Abstract
Despite a long history of community-directed treatment with ivermectin (CDTI), a high ongoing Onchocerca volvulus transmission is observed in certain onchocerciasis-endemic regions in Africa with a high prevalence of epilepsy. We investigated factors associated with higher microfilarial (mf) density after ivermectin treatment. Skin snips were obtained from O. volvulus-infected persons with epilepsy before, and 3 to 5 months after ivermectin treatment. Participants were enrolled from 4 study sites: Maridi (South Sudan); Logo and Aketi (Democratic Republic of Congo); and Mahenge (Tanzania). Of the 329 participants, 105 (31.9%) had a post-treatment mf density >20% of the pre-treatment value. The percentage reduction in the geometric mean mf density ranged from 69.0% (5 months after treatment) to 89.4% (3 months after treatment). A higher pre-treatment mf density was associated with increased probability of a positive skin snip after ivermectin treatment (p = 0.016). For participants with persistent microfiladermia during follow-up, a higher number of previous CDTI rounds increased the odds of having a post-treatment mf density >20% of the pre-treatment value (p = 0.006). In conclusion, the high onchocerciasis transmission in the study sites may be due to initially high infection intensity in some individuals. Whether the decreasing effect of ivermectin with increasing years of CDTI results from sub-optimal response mechanisms warrants further research.
Keywords: ivermectin, sub-optimal response, onchocerciasis, Onchocerca volvulus, epilepsy
1. Introduction
With an estimated 20.9 million people infected worldwide, of whom over 99% live in sub-Saharan Africa [1], Onchocerca volvulus (O. volvulus) infections are an important global health problem. O. volvulus is a filarial nematode causing onchocerciasis and is transmitted by blackflies (Simuliidae). Clinical manifestations associated with onchocerciasis include skin and eye disease, nodding syndrome, and other forms of epilepsy (hereafter referred to as onchocerciasis-associated epilepsy (OAE)) [2]. Adult female worms reside in subcutaneous nodules and produce larvae, called microfilariae (mf), that migrate under the skin.
Ivermectin, a broad-spectrum anti-helmintic drug, rapidly kills mf. A single dose of ivermectin leads to a decline in skin mf density of approximately 99% within the first two months [3]. Unfortunately, ivermectin does not kill the adult worms, it only temporarily represses mf production by the adult female worms and therefore mf production resumes at a slow rate after approximately 3 to 6 months [4,5]. Nonetheless, mf loads are expected to remain below 20% of the pre-ivermectin levels for up to 10 months following a single dose of ivermectin [3]. Therefore, many onchocerciasis endemic areas have adopted annual or bi-annual community-directed treatment with ivermectin (CDTI) as a strategy to decrease onchocerciasis transmission [6,7].
Onchocerciasis elimination programs using CDTI have significantly reduced O. volvulus transmission and onchocerciasis-related blindness in many African countries [7]. Nevertheless, despite a long history of CDTI, there is still active onchocerciasis transmission in many endemic areas in Ghana [8], Cameroon [9], Democratic Republic of Congo (DRC) [10], and Tanzania [11]. Moreover, such meso- and hyperendemic settings have also been noted to harbor a high burden of OAE [10,11,12,13]. In Ghana and Cameroon, an O. volvulus phenotype with sub-optimal ivermectin response has been documented [4,14] which may contribute, at least partly, to the persistence of O. volvulus transmission. To determine host factors associated with the high onchocerciasis prevalence and transmission observed in OAE hotspots, we conducted a study assessing individual mf density before and after ivermectin administration.
2. Materials and Methods
2.1. Study Participants and Study Sites
We performed the study among persons with epilepsy (PWE) with the intention to investigate the effect of ivermection on microfilarial (mf) density but also to evaluate whether ivermectin could decrease the frequency of seizures in PWE infected with O. volvulus. Study participants were PWE that were identified during door-to-door surveys in four onchocerciasis endemic study sites. The four study sites were the Aketi health zone, Bas Uélé province and the Logo health zone, Ituri province in the Democratic Republic of Congo (DRC), Mahenge, Ulanga district in Tanzania and Maridi in South Sudan. In Maridi, Aketi and Mahenge participants were enrolled only for a short follow up study, while in the Logo health zone, the 132 participants were enrolled in a randomized clinical trial to evaluate the potential added value of ivermectin in decreasing the frequency of seizures in PWE with O. volvulus infection treated with phenobarbital [15]. Right and left skin snips were obtained from each participant before ivermectin treatment intake. Participants with detectable mf in their skin snips were asked to have the skin snip testing repeated 3 to 5 months after ivermectin treatment. Results of the effect of ivermectin on the frequency of seizures will be published elsewhere. All study sites had a different history of CDTI and a different schedule of skin snip testing, as depicted in Table 1.
Table 1.
Years of community-directed treatment with ivermectin (CDTI) and timing of skin snip testing per study site.
| Characteristic | South Sudan | DRC | Tanzania | |
|---|---|---|---|---|
| Maridi | Logo | Aketi | Mahenge | |
| Years of CDTI | 1 | 0 | 14 | 21 |
| Number of months since last CDTI | 12 | NA | 12 | 5 |
| Number of months between pre-treatment skin snip (followed by ivermectin administration) and post-treatment skin snip | 5 | 4 | 3 | 3 |
NA = not applicable because of no previous CDTI round.
2.2. Aketi Health Zone, DRC
The Aketi health zone is an onchocerciasis hyper-endemic area in Bas-Uélé province in the DRC where CDTI was initiated 14 years ago [16]. Despite this long history of CDTI, an epilepsy prevalence of 5.7% was reported in Aketi rural town during a door-to-door household survey in 2017. In addition, a seroprevalence of OV16 IgG4 antibodies against O. volvulus of 64.5% was reported in children aged 7–10 years old, suggesting a high ongoing onchocerciasis transmission [10]. In January 2018, skin snips were obtained from selected PWE from Wela, Makoko, and Aketi rural town. Eighty-one skin snip positive PWE [17] were asked to participate in a follow up study and to be skin snipped again three months after ivermectin intake. Post-ivermectin skin snips could be obtained from 74 of these PWE.
2.3. Logo Health Zone, DRC
In October 2017, a proof-of-concept randomized clinical trial investigating the effect of ivermectin on seizure frequency in O. volvulus infected PWE was initiated in the Logo health zone, an onchocerciasis-endemic area with a high epilepsy prevalence (4.6%) where ivermectin had never been distributed before [13]. In February 2018, PWE were asked to participate in a randomized clinical trial to evaluate the effect of ivermectin on the frequency of seizures in O. volvulus infected PWE [15]. In 392 PWE, skin snips were obtained and 143 (36.5%) of them had detectable mf [17]. These 143 skin snip positive PWE were asked to participate in a follow up study and to be skin snip tested again four months after ivermectin intake. In 136 (95%) of them, pre-and post-ivermectin skin snip results were obtained.
2.4. Maridi, South Sudan
In May 2018, a door-to-door household survey in eight villages in an onchocerciasis endemic area in Maridi county showed an overall epilepsy prevalence of 4.4%, reaching up to 11.9% in the Kazana II village, which is located closest to the Maridi dam, a blackfly breeding site [12]. CDTI had been interrupted for at least ten years in this region and was re-initiated in 2017. During a CDTI round in December 2018, PWE identified in the initial survey were asked to participate in a study to determine the prevalence of O. volvulus infection among PWE. Of the 318 PWE who agreed to participate, 270 (84.9%) had detectable mf in their skin snips pre-ivermectin [18]. These 270 skin snip positive PWE were asked to participate in a follow up skin snip study 5 months after ivermectin intake. In 102 (38%) of them, pre- and post-ivermectin skin snip results were obtained.
2.5. Mahenge, Tanzania
Located in the Ulanga district, Mahenge is a known onchocerciasis-endemic focus. The Tanzanian National Onchocerciasis Control Programme initiated annual CDTI since 1997 and bi-annual CDTI in 2019. In January 2017, we conducted an epilepsy prevalence survey in two rural villages in the Mahenge area (Mdindo, Msogezi), and documented a high prevalence of epilepsy (3.5%) among the general population in these villages as well as a high prevalence of OV16 antibodies in children 6–10 years (42.5%) [11]. PWE identified during the initial survey were asked to participate in a study to determine the prevalence of O. volvulus infection among PWE. Of the 42 PWE who agreed to participate, 22 (52.4%) presented mf in skin snips (Bhwana D., personal communication). In April 2019, these 22 PWE with positive skin snip were asked to participate in a follow up study which entailed collecting a second skin snip 3 months after ivermectin intake. In 17 (77%) of them, pre- and post-ivermectin skin snip results were obtained.
2.5.1. Participant Data
Upon enrollment in the study, we obtained the following data from all study participants: age, gender, weight, height, and town or village of residence. In addition, we obtained from the health authorities the exact number of previous rounds of CDTI in each study site (Table 1).
2.5.2. Skin Snip Testing
Skin snips were obtained from each posterior iliac crest of eligible participants using a Holt-type punch. Snips were immediately placed in two wells of a microtitre plate containing 3 drops of normal saline solution and incubated for 24 hours at room temperature to allow mf to emerge into the fluid. After the incubation period, mf in the solution was examined microscopically under a x40 magnification and counted by a trained technician. Mf densities were expressed as arithmetic means between mf count at right skin snips and mf count at left skin snips. One punch was used per subject and punches were sterilized between subjects using steam under pressure (autoclave).
2.5.3. Ivermectin Treatment
Ivermectin was given under direct observation at approximately 150 µg per kg of body weight or using a height equivalent, as prescribed by the World Health Organization guidelines [6]. Our primary indicator of ivermectin treatment efficacy was the reduction in skin mf densities in O. volvulus-infected PWE measured 3–5 months after ivermectin treatment, both at the individual level and per study site. We also investigated host-factors associated with a persistent microfiladermia in the post-ivermectin skin snip samples.
2.5.4. Data Processing
The mf density of participants before and after ivermectin treatment were entered into electronic spreadsheets. Given that mf density was recorded as mf per skin snip, and that mf density per skin snip in a small body surface area (BSA) (mostly for children) is likely to be higher than in a large BSA, we calculated participants’ BSA using Boyd’s formula:
where W = weight of the participant (in kg) and H = height of the participant (in m). We then used the individual BSA to normalize individual O. volvulus skin mf densities [19,20]. A BSA of 1.73 is mostly used in medical physiology to normalize a number of physiological variables [21]. The absolute value of individual pre-ivermectin skin snip mf density was therefore normalized as: pre-/post-ivermectin skin mf density*individual rBSA with relative BSA (rBSA) equal to BSA/1.73.
2.5.5. Statistical Analysis
Baseline characteristics of all participants within each study site were summarized using the absolute and relative frequency for categorical variables, and median (with interquartile range, IQR) for continuous variables. Mf density before and after ivermectin for each study site were summarized with geometric mean (GM) calculated as the nth root of the product of individual mf density (to which 1 was added so as to include post-ivermectin negative skin snips), with n equal to number of PWE per study site. The percentage reduction in GM mf density per study site was calculated as the difference between pre- and post-ivermectin GM mf density divided by pre-ivermectin GM density multiplied by 100. The distribution of post-ivermectin O. volvulus mf density was skewed to the right and a large proportion of O. volvulus mf density values was zero. Therefore, post-ivermectin O. volvulus mf density data were modeled using Poisson and negative binomial hurdle models and zero-inflated Poisson and negative binomial models [22]. Akaike’s Information Criteria (AIC) was used to select the ‘best’ model among all candidate models [23]. Parameter estimation in the different models was done using maximum likelihood estimation. In a hurdle model, the analysis consists of a separate logistic regression model and a truncated Poisson (or negative binomial) regression model. The logistic regression model is used to assess the effect of predictors on the probability of having a positive skin snip after ivermectin treatment, whereas the truncated Poisson (or negative binomial) regression approach studies the effects of covariates on post-ivermectin O. volvulus mf densities conditional on having a positive skin snip after ivermectin treatment. All possible two-way interactions between baseline characteristics were tested to be included in the final model using the likelihood ratio test. Data were analyzed using SAS 9.4 (SAS Institute Inc.) and two-sided tests at a significance level of 5%.
3. Results
A total of 329 O. volvulus-infected PWE from four sites participated in the study (Table 2). Study sites were comparable in terms of gender and age, with the exception of Mahenge where participants appeared to be older (Kruskal–Wallis chi-square test = 41.8, and degree of freedom (df) = 3, p < 0.001)
Table 2.
Characteristics of study participants in the four study sites.
| Participant Characteristic | South Sudan | DRC | Tanzania | |
|---|---|---|---|---|
| Maridi (n = 102) | Logo (n = 136) | Aketi (n = 74) | Mahenge (n = 17) | |
| Age (years), Median (IQR) | 16.5 (14.0–18.0) | 22.0 (17.0–30.5) | 17.0 (15.0–20.0) | 35.0 (25.0–45.0) |
| Male gender: n (%) | 53 (50) | 69 (49) | 40 (49) | 11 (46) |
| Weight (kg), Median (IQR) | 38.0 (30.0–48.0) | 45.0 (38.5–50.0) | 37.5 (43.5–54.5) | 45.0 (40.0–51.0) |
| Height (cm), Median (IQR) | 158.0 (154.5–162.0) | 154.0 (147.0–160.0) | 154.0 (138.0–156.0) | 154.0 (146.0–160.0) |
| Pre-ivermectin mf density per skin snip, Median (IQR) | 22.7 (12.0–44.5) | 28.0 (7–93.0) | 9.4 (3.0–52.5) | 2 (2.0–20.6) |
| Pre-ivermectin mf density, GM (SE) | 15.0 (1.1) | 24.7 (3.2) | 12.9 (2.1) | 5.7 (1.6) |
N: number; mf: microfilariae; IQR = Interquartile range; GM: Geometric mean; SE: Geometric mean standard error.
3.1. Effects of Ivermectin Treatment on the Mf Density of Participants
Of the 329 PWE, 105 (31.9%) experienced a <80% decrease in mf density in their post-treatment skin snips (implying that the post-treatment mf density was >20% of the pre-treatment value). The proportion of PWE with <80% decrease in mf density increased with the number of months before the second skin snip was obtained (Mantel–Haenszel chi-square: 48.8, df =1, p < 0.001), being highest in Maridi (second skin snips after 5 months) and lowest in Aketi (second skin snips after 3 months). At the community level, reduction in geometric mean mf density was highest in the Aketi study site (89% reduction) followed by Logo (86% reduction) (Table 3).
Table 3.
Post-ivermectin parasitological data of study participants.
| Maridi (n = 102) | Logo (n = 136) | Aketi (n = 74) | Mahenge (n = 17) | |
|---|---|---|---|---|
| Months post-ivermectin intake: n | 5 | 4 | 3 | 3 |
| Positive skin snip: n (%) | 82 (80) | 66 (48) | 18 (24) | 9 (53) |
| <80% decrease in individual mf density: n (%) | 52 (51) | 38 (28) | 8 (11) | 7 (41) |
| Overall Mf per skin snip, Median (IQR) | 4.5 (1.0–17.5) | 0.0 (0.0–9.0) | 0.0 (0.0–0.5) | 0.5 (0.0–1.0) |
| Mf per skin snip of PWE with post-ivermectin positive skin snip; Median (IQR) | 5.0 (1.5–19.0) | 10.5 (2.5–38.0) | 2.0 (0.5–5.5) | 1 (0.5–1.0) |
| Post-ivermectin GM mf density per skin snip, GM (SE) | 4.7 (0.6) | 3.4 (0.5) | 1.4 (0.2) | 1.6 (0.3) |
| Overall percentage reduction in GM mf density per skin snip (%) | 69 | 86 | 89 | 72 |
n: number; mf: microfilariae density, IQR = Interquartile range; GM: Geometric mean; SE: Geometric mean standard error.
3.2. Host Factors Associated with Post-Ivermectin Mf Density Values Given a Post-Ivermectin Positive Skin Snip Test
To investigate host factors associated with post-ivermectin O. volvulus mf density, we described the average post-ivermectin mf density value, conditional on having a positive skin snip after ivermectin, while adjusting for gender, age, height, number of previous CDTI rounds in the site, duration since last ivermectin intake, and mf density before ivermectin treatment. The negative binomial hurdle model, for which results are presented in Table 4, outperformed other hurdle and zero-inflated models in terms of AIC (Table A1). A higher pre-ivermectin O. volvulus mf density was associated with an increased probability of having detectable mf in the follow-up skin snip (Table 4). The effect of pre-ivermectin O. volvulus mf density did not change when BSA was included as an offset in the model.
Table 4.
Negative binomial hurdles model to assess host factors associated with post-ivermectin O. volvulus microfilarial density.
| Post-Ivermectin O. volvulus Mf Density | Probability of Positive Skin Snip after Ivermectin Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Coeff | 95% CI | p-Value | Coeff | 95% CI | p-Value | ||
| Female gender | −0.312 | −0.946 | 0.323 | 0.336 | −2.518 | −4.913 | −0.124 | 0.039 |
| Male gender (reference) | ||||||||
| Age (years) | 0.012 | −0.041 | 0.064 | 0.661 | −0.391 | −0.656 | −0.125 | 0.004 |
| Age*age (years) | −0.001 | −0.003 | 0.001 | 0.349 | 0.012 | 0.003 | 0.020 | 0.007 |
| Height in cm | −0.007 | −0.055 | 0.041 | 0.773 | 0.091 | −0.010 | 0.192 | 0.076 |
| Number of previous CDTI rounds in site | −0.216 | −0.515 | 0.083 | 0.157 | −0.591 | −1.252 | 0.069 | 0.079 |
| >1 year since last ivermectin dose | 0.455 | −0.476 | 1.386 | 0.338 | −2.020 | −4.137 | 0.097 | 0.062 |
| <1 year since last ivermectin dose (reference) | ||||||||
| Follow-up skin snip at month 3 | 2.734 | −1.823 | 7.290 | 0.240 | ||||
| Follow-up skin snip at month 4 | 0.533 | −0.384 | 1.450 | 0.255 | ||||
| Follow-up skin snip at month 5 (reference) | ||||||||
| Pre-ivermectin microfilarial density # | 0.337 | 0.064 | 0.611 | 0.016 | 1.187 | 0.161 | 2.213 | 0.023 |
#: Log-transformed; Coeff = Negative binomial hurdle model coefficients, CI = Confidence interval; Age*age = Quadratic effect of age.
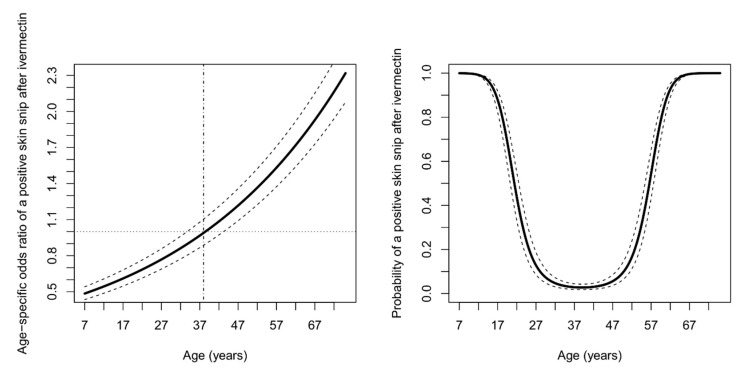
In Figure 1, we graphically display the quadratic effect of age on the probability of a positive skin snip after ivermectin treatment. More specifically, a plot of age-dependent odds ratio for a positive follow-up skin snip reveals that 38 years is the transition age before which the probability of a positive skin snip after ivermectin treatment decreases with increasing age, whereas the reverse is true for individuals aged 38 years or more (Figure 1).
Figure 1.
The age-dependent odds ratio of having a positive skin snip after ivermectin for a unit increase in age (left panel). Probability of having a positive skin snip after ivermectin treatment as function of age for female persons of height 180 cm with more than a year since last CDTI round, with 175 mf per skin snip before ivermectin and living in a community where CDTI has been implemented for at least 10 years (right panel). Solid black lines represent the point estimates and dashed lines indicate pointwise 95% confidence bands based on the delta method.
A higher number of previous CDTI rounds in the study site and a longer time lapse between ivermectin intake and the follow-up skin snip increased the odds for <80% individual skin mf reduction (Table 5).
Table 5.
Logistic regression to assess the factors associated with O. volvulus mf density reduction of <80% following ivermectin treatment in the four study sites.
| Factors | Coeff | 95% CI | p-Value | |
|---|---|---|---|---|
| Female gender | −0.058 | −0.568 | 0.453 | 0.825 |
| Male gender (reference) | ||||
| Age (years) | −0.097 | −0.192 | −0.002 | 0.044 |
| Age*age (years) | 0.001 | 0.001 | 0.003 | 0.045 |
| Height in cm | −0.019 | −0.048 | 0.010 | 0.207 |
| Number of previous CDTI rounds in site | 0.282 | 0.080 | 0.484 | 0.006 |
| Follow-up skin snip at month 3 | −6.943 | −10.064 | −3.822 | <0.001 |
| Follow-up skin snip at month 4 | −1.101 | −1.850 | −0.351 | 0.004 |
| Follow-up skin snip at month 5 (reference) | ||||
| Pre-ivermectin microfilarial density # | 0.202 | 0.012 | 0.392 | 0.037 |
#: Log-transformed; Coeff = Logistic regression model coefficients, CI = Confidence interval; Age*age = Quadratic effect of age.
4. Discussions
In this study, we investigated treatment response to ivermectin in 329 O. volvulus-infected PWE living in four onchocerciasis-endemic areas in sub-Saharan Africa. The post-ivermectin mf density was >20% the pre-treatment value in 105 (31.9%) participants, suggesting that their response to ivermectin warrants further investigation [8]. We observed the highest GM mf reduction of 89.4% three months after ivermectin treatment, as compared to 69.0% GM mf reduction five months post-treatment. Previous studies reported up to 98% reduction of GM mf density, 14 to 90 days after a single dose of ivermectin, with a recuperation of adult worm fertility starting around the third month resulting in a reduction in GM mf density of about 84%, four months post-ivermectin [3].
In the three study sites where the second skin snip was obtained within four months, the percentage of PWE with positive skin snips after ivermectin was lower when compared with a study in Ghana, where 70% of participants had detectable mf four months post-ivermectin [8]. This was not the case with participants from Maridi (South Sudan) whose skin snips were obtained 5 months after treatment and up to 80.4% of participants still presented with microfiladermia. The proportion of participants with positive skin snips post-treatment was higher in our study than observed in moxidectin-treated participants during a comparative trial with ivermectin in the Logo health zone. In this trial, only 8% of the participants who received moxidectin still had detectable mf six months after treatment [24]. In addition to being more potent in reducing mf density, the microfilaricidal effects of moxidectin also last longer than ivermectin [25]. Therefore annual moxidectin or bi-annual ivermectin treatment should be considered for mass treatment in hyperendemic settings as it results in a long-lasting suppression of mf compared to ivermectin once a year.
In our study, we observed that a high pre-ivermectin mf density was significantly associated with a lower mf reduction in the follow-up skin snip. This was previously reported by Pion et al. [26] and in a meta-analysis by Churcher et al. [27], possibly because of the presence of a higher adult worm burden and/or higher numbers of newly patent adult worms in individuals with higher mf density. Therefore, we assume that almost all of the post-treatment positive skin snips in our study can be explained by skin repopulation, which is in line with our observation that post-ivermectin skin snip positivity increased with increasing time between ivermectin treatment and follow-up skin snipping.
We found that in younger persons, the post-ivermectin O. volvulus mf densities initially decreased with an increase in age as previously documented by Pion et al. [26]. A potential explanation is that skin repopulation may be more rapid in younger individuals because the adult worms are still maturing (lifespan of about 9–11 years) and rapidly releasing huge numbers of mf in the early phase of the infection [26,28]. In the older participants (>38 years) however, post-ivermectin O. volvulus mf densities increased as they got older (Figure 1). A possible explanation for the reversal in trends observed after 38 years could be the absence of adjustments for the number of nodules in our multivariate model [26,29].
An increasing number of previous CDTI rounds was associated with higher probability of achieving <80% mf reduction after ivermectin treatment (Table 5). This finding concurs with previous reports from Cameroon [26,29] where the embryostatic effect of ivermectin was reduced among individuals who had been treated several times with ivermectin in the past, compared to ivermectin-naïve individuals. In contrast, a longer history of CDTI tended to decrease the chances of having a positive skin snip post-ivermectin, although this trend was not statistically significant (p = 0.079; Table 4). This could be due to a very low pre-treatment mf density among participants who had been receiving ivermectin for several years prior to our study.
Our study has several limitations. Firstly, our study sites were very different in many aspects, including the time between ivermectin treatment and follow-up skin snipping, and the number of previous CDTI rounds. Secondly, we only evaluated mf densities at two time points and given that we did not collect adult female worms at either of the four sites, we were not able to assess the fecundity and mf density dynamics immediately after ivermectin use, which are needed to confirm sub-optimal treatment response. Moreover, the number of palpable nodules, as a proxy for the total number of adult worms, was not assessed in our study participants. Finally, PWE who take anti-epileptic drugs may experience decreased ivermectin drug levels. It is well known that phenobarbital can influence the p-glycoprotein (MDR1) transporter, which plays an important role in the elimination of ivermectin. Unfortunately, ivermectin plasma concentrations were not measured in this study.
In conclusion, our study shows that ivermectin effectively reduces mf density in our study participants, similar to what was observed in other onchocerciasis endemic areas. The fact that almost one third of our participants still had >20% of their pre-treatment mf density, as well as the high ongoing O. volvulus transmission observed at our study sites are most likely related to elevated mf densities at baseline as a result of ineffective or inexistent onchocerciasis elimination programs. While we clearly demonstrate that post-ivermectin parasitic load depends on pre-treatment infection intensity, it is unclear whether some study participants exhibit a sub-optimal response to ivermectin. Resistance to the ivermectin treatment has been reported in several parasites in veterinary studies [30,31,32]. Given the rapid rise and spread of ivermectin resistance in the veterinary field, studies should also investigate the possibility of ivermectin resistance in human medicine. In the light of the apparent decreasing effect of ivermectin as the number years of CDTI increases, studies need to investigate the number/fecundity of adult female worms that may be contributing to skin repopulation, and whether a sub-optimal response genotype is present in these areas as was the case in Ghana [14].
Acknowledgments
We thank the population of the four study sites for their participation in the study. We also thank Stijn Van Hees for critically reviewing the manuscript.
Appendix A
Table A1.
Model comparison information’s with relative rankings.
| Model | −2 LL | AIC | Pearson Chi-Square | Ranking |
|---|---|---|---|---|
| Poisson | 4907.6 | 8732.6 | 8566.8 | 5 |
| Negative Binomial | 4270.8 | 1467.6 | 536.4 | 3 |
| Poisson Hurdle | 5115.7 | 5157.7 | 1320.2 | 4 |
| ZINB | 1432.4 | 1472.4 | 465.7 | 2 |
| ZIP | 6303.1 | 5961.7 | 2200.8 | 6 |
| Negative Binomial Hurdle | 1408.8 | 1448.8 | 343.7 | 1 |
LL: Log-likelihood; AIC: Akaike information criterion.
Author Contributions
Conceptualization, R.C. and A.D.; Methodology, R.C. and A.D.; Software, A.D.; Validation, R.C. and S.A.; Formal Analysis, A.D. and S.A.; Investigation, S.R., D.B., B.M., F.T., M.M., J.N.S.F.; Resources, R.C.; Data Curation, A.D., D.B., B.P.M., M.M., and S.R.; Writing – Original Draft Preparation, A.D. and R.C.; Writing–Review & Editing, A.D., R.C., D.B., S.R., B.P.M., S.A., A.H., F.T. and J.N.S.F.; Visualization, A.D., J.N.S.F. and A.H.; Supervision, R.C., S.A.; Project Administration, R.C., M.M., F.T. and B.P.M.; Funding Acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by a grant from the European Research Council (ERC 671055) and VLIR-UOS. The study sponsors, had no role in the design, execution, interpretation, or writing of the study.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics committee of the School of Public Health in Kinshasa, (Aketi: June 2017, ESP/CE/036/2017) and (Logo: January 2017, ESP/CE/006/2017), the ethical committee of the Ministry of Health of South Sudan (September 2018, MOH/ERB 3/2018), the ethical committee of the National Institute for Medical Research, Tanzania (October 2018, NIMR/HQ/R.8a/Vol.IX/2931) and the Ethics Committee of the Antwerp University Hospital (May 24, 2017, B300201733011). Informed consent was obtained from all study participants.
References
- 1.NTD Modelling Consortium Onchocerciasis Group The World Health Organization 2030 goals for onchocerciasis: Insights and perspectives from mathematical modelling: NTD Modelling Consortium Onchocerciasis Group. Gates Open Res. 2019;3:1545. doi: 10.12688/gatesopenres.13067.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colebunders R., Nelson Siewe F.J., Hotterbeekx A. Onchocerciasis-Associated Epilepsy, an Additional Reason for Strengthening Onchocerciasis Elimination Programs. Trends Parasitol. 2018;34:208–216. doi: 10.1016/j.pt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Basáñez M.G., Pion S.D.S., Boakes E., Filipe J.A., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet Infect. Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 4.Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remme J.H. Research for control: The onchocerciasis experience. Trop. Med. Int. Health. 2004;9:243–254. doi: 10.1046/j.1365-3156.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Onchocerciasis and Its Control. World Health Organisation; Geneva, Switzerland: 1995. (Technical Report Series No.852). [Google Scholar]
- 7.Tekle A.H., Zoure H.G., Noma M., Boussinesq M., Coffeng L.E., Stolk W.A., Remme J.H. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty. 2016;5:66. doi: 10.1186/s40249-016-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osei-Atweneboana M.Y., Eng J.K., Boakye D.A., Gyapong J.O., Prichard R.K. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: A two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 9.Siewe Fodjo J.N., Tatah G., Tabah E.N., Ngarka L., Nfor L.N., Chokote S.E., Mengnjo M.K., Dema F., Sitouok A.T., Nkoro G., et al. Epidemiology of onchocerciasis-associated epilepsy in the Mbam and Sanaga river valleys of Cameroon: Impact of more than 13 years of ivermectin. Infect. Dis. Poverty. 2018;7:114. doi: 10.1186/s40249-018-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukendi D., Tepage F., Akonda I., Siewe J.N.F., Rotsaert A., Ndibmun C.N., Laudisoit A., Couvreur S., Kabutako B., Menon S., et al. High prevalence of epilepsy in an onchocerciasis endemic health zone in the Democratic Republic of the Congo, despite 14 years of community-directed treatment with ivermectin: A mixed-method assessment. Int. J. Infect. Dis. 2019;79:187–194. doi: 10.1016/j.ijid.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mmbando B.P., Suykerbuyk P., Mnacho M., Kakorozya A., Matuja W., Hendy A., Greter H., Makunde W.H., Colebunders R. High prevalence of epilepsy in two rural onchocerciasis endemic villages in the Mahenge area, Tanzania, after 20 years of community directed treatment with ivermectin. Infect. Dis. Poverty. 2018;7:64. doi: 10.1186/s40249-018-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colebunders R., Cater J.Y., Olore P.C., Puok K., Bhattacharyya S., Menon S., Abd-Elfarag G., Ojok M., Ensoy-Musoro C., Lako R., et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure. 2018;63:93–101. doi: 10.1016/j.seizure.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenaerts E., Mandro M., Mukendi D., Suykerbuyk P., Dolo H., Wonya’Rossi D., Ngave F., Ensoy-Musoro C., Laudisoit A., Hotterbeekx A., et al. High prevalence of epilepsy in onchocerciasis endemic health areas in Democratic Republic of the Congo. Infect. Dis. Poverty. 2018;7:68. doi: 10.1186/s40249-018-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle S.R., Bourguinat C., Nana-Djeunga H.C., Kengne-Ouafo J.A., Pion S.D.S., Bopda J., Kamgno J., Wanji S., Che H., Kuesel A.C., et al. Genome-wide analysis of ivermectin response by Onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl. Trop. Dis. 2017;11:e0005816. doi: 10.1371/journal.pntd.0005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandro M., Siewe Fodjo J.N., Dusabimana A., Mukendi D., Haesendonckx S., Lokonda R., Nakato S., Nyisi F., Abhafule G., Wonya’rossi D., et al. Single versus Multiple Dose Ivermectin Regimen in Onchocerciasis-Infected Persons with Epilepsy Treated with Phenobarbital: A Randomized Clinical Trial in the Democratic Republic of Congo. Pathogens. 2020;9:205. doi: 10.3390/pathogens9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levick B., Laudisoit A., Tepage F., Ensoy-Musoro C., Mandro M., Bonareri Osoro C., Suykerbuyk P., Kashama J.M., Komba M., Tagoto A., et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2017;11:e0005732. doi: 10.1371/journal.pntd.0005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fodjo J.N.S., Mandro M., Mukendi D., Tepage F., Menon S., Nakato S., Nyisi F., Abhafule G., Wonya’rossi D., Anyolito A. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLoS Negl. Trop. Dis. 2019;13:e0007300. doi: 10.1016/j.ibror.2019.07.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abd-Elfarag G., Carter J.Y., Raimon S., Sebit W., Suliman A., Fodjo J.N.S., Olore P.C., Biel K.P., Ojok M., Logora M.Y. Persons with onchocerciasis-associated epilepsy and nodding seizures have a more severe form of epilepsy with more cognitive impairment and higher levels of Onchocerca volvulus infection. Epileptic Disord. 2020;22:301–308. doi: 10.1684/epd.2020.1164. [DOI] [PubMed] [Google Scholar]
- 19.Boyd E. The Growth of Surface Area o! the Human Body. 3rd ed. University of Minnesota Press; Minneapolis, MN, USA: 1935. [Google Scholar]
- 20.Sharkey I., Boddy A.V., Wallace H., Mycroft J., Hollis R., Picton S., Chemotherapy Standardisation group of the United Kingdom Children’s Cancer Study Group Body surface area estimation in children using weight alone: Application in paediatric oncology. Br. J. Cancer. 2001;85:23–28. doi: 10.1054/bjoc.2001.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaf J.G. The origin of the 1 x 73-m2 body surface area normalization: Problems and implications. Clin. Physiol. Funct. Imaging. 2007;27:135–137. doi: 10.1111/j.1475-097X.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu M.C., Pavlicova M., Nunes E.V. Zero-inflated and hurdle models of count data with extra zeros: Examples from an HIV-risk reduction intervention trial. Am. J. Drug Alcohol Abus. 2011;37:367–375. doi: 10.3109/00952990.2011.597280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding J., Tarokh V., Yang Y. Model selection techniques: An overview. IEEE Signal Process. Mag. 2018;35:16–34. doi: 10.1109/MSP.2018.2867638. [DOI] [Google Scholar]
- 24.Opoku N.O., Bakajika D.K., Kanza E.M., Howard H., Mambandu G.L., Nyathirombo A., Nigo M.M., Kasonia K., Masembe S.L., Mumbere M., et al. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: A randomised, controlled, double-blind phase 3 trial. Lancet. 2018;392:1207–1216. doi: 10.1016/S0140-6736(17)32844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: Consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pion S.D., Grout L., Kamgno J., Nana-Djeunga H., Boussinesq M. Individual host factors associated with Onchocerca volvulus microfilarial densities 15, 80 and 180 days after a first dose of ivermectin. Acta Trop. 2011;120(Suppl 1):S91–S99. doi: 10.1016/j.actatropica.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Churcher T.S., Pion S.D., Osei-Atweneboana M.Y., Prichard R.K., Awadzi K., Boussinesq M., Collins R.C., Whitworth J.A., Basanez M.G. Identifying sub-optimal responses to ivermectin in the treatment of River Blindness. Proc. Natl. Acad. Sci. USA. 2009;106:16716–16721. doi: 10.1073/pnas.0906176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnham G. Onchocerciasis. Lancet. 1998;351:1341–1346. doi: 10.1016/S0140-6736(97)12450-3. [DOI] [PubMed] [Google Scholar]
- 29.Pion S.D., Nana-Djeunga H.C., Kamgno J., Tendongfor N., Wanji S., Njiokou F., Prichard R.K., Boussinesq M. Dynamics of Onchocerca volvulus microfilarial densities after ivermectin treatment in an ivermectin-naive and a multiply treated population from Cameroon. PLoS Negl. Trop. Dis. 2013;7:e2084. doi: 10.1371/journal.pntd.0002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: A global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]