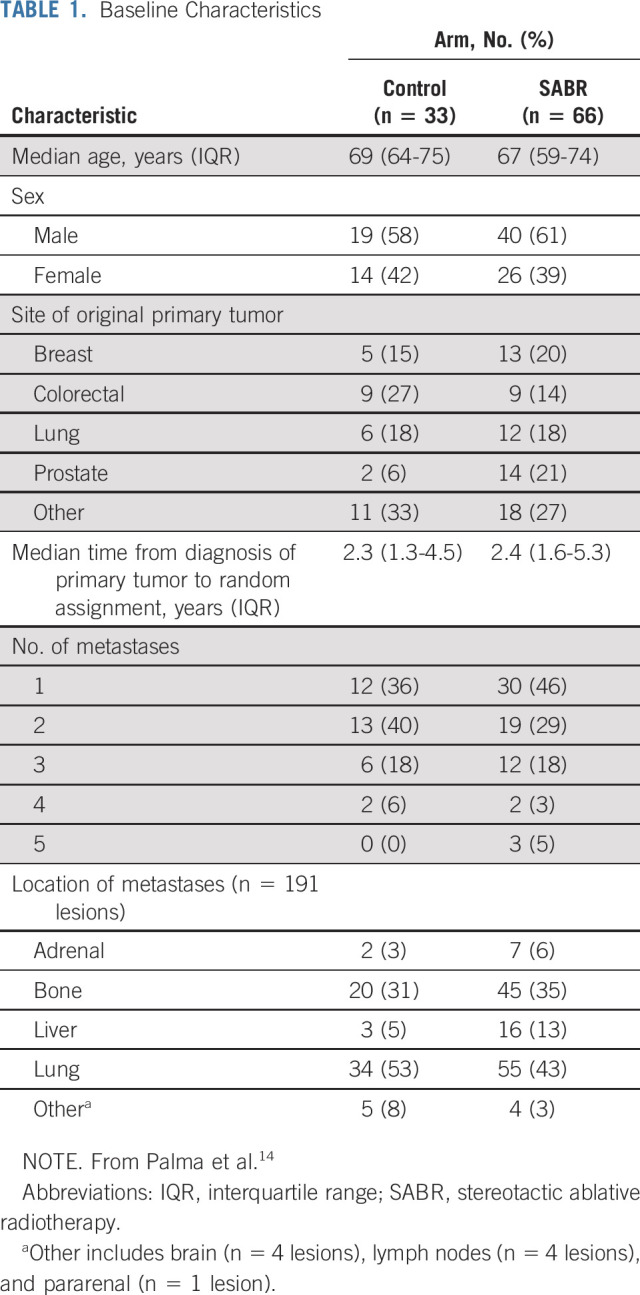
Between February 2012 and August 2016, 99 patients were enrolled at 10 centers: 33 in the control arm and 66 in the SABR arm (Fig 1). Baseline characteristics are listed in Table 1. Of note, the SABR group contained the preponderance of patients with prostate cancer and all the patients with 5 metastases.
FIG 1.
CONSORT diagram. SABR, stereotactic ablative radiotherapy.
TABLE 1.
Baseline Characteristics
After random assignment, 57 (58%) of 99 patients received palliative systemic therapy, and 39 (39%) of the 99 received palliative radiotherapy. Use of palliative radiotherapy was higher in the control arm (delivered to 23 [70%] of 33 patients) than in the SABR arm (delivered to 16 [24%] of 66 patients; P < .001). There were no differences between arms in use of systemic therapy (21 [64%] of 33 v 36 [55%] of 66, respectively; P = .39). Since the original report, 1 patient in the control arm received curative-intent SABR for a solitary liver lesion that had initially responded to targeted therapy but progressed with no new sites of disease. This patient remains alive and free of disease and is analyzed on the control arm. Nine patients in the SABR arm received salvage SABR for new metastases, including 3 (30%) of 10 patients who survived beyond 5 years.
The median follow-up was 51 months (95% CI, 46 to 58 months). The primary outcome event, death as a result of any cause, occurred in 24 (73%) of 33 patients in the control arm and 35 (53%) of 66 patients in the SABR arm. Median OS was 28 months in the control arm (95% CI, 18 to 39 months) v 50 months in the SABR arm (95% CI, 29 to 83 months; stratified log-rank test P = .006; HR, 0.47; 95% CI, 0.27 to 0.81; Fig 2A). Five-year OS rates were 17.7% (95% CI, 6% to 34%) v 42.3% (95% CI, 28% to 56%), respectively. A post hoc sensitivity analysis that excluded patients with prostate cancer was consistent with a treatment benefit, with 5-year OS rates of 16.2% (95% CI, 5% to 32%) v 33.1% (95% CI, 20% to 47%), respectively (stratified log-rank test P = .085). The histologic subtypes of patients surviving ≥ 5 years are as follows: arm 1 (n = 2), breast (n = 1) and kidney (n = 1); arm 2 (n = 10), breast (n = 4), prostate (n = 3), and other (n = 3, including 1 each with esophageal, skin, and colorectal cancer).
FIG 2.
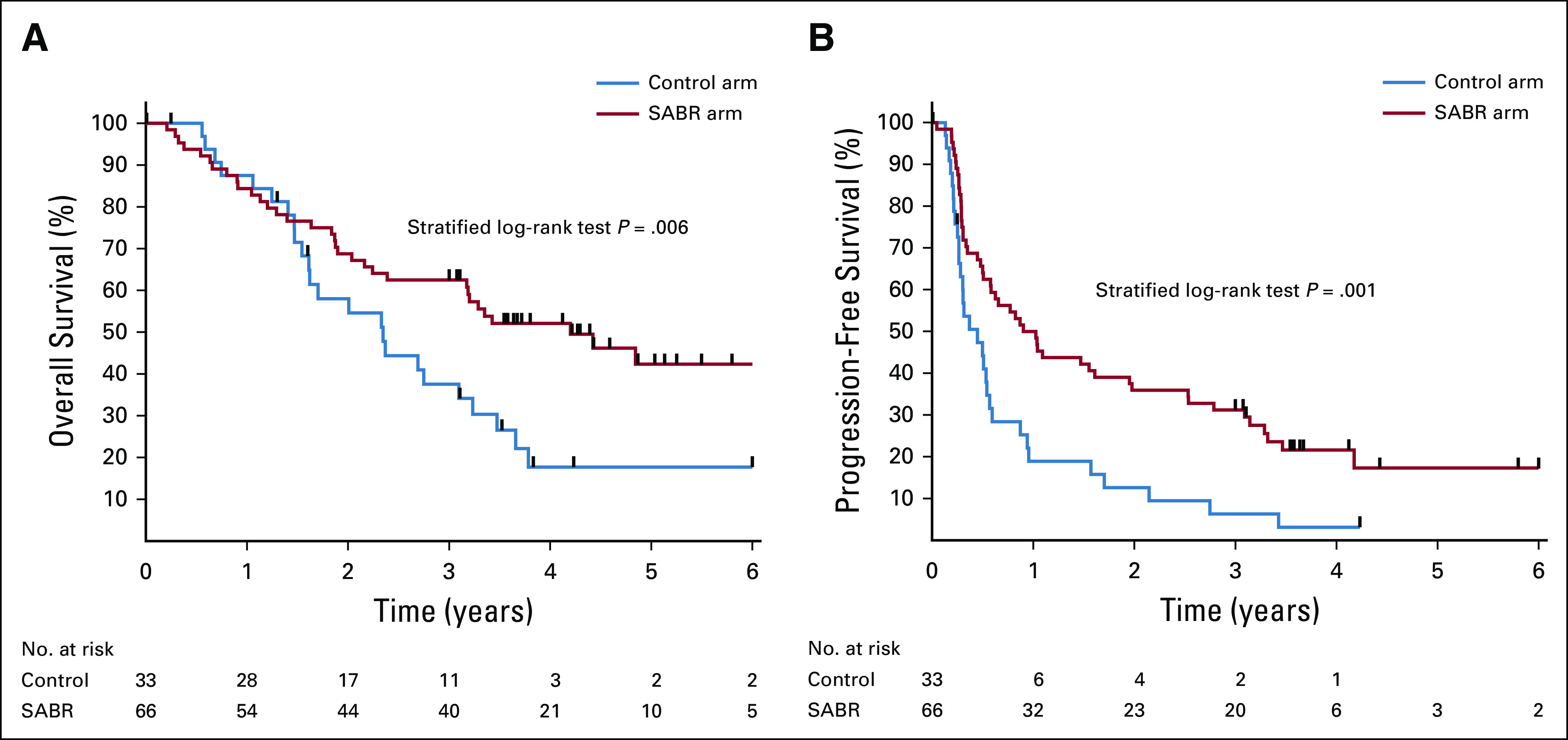
Kaplan-Meier plots for (A) overall survival and (B) progression-free survival. SABR, stereotactic ablative radiotherapy.
Progression events occurred in 74 patients: 29 (88%) of 33 patients in the control arm and 45 (68%) of 66 patients in the SABR arm. Median PFS was 5.4 months in arm 1 (95% CI, 3.2 to 6.8 months) and 11.6 months in arm 2 (95% CI, 6.1 to 23.4 months; stratified log-rank test P = .001; HR, 0.48; 95% CI, 0.31 to 0.76; Fig 2B). In arm 1, no patients survived 5 years without progression, and the PFS rate at 4 years was 3.2% (95% CI, 0% to 14%), with the last patient censored. In arm 2, the 4-year PFS rate was 21.6% (95% CI, 12% to 33%), and the 5-year PFS rate was 17.3% (95% CI, 8% to 30%).
The overall long-term LC rate, defined as the absence of progression in the lesions initially present at random assignment on the basis of RECIST version 1.1, was 46% (26 of 57 assessable lesions) in the control arm and 63% (65 of 104 assessable lesions) in the SABR arm (P = .039), corresponding to an absolute increase of 17% (95% CI, 1% to 33%). After SABR, there were significant differences in LC rates on the basis of lesion location (adrenal, 100%; bone, 72%; lung, 51%; liver, 50%; P = .04).
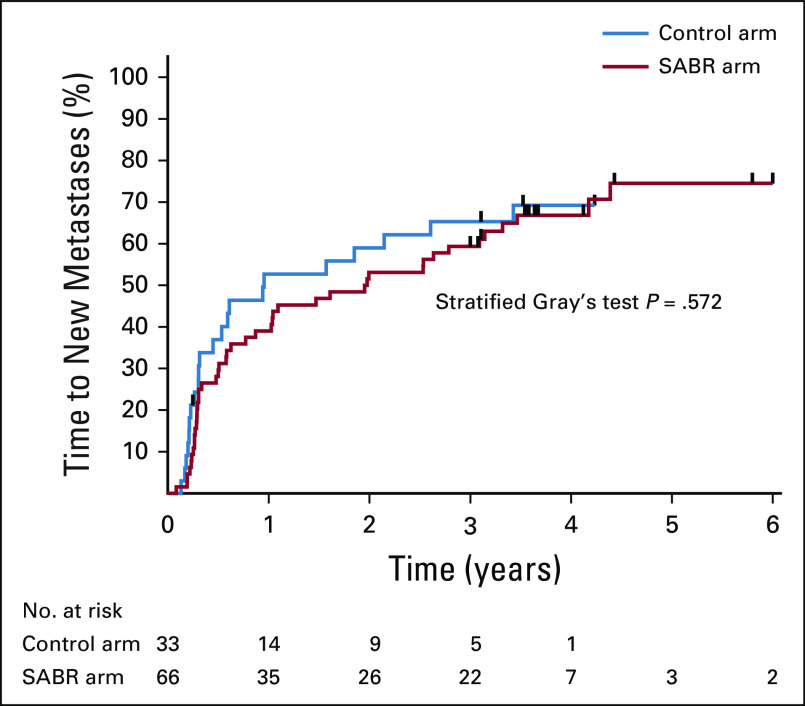
The long-term analysis of FACT-G scores over time are shown in Figure 3, with no differences in total QOL scores, or subscale scores, between arms over time. There were no new grade 2-5 adverse events, and therefore, the overall rates of grade ≥ 2 adverse events related to treatment remained at 9% (3 of 33 patients) in the control arm and 29% (19 of 66 patients) in the SABR arm (P = .03), an absolute increase of 20% (95% CI, 5% to 34%). Of note, as reported previously, there were 3 deaths (4.5%) in the SABR arm that were possibly, probably, or definitely related to treatment. The cumulative incidence of new metastases adjusted for death as a competing event is shown in Figure 4, with no differences between arms detected (stratified Gray’s test P = .57).
FIG 3.
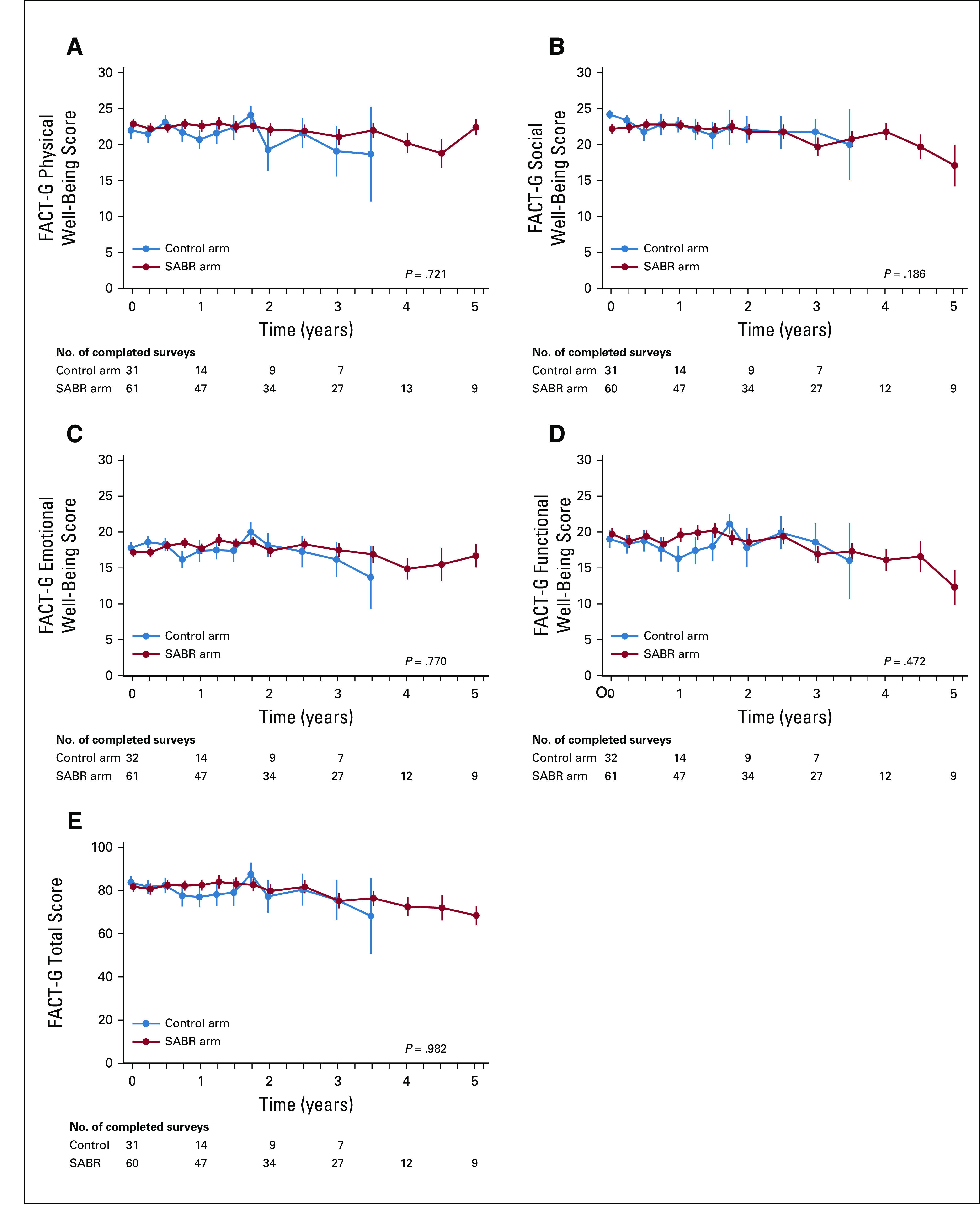
Functional Assessment of Cancer Therapy: General (FACT-G) mean ± SE quality-of-life scores over time, including (A) physical well-being, (B) social well-being, (C) emotional well-being, (D) functional well-being, and (E) total. SABR, stereotactic ablative radiotherapy.
FIG 4.
Kaplan-Meier plot for development of new metastases over time on the basis of cumulative incidence function, with death as a competing event.
DISCUSSION
At the time of initial publication, SABR-COMET was the first randomized trial to demonstrate an impact of any ablative therapy on a primary end point of OS in patients with oligometastases. In this long-term analysis, the effects of SABR on OS were larger in magnitude than previously reported, with a median OS benefit of 22 months (v 13 months in the original analysis), which corresponds to an absolute benefit of 24.6% at 5 years. SABR did not result in a detriment in QOL, and no new safety signals were apparent. The increasing magnitude of benefit over time suggests that long-term follow-up is required for any randomized trials in patients with oligometastases to fully ascertain the impact of ablative therapies on OS.
It is also apparent from this analysis that most patients with oligometastases have undetectable micrometastases at the time of enrollment, but with close surveillance and further SABR to subsequent developing sites of metastasis, some patients can be successfully treated and again be rendered disease free. Three lines of evidence support this conclusion. First, there was no significant difference between arms in time to development of new metastases, which suggests that these new metastatic lesions were seeded before SABR was delivered and grew in the months after randomization. Second, a substantial number of long-term survivors (30% of those alive beyond 5 years) required salvage SABR for new metastases. Third, a finding of a comparatively short median PFS benefit (6 months in this trial) in the setting of a longer median OS benefit generally indicates that post-progression treatment is influencing the OS benefit. Because there were no differences in use of systemic therapy between arms, it is likely that postprogression SABR is the main contributing factor to this difference. Taken together, these findings suggest that patients treated with SABR for oligometastases should undergo imaging surveillance with salvage SABR used if safe, as was done in this trial. Additional studies are required to determine the optimal imaging surveillance strategy and the maximum number of new lesions treatable with SABR.
Our long-term findings add to a growing body of evidence that supports the use of ablative therapies for oligometastatic cancers. Other phase II trials have suggested benefits of ablative therapies in the setting of colorectal cancer liver metastases,21,22 in non–small-cell lung cancer (NSCLC),13,15,17 and in prostate cancer.16,18 As a notable exception, the PulMiCC phase III trial failed to show a benefit for surgical resection of pulmonary metastases from colorectal cancers, although the trial closed early and reported on only 21% of target accrual (65 patients).23 Overall, however, the preponderance of randomized evidence suggests that patients with oligometastases benefit from ablative therapies, but larger phase III trials, with sufficient power to examine histologic subgroups separately, would be ideal to conclusively prove the survival benefit.
Such phase III trials are under way. SABR-COMET-3 (ClinicalTrials.gov identifier: NCT03862911) and SABR-COMET-10 (ClinicalTrials.gov identifier: NCT03721341) are assessing the impact of SABR on OS in patients with 1-3 and 4-10 metastases, respectively, accruing patients with a controlled primary tumor of any solid tumor histology.24,24a The CORE trial (ClinicalTrials.gov identifier: NCT02759783) is a phase II/III trial that includes patients with breast, NSCLC, or prostate histology with a controlled primary tumor and 1-3 metastatic lesions. Large cooperative group trials specific to lung cancer (NRG-LU002) and breast cancer (NRG-BR002) oligometastases are also under way and accruing well.
Predictive biomarkers would be a major asset to help to guide treatment decisions for patients with oligometastases, but currently, no validated biomarkers are available for clinical use. Biomarkers could allow physicians to tailor treatment and surveillance intensity to the risk of further metastatic recurrence. For example, patients with oligometastases predicted to be at high risk of rapid widespread metastatic progression after SABR may be best served by effective systemic therapy rather than by SABR (or both treatments in sequence). Efforts to develop biomarkers that are prognostic and predictive are under way as part of ongoing clinical trials; for example, SABR-COMET-3 and SABR-COMET-10 are both collecting samples to assess for circulating biomarkers, including circulating tumor DNA and circulating tumor cells.24
The possible toxicities of SABR must be borne in mind for patients and physicians considering treatment. SABR was well tolerated in the majority of patients, with a rate of grade ≥ 2 toxicity of only 29%. However, the grade 5 toxicity rate of 4.5% (despite strict dose constraints and peer review of all radiation plans) is higher than reported in other studies. This suggests that SABR delivery should continue to focus on minimization of toxicity, and additional studies are needed to determine the optimal SABR doses, balancing the competing considerations of maximizing LC while minimizing toxicity.
This trial has limitations that must be considered when interpreting its findings. Many of the limitations were discussed in detail in the original trial report,14 including the inclusion of multiple histologies (a common approach in stereotactic radiation trials for metastases). The large majority of patients with prostate cancer were assigned to the SABR arm, but our sensitivity analysis does not suggest that the results are merely due to the allocation of these patients. Despite the reduced power after excluding patients with prostate cancer, a benefit was still demonstrated that would meet the cutoff for a randomized phase II screening trial. The most favorable histologies in patients with oligometastatic cancers treated with SABR are breast, prostate, and kidney,25 and these groups are highly represented in the long-term survivors (100% in arm 1 and 70% in arm 2), but in arm 2, some patients with unfavorable histologies also achieved long-term survival. Evaluation of local control after SABR is difficult because focal fibrosis can present as an enlarging mass; this may explain the relatively low local control rates reported for lung lesions using RECIST version 1.1.26 Patients with 4-5 metastases are under-represented in this trial, which led to the development of separate trials for patients with 1-3 and 4-10 metastases, as described. This trial was launched before the immunotherapy era, and immunotherapeutic options may change the impact of SABR on long-term outcomes.
In conclusion, with longer-term follow-up, SABR achieved a 22-month median OS benefit in patients with a controlled primary tumor and 1-5 oligometastases. Even with SABR, many patients progress with new metastases, likely because of the presence of occult micrometastatic disease at presentation, but some can receive salvage therapy with repeat SABR. Phase III trials currently underway aim to confirm the OS benefits and to develop biomarkers predictive of benefit with SABR.
SUPPORT
Supported by the Ontario Institute for Cancer Research and London Regional Cancer Program Catalyst Grant.
AUTHOR CONTRIBUTIONS
Conception and design: David A. Palma, Stewart Gaede, Cornelis Haasbeek, Michael Lock, George B. Rodrigues, Belal Ahmad, Suresh Senan
Financial support: David A. Palma
Administrative support: David A. Palma
Provision of study material or patients: David A. Palma, Robert Olson, Stephen Harrow, Cornelis Haasbeek, Liam Mulroy, Michael Lock, George B. Rodrigues, Brian P. Yaremko, Devin Schellenberg, Mitchell Liu, Suresh Senan
Collection and assembly of data: David A. Palma, Robert Olson, Stephen Harrow, Alexander V. Louie, Liam Mulroy, Michael Lock, Brian P. Yaremko, Devin Schellenberg, Sashendra Senthi, Neil Kopek, Mitchell Liu, Karen Moore, Suzanne Currie, Roel Schlijper, Glenn S. Bauman, Joanna Laba, X. Melody Qu
Data analysis and interpretation: David A. Palma, Robert Olson, Stephen Harrow, Alexander V. Louie, Cornelis Haasbeek, Michael Lock, George B. Rodrigues, Brian P. Yaremko, Devin Schellenberg, Sashendra Senthi, Anand Swaminath, Mitchell Liu, X. Melody Qu, Andrew Warner, Suresh Senan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert Olson
Research Funding: Varian Medical Systems
Stephen Harrow
Honoraria: AstraZeneca, Pfizer, Takeda Pharmaceuticals
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Takeda Pharmaceuticals
Alexander V. Louie
Honoraria: Varian Medical Systems, Reflexion Medical, AstraZeneca
Consulting or Advisory Role: AstraZeneca
Speakers’ Bureau: Varian Medical Systems
Michael Lock
Consulting or Advisory Role: Sanofi Canada
Speakers’ Bureau: Ferring, AbbVie
Devin Schellenberg
Honoraria: AstraZeneca, Merck, Pfizer
Research Funding: Varian Medical Systems
Belal Ahmad
Consulting or Advisory Role: Ferring, AbbVie, Genentech
Anand Swaminath
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca
Karen Moore
Travel, Accommodations, Expenses: Takeda Pharmaceuticals, Roche
Suzanne Currie
Travel, Accommodations, Expenses: Varian Medical Systems
Glenn S. Bauman
Honoraria: Bayer AG
Suresh Senan
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Celgene, Varian Medical Systems, MSD Oncology
Speakers’ Bureau: AstraZeneca
Research Funding: ViewRay (Inst), AstraZeneca (Inst), Varian Medical Systems (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Barney J, Churchill E. Adenocarcinoma of the kidney with metastasis to the lung cured by nephrectomy and lobectomy. J Urol. 1939;42:269–276. [Google Scholar]
- 2.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 3.Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer. 2015;121:747–757. doi: 10.1002/cncr.29134. [DOI] [PubMed] [Google Scholar]
- 5.Palma DA, Salama JK, Lo SS, et al. The oligometastatic state—Separating truth from wishful thinking. Nat Rev Clin Oncol. 2014;11:549–557. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 6.Correa RJ, Salama JK, Milano MT, et al. Stereotactic body radiotherapy for oligometastasis: Opportunities for biology to guide clinical management. Cancer J. 2016;22:247–256. doi: 10.1097/PPO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 7.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turajlic S, Xu H, Litchfield K, et al: Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 173:581-594.e12, 2018. [DOI] [PMC free article] [PubMed]
- 9. doi: 10.1038/nature22364. Abbosh C, Birkbak NJ, Wilson GA, et al: Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545:446-451, 2017 [Erratum: Nature 554:264, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.1097/COC.0000000000000169. Lewis SL, Porceddu S, Nakamura N, et al: Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: An international survey of >1000 radiation oncologists. Am J Clin Oncol 40:418-422, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 15.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 17.Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips R, Lim SJ, Shi WY, et al: Primary outcomes of a phase II randomized trial of observation versus stereotactic ablative radiation for oligometastatic prostate cancer (ORIOLE). Int J Radiat Oncol Biol Phys 105:681, 2019.
- 19.Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): Study protocol for a randomized phase II trial. BMC Cancer. 2012;12:305. doi: 10.1186/1471-2407-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 21.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: A randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23:2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst. 2017;109:djx015. doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treasure T, Farewell V, Macbeth F, et al. Pulmonary metastasectomy versus continued active monitoring in colorectal cancer (PulMiCC): A multicentre randomised clinical trial. Trials. 2019;20:718. doi: 10.1186/s13063-019-3837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): Study protocol for a randomized phase III trial. BMC Cancer. 2019;19:816. doi: 10.1186/s12885-019-5977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1–3 Oligometastatic tumors (SABR-COMET-3): Study protocol for a randomized phase III trial. BMC Cancer. 2020;20:380. doi: 10.1186/s12885-020-06876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JC, Ayala-Peacock DN, Lee J, et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PLoS One. 2018;13:e0195149. doi: 10.1371/journal.pone.0195149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)—Can we distinguish fibrosis from recurrence? A systematic review of the literature. Pract Radiat Oncol. 2013;3:S11–S12. doi: 10.1016/j.prro.2013.01.039. [DOI] [PubMed] [Google Scholar]