Abstract
Noonan syndrome (NS) is a congenital autosomic dominant condition characterized by a variable spectrum from a clinical and genetical point of view. Germline mutations in more than ten genes involved in RAS–MAPK signal pathway have been demonstrated to cause the disease. An higher risk for leukemia and solid malignancies, including brain tumors, is related to NS. A review of the published literature concerning low grade gliomas (LGGs) in NS is presented. We described also a 13-year-old girl with NS associated with a recurrent mutation in PTPN11, who developed three different types of brain tumors, i.e., an optic pathway glioma, a glioneuronal neoplasm of the left temporal lobe and a cerebellar pilocytic astrocytoma. Molecular characterization of the glioneuronal tumor allowed to detect high levels of phosphorylated MTOR (pMTOR); therefore, a therapeutic approach based on an mTOR inhibitor (everolimus) was elected. The treatment was well tolerated and proved to be effective, leading to a stabilization of the tumor, which was surgical removed. The positive outcome of the present case suggests considering this approach for patients with RASopathies and brain tumors with hyperactivated MTOR signaling.
Keywords: pediatric brain tumor, cancer predisposition, Noonan syndrome, mTOR signaling, everolimus
1. Introduction
Noonan syndrome (NS, OMIM PS163950) is a genetically heterogeneous condition characterized by wide clinical variability. It is considered one of the prevalent non-chromosomal disorders affecting development and growth [1].
NS is characterized by reduced postnatal growth, distinctive facial traits (hypertelorism, downslanting palpebral fissures, ocular ptosis, high forehead, triangular face, low-set and posteriorly rotated ears and short and/or webbed neck), a variable spectrum of congenital heart defects (CHDs) and hypertrophic cardiomyopathy, learning difficulties, renal anomalies, lymphatic malformations, bleeding disorders and skeletal malformations [1,2,3]. The disorder overlaps clinically with Costello syndrome (CS), cardio–facio–cutaneous syndrome (CFCS), Noonan syndrome with multiple lentigines (NSML, known also as LEOPARD syndrome), Mazzanti syndrome, neurofibromatosis Type 1 (NF1) and Legius syndrome [2]. These disorders are related to germline mutations in the genes encoding proteins within the RAS-mitogen-activated protein kinase (MAPK) signaling pathway and have therefore been referred to as RASopathies [2,4]. NS is heterogeneous disorder, with causative germline mutations been reported in the “PTPN11, SOS1, KRAS, NRAS, RAF1, BRAF, MEK1, RIT1, SOS2, LZTR1, MRAS and RRAS2 genes”. RASopathies are considered as cancer-prone disorders [5]. In NS, childhood juvenile myelomonocytic leukemia and other hematologic malignancies most frequently occur, followed by neuroblastoma, primary brain tumors and rhabdomyosarcoma. In the present work, we report the clinical management of a pediatric patient with PTPN11-related NS carrying three different low grade gliomas (LGGs) and reviewed the published cases in literature about pediatric LGGs in NS.
2. Case Presentation
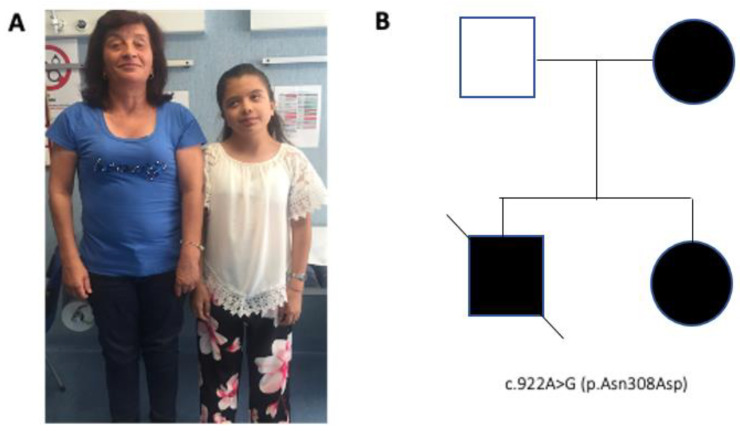
The patient was born to non-consanguineous parents at 33 weeks after an non pathologic gestation. No abnormality was noted at birth; however, since the first month of life she showed slight development delay, reduced growth and dysmorphic facial features. Due to a holosystolic heart murmur detected in the second week of life, an echocardiography was performed revealing pulmonary valve stenosis. At one month, based on these clinical features, the patient was suspected to have NS and a genetic analysis performed using a dedicated gene panel (BRAF, CBL, CDC42, HRAS, KRAS, MAP2K1, MAP2K2, NF1, NRAS, PTPN11, RAF1, RIT1, RRAS, SHOC2, SOS1, SOS2 and SPRED1) revealed the pathogenic PTPN11 variant NM_002834.3 (c.922A>G, p.Asn308Asp), one of the most common mutations associated with NS [3]. Sanger sequencing detected the same variant in her mother, confirming cosegregation of the mutation with disease in this family (Figure 1A,B). In addition, a maternally transmitted missense variant in SPRED1 (c.182G>A, p.Arg61His; NM_152594.3) was identified. This missense change was classified as “likely benign”, based on the American College of Medical Genetics criteria (PM1, BS2, BP1, BP4). Family history was positive for pulmonary stenosis in one brother, who died at the age of 13 years for acute lymphoblastic leukemia. No clinical report or biologic material to be analyzed was available.
Figure 1.
(A) Patient (right) at age 13 years and her mother (left) show typical features of NS, note the short stature and common facial features (ptosis, hypertelorism, downslanting palpebral fissures and low-set ears) and a short webbed neck; (B) pedigree of the family in which NS cosegregated with the recurrent PTPN11 mutation.
The patient′s family has provided informed consent to the sharing of clinical information and images for research purposes. Such consent has been designed in accordance with the internal policy approved by the ethical committee of the Bambino Gesù Hospital (approval code 347/RA, n°1353/2017).
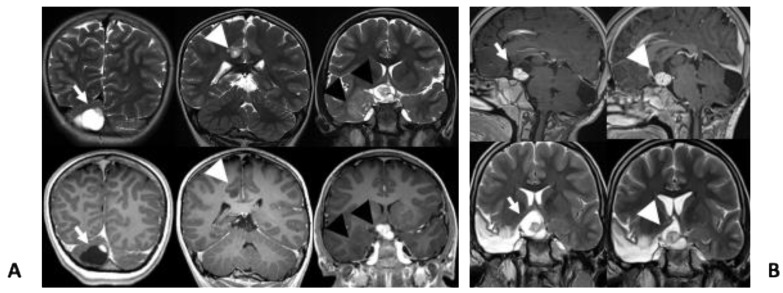
The proband was hospitalized at 18 months of age because of a prolonged febrile seizure. At admission, brain magnetic resonance imaging (MRI) revealed few small areas of hyper-intense signal on T2-weighted images without contrast enhancement after gadolinium injection involving the frontal and temporal right lobes and the splenium of corpus callosum. The patient underwent periodic follow-up, during which a second MRI analysis showed a new suprasellar–chiasmatic lesion suspected of optic pathway glioma; subsequently, a further small lesion with cystic component and a solid enhancing part on the right cerebellar hemisphere was also reported (Figure 2A).
Figure 2.
(A) Coronal T2w (top row) and T1w (bottom row) images show cystic lesion in the cerebellum with a peripheral enhancing nodule (arrows), a small hyperintense lesion without contrast-enhancement involving the right gyrus cinguli (arrow heads). There is also a suprasellar/chiasmatic lesion (black arrows) with avid contrast-enhancement and cystic components, consistent with optic pathway glioma and a hyperintense and non enhancing lesion involving the right mesial temporal lobe (black arrow heads); (B) sagittal GdT1w (top row) and coronal T2w (bottom row) images show optic pathway glioma before (arrows) and during treatment (arrow heads).
When the patient was 6 years old, she presented with a cluster of focal seizures characterized by sudden abdominal pain, vomiting, oro-alimentary automatism with subsequent stiffening of the left hemisoma and then sleepiness in the post-ictal period. She was therefore referred to our center where video-electroencephalography documented right temporo-mesial seizures. MRI confirmed the presence of multiple lesions, hyperintense in T2 derived images, mainly affecting the optic diencephalic region, the right temporo–mesial structure, the motor cingulate and right cerebellum (Figure 2A). Treatment with valproic acid and lacosamide did not allow seizure control. A new MRI showed progression of the cerebellar lesion (Figure 2A).
The appearance of multiple café-au-lait spots, increased in number and size with age, multiple lentigines and the presence of an optical–diencephalic lesion detected by MRI, led to suspect the possibility of concomitant NF1. Molecular data were reevaluated and an MLPA test for detection of intragenic NF1 structural rearrangements was performed with negative result. A clinical exome sequencing of the proband and her parents was also performed using DNA obtained from peripheral blood to exclude additional germline variants in genes associated with cancer predisposition syndromes. Library preparation and exome capture of patient with her parents were performed by using a custom gene panel (Twist Bioscience). The “BaseSpace pipeline” (Illumina, https://basespace.illumina.com/) and the “TGex software” (LifeMap Sciences, Inc., Alameda, CA 94501, USA) were used for the variant calling and annotating variants, respectively. Data analysis did not reveal any clinically/functionally variant related to cancer-prone conditions apart from the previously identified sequence changes in PTPN11 and SPRED1.
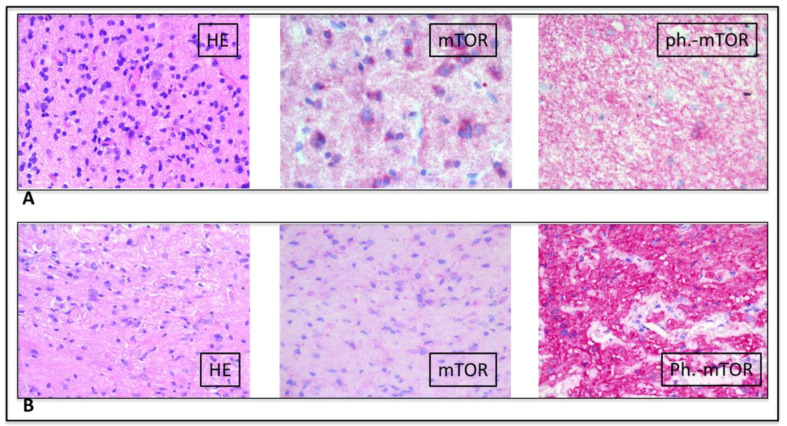
Due to the persistence of seizures, at the age of 9 years, the patient underwent right craniotomy and standard anterior temporal resection, including mesial structures (uncus, amygdala and hippocampus). At the end of the resection, the lateral chiasmatic region was exposed and a limited biopsy of the optic pathway lesion was performed. No complication occurred in the postoperative period; currently (5 years after surgery), the patient is seizure-free, without requirement of anti-epileptic therapy. Histology of the temporal lesion was diagnostic for a glioneuronal neoplasm (WHO I). Immunohistochemistry (ICH) was negative for the BRAF V600E point mutation and positive for phosphorylated MTOR (Ser2448), indicating the activation of the MTOR pathway (Figure 3A).
Figure 3.
(A) (upper raw) (low-grade glioneural tumor): HE: Low intensity glial proliferation with intermingled few dysplastic ganglion cells. Mild nuclear atypia and low proliferation index. mTOR: mild cytoplasmic positivity of neoplastic cells. Phospho–mTOR: strong cytoplasmic positivity of neoplastic cells; (B) (lower raw) (pilocytic astrocytoma): HE: Pilocytic glial proliferation in a fibrillary background with evidence of some Rosenthal fibers. Mild nuclear atypia and low proliferation index. mTOR: mild cytoplasmic positivity of neoplastic cells. Phospho–mTOR: Strong cytoplasmic positivity of neoplastic cells.
After few months, due to the increased volume of the cerebellar lesion, with initial hydrocephalus, occipital craniotomy was performed with excision of the cerebellar cystic neoplasm that was histologically characterized as a cerebellar pilocytic astrocytoma (WHO I) (Figure 3B). At 11 years, brain MRI showed a volume increase of the chiasmatic lesion adjacent to the temporal resection margin, associated with visual impairment. Therefore, treatment with the MTOR inhibitor, everolimus, was started at the dose of 5 mg/day [4], subsequently reduced to 2.5 mg/day due to signs of toxicity (mouth ulcers). Follow-up MRI were performed every six months; the last one, carried out when she was 13-year-old, showed stable disease at 27 months since the beginning of therapy (Figure 2B). Frequent blood test evaluations were performed to exclude the development of leukemia.
3. Discussion
Similar to other RASopathies, certain mutations causing NS may predispose to cancer [5]. The risk of cancer in NS is evaluated at 4% by the age of 20 [6,7,8]. The underlying molecular mechanism involves the upregulation of the RAS–MAPK signaling pathway; such dysregulation leads to increased cellular proliferation which can lead to tumor development in those cells where additional driver somatic mutations occur. Somatic mutations in the PTPN11 gene are found in 35% of sporadic juvenile myelomonocytic leukemia (JMML) and in other hematological malignancies [9,10]. Similarly, somatic PTPN11 mutations have been detected with variable prevalence in some solid tumors, such as lung, liver and colorectal cancer, thyroid carcinoma, bladder carcinoma, neuroblastoma, rhabdomyosarcoma, melanoma and brain tumors [11,12].
NS can be caused by germline mutations in several genes related to the RAS–MAPK pathway cascade (i.e., PTPN11, SOS1, SOS2, RAF1, KRAS, NRAS, RIT1, MRAS, RRAS2 and LZTR1). A gain-of-function mutations in PTPN11, which encodes the SHP2 protein, causes 50% of cases of NS. SHP2 is a non-receptor protein tyrosine phosphatase (PTP), positively controlling RAS function. The protein has a complex regulatory mechanism controlling its subcellular localization and activation. Both germline and somatic PTPN11 mutations are activating and enhance SHP2 function by destabilizing the catalytically inactive conformation of the phosphatase, resulting in increased signal flow through the RAS–MAPK pathway [13].
Patients with NS have a significant risk to develop JMML, embryonal rhabdomyosarcoma and brain tumors [7]. In particular, central nervous system (CNS) glial tumors are known to be a result of RASopathies [5,7]. A recent study conducted in France showed the correlation between JMML and NS in a cohort of 641 patients with germline PTPN11 mutations. Somatic mutations in those genes are related to up to 20% of all sporadic hematologic and solid malignancies [14]. Germline mutations in those genes underlying tumor formation seams to determine enhanced signaling activity, although in a less consistent way than seen in corresponding somatic mutations. It is hypothesized that those activating somatic gene mutations causing cancer are not seen in the germline because they may cause embryonal lethality [15].
We reviewed the literature for cases of pediatric LGGs reported in patients with NS. PubMed and Google scholar were used for publications. Investigated terms included “Noonan” OR “PTPN11” AND “dysembryoplastic,” “pilocytic,” “medulloblastoma,” “oligodendroglioma,” “glioneuronal,” “astrocytoma,” “glioma,” “ependymoma,” “craniopharyngioma,” “papilloma,” or “tumor”. We included reviews, case reports, case series and case report abstracts.
We analyzed 30 cases of patients with NS and brain tumors carrying PTPN11 mutation (Table 1). Most of them were glial or glioneuronal tumors. In a recent review the authors identified 22 cases of PTPN11-driven NS patients with brain tumors: mainly “dysembryoplasic neuroepithelial tumors” (DNETs), but also “medulloblastomas”, “oligodendrogliomas”, “astrocytomas”, “pilocytic astrocytomas (PA)”, “gliomas” and “mixed glioneuronal tumors” [16]. Siegfried at al. updated with 25 identified brain tumors [17]. Two other patients were subsequently reported [18], the first showing a dysembryoplastic neuroepithelial tumor, the second with a pilocytic astrocytoma. Finally, E-Ayadi et al. described two cases of anaplastic astrocytoma [19]. Out of the twenty-eight published cases, twenty-four were LGGs and glioneuronal tumors, most of which were DNETs. Of note, while PA are estimated the most common pediatric brain tumors and glioneuronal tumors are 30% of pediatric brain neoplasms, DTNs are rare (<1%), but account for approximately 40% of the brain tumors reported in NS [20]. Despite these findings, El-Ayadi et al. have proved that HGG may occur in NS [19]. Although our patient had low-grade gliomas, the peculiarity of our case is that she developed three different types of brain tumors at the same time, without any hematological disease. Another particular feature of our patient is that she had a positive genetic test for NS and, at the same time, she developed phenotypic characteristics also present in NF1, such as café-au-lait spots see [21], lentigines, in association with an optical-diencephalic lesion, although phenotypic overlapping is not uncommon for patients affected with RASopathies [22,23]. Nevertheless, the case was negative on clinical exome sequencing including NF1, but though she could have an undetected mutation that does not affect the coding part of the gene. To the best of our knowledge, no similar cases have been reported in the literature. The evidence provided by this and previous similar cases suggests the need for a close follow-up of patients affected by RASopathies starting from an accurate medical history and clinical examination aimed at identifying any characteristics referring to the RASopathy. Patients with a cancer predisposition syndrome should be evaluated periodically for specific types of tumors known to be frequent in this population (such as leukemia, lymphoma, neuroblastoma, rhabdomyosarcoma, brain tumors and bladder carcinoma) based on clinical symptoms, regular physical examinations and complete blood counts and, considering the high brain tumor risk, a regular screening protocol with brain MRIs may be implemented [24]. The early detection of cancer should positively influence patient outcome, just as a long follow-up is essential for those patients who have already developed a tumor. Characterizing the underlying tumor molecular alterations can facilitate the development of targeted therapies to improve efficacy of the treatment, quality of life and long-term survival in those patients.
Table 1.
Primary brain tumors reported in Noonan syndrome.
| No. | Reference | Age | Gender | Noonan Syndrome Diagnosis | Brain Tumor Diagnosis | Location |
|---|---|---|---|---|---|---|
| 1 | McWilliams et al. [16] | 8 | M | PTPN11 p.Glu139Asp | DNET | Temporal lobe and cerebellum |
| 2 | Jongmans et al. [6] | 10 | Ukn | PTPN11 c.179G > C; p.Gly60Ala | DNET | Temporal lobe |
| 3 | Selter et al. [25] Pellegrin et al. [17] |
13 | M | PTPN11 exon 3 ** | DNET | Left parietal lobe |
| 4 | Bendel et al. [26] | 17 | M | PTPN11 c.174C > G; p.Asn58Lys | DNET | Occipital cortex |
| 5 | Bendel et al. [26] | 37 | M | Maternal uncle of Case 4 | DNET | Unknown |
| 6 | Delisle et al. [27] | 12 | M | PTPN11 mutation ** | DNET | Temporal lobe and thalamus |
| 7 | Krishna et al. [18] | 9 | M | PTPN11 p.Asp61Gly | DNET | Temporal lobe and cerebellum |
| 8 | Pellegrin et al. [17] | 13 | M | N/A | DNET | Right parieto-occipital cortex |
| 9 | Pellegrin et al. [17] | 13 | M | PTPN11 exon 3 mutation ** | DNET (MRI) | Left parietal lobe |
| 10 | Kratz et al. [8] | 6 | F | PTPN11 p.Asn308Asp | DNET | Unknown |
| 11 | Siegfried et al. [17] | 16 | M | PTPN11 c.922A > G; p.Asn308Asp | DNET | Left temporal and frontal lobe, right thalamus |
| 12 | Rankin et al. [26] | 10 | M | PTPN11 c.1403C > T; p.Thr468Met | Medulloblastoma | Cerebellum |
| 13 | Jongmans el al [6] | 18 | F | PTPN11 c.417G > C; p.Glu139Asp | Oligodendroglioma | Hypothalamus |
| 14 | Sherman et al. [28] | 6 | M | PTPN11 c.172A > G; p.Asn58Asp | Low-grade mixed glioneuronal tumor | Suprasellar cisterns, sella turcica and hypothalamus and diffuse leptomeningeal disease |
| 15 | Schuettpelz et al. [29] | 8 | M | PTPN11 c.1471C > T and c.1472C > T; p.Pro491Phe | Pilocytic astrocytoma | Sellar/suprasellar with extension to prepontine region to the level of the pontomedullary junction |
| 16 | Fryssira et al. [30] | 11 | F | Clinical diagnosis | Pilocytic astrocytoma | Sellar/suprasellar |
| 17 | De Jongo et al. [31] | 21 | M | PTPN11 mutation ** | Multiple indeterminate lesions on MRI | Multiple: supratentorial, infratentorial, cortical and subortical, thalamus |
| 18 | Karafin et al. [32] | 18 | M | Clinical diagnosis | Rosette forming glioneuronal tumor | Fourth ventricle |
| 19, 20, 21 | Rush et al. * [17] | Ukn | M | PTPN11 mutation ** | Low grade astrocytoma | Suprasellar and thalamic region |
| 22 | Kratz et al. [19] | 7 | M | PTPN11 p.Gly60Ala | Pilocytic astrocytoma | Right optic nerve |
| 23 | Takagi at al. [33] | 20 | M | Clinical diagnosis | Glioma | Unknown |
| 24 | Standford et al. [34] | 16 | M | Clinical diagnosis | Pilocytic astrocytoma | Intramedullary spinal cord lesion involving the cervical medullary junction and descending to the C2-C3 disc space level |
| 25 | Nair et al. [35] | 14 | M | PTPN11 c.417G > C in exon 4 | Pilomyxoid astrocytoma | Right optic nerve |
| 26 | Bendel and Pond. [19] | 14 | F | PTPN11 c.922A > G; p.Asn308Asp | High grade glioma | Left brainstem/cerebellar peduncle |
| 27 | Martinelli et al. [36] | 24 | Ukn | PTPN11 c.64A > G; pThr22Ala | Oligodendroglioma grade II | Unknown |
| 28 | El Ayadi et al. [19] | 14 | F | PTPN11 c.922A > G; p.Asn308Asp | Anaplastic astocytoma | Left brainstem/cerebellar peduncle |
| 29 | El Ayadi et al. [19] | 9 | M | PTPN11 c.5C > T; p.Thr2lle | Anaplastic astocytoma | Third ventricle, cerebellum and fornix |
| 30 | Our case | 9 | F | PTPN11 c.922A > G; p.Asn308Asp | Pilocytic astrocitoma and glioneuronal tumor | Cerebellum Right temporal lobe |
* patients 19, 20 and 21 were from a case series abstract of two males and one female. Two patients were deaf and possibly had NSML. DNET—dysembryoplasic neuroepithelial tumor. ** Mutation unknown.
Syndromic patients represent a particular challenge to the neurosurgeon. When defining the surgical flap to approach to a specific lesion the possibility of further surgeries for new tumors must be considered setting a global strategy to possibly access the whole brain limiting the need for inappropriate subsequent skin incisions. Our patient presented a temporal lobe lesion and a separate optic pathway tumor on the same side. Biopsy of optic pathway lesions is not generally encouraged unless imaging data suggest atypical features or there is need to explore molecular targets [37]. Morbidity of surgical debulking or biopsy of optic pathway lesions has been shown to be acceptable with modern surgical techniques [38].
Considering the overall growth of all intracranial tumors in our patient, the high value of a targeted therapy approach in a syndromic child with higher chance to suffer from chemotherapy toxicity [39], reference and the chiasmatic lesion needed treatment, we decided to perform a limited biopsy to confirm histology and possible molecular targets. Performing the biopsy at the end of the temporal resection not only avoided a second craniotomy later on, but also allowed us to take advantage of the temporal mesial resection that exposed the area of interest avoiding additional dissection. This case demonstrates the benefit of a dedicated multidisciplinary team including neurosurgeons, neuro-oncologists, neuroradiologists and pathologists in the optimal management of such complex cases.
Another important aspect of our case is the positive response to an mTOR inhibitor. While the functional link between the identified PTPN11 mutation and MTOR hyperactivation needs further study, the present finding suggests a possible molecular target for a new therapeutic strategy and that assessment of MTOR activation should be evaluated in brain tumors of patients with NS or a related RASopathy. Our findings are in line with previous reports suggesting that the PI3K/mTOR signaling may mediate the oncogenic signal elicited by enhanced SHP2 function. These findings indicate a therapeutic potential of rapamycin analogs for PTPN11 mutation-associated cancers [39,40].
4. Conclusions
The success of the rapalog everolimus in treating subependymal giant cell astrocytomas (SEGAs) associated with tuberous sclerosis proved that this drug could shrink mTOR-driven tumors [15,41]. Everolimus may have activity in other pLGG. However, it is advisable to confirm the improper activation of the AKT–mTOR signaling pathway by molecular test before administering an mTOR inhibitor, because children with NS respond to chemotherapy treatment, but they are more likely to experience severe toxicity [42]. In our case, everolimus was well tolerated. For this reason, the use of everolimus and other targeted therapies in the treatment of genetic cancer syndromes provides significant opportunities for improved quality of life for patients with RASopathies. However, further research is needed to discern appropriate use of available pharmacologic agents as well as to develop more effective targets with reliable, durable responses for each of these conditions. We acknowledge that supplemental considerations may stem from further characterizations of LGGs in patients with NS and PTPN11 variants, however, we feel that the frequency of these tumors in NS is still too low to allow solid conclusions. The positive response to everolimus observed in our patient may provide an ex adiuvantibus piece of evidence for a pathogenic link between the RAS–MAPK and PI3K/mTOR pathways, activated by PTPN11 variants, but, as we stated in our Discussion, the functional link between the identified PTPN11 mutation and MTOR hyperactivation needs further study.
Acknowledgments
The authors thank the participating family and HEAL association for their support. AIRC (IG21614 to M.T.) and the Italian Ministry of Health (Ricerca Corrente 2019 and 2020 to M.T.; Ricerca Corrente 2019 and 2020 to ADL) and Casa Sollievo della Sofferenza Hospital are acknowledged.
Author Contributions
A.M. coordinated the study; M.L. reviewed the literature and wrote the manuscript; L.B., A.M. and E.A. reviewed the manuscript; F.D.C. performed the pathologic findings; A.C. (Antonella Cacchione) and L.D.P. contributed to patient management and revision; A.C. (Andrea Carai) and A.D.B. contributed to neurosurgery and revision; G.S.C. acquired and elaborated the images; E.M., G.C. and A.P. performed the bioinformatics analysis; F.R.L., M.R. performed the genetic analysis; F.L., M.T., E.F. and A.D.L. critically revised the manuscript for intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Roberts A.E., Allanson J.E., Tartaglia M., Gelb B.D. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinelli S., de Luca A., Stellacci E., Rossi C., Checquolo S., Lepri F., Caputo V., Silvano M., Buscherini F., Consoli F., et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am. J. Hum. Genet. 2010;87:250–257. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athota J.P., Bhat M., Nampoothiri S., Gowrishankar K., Narayanachar S.G., Puttamallesh V., Farooque M.O., Shetty S. Molecular and clinical studies in 107 Noonan syndrome affected individuals with PTPN11 mutations. BMC Med. Genet. 2020;21:50. doi: 10.1186/s12881-020-0986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz D.N., Belousova E., Sparagana S.P., Bebin E.M., Frost M., Kuperman R., Witt O., Kohrman M., Flamini J.R., Wu J.Y., et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 5.Smpokou P., Zand D.J., Rosenbaum K.N., Summar M.L. Malignancy in Noonan syndrome and related disorders. Clin. Genet. 2015;88:516–522. doi: 10.1111/cge.12568. [DOI] [PubMed] [Google Scholar]
- 6.Jongmans M.C.J., van der Burgt I., Hoogerbrugge P.M., Noordam K., Yntema H.G., Nillesen W.M., Kuiper R.P., Ligtenberg M.J.L., van Kessel A.G., van Krieken J.H., et al. Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur. J. Hum. Genet. 2011;19:870–874. doi: 10.1038/ejhg.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kratz C.P., Rapisuwon S., Reed H., Hasle H., Rosenberg P.S. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am. J. Med. Genet. C Semin Med. Genet. 2011;157C:83–89. doi: 10.1002/ajmg.c.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kratz C.P., Franke L., Peters H., Kohlschmidt N., Kazmierczak B., Finckh U., Bier A., Eichhorn B., Blank C., Kraus C., et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br. J. Cancer. 2015;112:1392–1397. doi: 10.1038/bjc.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh M.L., Vattikuti S., Schubbert S., Reynolds M.G., Carlson E., Lieuw K.H., Cheng J.W., Lee C.M., Stokoe D., Bonifas J.M., et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood. 2004;103:2325–2331. doi: 10.1182/blood-2003-09-3287. [DOI] [PubMed] [Google Scholar]
- 10.Tartaglia M., Martinelli S., Iavarone I., Cazzaniga G., Spinelli M., Giarin E., Petrangeli V., Carta C., Masetti R., Arico M., et al. Somatic PTPN11 mutations in childhood acute myeloid leukaemia. Br. J. Haematol. 2005;129:333–339. doi: 10.1111/j.1365-2141.2005.05457.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto D., Miyamoto M., Takahashi A., Yomogita Y., Higashi H., Kondo S., Hatakeyama M. Isolation of a distinct class of gain-of-function SHP-2 mutants with oncogenic RAS-like transforming activity from solid tumors. Oncogene. 2008;27:3508–3515. doi: 10.1038/sj.onc.1211019. [DOI] [PubMed] [Google Scholar]
- 12.Bentiresalj M., Paez J.G., David F.S., Keilhack H., Halmos B., Naoki K., Maris J.M., Richardson A.L., Bardelli A., Sagarbaker D.J., et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 13.Tartaglia M., Mehler E.L., Goldberg R., Zampino G., Brunner H.G., Kremer H., van der Burgt I., Crosby A.H., Ion A., Jeffery S., et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 14.Bos J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 15.Tartaglia M., Martinelli S., Stella L., Bocchinfuso G., Flex E., Cordeddu V., Zampino G., van der Burgt I., Palleschi A., Petrucci T.C., et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am. J. Hum. Genet. 2006;78:279–290. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWilliams G., SantaCruz K., Hart B., Clericuzio C. Occurrence of DNET and other brain tumors in Noonan syndrome warrants caution with growth hormone therapy. Am. J. Med. Genet. 2015;170 Pt A:195–201. doi: 10.1002/ajmg.a.37379. [DOI] [PubMed] [Google Scholar]
- 17.Siegfried A., Cances C., Denuelle M., Loukh N., Tauber M., Cave H., Delisle M. Noonan syndrome, PTPN11 mutations, and brain tumors. A clinical report and review of the literature. Am. J. Med. Genet. 2017;173:1061–1065. doi: 10.1002/ajmg.a.38108. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore Krishna K., Pagan P., Escobar O., Popovic J. Occurrence of Cranial Neoplasms in Pediatric Patients with Noonan Syndrome Receiving Growth Hormone: Is Screening with Brain MRI prior to Initiation of Growth Hormone Indicated? Horm. Res. Paediatr. 2017;88:423–426. doi: 10.1159/000479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elayadi M., Ansari M., Kuhnol C.D., Bendel A., Sturm D., Pietsch T., Kramm C.M., von Bueren A.O. Occurrence of high-grade glioma in Noonan syndrome: Report of two cases. Pediatr. Blood Cancer. 2019;66:e27625. doi: 10.1002/pbc.27625. [DOI] [PubMed] [Google Scholar]
- 20.Rickert C.H., Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv. Syst. 2001;17:503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 21.Bessis D., Miquel J., Bourrat E., Chiaverini C., Moricepicard F., Abadie C., Manna F., Baumann C., Best M., Blanchet P., et al. Dermatological manifestations in Noonan syndrome: A prospective multicentric study of 129 patients positive for mutation. Br. J. Dermatol. 2019;180:1438–1448. doi: 10.1111/bjd.17404. [DOI] [PubMed] [Google Scholar]
- 22.Digilio M.C., Sarkozy A., de Zorzi A., Pacileo G., Limongelli G., Mingarelli R., Calabro R., Marino B., Dallapiccola B. Leopard syndrome: Clinical diagnosis in the first year of life. Am. J. Med. Genet. A. 2006;140:740–746. doi: 10.1002/ajmg.a.31156. [DOI] [PubMed] [Google Scholar]
- 23.Digilio M.C., Lepri F., Baban A., Dentici M.L., Versacci P., Capolino R., Ferese R., de Luca A., Tartaglia M., Marino B., et al. RASopathies: Clinical Diagnosis in the First Year of Life. Mol. Syndromol. 2011;1:282–289. doi: 10.1159/000331266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villani A., Greer M.C., Kalish J.M., Nakagawara A., Nathanson K.L., Pajtler K.W., Pfister S.M., Walsh M.F., Wasserman J.D., Zelley K., et al. Recommendations for Cancer Surveillance in Individuals with RASopathies and Other Rare Genetic Conditions with Increased Cancer Risk. Clin. Cancer Res. 2017;23:e83–e90. doi: 10.1158/1078-0432.CCR-17-0631. [DOI] [PubMed] [Google Scholar]
- 25.Selter M., Dresel R., Althaus J., Bartels M.B., Dittrich S., Geb S., Hoche F., Qirshi M., Vlaho S., Zielen S., et al. Dysembryoplastic neuroepithelial tumor (DNET) in a patient with Noonan syndrome. Neuropediatrics. 2010;41:P1356. doi: 10.1055/s-0030-1265602. [DOI] [Google Scholar]
- 26.Bendel A., Hansen M., Dugan S., Mendelsohn N. Dysembyoplastic Neuroepithelial Tumor in Two Relatives with Noonan Syndrome And A PTPN 11 Mutation. Neuro. Oncol. 2012;14:156. doi: 10.1093/neuonc/nos108. [DOI] [Google Scholar]
- 27.Delisle M., Siegfried A., Tauber M., Cave H., Loukh N., Boetto S., Bertozzi A., Urocoste E., Cances C. Dysembryoplastic neuroepithelial tumor (DNET) and Noonan syndrome; Proceedings of the Brain Pathology. Abstracts of the XVIII International Congress of Neuropathology; Rio de Janeiro, Brazil. 14–18 September 2014. A case report. [Google Scholar]
- 28.Sherman C.B., Ali-Nazir A., Gonzales-Gomez I., Finlay J.L., Dhall G. Primary mixed glioneuronal tumor of the central nervous system in a patient with noonan syndrome: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2009;31:61–64. doi: 10.1097/MPH.0b013e31818ab2cf. [DOI] [PubMed] [Google Scholar]
- 29.Schuettpelz L.G., Mcdonald S., Whitesell K., Desruisseau D.M., Grange D.K., Gurnett C.A., Wilson D.B. Pilocytic astrocytoma in a child with Noonan syndrome. Pediatr. Blood Cancer. 2009;53:1147–1149. doi: 10.1002/pbc.22193. [DOI] [PubMed] [Google Scholar]
- 30.Fryssira H., Leventopoulos G., Psoni S., Kitsioutzeli S., Stavrianeas N.G., Kanavakis E. Tumor development in three patients with Noonan syndrome. Eur. J. Pediatr. 2008;167:1025–1031. doi: 10.1007/s00431-007-0636-3. [DOI] [PubMed] [Google Scholar]
- 31.de Jong M., Schieving J., Goraj B. Remarkable intra-cerebral lesions on MRI in a patient with Noonan syndrome. Eur. J. Radiol. Extra. 2011;78:e17–e19. doi: 10.1016/j.ejrex.2011.01.005. [DOI] [Google Scholar]
- 32.Karafin M., Jallo G.I., Ayars M., Eberhart C.G., Rodriguez F.J. Rosette forming glioneuronal tumor in association with Noonan syndrome: Pathobiological implications. Clin. Neuropathol. 2011;30:297–300. doi: 10.5414/NP300374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takagi M., Miyashita Y., Koga M., Ebara S., Arita N., Kasayama S. Estrogen deficiency is a potential cause for osteopenia in adult male patients with Noonan’s syndrome. Calcif. Tissue Int. 2000;66:200–203. doi: 10.1007/s002230010040. [DOI] [PubMed] [Google Scholar]
- 34.Sanford R.A., Bowman R., Tomita T., De Leon G., Palka P. A 16-year-old male with Noonan’s syndrome develops progressive scoliosis and deteriorating gait. Pediatr. Neurosurg. 1999;30:47–52. doi: 10.1159/000028761. [DOI] [PubMed] [Google Scholar]
- 35.Nair S., Fort J.A., Yachnis A.T., Williams C.A. Optic nerve pilomyxoid astrocytoma in a patient with Noonan syndrome. Pediatr. Blood Cancer. 2015;62:1084–1086. doi: 10.1002/pbc.25382. [DOI] [PubMed] [Google Scholar]
- 36.Martinelli S., Carta C., Flex E., Binni F., Cordisco E.L., Moretti S., Puxeddu E., Tonacchera M., Pinchera A., Mcdowell H.P., et al. Activating PTPN11 mutations play a minor role in pediatric and adult solid tumors. Cancer Genet. Cytogenet. 2006;166:124–129. doi: 10.1016/j.cancergencyto.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Gnekow A., Kandels D., van Tilburg C.M., Azizi A., Opocher E., Stokland T., Driever P.H., Meeteren A.Y.N.S., Thomale U.W., Schuhmann M.U., et al. SIOP-E-BTG and GPOH Guidelines for Diagnosis and Treatment of Children and Adolescents with Low Grade Glioma. Klin. Padiatr. 2019;231:107–135. doi: 10.1055/a-0889-8256. [DOI] [PubMed] [Google Scholar]
- 38.Goodden J., Pizer B., Pettorini B., Williams D., Blair J., Didi M., Thorp N., Mallucci C. The role of surgery in optic pathway/hypothalamic gliomas in children. J. Neurosurg. Pediatr. 2014;13:1–12. doi: 10.3171/2013.8.PEDS12546. [DOI] [PubMed] [Google Scholar]
- 39.Liu W., Yu W., Zhang J., Chan R.J., Loh M.L., Zhang Z., Bunting K.D., Qu C. Inhibition of the Gab2/PI3K/mTOR signaling ameliorates myeloid malignancy caused by Ptpn11 (Shp2) gain-of-function mutations. Leukemia. 2017;31:1415–1422. doi: 10.1038/leu.2016.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catanzaro G., Besharat Z.M., Miele E., Chiacchiarini M., Po A., Carai A., Marras C.E., Antonelli M., Badiali M., Raso A., et al. The miR-139-5p regulates proliferation of supratentorial paediatric low-grade gliomas by targeting the PI3K/AKT/mTORC1 signalling. Neuropath. Appl. Neuro. 2018;44:687–706. doi: 10.1111/nan.12479. [DOI] [PubMed] [Google Scholar]
- 41.Krueger D.A., Care M.M., Holland K.D., Agricola K., Tudor C., Mangeshkar P., Wilson K., Byars A.W., Sahmoud T., Franz D.N. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 42.Tartaglia M., Gelb B.D., Zenker M. Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]