BACKGROUND
Considerable advances in early detection and combination therapy during the past 70 years1 have contributed to significant cancer-specific survival gains, with an estimated 17 million individuals currently living with a history of cancer in the United States, a number expected to reach 26 million by the year 2040.2 The financial investment to achieve such progress, of course, has been enormous.3,4 Intriguingly, despite the increasing sophistication and cost of contemporary cancer care,5,6 evaluation of patient performance status (PS)—an integral aspect of treatment selection, toxicity monitoring, and clinical trial eligibility—has remained essentially unchanged since 1948.7 In this commentary, we provide a historical overview and critical evaluation of PS assessment in oncology. We also discuss alternative approaches to PS assessment that may improve prognostication and risk stratification in research and clinical practice.
PS SCALES: 72 YEARS AND COUNTING
In 1948, Karnofsky and colleagues7 used three criteria to evaluate nitrogen mustard efficacy in lung cancer: objective improvement (eg, decrease in lesion size), subjective improvement (eg, patient-reported symptoms), and PS (ie, patients’ ability to participate in activities of daily life). Evaluation of PS was standardized using a physician-rated scale, now known as Karnofsky Performance Status (KPS), ranging from 0 (dead) to 100 (well functioning) with 10-point increments.7 The simpler 6-point Eastern Cooperative Oncology Group (ECOG) PS scale (0 [fully active] to 5 [dead]) was introduced by Zubrod and colleagues8 in 1960 as one of 15 standardized assessments for all ECOG multicenter clinical trials. The intended purpose of the ECOG PS scale was to evaluate patient reaction to chemotherapy, along with patient-reported pain, nausea, and appetite.8 Both clinician-administered scales were used primarily to evaluate therapeutic efficacy until 1973, when Zelen concluded that failure to consider PS in clinical trial eligibility “will introduce so much variability and bias into the trial that real differences between therapies are likely to be missed altogether.”9(p34) As a result, subsequent use of PS scales in clinical trials transitioned from the assessment of therapeutic efficacy to eligibility (ie, KPS ≤ 60 or ECOG ≥ 2 ineligible)10-13 and stratification (ie, more homogenous subgroups).14-16 Both the KPS and ECOG scales are strong independent predictors of clinical outcome in numerous oncology populations.17-19 They are also inexpensive and feasible to implement in all oncology clinical settings. Consequently, the KPS and ECOG scales, and therefore the assessment of PS, remain integral tools in contemporary practice incorporated into virtually every clinical visit across the entire cancer care continuum.
KPS AND ECOG: ROOM FOR IMPROVEMENT
The demonstrated clinical value of KPS and ECOG directly contradicts why one would advocate for their replacement. Nevertheless, closer inspection of the KPS and ECOG scales reveal significant limitations. First, these scales are clinician based and therefore subjective, with poor reliability and validity.20 In a meta-analysis of 15 studies representing 2,808 patients, agreement between clinicians on KPS/ECOG scores was deemed moderate (Pearson correlation coefficients, 0.71 to 0.78).21 In addition, patient and clinician PS scores have low agreement,22 with up to 50% of patient-reported functional limitations missed by clinicians23 and adverse PS change (ie, KPS < 60) reported approximately 15 months earlier by patients than clinicians.24 Misclassification of PS has obvious implications for clinical trial eligibility as well as planned best practice therapy, with some patients classified as having sufficient PS to tolerate therapy but having nascent impairment, and conversely, another proportion classified as having insufficient PS yet with considerable reserve capacity and able to tolerate therapy.25-27 Second, poor KPS and ECOG scores are strong predictors of prognosis,17-19 which is not surprising when impairment is obvious; however, the prognostic value in patients with good PS is limited. A meta-analysis of 66 phase II and III randomized controlled trials (n = 44,511 patients with ECOG 0-2)18 found no differences in clinical outcomes between patients with either ECOG scores of 0, 1, or 2, suggesting that ECOG provides essentially no additional prognostic information for patients with no obvious physical impairments. Third, KPS/ECOG may have limited use for toxicity risk stratification in the era of contemporary practice. Use of PS scales for toxicity risk prediction is based on work in the 1980s that showed a higher incidence of chemotherapy toxicity in patients classified with poor PS (ie, KPS ≤ 60; ECOG ≥ 2).11,26,28 The safety profile of current treatment regimens, however, has evolved considerably, in part because of the use of supportive polytherapy care, the safety profile of modern scheduling approaches/lower-dose combinations, and the use of molecularly targeted agents.29 Indeed, a retrospective study of 16,233 patients with solid tumors receiving contemporary chemotherapy regimens found negligible differences in relative dose intensity between patients with ECOG PS of 0 versus 1-3.30 Finally, KPS/ECOG scales are only administered during in-person clinic visits, often weeks, if not months, apart; therefore, a snapshot of PS is captured, which limits the ability to detect more subtle real-time changes of potential clinical importance.
Overall, given the highlighted limitations, it is somewhat remarkable that PS scales exhibit any clinical value. We posit that the reason KPS/ECOG have widespread applicability is because they provide insight into PS, a metric with substantial importance in clinical populations. PS measurement, albeit using a variety of different assessment tools, is of central importance in virtually every area of clinical disease management.31 We further suggest that more objective, discriminatory, and dynamic tools may actually augment the value of PS assessment in the oncology setting beyond that currently possible with KPS and ECOG scales.
ALTERNATIVE PS MEASURES
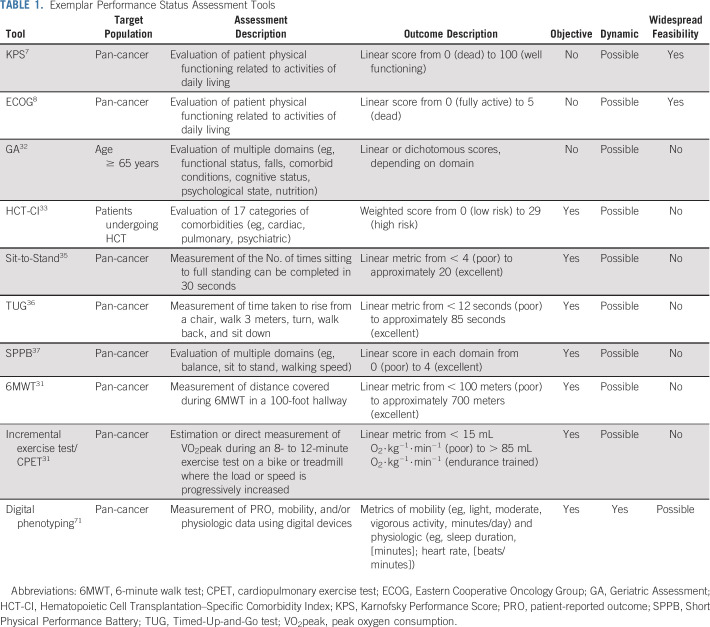
Alternative tools with which to assess PS or aspects of PS have been explored in the oncology setting (Table 1). For instance, the geriatric assessment32 (patients age > 65 years) and the hematopoietic cell transplantation–specific comorbidity index33 (hematologic malignancies) are multiparametric tools that evaluate different PS domains (eg, activities-of-daily-living questionnaire) and/or comorbidity indices (eg, cardiac disease). Preliminary data suggest that both tools improve toxicity and survival prediction beyond PS,32,33 leading to widespread clinical uptake, at least in certain oncology settings. Both tools are limited, however, as they are population specific and typically assessed at one time point.34 Therefore, pan-cancer assessment tools that provide an objective and dynamic PS evaluation must be identified.
TABLE 1.
Exemplar Performance Status Assessment Tools
Numerous standardized tests that include counting repetitions or timing an activity have been developed to evaluate PS in noncancer clinical populations. The Sit-to-Stand Test,35 Timed-Up-and-Go test (TUG)36 and Short Physical Performance Battery37 were developed to assess lower-extremity strength, balance, and mobility in frail elderly individuals. In patients with cancer age ≥ 70 years, both poor TUG test38 and Short Physical Performance Battery39 have been associated with an approximately two-fold increased risk of mortality. The 6-minute walk test, originally developed for chronic obstructive pulmonary disease,31,40 is a robust predictor of mortality in respiratory and cardiac diseases,31,41 whereas preliminary data in patients with cancer indicate a 6-minute walk test result of < 350 meters predicts mortality risk, even after adjustment for KPS.42,43 However, these tools fail to discriminate between patients who are classified with good PS44 and will likely not detect or identify therapy-related toxicity.41,45
Cardiorespiratory fitness (CRF), first quantified by Hill and Lupton in 1923,46 provides an objective assessment of the integrative capacity of the cardiovascular, pulmonary, hematopoietic, and musculoskeletal systems to transport and use oxygen.31 The gold standard assessment of CRF is a cardiopulmonary exercise test coupled with automated gas exchange assessment, which provides direct quantification of submaximal and peak oxygen consumption (VO2peak).31 Prediction equations that are based on achieved exercise workload can also estimate VO2peak. In chronic lung and cardiac conditions, CRF is considered a clinical vital sign, with assessment recommended across all phases of clinical decision making.31,47,48 In cancer, Reichel49 first reported in 1972 that presurgical estimated CRF was a stronger predictor of postoperative complications compared with standard clinical metrics (eg, age and pulmonary function) in lung cancer, concluding that CRF “should be used routinely in the evaluation of the candidate for pneumonectomy.”49(p576) Subsequently, the tolerability (ie, more than 80% of patients achieve peak criteria) and safety (ie, no exercise-related deaths and an approximate 15% nonserious adverse event rate) of CRF was established in a systematic review of 90 studies representing approximately 5,200 patients.45 Work in lung,50,51 GI,52 hepatobiliary and pancreatic,53 and hematologic malignancies54 show that CRF is an independent predictor of postoperative complications and mortality.55-59 Presurgical CRF stratification values are defined for lung47 and colorectal cancer,58 with less than 15 mL O2⋅kg−1⋅min−1 associated with an elevated risk of complications, whereas less than 10 mL O2⋅kg−1⋅min−1 is considered to be associated with a high risk of complications.47 Poor CRF is also associated with a higher prevalence of acute and chronic treatment-related late effects55-59 and all-cause, cardiovascular, and cancer mortality.60 Despite major advantages, CRF requires specialized equipment and trained personnel, likely limiting its widespread clinical application.48,61
In summary, despite promising findings, none of the aforementioned tools is currently incorporated into routine clinical practice. In addition to the dearth of evidence that supports the clinical use of such tools, a fundamental weakness of all tools is the stark discrepancy in practicality compared with the KPS/ECOG scales. There is a critical need for tools that can dynamically and objectively assess PS with the widespread feasibility of the KPS/ECOG scales.
THE DIGITAL FRONTIER
Advances in health technology may provide unprecedented opportunities to improve PS assessment in cancer. Most previous and ongoing studies leveraging technology focus on monitoring objective mobility via wearable devices. For instance, accelerometers are small, wireless, digital devices providing objective mobility measurement (eg, steps per day and/or minutes per day of light, moderate, and vigorous activity).62,63 Saint-Maurice et al64 found that, compared with less than 4,000 steps per day, more than 8,000 steps per day was associated with a lower risk of all-cause and cancer-specific mortality in 4,840 apparently healthy individuals. Preliminary findings from two studies in patients with advanced disease65 and those undergoing surgical tumor resection66 indicate that lower steps per day and total activity per day are associated with increased risk of death and postsurgical complications, respectively. Few studies are performing deep, dynamic phenotyping using multiparametric platforms; however, the rapid expansion of consumer-grade digital mobile devices—for example, Fitbit, Apple iWatch, smartphones—with integrated multisensory systems may offer a unique opportunity for digital phenotyping. Such devices dynamically generate an abundance of unlabeled sensor data points per day, including mobility and physiologic data, such as heart rate and sleep.67-70 The feasibility of digital phenotyping was recently demonstrated in work showing that 250,000 daily measurements in 43 individuals over 11 months were used to first develop personalized, activity-based baseline normative data, and abnormal phys-iologic signals from longitudinal data were subsequently used to identify early signs of disease.71 An ongoing prospective cohort study in 2,500 patients with heart failure (ClinicalTrials.gov identifier: NCT03810638) will create a digital registry that combines patient-reported outcomes (PROs), mobility, and electronic health record data to monitor changes in quality of life and other clinical outcomes. These are examples of the type of digital phenotyping studies needed in oncology. We posit that the integration of digital technologies assessing physiologic (eg, heart rate), mobility (eg, steps per day), and PRO (eg, PRO-Common Terminology Criteria for Adverse Events),72 as well as electronic health record data, may be of significant value for treatment selection, clinical trial eligibility, and toxicity monitoring in the oncology setting.
Several challenges remain to realize the potential of digital phenotyping in the oncology setting. Policies governing the privacy and security of patient data captured from digital mobile devices will need to be defined. Research efforts will require development and validation data sets and robust statistical tools, such as discrimination, calibration, and reclassification, to discern whether digital phenotyping–related data provide incremental value beyond the KPS/ECOG scales. Subsequent randomized trials that assess whether baseline/change in digital biomarkers improve current patient stratification approaches for informing clinical decision making are needed. Data repositories that allow for the simultaneous integration, query, and visualization of large amounts of heath data with artificial intelligence tools73,74 are needed to seamlessly integrate digital phenotyping data into clinical workflows.75 Overcoming such challenges is a US Food and Drug Administration priority, with efforts underway to advance the standardization of PS data in oncology.76
In conclusion, the prognostic utility and widespread applicability of the KPS/ECOG scales reflect the central importance of PS in patients with cancer. Nevertheless, the complex and heterogeneous nature of cancer management has accentuated the need for the development and validation of objective and dynamic measures of PS that can accurately discriminate between patients across the continuum of cancer care in all settings. The clinical impact of such measures would be considerable.
Footnotes
Supported by research grants from the National Cancer Institute (J.M.S. and L.W.J.), AKTIV Against Cancer and the Kavli Trust (J.M.S., G.S., E.E., and L.W.J.), and Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30-CA008748 (J.M.S. and L.W.J.).
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Performance Status in Cancer: Not Broken, But Time for an Upgrade?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elisabeth Edvardsen
Employment: GlaxoSmithKline
Lee W. Jones
Stock and Other Ownership Interests: Pacylex
No other potential conflicts of interest were reported.
REFERENCES
- 1.DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA. 2020;323:844–853. doi: 10.1001/jama.2020.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgardner JR, Shahabi A, Linthicum MT, et al. Share of oncology versus nononcology spending in episodes defined by the Centers for Medicare & Medicaid Services oncology care model. J Oncol Pract. 2018;14:e699–e710. doi: 10.1200/JOP.18.00309. [DOI] [PubMed] [Google Scholar]
- 6.Manz CR, Porter DL, Bekelman JE. Innovation and access at the mercy of payment policy: The future of chimeric antigen receptor therapies. J Clin Oncol. 2020;38:384–387. doi: 10.1200/JCO.19.01691. [DOI] [PubMed] [Google Scholar]
- 7.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 8.Zubrod CG, Schneiderman MA, Frei E, III, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 9.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 10.Selawry OS. Initial therapeutic trial of new drugs in lung cancer. Cancer Chemother Rep 3. 1973;4:215–225. [PubMed] [Google Scholar]
- 11.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 12.Vincent RG, Pickren JW, Fergen TB, et al. Evaluation of methotrexate in the treatment of bronchogenic carcinoma. Cancer. 1975;36:873–880. doi: 10.1002/1097-0142(197509)36:3<873::aid-cncr2820360308>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Baker LH, Vaitkevicius VK, Gehan E. Randomized prospective trial comparing 5-fluorouracil (NSC-19893) to 5-fluorouracil and methyl-CCNU (NSC-95441) in advanced gastrointestinal cancer. Cancer Treat Rep. 1976;60:733–737. [PubMed] [Google Scholar]
- 14.Wolf J. Nitrosoureas as single agents in the treatment of pulmonary cancer. Cancer Treat Rep. 1976;60:753–756. [PubMed] [Google Scholar]
- 15.Ahmann DL, Bisel HF, Hahn RG, et al. An analysis of a multiple-drug program in the treatment of patients with advanced breast cancer utilizing 5-fluorouracil, cyclophosphamide, and prednisone with or without vincristine. Cancer. 1975;36:1925–1935. doi: 10.1002/cncr.2820360901. [DOI] [PubMed] [Google Scholar]
- 16.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 17.West HJ, Jin JO. Performance status in patients with cancer. JAMA Oncol. 2015;1:998. doi: 10.1001/jamaoncol.2015.3113. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S, Qureshi M, Pullenayegum E, et al. Do patients with reduced or excellent performance status derive the same clinical benefit from novel systemic cancer therapies? A systematic review and meta-analysis. ESMO Open. 2017;2:e000225. doi: 10.1136/esmoopen-2017-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renfro LA, Goldberg RM, Grothey A, et al. Clinical calculator for early mortality in metastatic colorectal cancer: An analysis of patients from 28 clinical trials in the Aide et Recherche en Cancérologie Digestive Database. J Clin Oncol. 2017;35:1929–1937. doi: 10.1200/JCO.2016.71.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørensen JB, Klee M, Palshof T, et al. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773–775. doi: 10.1038/bjc.1993.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow R, Bruera E, Temel JS, et al. Inter-rater reliability in performance status assessment among healthcare professionals: An updated systematic review and meta-analysis. Support Care Cancer. 2020;28:2071–2078. doi: 10.1007/s00520-019-05261-7. [DOI] [PubMed] [Google Scholar]
- 22.Basch E, Wood WA, Schrag D, et al. Feasibility and clinical impact of sharing patient-reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin Trials. 2016;13:331–337. doi: 10.1177/1740774515615540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roman MA, Koelwyn GJ, Eves ND, et al. Comparison of performance status with peak oxygen consumption in operable patients with non-small-cell lung cancer. Respirology. 2014;19:105–108. doi: 10.1111/resp.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 27.West HJ. Patients with advanced non-small-cell lung cancer and marginal performance status: Walking the tight rope towards improved survival. J Clin Oncol. 2013;31:2841–2843. doi: 10.1200/JCO.2013.50.1502. [DOI] [PubMed] [Google Scholar]
- 28.Pater JL, Loeb M. Nonanatomic prognostic factors in carcinoma of the lung: A multivariate analysis. Cancer. 1982;50:326–331. doi: 10.1002/1097-0142(19820715)50:2<326::aid-cncr2820500227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Meijers WC, Moslehi JJ. Need for multidisciplinary research and data-driven guidelines for the cardiovascular care of patients with cancer. JAMA. 2019;322:1775–1776. doi: 10.1001/jama.2019.17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13:1383–1393. doi: 10.6004/jnccn.2015.0166. [DOI] [PubMed] [Google Scholar]
- 31.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign—A scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 32.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36:2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 35.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 38.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 39.Cesari M, Cerullo F, Zamboni V, et al. Functional status and mortality in older women with gynecological cancer. J Gerontol A Biol Sci Med Sci. 2013;68:1129–1133. doi: 10.1093/gerona/glt073. [DOI] [PubMed] [Google Scholar]
- 40.Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 42.Kasymjanova G, Correa JA, Kreisman H, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- 43.Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granger CL, Denehy L, Parry SM, et al. Which field walking test should be used to assess functional exercise capacity in lung cancer? An observational study. BMC Pulm Med. 2015;15:89. doi: 10.1186/s12890-015-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 46.Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. QJM. 1923;16:135–171. [Google Scholar]
- 47.American Thoracic Society. American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 48.Myers J, Forman DE, Balady GJ, et al. Supervision of exercise testing by nonphysicians: A scientific statement from the American Heart Association. Circulation. 2014;130:1014–1027. doi: 10.1161/CIR.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichel J. Assessment of operative risk of pneumonectomy. Chest. 1972;62:570–576. doi: 10.1378/chest.62.5.570. [DOI] [PubMed] [Google Scholar]
- 50.Roman MA, Koelwyn GJ, Eves ND, et al. Comparison of performance status with peak oxygen consumption in operable patients with non-small-cell lung cancer. Respirology. 2014;19:105–108. doi: 10.1111/resp.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones LW, Watson D, Herndon JE, II, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marshall CH, Al-Mallah MH, Dardari Z, et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: Results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019;125:2594–2601. doi: 10.1002/cncr.32085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar R, Garcea G. Cardiopulmonary exercise testing in hepato-biliary & pancreas cancer surgery: A systematic review—Are we any further than walking up a flight of stairs? Int J Surg. 2018;52:201–207. doi: 10.1016/j.ijsu.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Kelsey CR, Scott JM, Lane A, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: A feasibility study. Bone Marrow Transplant. 2014;49:1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones LW, Haykowsky M, Pituskin EN, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor: Positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 56.West MA, Parry MG, Lythgoe D, et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg. 2014;101:1166–1172. doi: 10.1002/bjs.9551. [DOI] [PubMed] [Google Scholar]
- 57.West MA, Lythgoe D, Barben CP, et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: A prospective blinded observational study. Br J Anaesth. 2014;112:665–671. doi: 10.1093/bja/aet408. [DOI] [PubMed] [Google Scholar]
- 58.West MA, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg. 2016;103:744–752. doi: 10.1002/bjs.10112. [DOI] [PubMed] [Google Scholar]
- 59.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 60.Groarke JD, Payne DL, Claggett B, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. doi: 10.1093/ehjqcco/qcaa015. [epub ahead of print on March 13, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 62.Peddle-McIntyre CJ, Cavalheri V, Boyle T, et al. A review of accelerometer-based activity monitoring in cancer survivorship research. Med Sci Sports Exerc. 2018;50:1790–1801. doi: 10.1249/MSS.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 63.Lee IM, Shiroma EJ, Evenson KR, et al. Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: The Women’s Health Study. Circulation. 2018;137:203–205. doi: 10.1161/CIRCULATIONAHA.117.031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saint-Maurice PF, Troiano RP, Bassett DR, Jr, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151–1160. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gresham G, Hendifar AE, Spiegel B, et al. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digit Med. 2018;1:27. doi: 10.1038/s41746-018-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panda N, Solsky I, Huang EJ, et al. Using smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg. 2019;155:1–7. doi: 10.1001/jamasurg.2019.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evangelista L, Steinhubl SR, Topol EJ. Digital health care for older adults. Lancet. 2019;393:1493. doi: 10.1016/S0140-6736(19)30800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veerabhadrappa P, Moran MD, Renninger MD, et al. Tracking steps on Apple Watch at different walking speeds. J Gen Intern Med. 2018;33:795–796. doi: 10.1007/s11606-018-4332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torkamani A, Andersen KG, Steinhubl SR, et al. High-definition medicine. Cell. 2017;170:828–843. doi: 10.1016/j.cell.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pew Research Center Demographics of mobile device ownership and adoption in the United States: Mobile fact sheet. http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/
- 71.Li X, Dunn J, Salins D, et al. Digital health: Tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol. 2017;15:e2001402. doi: 10.1371/journal.pbio.2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basch E, Dueck AC, Rogak LJ, et al. Feasibility of implementing the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events in a multicenter trial: NCCTG N1048. J Clin Oncol. 2018;36:3120–3125. doi: 10.1200/JCO.2018.78.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott JM, Dolan LB, Norton L, et al. Multisystem toxicity in cancer: Lessons from NASA’s Countermeasures Program. Cell. 2019;179:1003–1009. doi: 10.1016/j.cell.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haendel MA, Chute CG, Robinson PN. Classification, ontology, and precision medicine. N Engl J Med. 2018;379:1452–1462. doi: 10.1056/NEJMra1615014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genes N, Violante S, Cetrangol C, et al. From smartphone to EHR: A case report on integrating patient-generated health data. NPJ Digit Med. 2018;1:23. doi: 10.1038/s41746-018-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.US Food and Drug Administration FDA-ASCO public workshop: 2019 clinical outcome assessments in cancer clinical trials—Fourth annual workshop. https://www.fda.gov/drugs/news-events-human-drugs/fda-asco-public-workshop-2019-clinical-outcome-assessments-cancer-clinical-trials