Abstract
Simple Summary
Assisted reproductive technologies (ARTs) involve an extraordinary change in the natural developmental trajectory of the mammalian embryo, incurring potential long-term and inheritable effects in the resulting offspring. The results of this study demonstrate, for the first time, that ex vivo embryo manipulations during fresh and vitrified embryo transfer are associated with paternally inherited bodyweight variation, but seemed not transmissible via the female germline. This asymmetry in the transmission of acquired features following ARTs suggests that embryo paternal and maternal genomes differ in their degree of susceptibility to the lasting effects of ARTs. This study would provide a novel view of developmental plasticity in the early mammalian embryo.
Abstract
The concept of developmental programming suggests that the early life environment influences offspring phenotype in later life, whose effects may also be manifested in further generations. Valuable pieces of evidence come from the fields applying assisted reproductive technologies (ARTs), which deprive embryos of their optimal maternal environment and were thus associated with subsequent developmental deviations. Recently, we demonstrated that the in vitro manipulations during a vitrified embryo transfer procedure incurs a cumulative and transgenerational decline in the growth performance of the resulting offspring. Here, we provide a longitudinal study to investigate whether previous developmental deviations could be indistinctly paternally or maternally transmitted using crossbred mattings. Our findings revealed that early embryo manipulations through fresh and vitrified embryo transfer incurred paternally transmissible effects over the growth pattern and adult body weight, which seemed not inheritable via the female germline. Similar inheritable effects were observed after fresh and vitrified embryo transfer, suggesting that disturbing optimal embryo development through in vitro manipulations was the principal trigger of transmissible effects, rather than embryo cryopreservation per se.
Keywords: assisted reproduction technology, paternal inheritance, maternal inheritance, embryo transfer, embryo cryopreservation, long-term effects, developmental plasticity, developmental programming
1. Introduction
Since the introduction and widespread use of assisted reproductive technologies (ARTs), alterations in embryo viability and quality, together with developmental deviations in the resulting offspring, are starting to emerge [1,2,3]. The underlying hypothesis is that suboptimal in vitro conditions during ARTs may induce stress responses and alterations on developing embryos that can persist in the long term after birth [4,5,6]. In this context, some approaches have been developed in an attempt to mimic in vivo maternal environment, leading to embryos with better quality and improved development [7,8,9]. However, embryo cryopreservation requires embryos to be exposed to environments in which they have no intrinsic ability to survive such as ultralow temperatures and toxic cryoprotectant solutions used to achieve the cryogenic suspension of life [10]. Although the innocuousness of this procedure has already been questioned decades ago, today, the information assessing the long-term effects following the transfer of cryopreserved embryos is very scarce [11]. Recently, we reported a longitudinal study in a rabbit model designed to unravel the effects across the life course following either fresh and vitrified embryo transfer [12]. Although embryo transfer techniques are mandatory in the revitalization of an organism from cryopreserved embryos and cannot be waived from the whole procedure, our findings showed that both ex vivo manipulations during embryo transfer and embryo cryopreservation per se are important cumulative triggers of developmental reshaping and phenotypic deviations that are maintained in adulthood, concordantly with the ARTs literature [1,2,3,13,14,15].
The concept of developmental programming suggests that the early life environment influences offspring characteristics in later life, but growing evidence also suggests that these effects may also be manifested in further generations without further suboptimal exposure [16,17,18,19,20]. Our recent study demonstrated that the transfer of cryopreserved embryos incurs transgenerationally inheritable effects over the offspring growth performance, adult body weight, phenotype of vital organs, and their molecular physiology and metabolism [20]. The developmental plasticity following ARTs is believed to be mediated by epigenetic changes [5,6,21]. However, the epigenetic landscape and remodeling differs both between the sperm and oocyte during gametogenesis and between male and female pronuclei during early embryo development [22,23]. Based on this marked asymmetry in the regulation of paternal and maternal alleles, the plasticity and susceptibility to ARTs of paternal and maternal genomes could differ. Therefore, we hypothesized that the transmission of heritable features after ARTs may be different between the paternally and maternally legacy. In the current study, we performed a longitudinal phenotypic study to evaluate the possible divergence between the maternal and paternal transmission of the growth deviations following fresh and vitrified embryo transfer in a large size rabbit cohort.
2. Materials and Methods
2.1. Animals and Ethical Statements
New Zealand rabbits belonging to the Universitat Politècnica de València were used throughout the experiment. All animals belonged to a synthetic line selected since the 1980s to increase litter size at weaning [24], with the current 44th generation selected. The animal study protocol was reviewed and approved by the “Universitat Politècnica de València” Ethical Committee (code: 2018/VSC/PEA/0116). All experiments were performed in accordance with the guidelines and regulations set forth in Directive 2010/63/EU EEC and were conducted in an accredited animal care facility (code: ES462500001091).
2.2. Experimental Design
Three experimental progenies were previously established to study the long-term effects of the successive ARTs used during a vitrified embryo transfer procedure: one from fresh-transferred (FT) embryos, one from vitrified-transferred (VT) embryos, and another through natural conception (NC) as the control reference [12]. Embryo transfer was performed as described by Besenfelder and Brem [15] and Garcia-Dominguez et al. [25]. Embryo vitrification-warming procedures were adapted from the protocol developed by Vicente and Garcia-Ximénez and described elsewhere using the Cryotop® methodology [12,25,26]. Briefly, 96 fresh and 101 vitrified embryos were transferred into six and seven foster mothers, generating 71 FT and 65 VT animals, respectively. In addition, 73 NC animals were obtained from six pregnant females without any embryonic manipulation. Once the animals reached adulthood, 10 males from each experimental group were used to generate seminal pools and we performed 225 (72 NC, 77 FT, and 76 VT) inseminations in the control females. Likewise, 10 control males were used to generate seminal pools and inseminated 46 (16 NC, 12 FT, and 18 VT) experimental females. Here, our purpose was to carry out a follow-up study of the offspring born after these mattings in order to elucidate whether the long-term effects of the embryo transfer and embryo cryopreservation procedures were transmissible through the male and/or female germ line. To evaluate the paternal transmission, offspring born from experimental males and control females (paternal crossbred animals; Pc) were tracked, while the maternal transmission was monitored in the offspring born from control males and experimental females (maternal crossbred animals; Mc). All rabbit colonies used along this study were housed at the Universitat Politècnica de València animal facilities in flat deck indoor cages (75 × 50 × 40 cm). After weaning, animals were caged collectively (eight rabbits per cage) until the ninth week. Thereafter, animals were individually kept in separate cages. All animals were tracked from birth until adulthood, comparing their growth performance and body weight at birth, weaning (fourth week), prepuberty (ninth week), puberty (fifteenth week), and adulthood (twentieth week).
2.3. Statistical Analyses
A general linear model (GLM) was fitted for body weight analysis including the experimental group as a fixed effect and common litter as a random effect. Litter size was used as a covariate for body weight correction, although it remained non-significant from the ninth week of age onward. Growth performance was estimated by nonlinear regression using the Gompertz equation y = a exp(−b exp(−kt)), well suited for rabbits as described before [14,18]. As to the meaning of parameters, a can be interpreted as the mature body weight maintained independently of short-term fluctuations; b is a timescale parameter related to the initial body weight; and k is a parameter related to the rate of maturing (growth rate). Growth [a exp(−b exp(−kt))] and maturity [exp(−b exp(−kt))] curves were plotted using the estimated parameters. A p-value of less than 0.05 was considered indicative of a statistically significant difference. The data are presented as least square mean ± standard error of the mean. All statistical analyses were performed with the SPSS 21.0 software package (SPSS Inc., Chicago, IL, USA).
3. Results
Compared to NC animals, both FT and VT animals from the Pc generation exhibited lower body weight at puberty age and adulthood (Table 1). In the Mc generation, FT and VT animals only showed a reduced body weight at weaning time in comparison with NC animals, but these differences were resolved from the age of prepuberty (Table 1).
Table 1.
Bodyweight comparison between naturally conceived (NC), fresh-transferred (FT), and vitrified-transferred (VT) progenies of the paternal crossbred generation and maternal crossbred generation.
| Body Weight (g) | Naturally-Conceived | Fresh-Transferred | Vitrified-Transferred |
|---|---|---|---|
| Paternal crossbred Animals (n) | (718) | (701) | (748) |
| Birth | 52.7 ± 0.51 | 53.3 ± 0.62 | 52.7 ± 0.50 |
| Weaning | 559.5 ± 5.59 | 569.8 ± 7.54 | 562.7 ± 4.53 |
| Prepuberty | 1476.8 ± 18.44 | 1494.6 ± 16.57 | 1468.3 ± 14.49 |
| Puberty | 3108.1 ± 31.01 a | 3011.3 ± 27.87 b | 3009.5 ± 30.49 b |
| Adulthood | 3924.2 ± 41.49 a | 3758.9 ± 35.75 b | 3769.1 ± 38.97 b |
| Maternal crossbred Animals (n) | (136) | (98) | (145) |
| Birth | 55.3 ± 0.93 | 54.6 ± 1.12 | 52.9 ± 0.90 |
| Weaning | 570.5 ± 10.233 a | 514.6 ± 11.05 b | 525.6 ± 10.62 b |
| Prepuberty | 1668.7 ± 20.58 | 1704.4 ± 22.18 | 1751.8 ± 20.85 |
| Puberty | 3124.0 ± 55.76 | 3162.6 ± 57.21 | 3133.5 ± 55.76 |
| Adulthood | 3655.2 ± 82.99 | 3707.3 ± 82.99 | 3628.0 ± 80.88 |
n is the number of animals. a,b Values with different superscripts within a row differ (p < 0.05).
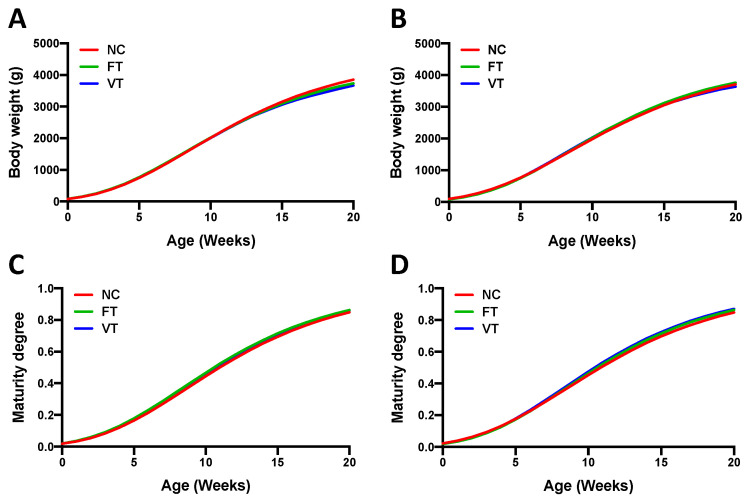
These results were also supported by the estimated Gompertz parameters (Table 2). Lower a values were noted for the FT and VT progenies of the Pc generation compared with the NC one, that of the Mc generation being similar for all progenies. No differences in the b and k parameters were observed among the experimental groups, either in the Pc or the Mc generation. Thus, growth and maturity curves showed that all the experimental progenies of the Pc and Mc generation exhibited a similar degree of growth and development, but those FT and VT animals of the Pc generation reached a lower mature body weight compared to the NC group (Figure A1).
Table 2.
Gompertz parameters of the naturally conceived (NC), fresh-transferred (FT), and vitrified-transferred (VT) progenies of the paternal crossbred generation and maternal crossbred generation.
| Gompertz Parameters * | Naturally-Conceived | Fresh-Transferred | Vitrified-Transferred |
|---|---|---|---|
| Paternal crossbred animals | |||
| a | 4536.6 ± 42.98 a | 4329.4 ± 44.83 b | 4349.6 ± 42.50 b |
| b | 4.1 ± 0.04 | 3.9 ± 0.05 | 3.9 ± 0.04 |
| k | 0.16 ± 0.002 | 0.16 ± 0.003 | 0.16 ± 0.003 |
| Maternal crossbred animals | |||
| a | 4372.7 ± 75.95 | 4354.1 ± 109.71 | 4157.6 ± 94.133 |
| b | 3.8 ± 0.07 | 4.0 ± 0.14 | 3.9 ± 0.13 |
| k | 0.16 ± 0.004 | 0.16 ± 0.007 | 0.17 ± 0.007 |
* Gompertz parameters: a can be interpreted as the mature body weight maintained independently of short-term fluctuations; b is a timescale parameter related to the initial body weight; and k is a parameter related to the rate of maturing (growth rate). a,b Values with different superscripts within a row differ (p < 0.05).
Overall, the results showed that the long-term effects caused by in vitro embryo manipulations are paternally, but not maternally transmitted to the offspring. Furthermore, no differences were observed between FT and VT animals, suggesting that ex vivo manipulation during the embryo transfer, rather than embryo cryopreservation stressors, are the principal cause of the inheritable effects following vitrified embryo transfer.
4. Discussion
Our findings revealed that growth deviations caused following an embryo transfer procedure are paternally transmissible, but did not seem to be inheritable via the female germline. Moreover, we found a comparable growth performance between FT and VT animals from both the Pc and Mc generations, suggesting that disturbing optimal embryo development through its recovery, in vitro handling, and mechanical manipulation during its transfer to a new foster mother was the principal trigger of transmissible effects, rather than embryo cryopreservation per se. Mounting evidence suggests that ARTs incur short- and long-term developmental deviations in the offspring, which are thought to be mediated by epigenetic mechanisms [1,2,3,13,14,15,17,23,27]. Recently, we demonstrated that both mandatory ARTs involved in a vitrified embryo transfer procedure (i.e., embryo transfer and embryo cryopreservation) incurred cumulative developmental effects, reducing the growth performance as in vitro manipulation increased [12]. Moreover, these alterations were transgenerationally propagated [20], which is in line with previous studies [18,19,27,28,29]. Here, we show that modifications in the growth performance is propagated with sex specificity. As a result, gametic transmission of the lower growth performance was propagated to the crossbred generations through the male germline, with indistinguishable effects via the female germline. This finding indicates that gametically programmed information survives to reprogram in the early embryo and persists into adulthood [30], but in a specific way, depending on the parental sex. Lower weaning weight was detected both in the FT- and VT-Mc offspring, but was erased in the long-term. We previously reported that females born following fresh and vitrified embryo transfer exhibited poorer lactation performance [12], which can incur a lower weaning weight in the Mc offspring that seemed to be restored later via compensatory growth, well described in rabbits [31].
In mammals, the maternal and the paternal genome are not functionally equivalent: the sperm and oocyte epigenetic profiles are very different from each other, and the epigenetic remodeling process of both pronuclei after fertilization exhibits allelic differences [22,23]. Considering this asymmetry, it is probable that the paternal and maternal genomes have a different sensitivity to ARTs stressors. Although we recognize that further molecular data are needed to validate this hypothesis, Kohda et al. demonstrated that intracytoplasmic sperm injection differentially impacts the gene expression of paternal and maternal alleles [22,32]. Along these lines, the present study provides evidence of gametic transmission of the ART-induced effects via the paternal germline. These findings emphasize the need to further characterize how ARTs lead to effects that can persist in the course of development and in subsequent generations, in order to better determine their biological relevance.
5. Conclusions
Here, we provide valuable information in a rabbit model about how the long-term effects caused during in vitro embryo manipulation can be transmitted to the next generation. In summary, our data revealed that embryo manipulation during fresh and vitrified embryo transfer procedures incur a lower growth performance that was propagated primarily through the male germline.
Acknowledgments
The authors would like to thank Neil Macowan Language Services for revising the English version of the manuscript, and the rabbit farmers from Altura for their support in this study.
Appendix A
Figure A1.
Gompertz curves comparing the growth between naturally conceived (NC), fresh-transferred (FT), and vitrified-transferred (VT) progenies of the (A) paternal crossbred generation and (B) maternal crossbred generation. Maturity curves of the NC, FT, and VT progenies of the (C) paternal crossbred generation and (D) maternal crossbred generation.
Author Contributions
Conceptualization, X.G.-D., J.S.V., and F.M.-J.; Methodology, X.G.-D., J.S.V., and F.M.-J.; Investigation, X.G.-D. and F.M.-J.; Resources, J.S.V. and F.M.-J.; Data curation, X.G.-D. and F.M.-J.; Writing—original draft preparation, X.G.-D.; Writing—review and editing, X.G.-D., J.S.V., M.P.V.-d.-C., and F.M.-J.; Project administration, M.P.V.-d.-C. and F.M.-J.; Funding acquisition, M.P.V.-d.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselleria d’Educació, Investigació, Cultura i Esport (Generalitat Valenciana, Spain), grant number AICO/2019/272. X.G.-D. was supported by a research grant from the Spanish Ministry of Economy, Industry, and Competitiveness, grant number BES-2015-072429.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Duranthon V., Chavatte-Palmer P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018;85:348–368. doi: 10.1002/mrd.22970. [DOI] [PubMed] [Google Scholar]
- 2.Vrooman L.A., Bartolomei M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017;68:72–84. doi: 10.1016/j.reprotox.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos-Ibeas P., Heras S., Gómez-Redondo I., Planells B., Fernández-González R., Pericuesta E., Laguna-Barraza R., Pérez-Cerezales S., Gutiérrez-Adán A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019;86:1292–1306. doi: 10.1002/mrd.23119. [DOI] [PubMed] [Google Scholar]
- 4.Canovas S., Ross P.J., Kelsey G., Coy P. DNA Methylation in Embryo Development: Epigenetic Impact of ART (Assisted Reproductive Technologies) BioEssays. 2017;39 doi: 10.1002/bies.201700106. [DOI] [PubMed] [Google Scholar]
- 5.Roseboom T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018;33:546–552. doi: 10.1093/humrep/dey034. [DOI] [PubMed] [Google Scholar]
- 6.Fleming T.P., Watkins A.J., Velazquez M.A., Mathers J.C., Prentice A.M., Stephenson J., Barker M., Saffery R., Yajnik C.S., Eckert J.J., et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet. 2018;391:1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canovas S., Ivanova E., Romar R., García-Martínez S., Soriano-Úbeda C., García-Vázquez F.A., Saadeh H., Andrews S., Kelsey G., Coy P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. Elife. 2017;6 doi: 10.7554/eLife.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Martínez S., Hurtado M.A.S., Gutiérrez H., Margallo F.M.S., Romar R., Latorre R., Coy P., Albors O.L. Mimicking physiological O 2 tension in the female reproductive tract improves assisted reproduction outcomes in pig. Mol. Hum. Reprod. 2018;24:260–270. doi: 10.1093/molehr/gay008. [DOI] [PubMed] [Google Scholar]
- 9.Campo H., García-Domínguez X., López-Martínez S., Faus A., Vicente Antón J.S., Marco-Jiménez F., Cervelló I. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 2019;89:126–138. doi: 10.1016/j.actbio.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Sparks A.E.T. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin. Reprod. Med. 2015;33:128–144. doi: 10.1055/s-0035-1546826. [DOI] [PubMed] [Google Scholar]
- 11.Dulioust E., Toyama K., Busnel M.C., Moutier R., Carlier M., Marchaland C., Ducot B., Roubertoux P., Auroux M. Long-term effects of embryo freezing in mice. Proc. Natl. Acad. Sci. USA. 1995;92:589–593. doi: 10.1073/pnas.92.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Dominguez X., Vicente J.S., Marco-Jiménez F. Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals. 2020;10:804. doi: 10.3390/ani10050804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuer S.K., Rinaudo P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017;8:403–410. doi: 10.1017/S204017441700023X. [DOI] [PubMed] [Google Scholar]
- 14.Feuer S., Rinaudo P. From Embryos to Adults: A DOHaD Perspective on In Vitro Fertilization and Other Assisted Reproductive Technologies. Healthcare. 2016;4:E51. doi: 10.3390/healthcare4030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Dominguez X., Marco-Jiménez F., Peñaranda D.S., Vicente J.S. Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals. 2020;10:1043. doi: 10.3390/ani10061043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiken C.E., Ozanne S.E. Transgenerational developmental programming. Hum. Reprod. Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 17.Novakovic B., Lewis S., Halliday J., Kennedy J., Burgner D.P., Czajko A., Kim B., Sexton-Oates A., Juonala M., Hammarberg K., et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019;10:3922. doi: 10.1038/s41467-019-11929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahsoudi B., Li A., O’Neill C. Assessment of the Long-Term and Transgenerational Consequences of Perturbing Preimplantation Embryo Development in Mice1. Biol. Reprod. 2007;77:889–896. doi: 10.1095/biolreprod.106.057885. [DOI] [PubMed] [Google Scholar]
- 19.Lavara R., Baselga M., Marco-Jiménez F., Vicente J.S. Long-term and transgenerational effects of cryopreservation on rabbit embryos. Theriogenology. 2014;81:988–992. doi: 10.1016/j.theriogenology.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Dominguez X., Marco-Jiménez F., Peñaranda D.S., Diretto G., García-carpintero V., Cañizares J., Vicente J.S., Marco-Jiménez F. Long-term and transgenerational phenotypic, transcriptional and metabolic effects in rabbit males born following vitrified embryo transfer. Sci. Rep. 2020;10:11313. doi: 10.1038/s41598-020-68195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laubach Z.M., Perng W., Dolinoy D.C., Faulk C.D., Holekamp K.E., Getty T. Epigenetics and the maintenance of developmental plasticity: Extending the signalling theory framework. Biol. Rev. 2018;93:1323–1338. doi: 10.1111/brv.12396. [DOI] [PubMed] [Google Scholar]
- 22.Kohda T., Ishino F. Embryo manipulation via assisted reproductive technology and epigenetic asymmetry in mammalian early development. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20120353. doi: 10.1098/rstb.2012.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zacchini F., Sampino S., Stankiewicz A.M., Haaf T., Ptak G.E. Assessing the epigenetic risks of assisted reproductive technologies: A way forward. Int. J. Dev. Biol. 2019;63:217–222. doi: 10.1387/ijdb.180402gp. [DOI] [PubMed] [Google Scholar]
- 24.Estany J., Baselga M., Blasco A., Camacho J. Mixed model methodology for the estimation of genetic response to selection in litter size of rabbits. Livest. Prod. Sci. 1989;21:67–75. doi: 10.1016/0301-6226(89)90021-3. [DOI] [Google Scholar]
- 25.Garcia-Dominguez X., Marco-Jimenez F., Viudes-de-Castro M.P., Vicente J.S. Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Vis. Exp. 2019;147:e58055. doi: 10.3791/58055. [DOI] [PubMed] [Google Scholar]
- 26.Vicente J.S., García-Ximénez F. Osmotic and cryoprotective effects of a mixture of DMSO and ethylene glycol on rabbit morulae. Theriogenology. 1994;42:1205–1215. doi: 10.1016/0093-691X(94)90869-9. [DOI] [PubMed] [Google Scholar]
- 27.Ventura-Juncá P., Irarrázaval I., Rolle A.J., Gutiérrez J.I., Moreno R.D., Santos M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015;48:68. doi: 10.1186/s40659-015-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez M.F., Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019;21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- 29.Calle A., Miranda A., Fernandez-Gonzalez R., Pericuesta E., Laguna R., Gutierrez-Adan A. Male Mice Produced by In Vitro Culture Have Reduced Fertility and Transmit Organomegaly and Glucose Intolerance to Their Male Offspring1. Biol. Reprod. 2012;87:1–9. doi: 10.1095/biolreprod.112.100743. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell E., Klein S.L., Argyropoulos K.V., Sharma A., Chan R.B., Toth J.G., Barboza L., Bavley C., Bortolozzi A., Chen Q., et al. Behavioural traits propagate across generations via segregated iterative-somatic and gametic epigenetic mechanisms. Nat. Commun. 2016;7:1–16. doi: 10.1038/ncomms11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gidenne T., Combes S., Feugier A., Jehl N., Arveux P., Boisot P., Briens C., Corrent E., Fortune H., Montessuy S., et al. Feed restriction strategy in the growing rabbit. 2. Impact on digestive health, growth and carcass characteristics. Animal. 2009;3:509–515. doi: 10.1017/S1751731108003790. [DOI] [PubMed] [Google Scholar]
- 32.Kohda T. Effects of embryonic manipulation and epigenetics. J. Hum. Genet. 2013;58:416–420. doi: 10.1038/jhg.2013.61. [DOI] [PubMed] [Google Scholar]