Abstract
Invasive species are the primary driver of island taxa extinctions and, among them, those belonging to the genus Rattus are considered as the most damaging. The presence of black rat (Rattus rattus) on Cyprus has long been established, while that of brown rat (Rattus norvegicus) is dubious. This study is the first to provide molecular and morphological data to document the occurrence of R. norvegicus in the island of Cyprus. A total of 223 black rats and 14 brown rats were collected. Each sample was first taxonomically attributed on the basis of body measurements and cranial observations. Four of the specimens identified as R. norvegicus and one identified as R. rattus were subjected to molecular characterization in order to corroborate species identification. The analyses of the mitochondrial control region were consistent with morphological data, supporting the taxonomic identification of the samples. At least two maternal molecular lineages for R. norvegicus were found in Cyprus. The small number of brown rats collected in the island, as well as the large number of samples of black rats retrieved in the past years might be an indication that the distribution of R. norvegicus is still limited into three out of the six districts of Cyprus.
Keywords: mitochondrial DNA, D-loop, brown rat, alien species, biological invasion
1. Introduction
Rattus rattus Linnaeus, 1758 (black rat) and Rattus norvegicus Berkenhaut, 1769 (brown rat) are considered as the most damaging and successful invasive species on the planet. Due to their commensality with humans they have spread in almost all regions of the world, with R. rattus occurring on all continents and R. norvegicus excluded only from Antarctica [1,2,3]. Invasive species, and more precisely species of the genus Rattus, are known to negatively affect island biota [4,5,6] and are the primary drivers of species extinctions on islands [7,8,9]. Due to the overall dramatic impact of rat invasions on autochthonous biota and on public health, the taxonomic identification of Rattus species spreading in specific locations, with particular reference to islands, are of pivotal importance for the conservation of local endemism [10,11]. The genus Rattus includes 66 species [3]; therefore, its taxonomy is complex being further complicated by a plethora of synonyms for different species [12]. Identification of individuals, even by experts, is often difficult [13], and for this reason the analysis of morphological traits combined with the genetic characterization of molecular markers is the most efficient system for a well addressed species taxonomic identification [3].
The island of Cyprus is situated at the eastern end of the Mediterranean Sea, south of Turkey, West of Syria, Lebanon and Israel, and North of Egypt, and it has been early and widely colonized by black rats since their spread from the Indian Peninsula [14,15]. On the contrary, the brown rat is not as frequent on the island and the earliest attempt to report species of Rattus for Cyprus was by Watson [16,17] who captured through trapping 150 rats from an area near Kyrenia. In addition, 1271 additional specimens were examined from various places along the north coastal region of the island. Watson concluded that “all the rats seen in Cyprus were the variety of brown back and white belly which is usually given the subspecies name of frugivorous” [17]. Nevertheless, Watson also stated that “the brown rat Rattus norvegicus also occasionally turns up in the ports but none were encountered elsewhere’. Similar outcomes were obtained by Spitzenberger [18] and Kourtellarides [19] who found R. rattus to be common in barn owl (Tyto alba) pellets in Cyprus, while no R. norvegicus were found in the island. In 1986–1987, one of the authors of the present study (E.H.) performed an extensive trapping of rats in Paphos Forest, an area of 620 km2, capturing only R. rattus (unpublished data). In 2003, in a list of the mammals of Cyprus, Hadjisterkotis [20,21] listed R. rattus and R. norvegicus as present on the island. However, the inclusion of R. norvegicus in this list was based strictly on oral information from Dr. A. Emanouel of the Central Veterinary Services of Nicosia, who noted that this species was present on the island only at the port of Limassol.
Within the frame of a study on pathogens transmitted by rodents in Cyprus, rats were collected between 2000 and 2003 for the analysis of fleas [22,23]. Authors reported the wide trapping of hundreds of R. norvegicus and R. rattus, but although fleas were counted and identified using accepted morphologic criteria, no molecular or morphological data were provided for the collected rats.
In 2009, Kryštufek and Vohralík [24] during their visit to Cyprus to study and record the small mammals of Cyprus, captured only black rats, in accordance with the findings reported by Watson [17]. Furthermore, these authors noted that R. rattus seems to be by far the most abundant small mammal in Cyprus, particularly so in the maquis and, to a lesser extent, in forests. Since they did not capture any R. norvegicus they noted that the presence of this species in Cyprus was dubious. Furthermore, Cucchi et al. [25] performed one of the most extensive live trappings for small mammals in Cyprus, noting the presence of only R. rattus, and concluded that this species was introduced with maritime traffic during historical times. From December 2006 to December 2009, the Research Promotion Foundation [26] of Cyprus, financed a project for the qualitative and quantitative study of wild small mammalian fauna in Natura 2000 sites of Cyprus, with the participation of the Wildlife Society of Cyprus, the University of Crete, and the Museum of Natural History of Crete, without finding any R. norvegicus on the island. In accordance, in 2016, Nicolaou et al. [27], after 9 years of live trapping of small mammals all over the island, including Limassol port, reported only the presence of R. rattus, and stated that “since the species lacks other rodent competitors in Cyprus, they also occur in areas remote from development”. A year later, Nicolaou [28] claimed that “in a number of projects referring to Cyprus, there are references for another rat species, R. norvegicus. However, there are no samples from this species until today to excuse its presence, although is possible that existed on the island in the past”. Accordingly, in the same year, Hadjisterkotis [29,30] found only R. rattus in the island, and in 2018, Moysi et al. [31] in a study on the diet of the barn owls on Cyprus, did not report any R. norvegicus, thus considering their presence on the island as dubious.
During the last few years, several pictures of rats taken by bird watchers in Cyprus appeared on social media. The rats were usually identified as R. rattus, and occasionally the same images were reported as R. norvegicus. However, according to Kryštufek and Vohralík [24] and Nagorsen [32], while brown rat adults differ clearly from R. rattus, more slender juveniles with a pointed muzzle and relatively larger ears can be misidentified as black rats. Relative tail length, however, remains constant regardless of age [24]. Since the age of these rats is unknown, their identification as R. norvegicus based only on photographs, may be misleading. Although there are studies presenting body measurements of black rats from Cyprus [24], to the best of our knowledge, there are no molecular and/or morphological characterization of specimens belonging to the species R. norvegicus in Cyprus.
In such a context, the primary aim of this study is the assessment of the presence of this species on the island, thus providing the first morphological characterization for individuals of R. norvegicus in Cyprus. In order to corroborate the morphological identification, a molecular taxonomic identification was also performed for several specimens, thus providing the first molecular data for this species in the island.
2. Materials and Methods
2.1. Sample Collection
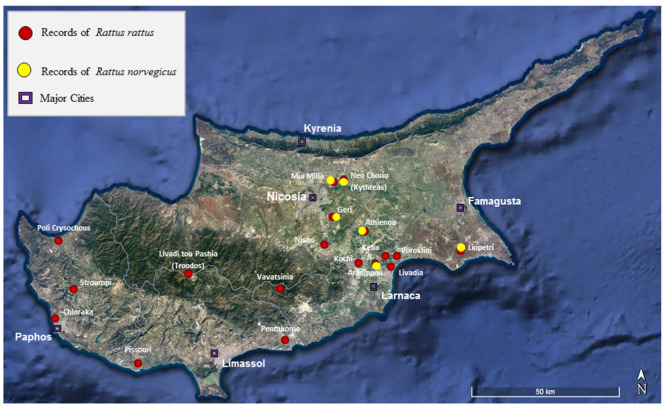
The number of rats collected and identified for each species is presented in Table 1, and the sampling sites are indicated in Figure 1. Rats were generally collected using snap traps and Herman live traps from January 2019 up to June 2020. Samples were also collected from animals found dead on the road. In addition, 18 skulls of R. rattus were collected from five barn owl nests, located at Xylophagou Larnaca, Machaeras Monastery, Kissonerga Paphos, Polis Paphos, and Vavatsinia Larnaca (Table 1).
Table 1.
Sampling plan for the brown rats and black rats collected in the present study. + visual observation, ++ visual observation, dead in the nest of a long-legged Buzzard (Buteo rufinus), * road killed, ** captured in snap traps or live traps, +++ skulls collected in barn owl pellets.
| Locality-Village/City and District | R. rattus | R. norvegicus | |
|---|---|---|---|
| Dead specimens | Aradippou-Larnaca | 18 * | 7 * |
| Kochi-Larnaca | 1 * | - | |
| Vavatsinia-Larnaka | 14 ** | - | |
| Pentakomo-Larnaka | 1 * | - | |
| Kelia-Larnaka | 1 * | - | |
| Livadia-Larnaka | 1 * | - | |
| Oroklini-Larnaka | 1 * | - | |
| Athienou-Larnaka | - | 1 ++ | |
| Geri-Nicosia | 4 ** | 3 ** | |
| Nissou-Nicosia | 1 * | - | |
| Neo Chorio Kythreas-Nicosia | - | 1 * | |
| Mia Milia-Nicosia (visual observations only) | - | 1 + | |
| Chloraka-Paphos | 160 ** | - | |
| Liopetri-Famagusta | 1 * | 1 * | |
| Pissouri-Limassol (Lemesos) | 1 * | - | |
| Troodos (locality Livadi tou Pashia)-Limassol (Lemesos) | 1 * | - | |
| Total number of collected specimens | 205 | 14 | |
| Skulls collected from owl pellets | Xylophagou-Larnaca | 1 +++ | - |
| Machaeras Monastery-Troodos mountains | 3 +++ | - | |
| Kissonerga-Paphos | 5 +++ | - | |
| Polis-Paphos | 4 +++ | - | |
| Vavatsinia-Larnaca | 5 +++ | - | |
| Total number of collected specimens and skulls | 223 | 14 |
Figure 1.
Map indicating the sampling sites in Cyprus where rats were collected.
Black rats were identified from brown rats based on the characteristics descripted by Kryštufek and Vohralík [24], Nagorsen [32], Qumsiyeh [33], and Yiğit et al. [34]. Since in most of the road-killed individuals part of the animal was smashed, one of the characteristics which remained unchanged and allowed instant identification of specimens was the colors of the tail. According to Kryštufek and Vohralík [24], in R. rattus the tail is uniformly black, whereas in R. norvegicus the tail is indistinctly bicolored, greyish brown above, pale below. For the identification of the species from the skulls found in owl pellets, only complete skulls were used, with intact parietal and interparietal ridges and occipital condyles. A mitochondrial DNA sequencing analysis was carried out in four rats collected between September and December 2019 from three different locations of Cyprus which showed a R. norvegicus phenotype (see Table 1 and Figure 1, Figure 2 and Figure 3 for more details) with the aim to support the species identification performed on morphological bases (Table 2). The same molecular analysis was also carried out for one of the samples of R. rattus in order to perform a comparative molecular phylogenetic analysis. Pictures of a brown rat and a black rat, respectively, taken in Cyprus during the present study, are shown in Figure 2 and Figure 3.
Figure 2.
An individual of Rattus norvegicus at Mia Milia Sewage Treatment Plant (Photo: E. Hadjisterkotis).
Figure 3.
An individual of Rattus rattus from Chloraka (Photo: E. Hadjisterkotis).
Table 2.
Samples selected for molecular analyses. The table reports collection date, code, and sampling location for each specimen whose D-loop sequence was acquired.
| Collection Date | Code | Sampling Site | Species |
|---|---|---|---|
| April 2019 | RnC1 | Neo Chorio-Kythrea | R. norvegicus |
| May 2019 | RnC2 | Geri-Nicosia | |
| June 2019 | RnC3 | Aradippou-Larnaca | |
| June 2019 | RnC4 | Geri-Nicosia | |
| September 2019 | RrC1 | Koshi-Larnaca | R. rattus |
2.2. Examination of Live Specimens and Carcasses
In April 2019, an adult male was collected dead on the road at the center of the village Neo Chorio near Kythrea. A hair sample was collected for molecular analysis (RnC1 in Table 2). The two specimens from Geri, one specimen from Kelia village and one from Geri livestock farms, were skinned in the standard museum manner. From a fifth specimen from Aradippou, the body was damaged and only the head was skinned and preserved. Examination of the stomachs of two specimens from Aradippou and Kelia, indicated that the animals fed on livestock food. These individuals were collected near cow farms. The specimen from Neo Chorio was in an advanced stage of decomposition and after taking body measurements and cleaning the skull, it was disposed. Body measurements (head and body and tail length) were taken using a Tiger 771,588 Vernier Caliper 0–300 mm. For the measurements of the ears and the hind foot, a powerfix digital caliper, version 11/2010 was used. For the weight a portable electronic balance was used, measuring to the nearest gram. For the observation of the color of the tail a Jeweller’s loupe (handheld lens) 30 × 21 mm was used.
One of the authors of the present study (G.K.) for the last few years has been visiting the Mia Milia Sewage Treatment ponds for wildlife photography. He observed that in the ponds there were rats having the characteristics of R. norvegicus, as described by Nagorsen [32]. These rats, when compared with R. rattus individuals, showed smaller ears and eyes, blunt nose, thick heavy body, and tail shorter than head and body (see Figure 2). G. Konstantinou and E. Hadjisterkotis (authors of this study) visited the area in order to photograph these rats and to visually compare their external characteristics with the rats collected at Neo Chorio and Geri (RnC1 and RnC2 in Table 2, respectively).
Sample identification was based on the following diagnostic external characteristics that allow to distinguish R. norvegicus from R. rattus [24,32,33,34]:
The tail is always shorter than head and body length, indistinctly bicolored, greyish brown above, pale below, ears do not reach the eyes when laid forwards: R. norvegicus, Norway rat, brown rat.
The tail length is always greater than head and body length, uniformly black. When the ears are drawn forward, they reach the eyes and usually cover them: R. rattus, black rat.
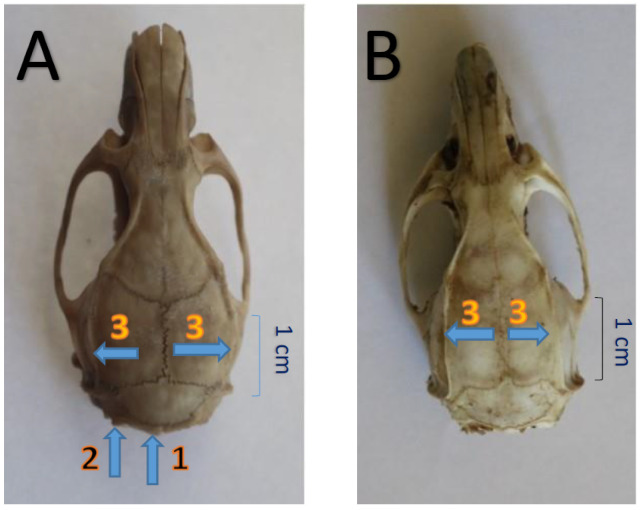
Species were also recognized based on the diagnostic skull characteristics of R. norvegicus and R. rattus [24,32,33,34], with particular reference to the shape of the supraorbital ridges. In R. rattus supraorbital ridges diverged posteriorly along parietals and close to occipital region they are slightly bent or curved, while in R. norvegicus they ran mainly parallel along parietals (Figure 4). According to Pimsai et al. [35] the skull ridges are well-defined in older individuals and characteristic in shape; they are relatively straight and situated close to one another, which gives the impression of a relatively narrow braincase. As it is described by Nagorsen [32], in the Norway rat the braincase is rectangular, temporal ridges straight and nearly parallel, whereas in black rat the braincase is rounded, and the temporal ridges curved.
Figure 4.
Picture showing the differences in cranial characteristics between the black and brown rats collected in Cyprus. (A) The skull of R. rattus dorsal view. (B) The skull of R. norvegicus dorsal view. 1. Supraoccipital ridges, 2. Occipital condyles, 3. Parietal and interparietal ridges.
The palate of R. norvegicus is broader behind, bullae are relatively smaller, and the incisive foramen slightly shorter than in R. rattus—(see [24]: p. 135, Figure 107 for R. norvegicus skull and p. 125, Figure 100 for R. rattus). In addition, the skull of R. rattus is also more elongated than that of R. norvegicus [33] and the occipital condyles are the most posteriorly projecting point of the skull [34].
2.3. DNA Extraction, Amplification, and Sequencing
Genomic DNA was extracted from the hairs of five samples (see Table 2 for details) by means of the InstaGene™ Matrix (Bio-Rad) according to the manufacturer’s protocol. Sample quality and DNA concentration were determined via spectrophotometry using a ND-8000 (NanoDrop Technologies, Thermo Fisher Scientific Inc., Wilmington, DE, USA). The DNA mean concentration obtained was 50 ng/μL.
A pair of primers was designed by the authors by means of the “Web Primer: DNA and Purpose Entry” bioinformatic tool available at http://www.candidagenome.org/cgi-bin/compute/web-primer [36] and used to amplify a region of the R. norvegicus mitogenome encompassing the first 434 base pairs of the D-loop region (HVS-I domain): Rat_DFw (5′-ccatcaacacccaaagctgat-3′), Rat_DRv (5′-cgagatgtcttatttaagggg-3′).
A standard 50 μL PCR mixture was used, including 250 ng DNA template, 2.5 mM MgCl2, 0.20 mM each dNTP, 0.20 μM each primer, 1× PCR buffer, and 2 units Taq DNA Polymerase (Sigma-Aldrich), furthermore, 25 μg of bovine serum albumin (BSA) (5 ng/mL) was also added to the reaction mixture. PCR amplifications were carried out on a GeneAmp PCR System 9700 (Applied Biosystems) under the following conditions: initial denaturation of 95 °C for 5 min, followed by 35 cycles of 95 °C for 50 s, 54 °C (annealing temperature) for 50 s, and 72 °C for 1 min; a final extension of 72 °C for 4 min was also applied. At the end, a post-treatment of 5 min at 72 °C and a final cooling at 4 °C were carried out. Both positive (i.e., a known sample of Rattus sp. DNA used to verify that the primers have attached to the DNA strand) and negative controls were used to test the effectiveness of the PCR protocols, and the absence of possible contamination. Electrophoresis was carried out on 2% agarose gels, prepared using 1× SBA buffer (Sodium Boric Acid, pH 8.2) and stained with Gel Red Nucleic Acid Stain (Biotium). PCR products were purified by ExoSAP-IT (USB Corporation) and sequenced for both forward and reverse strands (by means of the same primers used for PCR), using an external sequencing core service (Macrogen The Netherlands). PCRs and sequencing were repeated twice for each sample in order to verify the reliability of results. The PCR products did not show occurrence of aspecificity, excluding the possibility of multiple nuclear mtDNA-like sequences, furthermore, dual peaks of similar height, which could be interpreted as evidence of possible heteroplasmy, were not observed in any of the electropherograms.
Dataset Creation, Sequence Comparisons, and Species Identification
The recovered sequences were identified through BLAST analysis in the GenBank nucleotide database (NCBI) (http://www.ncbi.nlm.nih.gov/) in order to estimate the statistical significance of matches and corroborate the taxonomic attribution performed for the specimens on morphological bases.
The amount of genetic variation among R. norvegicus sequences, including the number of polymorphic sites (S) and haplotypes (H), the haplotype diversity (Hd), and nucleotide diversity (π), were estimated using DnaSP 6.10.03 [37].
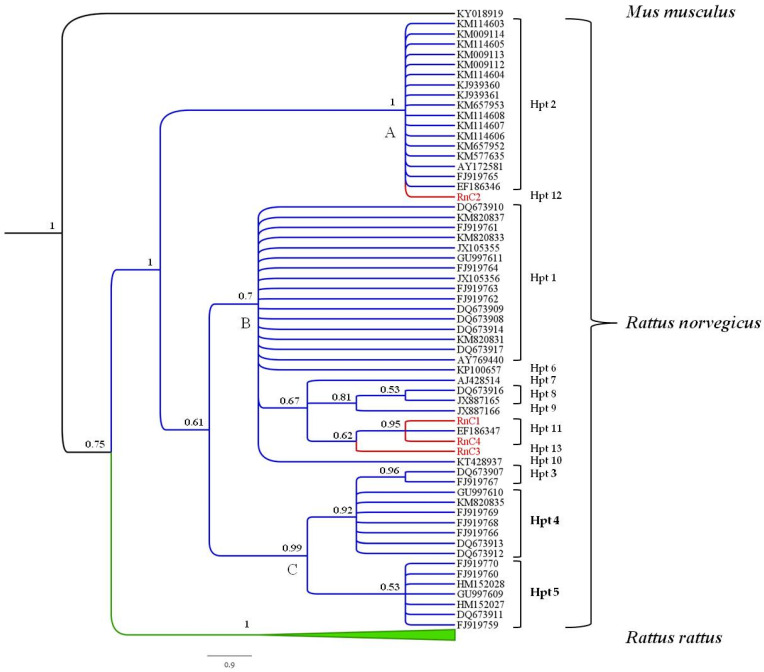
Sequences were aligned using the software Clustal X2 [38] and a dataset including the sequences obtained in the present study for R. norvegicus (4) and R. rattus (1) along with the haplotypes deposited in GenBank for these species which showed the higher levels of similarity at the BLAST analysis (56 for R. norvegicus and 39 for R. rattus, see Figure 5 for GenBank accession numbers) was constructed.
Figure 5.
Bayesian phylogenetic rooted tree showing the relationships between the sequences obtained in this study and the mitochondrial D-loop haplotypes available in GenBank for R. norvegicus and R. rattus. The list of haplotypes grouped within the collapsed clade of R. rattus are reported in supplementary Table S1.
A phylogenetic tree analysis was performed using the Bayesian Inference (BI) method and a Mus musculus sequence (GB # KY018919) was used as outgroup [39]. MEGA 7.0.14 [38] was used to infer the best nucleotide substitution model. The Bayesian tree was inferred using Mr Bayes 3.2.5 [40] via Markov chain Monte Carlo (MCMC). Samples were drawn every 1000 steps over 20,000,000 MCMC steps. The first 10% were discarded as burn-in. Acceptable sampling and convergence to the stationary distribution were checked by inspection of traces using Tracer 1.7 [41] and tree topology was edited in FigTree 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
3. Results
3.1. Morphological Analysis
The preliminary body measurements, the shape of the skulls, the robustness of the body, and the length and color of the tail of each individual allowed to discriminate between R. norvegicus and R. rattus, the latter being slimmer with relatively larger ears and a longer black tail (Table 3).
Table 3.
This table reports the morphological diagnostic characters that were considered for the analysis. Body measurements in millimetres of nine R. norvegicus from Turkey and 63 R. rattus from Turkey and Cyprus, respectively ([24] and references therein), were compared with the specimens from Cyprus here analyzed. The measurements of weight in R. norvegicus reported by Kryštufek and Vohralik [24] refer to one specimen only, therefore, no range of values (Min–Max) was provided.
| [24] | Present Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R. rattus | R. norvegicus | ||||||||
| N | Mean | Min–Max | N | Mean | Min–Max | N | Mean | Min–Max | |
| Head and body | 63 | 182.7 | 155–234 | 9 | 222.9 | 192–255 | 7 | 195.3 | 165–230 |
| Tail | 60 | 216.9 | 180–269 | 9 | 187.9 | 145–216 | 7 | 175.4 | 160–205 |
| Hind foot | 40 | 36.2 | 33.0–42.0 | 9 | 41.6 | 38.0–44.0 | 7 | 40.8 | 36.5–43.4 |
| Ear | 30 | 23.7 | 20.0–26.0 | 9 | 21.5 | 20.0–23.0 | 7 | 18.5 | 16.5–18.4 |
| Weight | 35 | 188.4 | 100–279 | 1 | 498 | - | 5 | 267 | 235–285 |
3.1.1. External Characteristics
The number of rats collected and identified for each species is presented in Table 1. In addition, 18 skulls of R. rattus were collected from five barn owl nests, located at Xylophagou village of Larnaca (one skull), Machaeras Monastery, Troodos mountains (four skulls), Kissonerga Paphos (six skulls), Polis tis Chrysochous Paphos (two skulls), and Vavatsinia Larnaca (five skulls).
The brown rats were robust and heavily built, compared to slender black rats. The tail length of the brown rats was less than head and body (Table 3), whereas in black rats was longer than head and body.
The color of the brown rats on the back is a mixture of dull ochraceous brown to dark brown and slate grey; it is interspersed by black tips, and occasional white and black hairs. Black hairs are coarser than the soft buff brown hairs. Head, shoulder, and spine are darker; flanks are more buff grey; belly is greyish white of varying intensity. The outer surface of the ears is covered with short, sparse, blackish brown hairs. The inner surface of the ear is naked, pinkish white, with the edges and inner surfaces covered with short, sparse, brownish or whitish hairs. The base of the ear is covered with longer brownish hairs. The upper sides of both the fore and hind feet are covered with tiny whitish hairs, the nails are not pigmented, and the soles of the fore and hind feet are completely naked. When the ear is bend forward towards the eye, is not reaching the eye, or sometimes just reaching the eye. The hairs on the belly, the chest, and the chin are buff white to grey, the bases are greyish to dirty white.
The color of the black rats resembled what Kryštufek and Vohralík [24] considers frugivorous or alexandrines type. In the frugivorous type has pale (white to light grey, yellowish white or pale buff) and sharply defined belly, and dark back which is blackish brown, dark grey brown, reddish brown, or light brown. In the alexandrinus type the back is dark to light brown or grey and belly is darker than in the previous type, albeit clearly paler than back; flanks are frequently grey and demarcation is faint. All possible intermediate stages connect these color types. The tail of the R. norvegicus is indistinctively bicolored, due to the tiny dark bristles above and whitish bristles below, which gives the bicolored appearance. The tail bristles of 205 specimens of R. rattus examined from the districts Famagusta, Larnaca, Nicosia, Limassol, and Paphos, were all dark, giving the one color appearance.
3.1.2. Cranial Characteristics
The skull of brown rats is considerably robust, the braincase is narrow and elongated. The rostrum is moderately long, and the nasals are rounded off posteriorly. The parietals and interparietal are bordered by well-defined ridges which are straight and almost parallel, which gives the impression of a relatively narrow braincase. The squamosal and maxillary process of the zygomatic arc are laterally widened. The lacrimals project slightly from behind the infraorbital foramen, which is laterally and vertically broadened. The supraoccipital is nearly vertical, and the exoccipital condyles form the most posteriorly projecting point of the skull. In the black rats the skull is strong and heavily built, and supraorbital ridges diverge posteriorly along parietals; close to occipital region they are slightly bent, forming a distinctive rounded braincase. A morphological comparison of the cranial characteristics between black and brown rats is provided in Figure 4.
3.2. DNA Analysis
A 483-base pair-long fragment of the hypervariable domain I of the mitochondrial D-loop region from four R. norvegicus and one R. rattus specimens were sequenced and deposited in GenBank (GB# MN115391-94). A BLAST search for homologous sequences corroborated the taxonomic attribution performed for these individuals on morphological bases. A dataset was constructed including all the haplotypes with higher level of similarity at the BLAST analysis so far available for R. norvegicus and R. rattus (see Figure 5 for GenBank accession numbers).
The Bayesian tree analysis (Figure 5) provided a topology where the two Rattus species formed two well-supported monospecific and monophyletic clades, representing R. norvegicus (branches colored in blue in the figure) and R. rattus (branches colored in green), respectively (Figure 5).
Three different maternal lineages (A, B, and C, see Figure 5) were detected for the R. norvegicus early radiation. Three sequences RnC1, RnC3, and RnC4 grouped within the clade B. RnC1 and RnC4 showed the same haplotype and clustered together with a sequence obtained from a wild brown rat captured in 2007 in French Polynesia (EF186347), while RnC3 showed a haplotype never described before. On the other hand, the Cypriot brown rat RnC2 was phylogenetically related to sequences belonging to the clade A. The Cypriot black rat RrC1 sequence was grouped within the R. rattus clade.
Overall, a total of 13 haplotypes, defined by 20 polymorphic sites, were detected for R. norvegicus (Table 4). Eight of them were previously reported by Liu et al. 2017 [42] in brown rats from China (D1, D2, D4, D5, D12, D23, D24, D25 in Table 4). The three haplotypes found for the individuals of R. norvegicus from Cyprus resulting in nine polymorphic sites and high levels of genetic divergence (Hd = 0.833 π = 0.011). Estimates of genetic diversity among haplotypes are reported in Table 4.
Table 4.
Sample distribution and genetic diversity indexes among the clades evidenced in Bayesian phylogenetic tree and among haplotypes. N: number of sequences composing each haplotype; S: number of polymorphic sites; h: number of haplotypes; Hd: haplotype diversity; π: nucleotide diversity.
| Clades | Haplotype | Composition | N | S | h | Hd | π |
| A | Hpt-2 | KM114603, KM009114, KM114605, KM009113, KM009112, KM114604, KJ939360, KJ939361, KM657953, KM114608, KM114607, KM114606, KM657952, KM577635, AY172581, FJ919765, EF186346 | 17 | ||||
| Hpt-12 | RnC2 | 1 | |||||
| B | Hpt-1 | DQ673910, KM820837, FJ919761, KM820833, JX105355, GU997611, DQ673908, DQ673914, KM820831, DQ673917 (D5), AY769440 FJ919764, JX105356, FJ919763, FJ919762, DQ673909 | 16 | ||||
| Hpt-6 | KP100657 | 1 | |||||
| Hpt-7 | AJ428514 (D12) | 1 | |||||
| Hpt-8 | DQ673916 (D1), JX887165 (D2) | 2 | |||||
| Hpt-9 | JX887166 (D4) | 1 | |||||
| Hpt-10 | KT428937 | 1 | |||||
| Hpt-11 | RnC1, EF186347, RnC4 | 3 | |||||
| Hpt-13 | RnC3 | 1 | |||||
| C | Hpt-3 | DQ673907 (D25), FJ919767 | 2 | ||||
| Hpt-4 | GU997610, KM820835, FJ919769, FJ919768, FJ919766, DQ673913, DQ673912 (D24) | 7 | |||||
| Hpt-5 | FJ919770, FJ919760, HM152028, GU997609, HM152027, DQ673911, FJ919759 (D23) | 7 | |||||
| Total | 13 | 60 | 20 | 13 | 0.829 | 0.001 |
4. Discussion
Identification of individuals belonging to species of Rattus, is often difficult [13], and for this reason the analysis of morphological traits combined with the genetic characterization of molecular markers represents the most efficient system for a well addressed species taxonomic identification [3]. Although the morphological criteria used for the first identification of an individual may vary over time, distinguishing among R. rattus and R. norvegicus is not so difficult, since according to Kryštufek and Vohralík [24], Nagorsen [32], Qumsiyeh [33], and Yiğit et al. [34], as well as our observations in this study, in R. norvegicus the tail is always shorter than head and body length, ears do not reach the eyes when laid forwards, and the tail is bicolored. Although Kryštufek and Vohralík [24], notes that the tail of R. norvegicus is indistinctly bicolored, grayish brown above, pale below, based on our observations the tail in this species is distinctively bicolored, greyish brown above, whitish below. The distinctive discrepancy with previous descriptions is due to the different color of the tail bristles above and below the tail. On the contrary, in all R. rattus individuals the tail bristles above and below were black.
This is the first confirmation based on cranial and body measurements as well as on molecular analysis of the presence of R. norvegicus on the island of Cyprus. We also present the first study on the variation of brown rats in Cyprus, providing the first preliminary molecular and morphological data on this alien invasive species, which can become devastating for agriculture and local wildlife. Cyprus is the only center of endemism for birds in Europe [43], a center of endemism for terrestrial mammals [27,29,30], reptiles [44], insects [45,46], terrestrial gastropods [46], and plants [47,48,49,50,51], and represents a biodiversity ‘hotspot’ [20,21,52,53,54]. Biological diversity faces many threats throughout the world, and one of the major threats to native biological diversity is now acknowledged by scientists and governments to be biological invasions caused by alien invasive species such as rats. The impact of invasive species is immense, insidious, and usually irreversible. They may be as damaging to native species and ecosystems on a global scale as the loss and degradation of habitats [53].
The limited number of brown rats so far collected in Cyprus is an indication that the distribution of R. norvegicus is limited in the district of Nicosia and Larnaca, around the cities of Aradippou, Geri, Nicosia, and Larnaca, as well as the village of Neo Chorio Kythrea, and the Sewage Treatment Ponds of Mia Milia (Figure 3). In these restricted areas brown rats were found in the sewage treatment plant canals suggesting the possibility that they might spread into new locations following the canals. A similar situation was observed in Israel, where brown rats are more commensal than their relative the black rat but are less widespread. They penetrated human habitations of port cities and managed to reach some cities of the interior. R. norvegicus shows to prefer more mesic areas than R. rattus. Barrows can be found along stream banks (including sewage streams) in many areas [33].
The ports that are near to the localities where brown rats were collected are the ports of Famagusta and Larnaca, which are about 65 km from Nicosia. Therefore, finding brown rats in the District of Nicosia is an indication that they are spreading toward urban areas where they live around buildings as it was observed in the city of Geri, as well as in the center of Neo Chorio near Kythrea.
The island of Cyprus represents a further case study, where an alien highly invasive species (whose presence on the island was considered as dubious by several researchers), which can be a significant driver of population declines and species extinctions was documented for the first time in the present study. Black rats, according to Hadjisterkotis [29,30], occupy just about every rural habitat in Cyprus. The same was reported by Krystufek and Vohralik [24] who noted that “throughout Turkey and Cyprus, the black rat occupies dwellings (houses, granaries, stables and farm buildings, mills and so forth). Along the Aegean and the Mediterranean coasts of Anatolia and in Cyprus it is abundant and widespread also outside buildings, either in urban and suburban areas (parks, gardens, orchards), in cultivated areas (hedgerows, along fields), and in various types of shrubland, but is most common in humid places and in high maquis”. Hadjisterkotis [55] also observed that black rat is common in forested areas with pine Pinus brutia and black pine Pinus nigra, as well as golden oak Quercus alnifolia, and in torrential river beds on the mountains, where it finds cover in bramble (Rubus sanctus), Smilax asperea, and myrtle Myrtus communis. This species is highly dispersed even on pine trees, where the species uses old nests of woodpigeons as platforms to brake pinecones to extract the seeds [55]. They are excellent climbers and occasionally they prefer to build large squirrel-like nests in dense bramble or even pine trees, and occasionally carob trees (Ceratonia ciliqua). Nests, generally at height of 2–3.5 m, are loosely made of leaves and twigs of the available trees or vegetation. The entrance is at one side of the nest, which is flatly domed like a squirrel’s drey, and, from casual inspection, appears to be nothing more than a bunch of dead leaves and twigs, which the thickness of the vegetation keeps in place. In Cyprus during the summer, black rats are a major destruction for carob trees since they eat the fruit and peel the branches, consequently killing the trees. For the first time we also observed them in 2019 and 2020 building roughly round bulky nests on olive trees (Olea europaea), by cutting and pilling large numbers of olive tree twigs. In addition, we observed them eating the cambium layer of olive trees, in a similar way that they do with carob trees. Particularly selected for attack are fresh shoots, stripping branches up to 45 mm in diameter. Caches of olives were found under thick vegetation of rock roses (Cistus sp.), under stones and wooden boards, in hollow olive trees, even in tunnels under dense grass. Cashes of almonds were found in the hollow branches of almond trees, in abandoned farm buildings, and underneath large rocks (E.H. personal observation).
Black rats are well adapted to small islands, whereas brown rats are mostly observed on the largest islands where humans also occur [56,57,58]. Considering that Cyprus is the third largest island in the Mediterranean, brown rats are expected to adapt and to compete well with black rats thus exerting a stronger impact on native species.
Besides the above reported destruction of trees and crops, rats are particularly harmful in and around farm facilities where they seek food and refuge indoors, gnaw on structural, mechanical, and electrical components, weaken concrete slabs and walkways with their burrowing activities [58]. If not promptly eradicated, they can reach huge numbers, as in the case of a chicken farm in Chloraka, where we trapped 160 black rats.
Rats are reservoirs and vectors of pathogens [55,59,60] that can infect livestock, wildlife species, and humans [58]. A costly disease that was found to infect farm animals and rats, is paratuberculosis that causes significant economic losses for the farm industry with annual estimates of millions of dollars around the world. It was estimated that paratuberculosis costs the U.S. dairy industry alone 200–250 million dollars annually [60]. The etiologic agent Mycobacterium avium ssp. paratuberculosis (MAP) has been isolated from Cypriot cattle, sheep, and goat populations and dairy food [61,62,63]. Several studies revealed MAP isolations from brown rats [64], foxes, and feral cats [65]. The latter two species are predators of rats on Cyprus, which can become infected by feeding on them. These species may live for several years with home ranges that cover areas large enough to include more than one farm, spreading the disease from farm to farm, and re-infecting farms which eliminated all infected livestock. Therefore, the role of wildlife species (also including both species of rats) in the spreading of MAP to cattle, sheep, and goats must be examined, in order to take the proper management measures for the protection of livestock and humans. Besides the spreading of MAP to cattle, sheep, and goats, epidemiological and clinical data of 193 human cases of murine typhus in Cyprus were recorded and analyzed during a 9-year period (2000–2008). The data collected enhance the belief that murine typhus is a serious public health problem in Cyprus [66].
In the future, extensive studies focused on the demographic patterns of dispersal and habitat selection of R. norvegicus in Cyprus, will be needed to assess proper management plans for the protection of public health, agriculture, livestock, and biodiversity, and to depict, if any, the competitive interactions with R. rattus.
Furthermore, the expansion of R. norvegicus in the island should be also investigated with molecular markers on a large number of individuals to shed light on the phylogeographic structure of this species in Cyprus.
Acknowledgments
We would like to thank the Director of the Agricultural Research Institute (ARI) Dora Chimonidou and Damianos Neocleous, Head of Section Natural Resources and Environment (ARI), for their support. We would like also to thank the Ilenia Azzena for her invaluable help in the molecular laboratory activities, Michael Hadjiconstantis for assisting with the design of the map, Andreas Demetriades for assisting with trapping of rats in Chloraka, Demetris Kokkinos for assisting with trapping of rats near Vavatsinia village, and Roula Panayiotou for assisting with the English language.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-1729/10/8/136/s1, Table S1: Rattus rattus sequences retrieved from GenBank and included in the phylogenetic analysis.
Author Contributions
E.H.: research conceptualization, sample design and methodology, specimen collection, investigation and data collection, morphological data analysis and interpretation, writing original draft, review, and editing. G.K.: research conceptualization, specimen collection, investigation, data collection, writing and review. D.S.: investigation and data collection, molecular data analysis and interpretation, writing review and editing. P.M.: sample design and methodology, writing original draft, review, and editing. M.P.: molecular data analysis and interpretation, writing original draft, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The present research was supported by the Department of Biomedical Sciences (University of Sassari-Italy) and the Agricultural Research Institute of the Ministry of Agriculture, Rural Development and Environment (Cyprus).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Nowak R.M. Walker’s Mammals of the World. Volume I Johns Hopkins University Press; Baltimore, MD, USA: London, UK: 1999. [Google Scholar]
- 2.Aplin K.P., Brown P.R., Jacob J., Krebs C.J., Singleton G.R. Field Methods for Rodent Studies in Asia and the Indo-Pacific. Australian Centre for International Agricultural Research (ACIAR); Canberra, Australia: 2003. [Google Scholar]
- 3.Musser G.G., Carleton M.D. Family Muridae. In: Wilson D.E., Reeder D.M., editors. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press; Baltimore, MD, USA: 2005. pp. 894–1531. [Google Scholar]
- 4.Atkinson I.A.E. The spread of commensal species of Rattus to oceanic islands and their effects on island fauna. In: Moors P., editor. Conservation of Island Birds: Case Studies for the Management of Threatened Island Species. Paston Press; Norwich, UK: 1985. pp. 35–81. [Google Scholar]
- 5.Courchamp F., Chapuis J.L., Pascal M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. Camb. Philos. Soc. 2003;78:347–383. doi: 10.1017/S1464793102006061. [DOI] [PubMed] [Google Scholar]
- 6.Jones H., Holmes N.D., Butchart S.H.M., Tershy B.R., Kappes P.J., Corkery I., Aguirre-Muñoz A., Armstrong D.P., Bonnaud E., Burbidge A.A., et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. USA. 2016;113:4033–4038. doi: 10.1073/pnas.1521179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburn T.M., Cassey P., Duncan R.P., Evans K.L., Gaston K.J. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305:1955–1958. doi: 10.1126/science.1101617. [DOI] [PubMed] [Google Scholar]
- 8.Doherty T.S., Glen A.S., Nimmo D.G., Ritchie E.G., Dickman C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA. 2016;113:11261–11265. doi: 10.1073/pnas.1602480113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellard C., Cassey P., Blackburn T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016;12:20150623. doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks P.B., Hughes N.K. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildl. Res. 2012;39:78–88. doi: 10.1071/WR11086. [DOI] [Google Scholar]
- 11.Shiels A.B., Pitt W.C., Sugihara R.T., Witmer G.W. Biology and impacts of Pacific island invasive species. 11. Rattus rattus, the black rat (Rodentia: Muridae) Pac. Sci. 2014;68:145–184. [Google Scholar]
- 12.Robins J.H., Hingston M., Matisoo-Smith E., Ross H.A. Identifying Rattus species using mitochondrial DNA. Mol. Ecol. Notes. 2007;7:717–729. doi: 10.1111/j.1471-8286.2007.01752.x. [DOI] [Google Scholar]
- 13.Taylor J.M., Calaby J.H., Van Deusen H.M. A revision of the genus Rattus (Rodentia, Muridae) in the New Guinean region. Bull. Am. Mus. Nat. Hist. 1982;173:177–336. [Google Scholar]
- 14.Audoin-Rouzeau E., Vigne J.D. La colonisation de l’Europe par le rat noir (Rattus rattus) Revue de Paléobiologie. 1994;3:125–145. [Google Scholar]
- 15.Vigne J.D., Valladas H. Small Mammal Fossil Assemblages as Indicators of Environmental Change in Northern Corsica during the Last 2500 Years. J. Archaeol. Sci. 1996;23:199–215. doi: 10.1006/jasc.1996.0018. [DOI] [Google Scholar]
- 16.Watson J.S. Tetrathyridium Larvae of Mesocestoides in Rodents in Cyprus. J. Parasitol. 1949;35:383–387. doi: 10.2307/3273428. [DOI] [PubMed] [Google Scholar]
- 17.Watson J.S. The Rat Problem in Cyprus: A Report of Investigations Made in Carob-Growing Areas. His Majesty’s Stationery Office; London, UK: 1951. Colonial Research Publications No. 9. [Google Scholar]
- 18.Spitzenberger F. Die Saugetierfauna Zyperns. Teil I: Insectivora and Rodentia. Ann. Nat. Hist. Mus. Wien. 1978;81:401–441. [Google Scholar]
- 19.Kourtellarides L. The Nesting Birds of Cyprus. Bank of Cyprus and Ornithological Society of Cyprus; Nicosia, Cyprus: 1997. p. 299. [Google Scholar]
- 20.Hadjisterkotis E. The terrestrial prehistoric and modern mammals and birds of Cyprus, with emphasis on the endemics. In: Hadjisterkotis E., editor. International Year of the Mountains, Peripheral Symposium on Pitsilia (27 December 2002) Ministry of Interior; Nicosia, Cyprus: 2003. pp. 23–38. [Google Scholar]
- 21.Hadjisterkotis E. The effect of corvid shooting on the population of owls, kestrels and cuckoos in Cyprus, with notes on corvid diet. Z. Jagdwiss. 2003;49:50–60. doi: 10.1007/BF02192013. [DOI] [Google Scholar]
- 22.Psaroulaki A., Antoniou M., Papaeustathiou A., Toumazos P., Loukaides F., Tselentis Y. First detection of Rickettsia felis in Ctenocephalides felis fleas parasitizing rats in Cyprus. Am. J. Trop. Med. Hyg. 2006;74:120–122. doi: 10.4269/ajtmh.2006.74.120. [DOI] [PubMed] [Google Scholar]
- 23.Antoniou M., Psaroulaki A., Toumazos P., Mazeris A., Ioannou I., Papaprodromou M., Georgiou K., Hristofi N., Patsias A., Loucaides F., et al. Rats as indicators of the presence and dispersal of pathogens in Cyprus: Ectoparasites, parasitic helminths, enteric bacteria, and encephalomyocarditis virus. Vector Borne Zoonotic Dis. 2010;10:867–873. doi: 10.1089/vbz.2009.0123. [DOI] [PubMed] [Google Scholar]
- 24.Kryštufek B., Vohralík V. Mammals of Turkey and Cyprus Rodentia II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae. Univerza na Primorskem; Koper, Slovenia: 2009. [Google Scholar]
- 25.Cucchi T., Orth A., Auffray J.C., Renaud S., Fabre L., Catalan J., Hadjisterkotis E., Bonhomme F., Vigne J.D. A new endemic species of the subgenus Mus (Rodentia, Mammalia) on the island of Cyprus. Zootaxa. 2006;1241:1–36. [Google Scholar]
- 26.Research Promotion Foundation . The Qualitative and Quantitative Approached of the Small Mammalian Fauna in Natura 2000 Sites of Cyprus. RPF; Nicosia, Cyprus: 2010. (Final Report, Project: AEIFO/056/09). [Google Scholar]
- 27.Nicolaou H., Hadjisterkotis E., Sparrow D.J., Sparrow R. Mammals. In: Sparrow D.J., John E., editors. An Introduction to the Wildlife of Cyprus. Terra Cypria; Limassol, Cyprus: 2016. [Google Scholar]
- 28.Nicolaou H. The Wild Mammals of Cyprus. Philodasikos Syndesmos Kyprou; Nicosia, Cyprus: 2017. [Google Scholar]
- 29.Hadjisterkotis E. The origin and extinction of the large Pleistocene endemic mammals of Cyprus. In: Demetriades K., Sofokleous A., Argyrides P., editors. Geography of Cyprus. Geographical Society of Cyprus; Nicosia, Cyprus: 2017. pp. 291–319. [Google Scholar]
- 30.Hadjisterkotis E. Terrestrial prehistoric and modern mammals of Cyprus. In: Demetriades K., Sofokleous A., Argyrides P., editors. Geography of Cyprus. Geographical Society of Cyprus; Nicosia, Cyprus: 2017. pp. 321–354. [Google Scholar]
- 31.Moysi M., Christou M., Goutner V., Kassinis N., Iezekiel S. Spatial and temporal patterns in the diet of barn owl (Tyto alba) in Cyprus. J. Biol. Res. (Thessalon.) 2018;25:9. doi: 10.1186/s40709-018-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagorsen D.W., Royal British Columbia Museum and British Columbia. Ministry of Sustainable Resource Management and British Columbia. Biodiversity Branch . An Identification Manual to the Small Mammals of British Columbia. Government of British Columbia; Victoria, Canada: 2002. [Google Scholar]
- 33.Qumsiyeh M.B. Mammals of the Holy Land. Texas Tech University Press; Lubbock, TX, USA: 1996. [Google Scholar]
- 34.Yiğit N., Çolak E., Sözen M. The Taxonomy and Karyology of Rattus norvegicus (Berkenhout, 1769) and Rattus rattus (Linnaeus, 1758) (Rodentia: Muridae) in Turkey. Turk. J. Zool. 1998;22:203–212. [Google Scholar]
- 35.Pimsai U., Pearch M.J., Satasook C., Bumrungsri S., Bates P.J.J. Murine rodents (Rodentia: Murinae) of the Myanmar-Thai-Malaysian peninsula and Singapore: Taxonomy, distribution, ecology, conservation status, and illustrated identification keys. Bonn. Zool. Bull. 2014;63:15–114. [Google Scholar]
- 36.Skrzypek M.S., Binkley J., Binkley G., Miyasato S.R., Simison M., Sherlock G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017;45:592–596. doi: 10.1093/nar/gkw924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozas J., Ferrer-Mata A., Sanchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S.E., Sanchez-Gracia A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 38.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Wallace I.M., Wilm A., Lopez R., Thompson J.D., et al. Clustal w and clustal x version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 39.Ronquist F., Huelsenbeck J.P. Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S., Stecher G., Tamura K. Mega 7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarisation in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D.-Y., Liu J., Liu B.-Y., Liu Y.-Y., Xiong H.-R., Hou W., Yang Z.-Q. Phylogenetic analysis based on mitochondrial DNA sequences of wild rats, and the relationship with Seoul virus infection in Hubei, China. Virol. Sin. 2017;32:235–244. doi: 10.1007/s12250-016-3940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bibby C.J., Crosby M.J., Health M.F., Johnson T.H., Long T.H., Sattersfield A.J., Thirgood S.J. Putting Biodiversity on the Map: Global Priorities for Conservation. International Council for Bird Preservation; Cambridge, UK: 1992. [Google Scholar]
- 44.Sparrow D.J., Baier F. Squamata and Testudines—Snakes, Lizzards, Terrapins and Turtles. In: Sparrow D.J., John E., editors. An Introduction to the Wildlife of Cyprus. Terra Cypria; Limassol, Cyprus: 2016. [Google Scholar]
- 45.Makris C. Butterflies of Cyprus. Cultural Foundation of the Bank of Cyprus; Nicosia, Cyprus: 2002. [Google Scholar]
- 46.John E. Butterflies. In: Sparrow D.J., John E., editors. An Introduction to the Wildlife of Cyprus. Terra Cypria; Limassol, Cyprus: 2016. [Google Scholar]
- 47.Gittenberger E. Non-Marine Snails and Slugs. In: Sparrow D.J., John E., editors. An Introduction to the Wildlife of Cyprus. Terra Cypria; Limassol, Cyprus: 2016. pp. 569–586. [Google Scholar]
- 48.World Conservation Monitoring Centre (WCMC) Global Biodiversity: Status of the Earth’s Living Resources. Chapman and Hall; London, UK: 1992. [Google Scholar]
- 49.Tsintides T.C. The Endemic Plants of Cyprus. Bank of Cyprus Group and Cyprus Association of Professional Foresters; Nicosia, Cyprus: 1998. [Google Scholar]
- 50.Tsintides T.C., Hadjikyriakou G.N., Christodoulou C.S. Trees and Shrubs in Cyprus. Foundation Anastasios G. Leventis and Cyprus Forest Association; Lefkosia, Cyprus: 2002. [Google Scholar]
- 51.Tsintides T.C., Christodoulou C.S., Georgiou K., Delipetrou P. The Red Data Book of the Flora of Cyprus. Cyprus Forest Association; Lefkosia, Cyprus: 2006. [Google Scholar]
- 52.Hadjisterkotis E., Masala B. Vertebrate extinction in Mediterranean islets: An example from Cyprus. Biogeographia. 1995;18:691–699. doi: 10.21426/B618110414. [DOI] [Google Scholar]
- 53.Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 54.Hadjisterkotis E. The Cyprus mouflon, a threatened species in a biodiversity “hotspot” area. In: Nahlik A., Uloth W., editors. Proceedings of the International Mouflon Symposium; Sopron, Hungary. 27–29 October 2000; Sopron, Hungary: Nygat-Magyarországi Egyetem; 2001. pp. 71–81. [Google Scholar]
- 55.Hadjisterkotis E. Breeding phenology and success of the Woodpigeon (Columba palumbus) in Cyprus. Game Wildl. Sci. 2000;17:81–92. [Google Scholar]
- 56.Chaval Y., Dobigny G., Michaux J., Pagès M., Corbisier C., Cosson J.F., Herbreteau V. A multiapproach survey as the most reliable tool to accurately assess biodiversity: An example of Thai murine rodents. Kasetsart J. (Nat. Sci.) 2010;44:590–603. [Google Scholar]
- 57.Canale D.E., Dio V., Massa B., Mori E. First successful eradication of invasive Norway rats Rattus norvegicus from a small Mediterranean island (Isola delle Femmine, Italy) Folia Zool. 2019;68:29–34. doi: 10.25225/fozo.060.2019. [DOI] [Google Scholar]
- 58.Angelici F.M., Laurenti A., Nappi A. A checklist of the mammals of small Italian islands. Hystrix. 2009;20:3–27. [Google Scholar]
- 59.IUCN Information Paper; Proceedings of the Fifth Meeting of the Conference of the Parties to the Conservation on Biological Diversity; Nairobi, Kenya. 15–16 May 2000. [Google Scholar]
- 60.Ott S.L., Wells S.J., Wagner B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999;40:179–192. doi: 10.1016/S0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 61.Liapi M., Leontides L., Kostoulas P., Botsaris G., Iacovou Y., Rees C., Georgiou K., Smith G.C., Naseby D.C. Bayesian estimation of the true prevalence of Mycobacterium avium subsp. paratuberculosis infection in Cypriot dairy sheep and goat flocks. Small Rumin. Res. 2011;95:174–178. doi: 10.1016/j.smallrumres.2010.09.010. [DOI] [Google Scholar]
- 62.Liapi M., Botsaris G., Slana I., Moravkova M., Babak V., Avraam M., Di Provvido A., Georgiadou S., Pavlik I. Mycobacterium avium subsp. paratuberculosis Sheep Strains Isolated from Cyprus Sheep and Goats. Transbound. Emerg. Dis. 2015;62:223–227. doi: 10.1111/tbed.12107. [DOI] [PubMed] [Google Scholar]
- 63.Botsaris G., Liapi M., Kakogiannis C., Dodd C., Rees C. Detection of Mycobacterium avium subsp. paratuberculosis in bulk tank milk by combined phage-PCR assay: Evidence that plaque number is a good predictor of MAP. Int. J. Food Microbiol. 2013;164:76–80. doi: 10.1016/j.ijfoodmicro.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 64.Florou M., Leontides L., Kostoulas P., Billinis C., Sofia M., Kyriazakis I., Lykotrafitis F. Isolation of Mycobacterium avium subspecies paratuberculosis from non-ruminant wildlife living in the sheds and on the pastures of Greek sheep and goats. Epidemiol. Infect. 2007;136:644–652. doi: 10.1017/S095026880700893X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer M.V., Stoffregen W.C., Carpenter J.G., Stabel J.R. Isolation of Mycobacterium avium subsp. paratuberculosis (Map) from feral cats on a dairy farm with Map-infected cattle. J. Wildl. Dis. 2005;41:629–635. doi: 10.7589/0090-3558-41.3.629. [DOI] [PubMed] [Google Scholar]
- 66.Psaroulaki A., Christou C., Chochlakis D., Tsiligianni I., Sandalakis V., Georgalis L., Ioannou I., Giorgalas G., Tselentis Y. Murine typhus in Cyprus: A 9-year survey. Trans. R. Soc. Trop. Med. Hyg. 2012;106:489–495. doi: 10.1016/j.trstmh.2012.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.