Abstract
PURPOSE
Treatment options are limited for patients with recurrent and/or metastatic (R/M) cutaneous squamous cell carcinoma (cSCC); mortality rates exceed 70% in patients with distant metastases. Here, we present the first interim analysis of the R/M cSCC cohort from the 2-cohort—locally advanced and R/M—phase II KEYNOTE-629 study.
PATIENTS AND METHODS
Patients with R/M cSCC not amenable to surgery or radiation received pembrolizumab 200 mg every 3 weeks. The primary end point was objective response rate per RECIST v1.1. Secondary end points were duration of response, disease control rate, progression-free survival, overall survival, and safety.
RESULTS
At data cutoff (April 8, 2019), median follow-up of 105 enrolled patients in the R/M cohort was 11.4 months (range, 0.4 to 16.3 months). Objective response rate was 34.3% (95% CI, 25.3% to 44.2%; 4 complete responses, 32 partial responses), and disease control rate was 52.4% (95% CI, 42.4% to 62.2%). Median duration of response was not reached (range, 2.7 to 13.1+ months; ‘+’ refers to ongoing response at data cutoff). Median progression-free survival was 6.9 months (95% CI, 3.1 months to 8.5 months). Median overall survival was not reached (95% CI, 10.7 months to not reached). Treatment-related adverse events occurred in 66.7% of patients (n = 70), the most common of which were pruritus (n = 15; 14.3%), asthenia (n = 14; 13.3%), and fatigue (n = 13; 12.4%). Grade 3 to 5 treatment-related adverse events occurred in 5.7% (n = 6) of patients. One patient died of treatment-related cranial nerve neuropathy.
CONCLUSION
Pembrolizumab demonstrated effective antitumor activity; clinically meaningful, durable responses; and acceptable safety in primarily elderly patients with R/M cSCC, supporting its use in clinical practice. Pembrolizumab adverse events in this study were consistent with its established safety profile.
INTRODUCTION
Cutaneous squamous cell carcinoma (cSCC) is the second most common nonmelanoma skin cancer,1 accounting for 20% of all skin cancer deaths.2,3 The estimated incidence of new cSCC cases is 15 to 35 per 100,000 people and is increasing.3 Mortality rates exceed 70% in patients with distant metastases,4 with recurrence rates from 15% to 28%.5,6 Until recently, there was no consensus on treatment recommendations for patients with unresectable, locally advanced (LA), recurrent, or metastatic cSCC. Treatment guidelines acknowledge the scarcity of efficacy data for patients with distant metastases and suggest using platinum-containing regimens or an epidermal growth factor receptor (EGFR) inhibitor —for example, cetuximab—and encourage participation in clinical trials involving immune checkpoint inhibitors.7,8
cSCC is considered an immunogenic cancer; it has the highest mutational burden exceeding that of classic head and neck squamous cell carcinoma (HNSCC), including mucosal tumors.9,10 In cSCC, tumor suppressor genes are most frequently altered, with the UV signature a key mutational difference.9 Long-term sun exposure leading to DNA damage is postulated to account for the high mutational burden.9,11,12 In addition, there is a positive correlation between the expression of programmed death ligand 1 (PD-L1) in patients with cSCC and pathologic findings related to metastatic risk.13 Therefore, cSCCs will likely respond to immune checkpoint inhibitor therapy, including programmed death 1 (PD-1) inhibitors.
The PD-1 inhibitor cemiplimab demonstrated antitumor activity and safety in patients with LA or recurrent and/or metastatic (R/M) cSCC in an open-label, single-arm, multicohort study. Cemiplimab was well tolerated, with an objective response rate (ORR) of 47% in the metastatic cSCC cohort of the phase II study.14
The PD-1 inhibitor pembrolizumab has demonstrated extended survival for advanced malignancies, including R/M HNSCC15-18 and Merkel cell carcinoma, another UV exposure–driven malignancy.19 KEYNOTE-629 is a 2-cohort study designed to assess pembrolizumab in patients with LA or R/M cSCC. Here, we present the first interim analysis results from the R/M cohort.
PATIENTS AND METHODS
Study Design and Patients
KEYNOTE-629 is an open-label, single-arm, phase II study of pembrolizumab in patients with LA or R/M cSCC. Patients in the R/M cohort were enrolled at 39 sites in 9 countries (Data Supplement). The study protocol (available at ascopubs.org/journal/jco) and amendments were reviewed and approved by the institutional review board or independent ethics committee of each participating institution. The study complied with the ethical principles originating from the Declaration of Helsinki, Good Clinical Practice requirements, and all local laws and regulations.
Eligible patients were aged 18 years and older with histologically confirmed cSCC, measurable disease per RECIST v1.1, Eastern Cooperative Oncology Group performance status of 0 or 1, adequate tissue sample for PD-L1 testing, and life expectancy greater than 3 months. Patients with measurable disease per RECIST v1.1 as assessed by digital photography were also eligible. Tissue biopsy was required within 30 days of investigator-determined complete response (CR) for central pathology review and confirmation CR for patients who were deemed eligible via digital photography by central review. For the R/M cohort, patients had locoregionally recurrent (disease not curable by surgery or radiation) and/or metastatic cSCC (disseminated disease distant to the initial primary site of diagnosis; the Data Supplement includes additional criteria). The R/M cohort consisted of 2 subgroups: distant metastatic cSCC and locoregionally recurrent. The distant metastatic subgroup included only patients with disseminated disease. Patients with locoregional lymph node metastasis were included in the locoregionally recurrent only subgroup. All patients provided written informed consent to participate in the study, which included consent to document all data, such as digital photography of tumor responses.
Procedures
Patients received intravenous pembrolizumab 200 mg every 3 weeks for 35 administrations—approximately 2 years—or until documented disease progression, unacceptable toxicity, or investigator’s or patient’s decision to withdraw. Clinically stable patients with first radiologic evidence of progressive disease per RECIST 1.1 were permitted to continue pembrolizumab treatment until confirmation of progressive disease (the Data Supplement includes details of treatment beyond progression).
Outcomes
The primary end point was ORR, as assessed by blinded independent central review using RECIST v1.1. Secondary end points included duration of response (DOR), disease control rate (DCR; CR, partial response [PR], or stable disease for ≥ 12 weeks), and progression-free survival (PFS), all of which were assessed by blinded independent central review using RECIST v1.1. Additional secondary end points were overall survival (OS) and safety and tolerability in the R/M cSCC cohort. Follow-up was defined as time from treatment start to data cutoff for all patients, regardless of death. Response was assessed every 6 weeks with radiologic/photographic imaging for year 1 and every 9 weeks thereafter until the end of treatment. Treatment decisions were made by investigators per immune-related RECIST (defined in the Data Supplement). Survival follow-up occurred every 12 weeks. Adverse events (AEs) were recorded throughout the study and for 30 days thereafter—90 days for serious AEs—and graded per Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analysis
The current open-label, phase II study was designed to allow periodic data monitoring by the sponsor to observe response rates and ensure adequate minimum follow-up time—6 months or more—at interim analyses. Results were based on assessing the maturity of the data in this open-label trial. Therefore, the first interim analysis occurred at approximately 18 months after study start, when 69% of responding patients had achieved 6 months or longer of durable response after confirmed response. Because of the single-arm design, no statistical hypothesis testing or multiplicity adjustment was planned. The current efficacy and safety analyses included all patients in the R/M cSCC cohort who received at least one dose of study treatment. For ORR or DCR, point estimates and 95% CIs were assessed using the Clopper and Pearson exact binomial method. With an expected proportion of objective response of at least 30% for patients with R/M cSCC, the study has greater than 95% power for the lower bound of the 95% CI to be more than 15%. DOR, PFS, and OS were estimated using the Kaplan-Meier method. Exploratory analyses of ORR, PFS, and OS by line of therapy, primary disease site, PD-L1 status, and disease status were also performed. Data cutoff was April 8, 2019.
Data-Sharing Statement
Merck Sharp & Dohme, a subsidiary of Merck & Co, Kenilworth, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized patient-level data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. The company is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The process includes submission of data requests to the MSD data-sharing website (available at: http://engagezone.msd.com/ds_documentation.php). Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing the requested data.
RESULTS
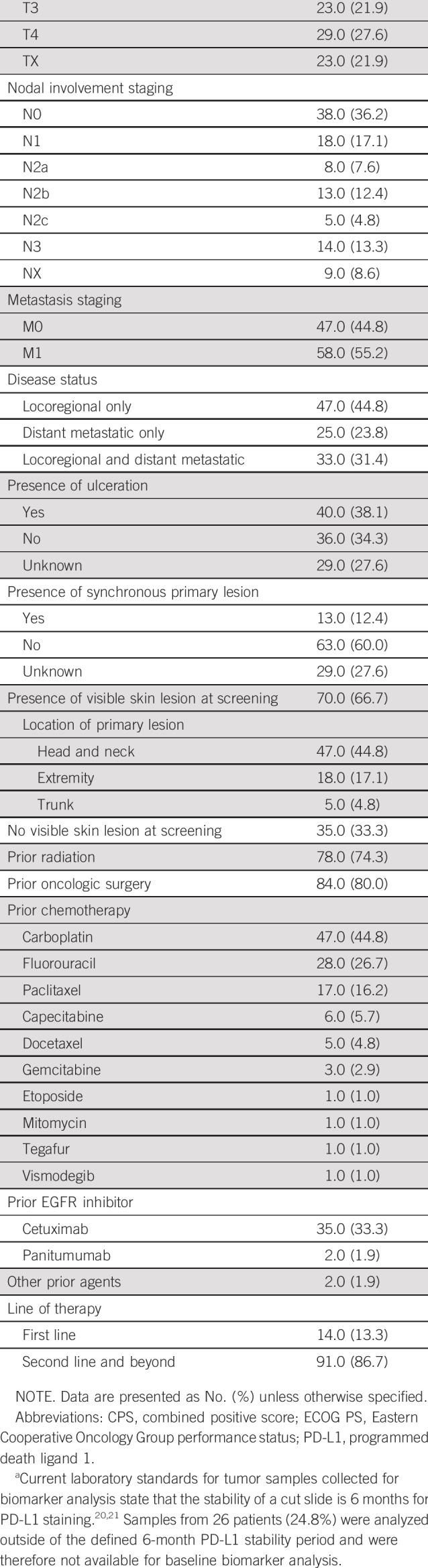
Between October 26, 2017, and June 15, 2018, 144 patients with R/M cSCC were screened, and 105 were enrolled and received at least one dose of pembrolizumab (Data Supplement). Median follow-up was 11.4 months (range, 0.4 to 16.3 months) for the overall population (N = 105). Sixty-three patients (60.0%) discontinued treatment, most commonly for disease progression (n = 37; 35.2%), reported as progressive disease per radiographic criteria RECIST v1.1 in 22 patients and clinical progression in 15 patients, and AEs (n = 13; 12.4%). At data cutoff, 42 patients (42.0%) continued to receive study treatment. Median duration of exposure was 5.8 months (range, 0.0 days to 16.1 months). Median age was 72 years (range, 29 to 95 years). Sixty-nine patients (65.7%) had a baseline Eastern Cooperative Oncology Group performance status of 1; 87 (82.9%) had stage IV disease; 78 (74.3%) previously received radiation therapy; 14 (13.3%) received first-line (1L) pembrolizumab (1L patients were allowed per protocol amendment 3), and 91 (86.7%) had previously received at least one systemic therapy20,21 (Table 1).
TABLE 1.
Baseline Characteristics in All Patients as Treated
Efficacy
Of 105 patients, 95 (90.5%) had baseline and at least 1 postbaseline imaging data. Eight patients (7.6%) had no postbaseline imaging assessment available, whereas 2 patients (1.9%) had postbaseline assessment that was not evaluable. The best overall confirmed response was CR in 4 patients (3.8%; 95% CI, 1.0% to 9.5%), and an additional 32 patients (30.5%; 95% CI, 21.9% to 40.2%) experienced confirmed PR. Thus, ORR was 34.3% (n = 36; 95% CI, 25.3% to 44.2%; Table 2). Stable disease was achieved in 31 patients (29.5%). DCR was 52.4% (n = 55; 95% CI, 42.4% to 62.2%; Table 2). Among the 76 patients with baseline and nonmissing postbaseline data for sum of the longest diameters of target lesions, 58 (76.3%) had a reduction from baseline in target lesion sizes, including 44 patients (57.9%) with at least a 30% reduction (Fig 1A).
TABLE 2.
Summary of Tumor Response in All Patients as Treated
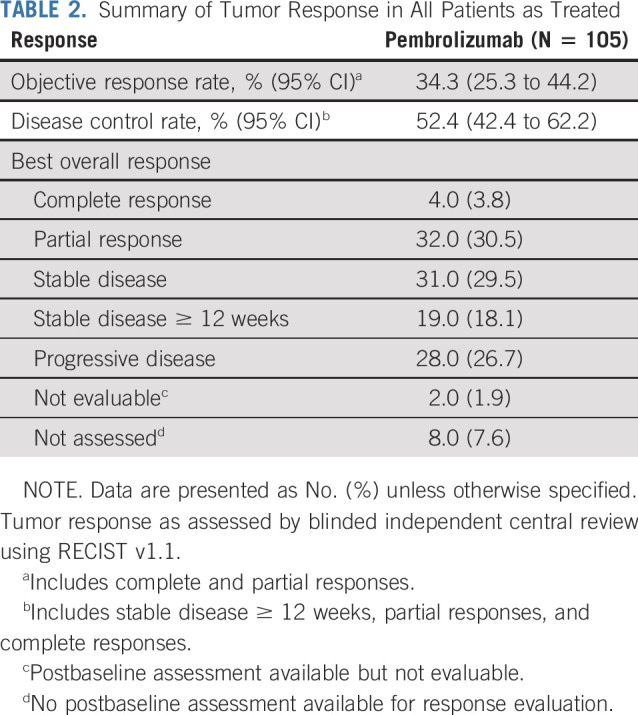
FIG 1.
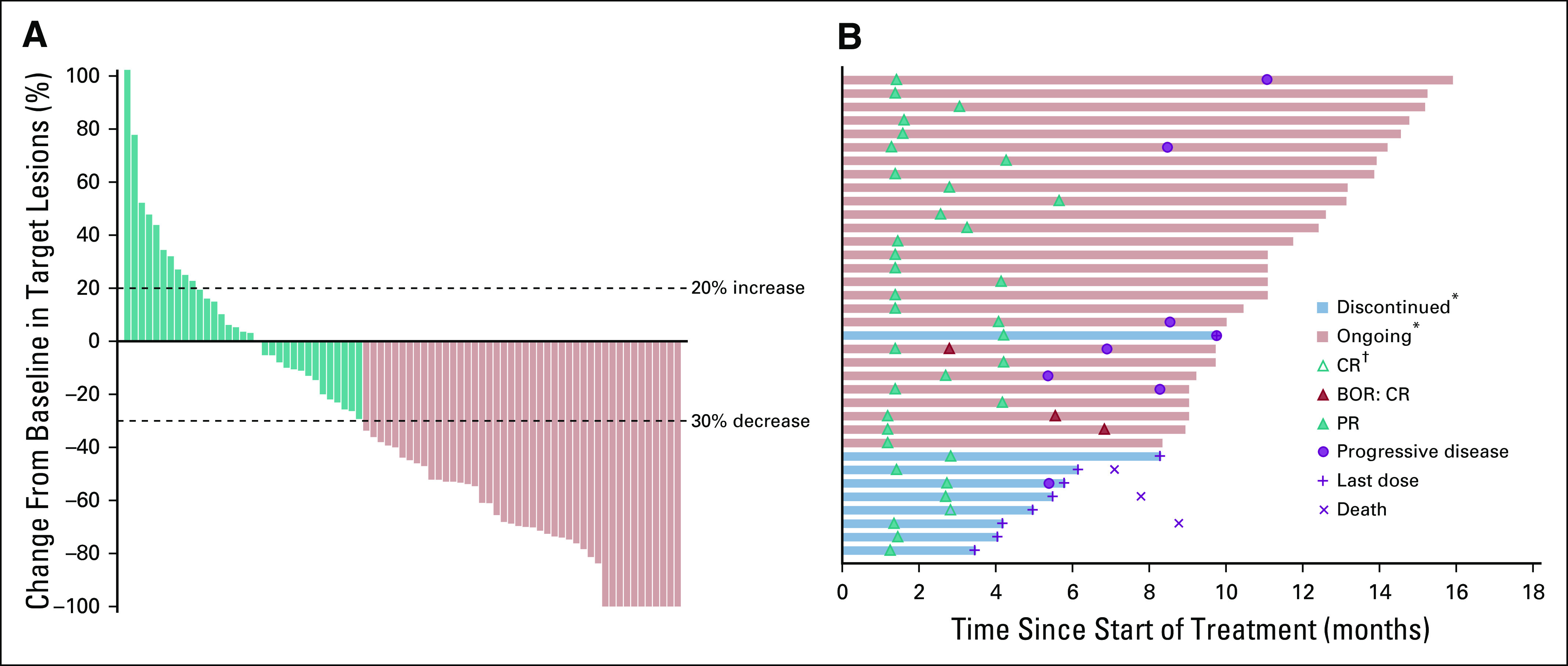
(A) Best percentage change from baseline in target lesion and (B) duration of study treatment and response in responders (n = 36) in all patients as treated. All patients had at least one postbaseline assessment of target lesion(s) (n = 76). Symbols for complete response (CR), partial response (PR), and progressive disease depict the first response to pembrolizumab. Symbols depict the timing of first objective response unless otherwise indicated. (*) Discontinued or ongoing refers to status in relation to study treatment. (†) Patient achieved a best overall response (BOR) of CR.
Median time to response was 1.5 months (range, 1.2 to 5.7 months; Fig 1B). Median DOR was not reached (NR; range, 2.7 to 13.1 + months; where ‘+’ indicates ongoing response at database cutoff; Data Supplement). Of the 36 responders, 31 and 7 had a minimum follow-up of 6 months and 12 months, respectively, after achieving response; 25 (79.5%) and 1 (65.6%) patients were estimated to have ongoing responses at 6 months or more and 12 months or more, respectively. Patients experienced rapid tumor reduction after 6 weeks of pembrolizumab treatment (Fig 2 and Data Supplement).
FIG 2.
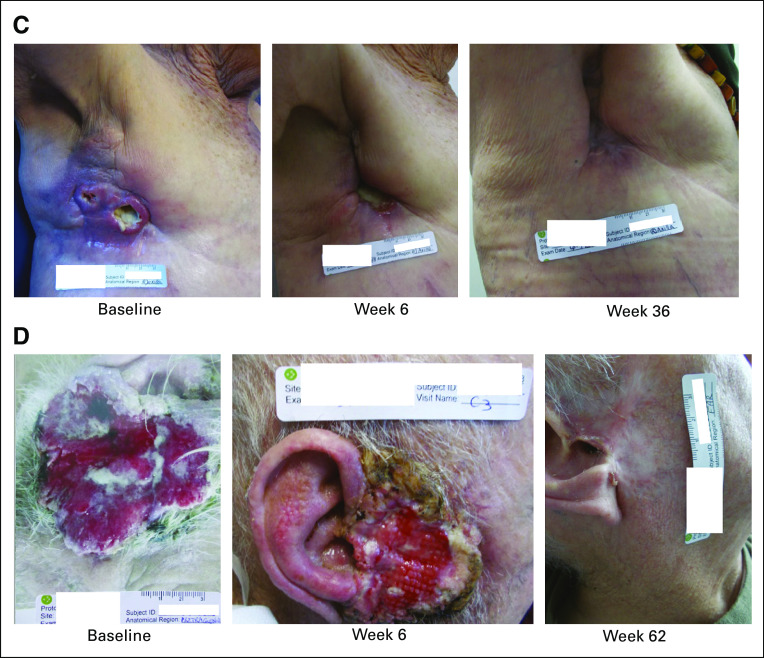
Effects of pembrolizumab monotherapy in patients with recurrent and/or metastatic cutaneous squamous cell carcinoma (cSCC). (A) An 80-year-old man with cSCC at the temple who previously underwent surgery at baseline (left), after 6 weeks of treatment (middle), and at the most recent follow-up (35 weeks; right). (B) An 87-year-old woman with cSCC at the jaw who previously received systemic therapy and radiation at baseline (left), after 6 weeks of treatment (middle), and at the most recent follow-up (37 weeks; right). (C) An 84-year-old woman with cSCC at the right axilla who previously underwent surgery and radiation at baseline (left), after 6 weeks of treatment (middle), and at the most recent follow-up (36 weeks; right). (D) A 92-year-old man with cSCC at the ear who previously received systemic therapy at baseline (left), after 6 weeks of treatment (middle), and at the most recent follow-up (62 weeks; right). Weeks are time since the date of the first dose of pembrolizumab.
At data cutoff, 64 patients (61.0%) had died or experienced disease progression. Median PFS was 6.9 months (95% CI, 3.1 months to 8.5 months; Fig 3A), and PFS rates were 50.4% (95% CI, 40.3% to 59.7%) at 6 months and 32.4% (95% CI, 22.6% to 42.5%) at 12 months. Of the 105 patients, 40 (38.1%) had died. Median OS was NR (95% CI, 10.7 months to NR; Fig 3B), and OS rates were 79.0% (95% CI, 69.9% to 85.7%) at 6 months and 60.3% (95% CI, 49.8% to 69.3%) at 12 months.
FIG 3.
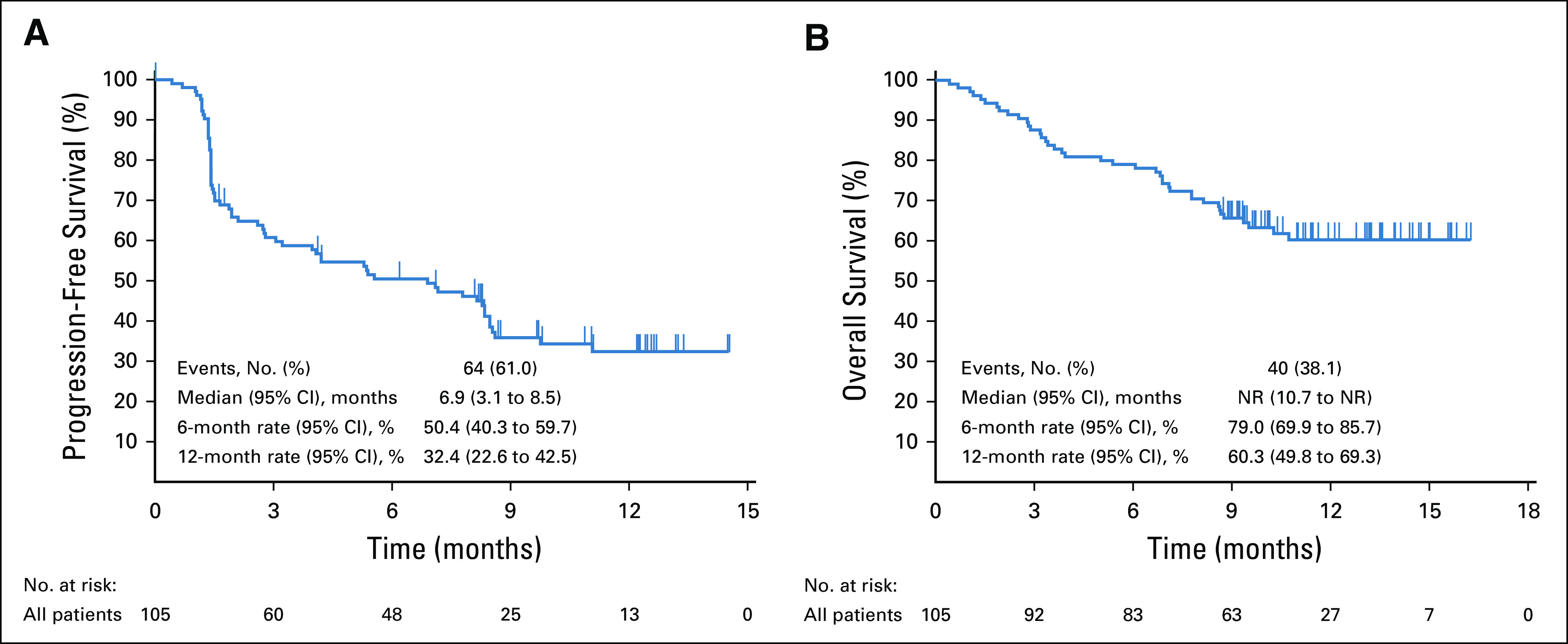
Kaplan-Meier estimates of (A) progression-free survival by blinded independent central review using RECIST v1.1 and (B) overall survival in all patients as treated. NR, not reached.
Treatment Beyond Initial RECIST v1.1 Disease Progression
Twenty-nine patients received pembrolizumab treatment beyond disease progression. Of these, 1 patient achieved a best overall response of CR and 7 had PR per immune-related RECIST. Response was ongoing in 4 patients (Data Supplement).
Subgroup Analysis of Efficacy
Exploratory subgroup analysis was conducted by line of therapy, disease extension, primary disease site, and PD-L1 combined positive score (CPS) status (Data Supplement).
At data cutoff, median follow-up was 9.2 months (range, 8.9 to 11.2 months) for 14 patients who received 1L pembrolizumab and were enrolled after protocol amendment 3. These 14 patients had shorter duration of follow-up than the remaining 91 patients. In the 1L subgroup, ORR was 50.0% (n = 7 [2 CR and 5 PR]; Data Supplement). Median PFS was 8.3 months, and PFS rates were 70.7% at 6 months and NR at 12 months. Median OS was NR, and OS rates were 78.6% at 6 months and NR at 12 months.
At data cutoff, median follow-up was 12.1 months (range, 9.0 to 16.2 months) for the 91 patients who received second-line (2L) or later pembrolizumab. In the 2L or later subgroup, ORR was 31.9% (n = 29 [2 CR and 27 PR]; Data Supplement). Median PFS was 5.4 months, and 6- and 12-month PFS rates were 47.3% and 32.2%, respectively. Median OS was NR, and 6- and 12-month OS rates were 79.1% and 61.3%, respectively.
In the 47 patients with locoregional-only disease, ORR was 36.2% (n = 17 [1 CR and 16 PR]), median PFS was 7.1 months, and median OS was NR. In the 58 patients with distant metastatic disease, ORR was 32.8% (n = 19 [3 CR and 16 PR]), median PFS was 5.4 months, and median OS was NR.
In the 47 patients who had cSCC tumors with primary head and neck location, ORR was 42.6% (n = 20 [1 CR and 19 PR]), median PFS was 8.5 months, and median OS was NR. In the 58 patients with cSCC tumors at other primary locations, ORR was 27.6% (n = 16 [3 CR and 13 PR]) and median PFS and OS were 4.2 months and 10.3 months, respectively.
In the 69 patients with PD-L1 CPS 1 or greater tumors, ORR was 33.3% (n = 23 [2 CR and 21 PR]), median PFS was 5.4 months, and median OS was NR. In the 10 patients with PD-L1 CPS less than 1 tumors, ORR was 20.0% (n = 2 [2 PR]), median PFS was 4.2 months, and median OS was NR.
Safety
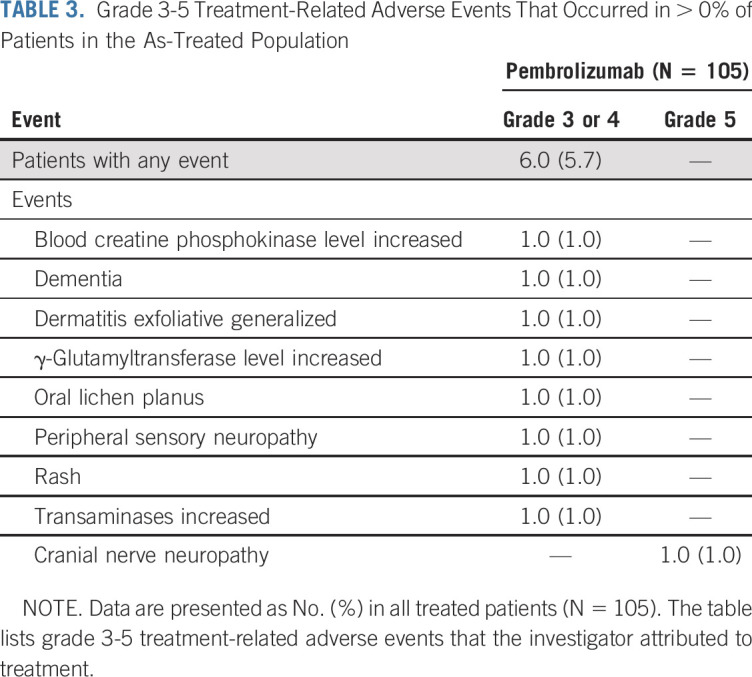
One hundred two patients (97.1%) experienced at least 1 AE (Data Supplement), and 70 patients (66.7%) experienced a treatment-related adverse event (TRAE). The most common any-grade TRAEs were pruritus (n = 15; 14.3%), asthenia (n = 14; 13.3%), and fatigue (n = 13; 12.4%; Data Supplement). Most TRAEs were mild to moderate; 6 patients (5.7%) experienced grade 3 to 5 TRAEs (Table 3). Treatment-related serious AEs occurred in 7 patients (6.7%; Data Supplement). One patient died of treatment-related cranial nerve neuropathy (details in the Data Supplement).
TABLE 3.
Grade 3-5 Treatment-Related Adverse Events That Occurred in > 0% of Patients in the As-Treated Population
Overall, AEs led to treatment interruption in 28 patients (26.7%) and treatment discontinuation in 13 patients (12.4%; Data Supplement). TRAEs led to treatment interruption in 9 patients (8.6%) and treatment discontinuation in 5 patients (4.8%; Data Supplement). Twenty-three patients (21.9%) experienced at least 1 immune-mediated AE. The most frequently reported immune-mediated AEs were hypothyroidism (n = 10; 9.5%), severe (grade ≥ 3) skin reactions (n = 5; 4.8%), pneumonitis (n = 4; 3.8%), adrenal insufficiency (n = 3; 2.9%), and hyperthyroidism (n = 3; 2.9%; Data Supplement). Most immune-mediated AEs were grade 1 or 2 and nonserious. There were no grade 4 to 5 immune-mediated AEs.
DISCUSSION
At the first interim analysis of KEYNOTE-629, pembrolizumab monotherapy demonstrated clinically meaningful antitumor activity and was generally well tolerated in patients with R/M cSCC. With 11.4 months of median follow-up, pembrolizumab demonstrated an ORR of 34.3%, and 76.3% of patients experienced a reduction in target lesion size from baseline. Disease control was maintained in 52.4% of patients. Pembrolizumab elicited rapid, durable responses, with a median time to response of 1.5 months. Median DOR was NR. Furthermore, in the 29 patients who received pembrolizumab treatment beyond progression, additional benefit was observed (ORR, 27.6%).
Subgroup analysis by line of therapy showed a promising response rate of 50.0% with 1L pembrolizumab and 31.9% in heavily pretreated patients receiving pembrolizumab as 2L or later therapy. Strikingly, with a median follow-up of 11.4 months, the PFS rate at 12 months was estimated to be 32.4% in the total population. The same 12-month PFS rate was observed with 2L or later pembrolizumab (32.2%). Because of the small sample size of patients on 1L therapy, the 12-month PFS rate was not evaluated. cSCC arising from the head and neck region has historically been recognized to be more aggressive and difficult to treat than other sites of the body.22 Of interest, pembrolizumab treatment demonstrated effective antitumor activity in patients who had tumors with a primary head and neck location; both median PFS and OS were longer than in patients with cSCC at other anatomic sites. Thus, whereas increased efficacy in this subgroup of patients with high-risk disease and worse outcomes is notable, additional data are required to validate whether the response to pembrolizumab therapy differs based on the anatomic location of the disease.22-24 Biomarker subgroup analysis by PD-L1 CPS showed pembrolizumab treatment benefit regardless of PD-L1 expression. Efficacy of pembrolizumab was comparable in locoregional-only and distant metastatic cSCC subgroups.
Pembrolizumab was well tolerated in this elderly population, including patients up to age 95 years. Most TRAEs were mild or moderate, and no new safety signals were identified. The safety profile was consistent with the well-established safety profile of pembrolizumab monotherapy in HNSCC.15-18
Pembrolizumab demonstrated comparable responses and similar or favorable safety compared with historical data from systemic therapies in R/M cSCC.25-28 Treatment with monotherapy agents, including EGFR inhibitors, in an R/M population offered lower response rates (11% to 31%), with a median DOR of 6 months. Overall, the side effects were more tolerable than those of chemotherapy.27,28 To our knowledge, the current report is the largest clinical trial (n = 105) in patients with R/M cSCC, with a population size greater than that of aforementioned chemotherapy studies (n = 3 to 7)25,26 or EGFR studies (n = 16 to 36).27,28 The ORR of pembrolizumab exceeded that of cetuximab or panitumumab.27,28 Of note, the median DOR with pembrolizumab was NR after 11.4 months of follow-up, which is uncommon with chemotherapy or EGFR inhibitors.25-28
Cemiplimab has demonstrated efficacy and safety in early-phase studies among patients with LA or metastatic cSCC. In the metastatic cSCC cohort (n = 59), 50 patients (85%) previously received radiation therapy and 56% (n = 33) previously received systemic therapy, whereas 44% (n = 26) previously received no therapy.14 The ORR was 47% (95% CI, 34% to 61%) and the DCR was 61% (95% CI, 47% to 74%), with a median follow-up of 7.9 months. The median DOR was NR; however, among the 28 responders, 16 patients (57%) had ongoing responses for 6 months or longer.14 Pembrolizumab was studied in a patient population in whom 86.7% had previously received 1 or more lines of systemic therapy. The 1L pembrolizumab subgroup more closely corresponds to the metastatic cohort in the cemiplimab study. In the 1L pembrolizumab subgroup of the current study, ORR reached 50.0%, which is similar to that of cemiplimab; however, the 1L pembrolizumab subgroup had a shorter median follow-up (9.2 months) than that of the overall population of the current study (11.4 months), and the 12-month PFS rate was not estimable in this small subgroup. Therefore, comparison of 12-month PFS rates of the 1L pembrolizumab subgroup with the cemiplimab results will require additional follow-up of the 1L subgroup. Collectively, although cross-trial comparisons should be cautiously interpreted, response results with pembrolizumab and cemiplimab monotherapy seem comparable.
Recently, the phase II CARSKIN study evaluated 1L pembrolizumab in 39 patients with chemotherapy-naive, unresectable locally or regionally advanced or metastatic cSCC, 21% of whom had metastatic disease.29 The 15-week response rate was 38.5% (95% CI, 24% to 55%), and at 15 weeks 2 patients achieved CR and 13 achieved PR.29 Median PFS was 8.4 months, and median OS was NR.29 These findings are comparable to the current results of 1L pembrolizumab—with a median PFS of 8.3 months and median OS NR. The safety profile from CARSKIN was consistent with that of the current study: TRAEs in 67% of patients.29
Although the current report involves R/M cSCC, pembrolizumab is also being evaluated in patients with unresectable LA disease for whom no available localized treatments are feasible. In addition, the ongoing randomized, double-blind, placebo-controlled phase III study KEYNOTE-630 (ClinicalTrials.gov identifier: NCT03833167) evaluates pembrolizumab as adjuvant therapy after surgery and radiation in patients with high-risk LA cSCC.
Study limitations include the single-arm design and duration of follow-up at this interim analysis. Despite limited follow-up, the promising antitumor activity, durable responses, and tolerability of pembrolizumab in this challenging setting of R/M cSCC, in which most patients were elderly, provide a compelling reason to report these results. Follow-up of patients is ongoing, and longer follow-up will be reported as results become available.
Overall, pembrolizumab provides a therapeutic benefit and offers an effective treatment option for patients with R/M cSCC. The observations in this phase II trial highlight the role of pembrolizumab monotherapy as an attractive treatment option in this patient population. While this article was in press, pembrolizumab was approved by the US Food and Drug Administration for the treatment of patients with recurrent or metastatic cSCC.30
ACKNOWLEDGMENT
The authors thank the patients and their families and all investigators and site personnel. Medical writing and/or editorial assistance was provided by Holly C. Cappelli, PhD, CMPP, and Doyel Mitra, PhD, CMPP, of the ApotheCom pembrolizumab team (Yardley, PA). This assistance was funded by Merck Sharp & Dohme, a subsidiary of Merck & Co, Kenilworth, NJ.
PRIOR PRESENTATION
Presented at the 2019 European Society for Medical Oncology Annual Meeting, Barcelona, Spain, September 27-October 1, 2019.
SUPPORT
Funded by Merck Sharp & Dohme, a subsidiary of Merck & Co, Kenilworth, NJ
AUTHOR CONTRIBUTIONS
Conception and design: Jean-Jacques Grob, Burak Gumuscu, Ramona F. Swaby
Provision of study materials or patients: Nicole Basset-Seguin, Olga Vornicova, Nicolas Meyer, Josep M. Piulats, Ramona F. Swaby, Brett G. M. Hughes
Collection and assembly of data: Jean-Jacques Grob, Olga Vornicova, Nicolas Meyer, Florent Grange, Josep M. Piulats, Jessica R. Bauman, Burak Gumuscu, Ramona F. Swaby, Brett G. M. Hughes
Data analysis and interpretation: Jean-Jacques Grob, Rene Gonzalez, Nicole Basset-Seguin, Olga Vornicova, Jacob Schachter, Abhishek Joshi, Nicolas Meyer, Josep M. Piulats, Jessica R. Bauman, Pingye Zhang, Burak Gumuscu, Ramona F. Swaby, Brett G. M. Hughes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jean-Jacques Grob
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma, Merck, Roche
Speakers Bureau: Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Nicole Basset-Seguin
Honoraria: Sun Pharma, MSD Oncology (Inst), Sanofi, Pierre Fabre
Consulting or Advisory Role: Sun Pharma, Sanofi
Travel, Accommodations, Expenses: Roche
Olga Vornicova
Consulting or Advisory Role: Novartis, Merck Serono
Speakers Bureau: MSD Oncology, Bristol-Myers Squibb, Novartis, Medison
Research Funding: MSD Oncology, Bristol-Myers Squibb, Novartis, Roche, Eli Lilly
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis, Medison, Pfizer, MSD Oncology
Jacob Schachter
Honoraria: Bristol-Myers Squibb, MSD Oncology
Consulting or Advisory Role: MSD Oncology, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Abhishek Joshi
Honoraria: AstraZeneca, Pfizer, Ipsen
Travel, Accommodations, Expenses: Roche, Novartis, Ipsen, Bristol-Myers Squibb, Pfizer, Merck Sharp & Dohme
Nicolas Meyer
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Genentech, Novartis, Pierre Fabre, AbbVie
Research Funding: Bristol-Myers Squibb (Inst), MSD Oncology (Inst)
Florent Grange
Consulting or Advisory Role: Novartis, MSD Oncology
Josep M. Piulats
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, VCN Biosciences, Clovis Oncology, Genentech, Bristol-Myers Squibb, Merck Sharp & Dohme, BeiGene
Research Funding: Bristol-Myers Squibb, AstraZeneca, MedImmune, Merck Sharp & Dohme, Pfizer, EMD Serono, Incyte, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology, Roche, Bristol-Myers Squibb
Jessica R. Bauman
Consulting or Advisory Role: Pfizer, Bayer, AstraZeneca, Kura
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Trident Therapeutics
Pingye Zhang
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Burak Gumuscu
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Ramona F. Swaby
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Brett G. M. Hughes
Consulting or Advisory Role: MSD Oncology, Bristol-Myers Squibb, Roche, Pfizer, Boehringer Ingelheim, AstraZeneca, Eisai, Sanofi, Regeneron
Research Funding: Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Gurney B, Newlands C. Management of regional metastatic disease in head and neck cutaneous malignancy. 1. Cutaneous squamous cell carcinoma. Br J Oral Maxillofac Surg. 2014;52:294–300. doi: 10.1016/j.bjoms.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: A review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491–508. doi: 10.1007/s40257-016-0207-3. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Veness MJ, Ch’ng S, et al. Distant metastases from cutaneous squamous cell carcinoma: Analysis of AJCC stage IV. Head Neck. 2013;35:72–75. doi: 10.1002/hed.22913. [DOI] [PubMed] [Google Scholar]
- 5.Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope. 2005;115:870–875. doi: 10.1097/01.MLG.0000158349.64337.ED. [DOI] [PubMed] [Google Scholar]
- 6.Veness MJ, Palme CE, Morgan GJ. High-risk cutaneous squamous cell carcinoma of the head and neck: Results from 266 treated patients with metastatic lymph node disease. Cancer. 2006;106:2389–2396. doi: 10.1002/cncr.21898. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network NCCN Practice Guidelines in Oncology: Squamous cell skin cancer (version 2.2019) https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf
- 8.Stratigos A, Garbe C, Lebbe C, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 9.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, He YY. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014;1:188–198. doi: 10.1016/j.gendis.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 13.Slater NA, Googe PB. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J Cutan Pathol. 2016;43:663–670. doi: 10.1111/cup.12728. [DOI] [PubMed] [Google Scholar]
- 14.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 15.Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 18.Rischin D, Harrington KJ, Greil G, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) J Clin Oncol. 2019;37(suppl; abstr 6000) [Google Scholar]
- 19.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dako North America PD-L1 IHC 22C3 pharmDx: 50 tests for use with Autostainer Link 48. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013c.pdf
- 21.Garon EB, Rizvi N, Hui R, et al. Efficacy of pembrolizumab (MK-3475) and validation of PD-L1 expression as a biomarker in patients with non-small cell lung cancer (NSCLC): findings from KEYNOTE-001. AACR 106th Annual Meeting; Philadelphia, PA. April 18-22, 2015; [Google Scholar]
- 22.Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013;149:541–547. doi: 10.1001/jamadermatol.2013.2139. [DOI] [PubMed] [Google Scholar]
- 23.Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: A systematic review and meta-analysis. JAMA Dermatol. 2016;152:419–428. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brougham ND, Dennett ER, Cameron R, et al. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106:811–815. doi: 10.1002/jso.23155. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie TH, Jr, McElveen LJ, Porubsky ES, et al. Cisplatin and doxorubicin. An effective chemotherapy combination in the treatment of advanced basal cell and squamous carcinoma of the skin. Cancer. 1985;55:1629–1632. doi: 10.1002/1097-0142(19850415)55:8<1629::aid-cncr2820550802>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Khansur T, Kennedy A. Cisplatin and 5-fluorouracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer. 1991;67:2030–2032. doi: 10.1002/1097-0142(19910415)67:8<2030::aid-cncr2820670803>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 28.Foote MC, McGrath M, Guminski A, et al. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann Oncol. 2014;25:2047–2052. doi: 10.1093/annonc/mdu368. [DOI] [PubMed] [Google Scholar]
- 29.Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): Phase 2 results from CARSKIN. J Clin Oncol. 2019;37(suppl; abstr 9547) doi: 10.1200/JCO.19.03357. [DOI] [PubMed] [Google Scholar]
- 30. Keytruda (pembrolizumab) injection, for intravenous use [package insert]. Whitehouse Station, NJ: Merck & Co, 2020.