Abstract
Myometrial invasion affects the prognosis of endometrial cancer. However, discrepancies exist between pre-operative magnetic resonance imaging staging and post-operative pathological staging. This study aims to validate the accuracy of artificial intelligence (AI) for detecting the depth of myometrial invasion using a deep learning technique on magnetic resonance images. We obtained 4896 contrast-enhanced T1-weighted images (T1w) and T2-weighted images (T2w) from 72 patients who were diagnosed with surgico-pathological stage I endometrial carcinoma. We used the images from 24 patients (33.3%) to train the AI. The images from the remaining 48 patients (66.7%) were used to evaluate the accuracy of the model. The AI then interpreted each of the cases and sorted them into stage IA or IB. Compared with the accuracy rate of radiologists’ diagnoses (77.8%), the accuracy rate of AI interpretation in contrast-enhanced T1w was higher (79.2%), whereas that in T2w was lower (70.8%). The diagnostic accuracy was not significantly different between radiologists and AI for both T1w and T2w. However, AI was more likely to provide incorrect interpretations in patients with coexisting benign leiomyomas or polypoid tumors. Currently, the ability of this AI technology to make an accurate diagnosis has limitations. However, in hospitals with limited resources, AI may be able to assist in reading magnetic resonance images. We believe that AI has the potential to assist radiologists or serve as a reasonable alternative for pre-operative evaluation of the myometrial invasion depth of stage I endometrial cancers.
Keywords: artificial intelligence, endometrial neoplasms, magnetic resonance imaging (MRI), neoplasm staging, neural networks (computer)
1. Introduction
Endometrial cancer is one of the leading gynecologic malignancies in industrialized countries. The incidence of endometrial cancer has been increasing significantly worldwide in the past 10 years [1,2]. Myometrium, the middle layer of the uterine wall, serves as a barrier to prevent further expansion of endometrial cancer [3,4]. When the disease is diagnosed at an advanced stage, poor prognoses can be expected. One of the key parameters used to determine the stage is the depth of myometrial invasion. This is a prognostic factor used to categorize patients into high or low–intermediate risk categories, leading to different postoperative treatment approaches [5]. Therefore, accurate diagnoses followed by appropriate treatments in the early stages are the keys to good prognoses [6,7,8]. Currently, magnetic resonance imaging (MRI) is the primary tool used to evaluate the depth of myometrial invasion in endometrial cancer [9,10,11].
With the development of artificial intelligence (AI), radiologists have begun to use this technology to read medical images for various diseases [12,13,14]. AI comprises a collection of algorithms, mathematical functions, interrelated practical approaches, and overlapping areas of mathematics and statistics, which are well-suited for radiology because the pixel values of an MRI image are quantifiable [14,15]. Artificial neural network (ANN), for instance, is one technique used in the subdiscipline of classification systems. In ANN, the idea of deep learning (DL) has gained considerable attention. Various types of sub-algorithms concerning advances in fast processing, memory enhancement, and new model features and designs are continually being developed and upgraded [15]. The most common ANN used by DL is the convolutional neural network (CNN), which is the most suitable neural network for radiology when images are the primary units of analyses [15,16]. CNN is biologically inspired network mimicking the behavior of the brain cortex, which contains a complex structure of cells sensitive to small regions of the visual field. CNN not only comprises a series of layers which maps the image inputs to desired end points, but also learns higher-level imaging features [17].
According to the International Federation of Gynecology and Obstetrics (FIGO) classification system, endometrial cancer can be categorized as Stage I to IV, and Stage I can be further separated into IA and IB, which are distinguished based on the depth of myometrial invasion (less than vs. more than 50% myometrial invasion) [6]. However, the ability to determine the pre-operative MRI stages based, mainly, on personal expertise and experience which vary dramatically from person to person [18]. Additionally, various pathological factors—such as hematometra, interference due to a large coexisting leiomyoma or adenomyosis, or differences in the histological subtypes of the endometrial carcinoma—may lead to incorrect myometrial invasion diagnoses [10,19]. Discrepancies often exist between the pre-operative MRI staging and the post-operative pathological staging.
The previous literature about AI assistance in endometrial cancer diagnosis focuses on the performance of “post-operative” diagnosis (histopathological hematoxylin and eosin image) made by CNN-based classifier [20], while research examines the “pre-operative” MRI staging and performance of AI interpretation on endometrial cancer is rare. Our study is the pilot one to examine whether AI has the ability to assist physicians in making diagnoses of MRI before invasive surgery, i.e., pre-operative diagnosis. To achieve this goal, we compared the myometrial invasion diagnostic accuracy rate of the DL model with that of radiologists. Here, we used CNN to identify the myometrial invasion depth of endometrial cancer at an early stage and discussed the implications using AI as an auxiliary resource for making more comprehensive judgements.
2. Materials and Methods
2.1. Study Population
This is a retrospective study examining data from 72 endometrial cancer patients who received surgical treatment. Originally, there were 262 patients who had surgeries at the Tri-Service General Hospital in Taipei, Taiwan from January 2014 to September 2018. However, since we were interested in examining the ability of AI to validate myometrial invasion in early stage cancer, we excluded patients whose endometrial cancer was staged based merely on post-operative pathology without preoperative MRI scans and patients with stage II, III, and IV cancer. Eventually, 72 patients were qualified for this study (see Table 1). Among these, 53 were diagnosed with stage IA cancer, and 19 were diagnosed with stage IB based on permanent pathology. The average age of the patients was 59.7, with a minimum age of 39 and maximum age of 85. In terms of menopausal status, 63 (87.5%) postmenopausal. In terms of histology grade, 27, 32, and 13 belonged to grades 1, 2, 3 (5 were serous carcinomas, 1 was clear cell, and 3 were mixed). Among all the patients, 29 (40.3%) had uterine leiomyomas, and 43 (59.7%) did not.
Table 1.
Clinical and pathologic characteristics of all patients.
| Characteristics | n = 72 |
|---|---|
| Age (year) [mean ± SD a] (range) | 59.7 ± 9.08 (39–85) |
| Menopausal status | |
| Postmenopausal | 63 (87.5%) |
| Premenopausal | 9 (12.5%) |
| ECOG b performance status | |
| 0 | 54 |
| 1 | 18 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| FIGO c Stage | |
| IA | 53 (73.6%) |
| IB | 19 (26.4%) |
| Histology subtype | |
| Type I | |
| Grade 1 endometrioid | 27 (37.5%) |
| Grade 2 endometrioid | 32 (44.4%) |
| Type II | |
| Grade 3 endometrioid | 4 (5.6%) |
| Serous | 5 (6.9%) |
| Clear cell | 1 (1.4%) |
| Mixed | 3 (4.2%) |
| Histology grade | |
| 1 | 27 (37.5%) |
| 2 | 32 (44.4%) |
| 3 | 13 (18.1%) |
| Uterine leiomyomas | |
| Present | 29 (40.3%) |
| Absent | 43 (59.7%) |
Characteristics are presented based on the pathology reports. a SD: standard deviation. b ECOG: Eastern Cooperative Oncology Group. c FIGO: International Federation of Gynecology and Obstetrics.
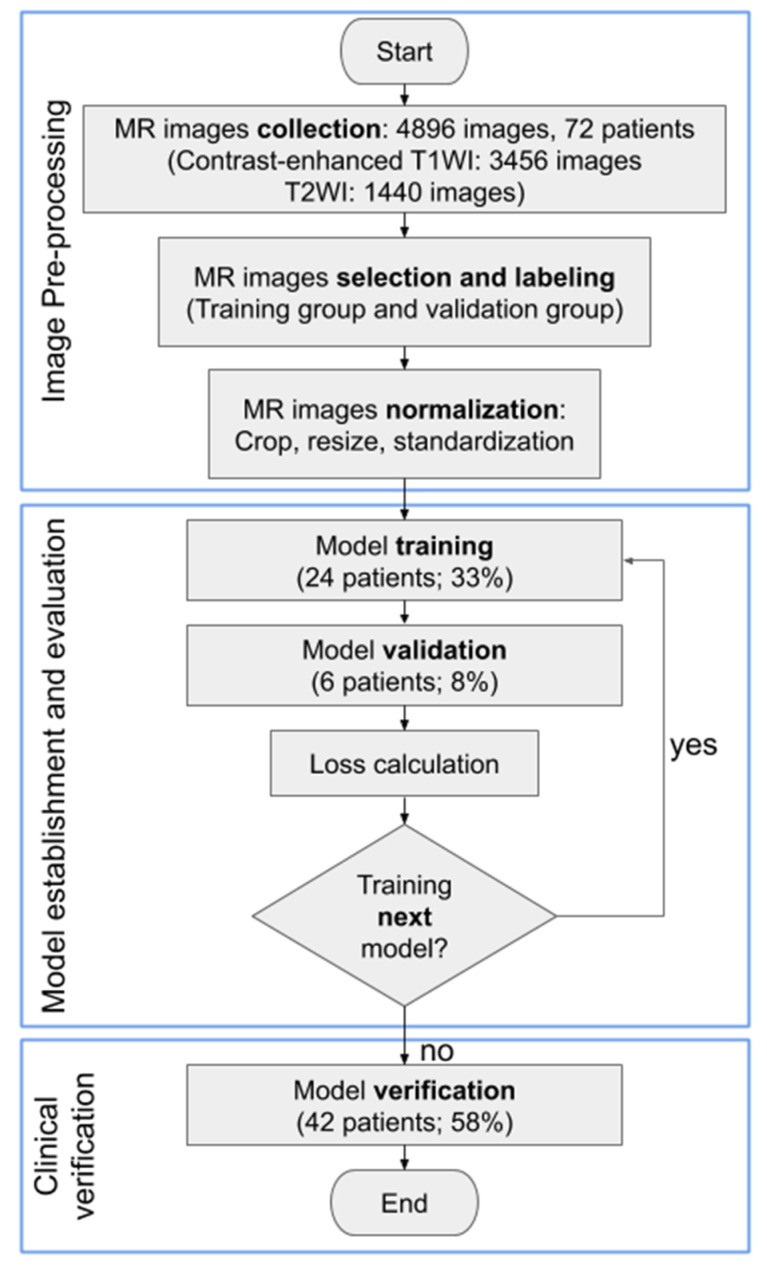
A total of 4896 MRI slices (3456 slices of contrast-enhanced T1w, and 1440 slices of T2w) with detailed preoperative radiology reports were collected from these 72 patients. Patients were divided into training, validation, and testing groups. The training group was comprised of patients whose results from the radiologists’ diagnoses and from the pathology reports were compatible. One third of the patients (24 patients) were selected as the training group that was used to train the DL model and generate the model parameters. Then, the performance of the model was checked by evaluating the error function using an independent validation group (6 patients). The model that generated the smallest error was selected as the final model. Finally, the test group was comprised of a dataset that was independent of the training group (42 patients plus the 6 patients in the validation group), and this group was used to appraise the accuracy rate of the novel AI-based system (see Figure 1). Two gynecologic oncologists with 25 and 14 years of clinical experience, respectively, were recruited to label the MRI images of each patient, including the contours of the uterus, lesion of the endometrium, and lining of the endometrium. Our research team then double-checked their work. Contrast-enhanced T1w and T2w images were both labeled to provide the AI model with appropriate information for image segmentation and training. Results of the histopathological report were used as a reference to calculate the accuracy rates.
Figure 1.
Workflow diagram demonstrating the process and preparation of the MR image dataset and training convolutional neural network models. The consecutive steps of MR image analysis include image upload, convolutional neural network model selection, and diagnosis output.
2.2. Artificial Intelligence Systems Selection
CNN is an efficient recognition algorithm that is frequently used in image processing and pattern recognition [21,22]. In this study, we used a deep neural network architecture known as U-Net as a model for segmentation of MR images, which consist of equal amount of up- and down-sampling layers. U-Net combines them with the so-called skip connections between opposing convolution and deconvolution layers. It concatenates a contracting path and expansive path while a large number of feature channels during up-sampling allows the propagation of the context information to higher resolution layers [17,23]. The U-Net architecture has proven to be useful for biomedical segmentation applications and medical image analyses [24,25,26,27]. Furthermore, using different methods of weights initialization within the same architecture (known as fine tuning) to initialize the weights for an encoder of the network, VGG11, VGG16, and ResNet34 pre-trained encoder models which converge considerably faster to a steady value and reduce training time in comparison to the non-pre-trained network, were used [28,29]. Based on the findings from previous research [30], the training settings of the aforementioned three architectures included hyperparameters such as batch size, epoch, learning rate, and optimizer that can be adjusted to enhance recognition accuracy. The training results of the AI models were evaluated using data from the validation group [31].
2.3. Images Processing and Analysis
In this study, MR images were obtained using 1.5T (Optima MR450W, GE Healthcare, Chicago, IL, USA) and 3T superconducting units (Discovery MR 750, GE Healthcare, Chicago, IL, USA). The imaging protocol for MR imaging scanners typically include sagittal, contrast-enhanced T1w (TR/TE, 501/Minimum ms; section thickness, 4 mm; gap, 1.2 mm; matrix, 288 × 192; and FOV, 280 mm), and sagittal T2w (TR/TE, 5000/90 ms; section thickness, 5 mm; gap, 1 mm; echo-train length, 19; matrix, 320 × 224; and FOV, 240 mm). Since the MR images have different formats and resolutions, initial quality control is important to filter out images with improper formats and low resolutions. We cropped all the raw images into 896 × 896 pixel resolution by using the equation for altered resolution: P′i = (Pi − Pmean)/Pstd. Here, Pi represents each pixel, Pmean and Pstd are the mean and standard deviation of all pixels, respectively, and P′i is the resulting altered pixel. Moreover, to improve DL efficiency, data augmentation was conducted by multiplying, horizontally flipping, vertically flipping, and using affine transformation on all MR images. Thus, multiple images were derived from the original ones used for AI model training. The augmented dataset was used only for training and not for validation or testing [32]. Thereafter, segmentation was carried out, which involves assigning a label to every pixel in an image such that pixels with the same label share certain characteristics [33]. After processing the MR images, the training and validation groups were used to establish and validate the models, respectively. We used Intersection over Union (IoU) to evaluate the performance of the AI model. IoU is an evaluation metric used to measure the accuracy of an object detector on a particular dataset, which is often used for evaluation the performance of CNN detectors.
2.4. Establishing AI Models
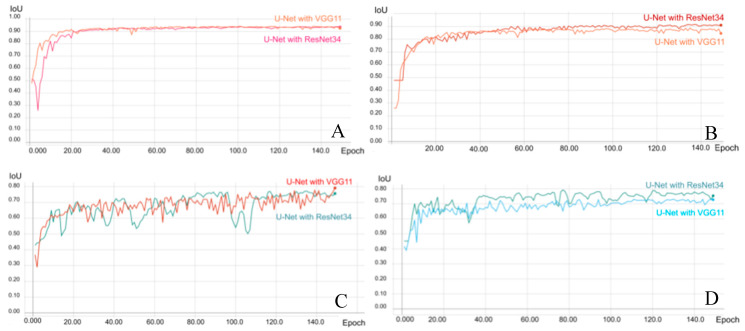
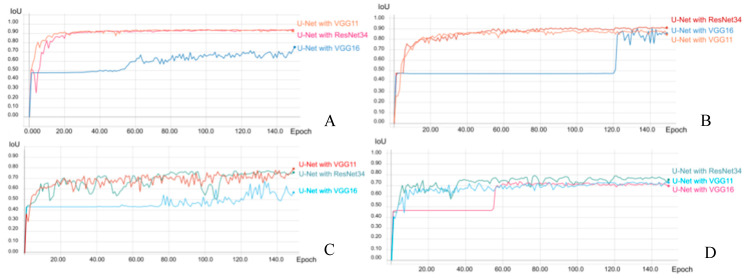
After testing several different models, the U-Net with ResNet34, VGG16, and VGG11 encoders, pre-trained on ImageNet architectures, was used to establish the CNN-based AI models. The above models were established using a QNAP TS-2888X Linux based server with an Intel Xeon CPU, four GPU cards, and 512 GB available RAM for training and validation. During the training process, the original MR and mask images were initially adjusted to have the same size and resolution as the training input images, and the MR images of endometrium and uterus were learned through AI training. The layers were trained by a stochastic gradient descent in a relatively small batch size (16 images) because of the variations in the size and shape of the uterus and endometrial lesions, with a learning rate of 0.001. To determine the best model, the training for all categories was performed for 150 epochs and the loss was calculated using the Dice-coefficient loss function, rather than by the cross entropy loss function, because of its advantage in solving the problem of disparity between the size of the endometrium and non-endometrial areas in the image (Figure A1). After adjusting the parameters, segmentation of the uterus and endometrial lesions in contrast-enhanced T1w exhibited significant and superior performance for the U-Net with VGG11 model and achieved 94.20% and 79.16% of the mean IoU, respectively (Table A1, Table A4 and Table A5). However, the U-Net with ResNet34 model for segmentation training of T2w exhibited better performance than the other models and achieved 91.66% and 79.31% of the mean IoU of the uterus and endometrial lesion, respectively (Table A2, Table A6 and Table A7). The parameters of the best model were selected (see Figure 2) and used for the validation, and these are listed in Table A3.
Figure 2.
Performance and validation curves for each architecture of the trained convolutional neural network (CNN) models (VGG11 and Resnet34) on MRI. The prediction (intersection over union) score of CNN models in reading the uterus on contrast-enhanced T1w (A) and T2w (B). The prediction score of CNN models in reading endometrium on contrast-enhanced T1w (C) and T2w (D).
2.5. Statistical Analysis
To determine the differences between diagnoses by the radiologists and AI interpretations, Chi-square tests were used to identify whether two categorical variables were independent of each other, including the “accuracy,” “over-staged/under-staged,” and “whether the concomitant conditions (coexisting uterine leiomyoma, different histology types) affect the diagnoses.” For continuous variables, Pearson correlation was applied to examine the positive or negative relationships between the degree (depth) of myometrial invasion from the pathology reports and the degree (depth) of myometrial invasion interpreted by AI. These two variables were both measured as fractions (myometrial invasion over myometrial thickness). Box and whisker plots were used to compare the accuracy of diagnoses made by radiologists and those made by AI (the center, spread, and overall range of the depth of myometrial invasion were used as determinants), and a one-way analysis of variance (ANOVA) was used to generate the F-statistics and p-value (α = 0.05 was the standard used to determine any significant differences). This study used STATA 14 software (StataCorp Limited Liability Company, College Station, TX, USA) for statistical analyses.
2.6. Ethical Approval
Our research was a retrospective study using the MRI images, pathological reports, and other demographic information of patients. All data/samples were fully anonymized before we accessed them. Only serial numbers were associated with the collected data/samples. We could not identify any individual based on the serial numbers. The data collection period was between January 2019 to November 2019. The IRB approved our study as “Low Risk” (IRB No.: 1-107-05-165).
3. Results
3.1. Verification of the Final Model
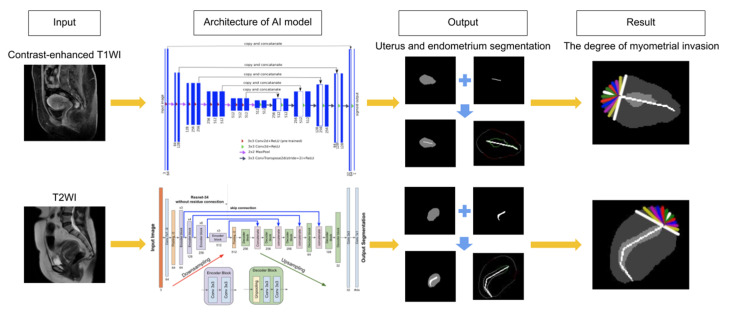
To verify the established AI model, a total of 48 patients were used, and the AI calculated the depth of myometrial invasion by endometrial cancer and then classified each as stage IA or IB. The architecture of our AI model was adopted from Iglovikov and Shvets [24] and modified from Shvets, Iglovikov, Rakhlin, and Kalinin [25] (see Figure 3). The results were compared with the surgico-pathological findings. The accuracy rates for the contrast-enhanced T1w, T2w, and radiologists were 79.2%, 70.8%, and 77.8%, respectively. The chi-square tests showed that there were no significant differences between the AI interpretations for both T1w and T2w and radiologist’s diagnoses (p = 0.856 and p = 0.392, respectively) (see Table 2). However, we did notice a relatively higher “over-diagnosis rate” from the radiologists. Among the incompatible cases in T1w, 7 out of 10 were over diagnosed (over-diagnosis rate: 70.0%). Among the incompatible cases in T2w, 9 out of 14 were over diagnosed (over-diagnosis rate: 64.3%). However, for the incompatible cases in radiologists’ diagnoses, 14 out of 16 were over diagnosed (over-diagnosis rate: 87.5%).
Figure 3.
Consecutive steps of MR image analysis include image upload, interpretation of image by AI, and diagnosis output. The architecture of our AI model includes convolutional neural networks consisting of convolution layers, max pooling layers, and a fully connected layer. Each layer extracts different image features; subsequently, all of the extracted features are integrated. The result includes the depth of myometrial invasion and stage classification (FIGO stage IA or IB).
Table 2.
Accuracy rates of AI and radiologists.
| Results | Pathology Report | Accuracy Rates | |
|---|---|---|---|
| IA | IB | ||
| AI Interpretation | |||
| Contrast-enhanced T1w | 79.2% (38/48) |
||
| <50% Invasion | 30 (compatible) | 3 (under diagnosed) | |
| ≥50% Invasion | 7 (over diagnosed) | 8 (compatible) | |
| T2w | 70.8% (34/48) |
||
| <50% Invasion | 29 (compatible) | 5 (under diagnosed) | |
| ≥50% Invasion | 9 (over diagnosed) | 5 (compatible) | |
| Radiologists’ Diagnoses | 77.8% (56/72) |
||
| IA | 39 (compatible) | 2 (under diagnosed) | |
| IB | 14 (over diagnosed) | 17 (compatible) | |
Chi-square test results: For Contrast-enhanced T1w and Radiologists: χ2 = 0.033, p = 0.856; For T2w and Radiologists: χ2 = 0.738, p = 0.392.
3.2. Effects of Concomitant Conditions on MR Image Interpretation
In addition to the aforementioned findings, we found that the MR images interpreted by AI were more likely to be inaccurate when the patients had coexisting uterine leiomyoma, (p = 0.027 for contrast-enhanced T1w and p = 0.12 for T2w). In contrast, coexisting leiomyoma usually did not affect the radiologist’s MRI interpretations (p = 0.140). Other than uterine leiomyoma, different histological subtypes did not affect the accuracy of the radiologists (p = 0.413) or the AI (p = 0.549 for contrast-enhanced T1w; p = 0.727 for T2w) (see Table 3).
Table 3.
Influence of concomitant conditions on the accuracy rates of AI and radiologists.
| Pathology Report | ||||||
|---|---|---|---|---|---|---|
| Results | IA | IB | IA | IB | Accuracy Rates | p-Value |
| Uterine leiomyoma | + | − | +/− | |||
| AI Interpretation | ||||||
| Contrast-enhanced T1w | 60%/87.9% | 0.027 | ||||
| <50% Invasion | 9 * | 1 | 21 * | 2 | ||
| ≥50% Invasion | 5 | 0 * | 2 | 8 * | ||
| T2w | 56.3%/78.1% | 0.115 | ||||
| <50% Invasion | 8 * | 1 | 21 * | 4 | ||
| ≥50% Invasion | 6 | 1 * | 3 | 4 * | ||
| Radiologists’ Diagnoses (MR stage) | 69%/83.7% | 0.140 | ||||
| IA | 16 ** | 1 | 23 ** | 1 | ||
| IB | 8 | 4 ** | 6 | 13** | ||
| Histology | Type I | Type II | Type I/II | |||
| AI Interpretation | ||||||
| Contrast-enhanced T1w | 81.1%/72.7% | 0.549 | ||||
| <50% Invasion | 26 * | 2 | 4 * | 1 | ||
| ≥50% Invasion | 5 | 4 * | 2 | 4 * | ||
| T2w | 71.1%/70% | 0.727 | ||||
| <50% Invasion | 25 * | 4 | 4 * | 1 | ||
| ≥50% Invasion | 7 | 2 * | 2 | 3 * | ||
| Radiologists’ Diagnoses (MR stage) | 79.7%/69.2% | 0.413 | ||||
| IA | 35 ** | 1 | 4 ** | 1 | ||
| IB | 11 | 12 ** | 3 | 5 ** | ||
* “compatible” between pathology report and AI interpretation; ** “compatible” between pathology report and radiologists’ diagnoses.
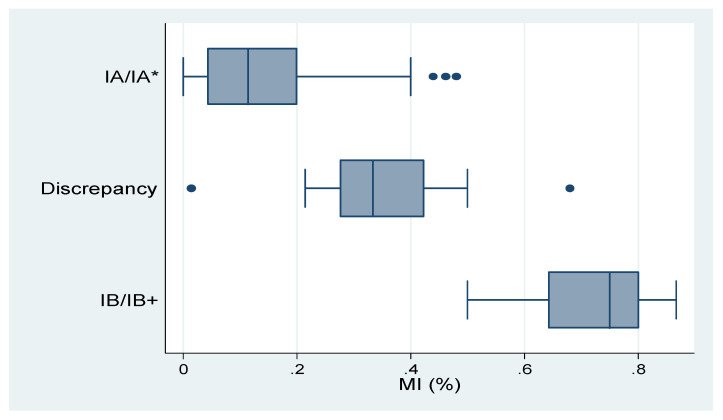
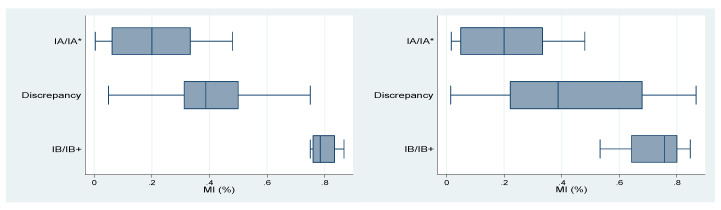
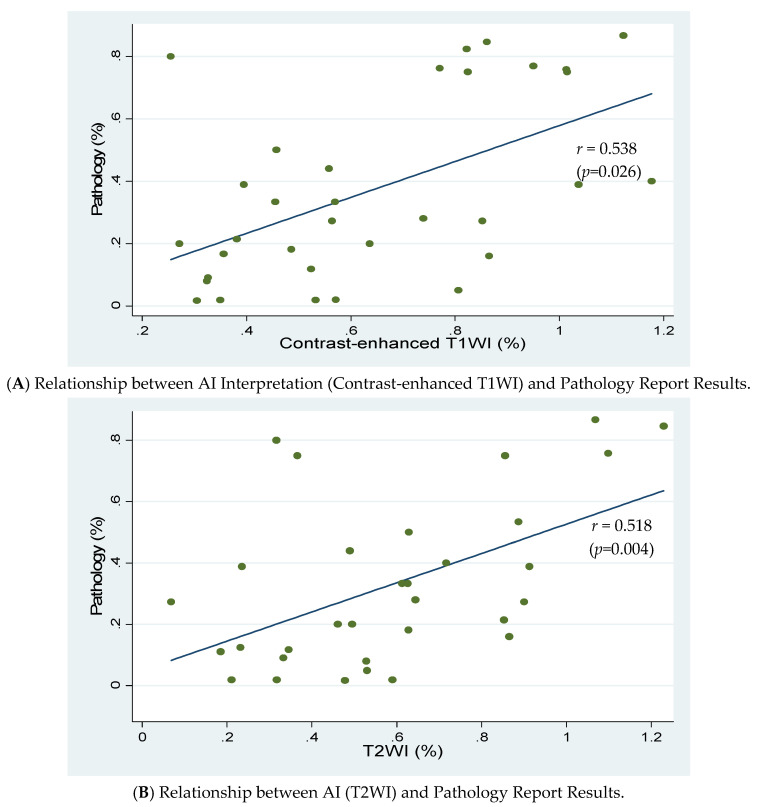
In addition, we found a positive correlation between the depth of myometrial invasion (the percentage of myometrial invasion over myometrial thickness) from the pathology report and that interpreted by AI (r = 0.54 for contrast-enhanced T1w, p = 0.026; r = 0.52 for T2w, p = 0.004) (see Figure A2). We also found that the distribution of the percentage of myometrial invasion may lead to discrepancies between the stages diagnosed by radiologists or AI. Results are presented in Table 4 and Table 5. Table 4 and Figure 4 show the results of radiologists’ diagnoses. As shown in the table, when the degree of myometrial invasion was relatively small or large, radiologists were less likely to make incorrect decisions. However, when the depth of myometrial invasion was around 50%, radiologists’ diagnoses tended to be incompatible with the pathology reports. The box and whisker plots display the distribution of myometrial invasion. Table 5 and Figure 5 show the results of AI interpretation. Similarly, when the degree of myometrial invasion was at the two extremes, AI was more likely to generate a correct answer; however, when the depth of myometrial invasion was in the middle range (50%), AI also generated incompatible results. Particularly, the range for AI to make discrepant results for the T2w (from 1.5% to 86.7%) was significantly wider than that for the T1w or for the radiologists. Results of the ANOVA showed that the closer the depth of myometrial invasion was to 50%, the easier it was for both radiologists (F-value = 99.06, p < 0.001) and AI (F-value = 44.46, p < 0.001 for contrast-enhanced T1w; F-value = 17.68, p < 0.001 for T2w) to provide incorrect diagnoses. We found the results reasonable since the ability to determine the pre-operative MRI stages based, mainly, on personal expertise and experience which vary from person to person. On the other hand, using AI to determine the depth of myometrial invasion may also be affected by various pathological factors, such as irregular endomyometrial junction inside single uterus, hematometra, endometrial polyps, exophytic tumor growth, adenomyosis or extensive leiomyomas. These factors may inevitably cause both human beings and AI to come up with incorrect diagnoses, especially when there is a clear cut-off value, below or above 50% myometrial invasion.
Table 4.
Results of radiologists′ diagnoses and pathological stages.
| Results | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|
| IA/IA * (compatible) | 0 | 0.043 | 0.114 | 0.2 | 0.48 |
| Discrepancy | 0.015 | 0.276 | 0.333 | 0.422 | 0.68 |
| IB/IB+ (compatible) | 0.5 | 0.643 | 0.75 | 0.8 | 0.867 |
IA/IA *: pathological stage/clinical stage; IB/IB+: pathological stage/clinical stage; MI: myometrial invasion; ANOVA: F-value = 99.06, p = 0.000.
Table 5.
Results of AI interpretation and pathological stages (contrast-enhanced T1w and T2w).
| Contrast-Enhanced T1w | T2w | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Min | Q1 | Median | Q3 | Max | ||
| IA/IA * (Compatible) |
0.004 | 0.063 | 0.2 | 0.333 | 0.48 | IA/IA * (compatible) |
0.017 | 0.05 | 0.2 | 0.333 | 0.48 |
| Discrepancy | 0.05 | 0.313 | 0.388 | 0.5 | 0.75 | Discrepancy | 0.015 | 0.222 | 0.388 | 0.68 | 0.867 |
| IB/IB+ (Compatible) |
0.75 | 0.76 | 0.785 | 0.835 | 0.867 | IB/IB+ (compatible) |
0.533 | 0.643 | 0.758 | 0.8 | 0.846 |
IA/IA *: pathological stage/clinical stage; B/IB+: pathological stage/clinical stage; MI: myometrial invasion; ANOVA: Contrast-enhanced T1w: F-value = 44.46, p = 0.000; T2w: F-value = 17.68, p = 0.000.
Figure 4.
Box and Whisker Plot of data from radiologists′ diagnoses and pathological stages.
Figure 5.
Box and Whisker Plot of data from AI interpretation and pathological stages (contrast-enhanced T1w and T2w).
4. Discussion
We compared the accuracy rates of the radiologists’ diagnoses and AI interpretations based on the depth of myometrial invasion. The results indicated that the AI interpretations for both contrast-enhanced T1w and T2w were similar to radiologists’ diagnoses. Although small differences exist, they were not statistically significant. However, we found that the closer the depth of myometrial invasion was to 50%, the easier it was for both the radiologists and AI to provide incorrect judgements. However, compared with AI, radiologists were more likely to “over-stage” the results from IA to IB. We believe these findings shed light on the fact that human beings tend to act conservatively when making critical decisions. In clinical practice, when facing with a situation where it is necessary to choose the lesser of two evils, radiologists would rather let the patients receive more evaluations or treatments than receive insufficient ones. More treatments usually include more extensive surgeries (lymph nodes dissection), radiation therapy, and/or chemotherapy. However, receiving more treatments are not always beneficial, and they also come with additional risks. Patients are more likely to suffer from surgical complications or therapy-related complications.
In addition, we found that when patients had coexisting leiomyoma, AI was more likely to provide incorrect interpretations. However, the coexisting leiomyoma did not affect the radiologists’ judgements. We believe that was because the myometrial compression from a leiomyoma or bulky polypoid tumor would lead to unclear boundaries between the tumor and myometrium, which would make it difficult for the AI to calculate the depth. The histological types of endometrial carcinoma, on the contrary, did not affect the radiologists or AI. Such findings suggested that the primary role of MRI used in gynecologic oncology is in delineating the extent of the disease, not for analyzing the morphological features or histological types [34].
Still, AI technology is potentially useful especially in hospitals without radiologists specializing in gynecology. There are several benefits of using AI to predict myometrial invasion before surgery. First, it can affect the choice of surgical approach methods, and second, it can be used to determine if lymphadenectomy is necessary. In the early stages, patients have the chance to choose either exploratory laparotomy or micro-invasive laparoscopic surgery. The result of a Gynecologic Oncology Group LAP2 trial for early stage endometrial cancer reported favorable recurrence and survival outcomes of laparoscopy surgical staging [35]. In another Cochrane review published in 2018 [36], the key results revealed no difference in perioperative mortality risk, overall survival, and disease-free survival between laparoscopy and laparotomy. Furthermore, laparoscopy is associated with significantly shorter hospital stays. In hospitals without radiologists specializing in gynecology, AI technology could help identify low-risk patients with stage IA disease, and therefore, the gynecological oncologist may feel more comfortable performing laparoscopic surgery.
In addition, the necessity of routine lymphadenectomy in staging surgery has been widely debated. The Mayo group described the criteria of patients with a low risk of nodal disease spread and a high disease-free survival rate: grade 1 to 2 tumors, less than 50% myometrial invasion, and tumor size less than 2 cm [37]. In GOG Lap 2 trial, in 971 patients with type 1 endometrioid carcinoma, 40% met Mayo low-risk criteria and only 0.8% (3/389) had positive nodes. Therefore, with careful selection of low risk patients, lymphadenectomy may be safely avoided, which reduces surgery-related morbidity such as lower limb lymphedema or intra-abdominal lymphocele formation. In hospitals without radiologists specializing in gynecology and gynecologists specializing in oncologic surgery, with help of AI pre-operative diagnosis, a general gynecological surgeon could perform simple hysterectomy, bilateral salpingo-oophorectomy without lymphadenectomy for selective low-risk patients. In remote area with limited medical resources, AI pre-operative diagnosis will reduce the need of transferring low-risk patients to tertiary medical center.
We noted a few potential limitations of our study. First, our datasets were built based on cases from one institution. The diagnoses made using MRI and pathology were based on personal expertise and experience, which exhibit individual differences. Although the Tri-Service General Hospital is a medical center in Taipei, we still cannot eliminate the possibility of that there is bias in the data. Second, the key imaging sequence used to assess uterine cavity tumors involves choosing a sagittal T2w. However, a multiparametric approach which combines T2w and contrast-enhanced T1w along with different planes may represent the most comprehensive approach to assess tumor spread. Also, our study did not analyze the diffusion-weighted imaging or the MR spectroscopy, which may could serve as one potential research subject in diagnosing endometrial cancer. Training AI from a two-dimensional approach (largest cross-sectional tumor area) to a three-dimensional approach (whole tumor) could lead to differences in establishing the AI-based model or affect the accuracy of the interpretation of the results, especially for results obtained by assessing only the sagittal plane of T2w and contrast-enhanced T1w [38,39]. Third, different CNN architectures might be suitable for the interpretation of different diseases’ images, and thereby selecting and establishing appropriate architectures might provide slightly different results [23,24,25,26]. Fourth, the impact of ethnic differences was not examined because all the patients in this study were Taiwanese. Therefore, using AI to determine the depth of myometrial invasion still has its weak spot given the limited cases using for AI training regarding the endometrial cancer. The AI’s decision cannot be final at this point. However, if more diverse cases can be used for deep learning in the future, or if we can develop a multicenter database for this purpose, we may further enhance the validity of the AI and improve the quality of our health care. Alternatively, we could further design a prospective randomized study to identify a population of patients with endometrial cancer to examine the efficacy of AI-assisted method. Our paper, as a pilot study in this uncharted territory, shows that using AI as an assist to interpret the depth of myometrial invasion of MRI is indeed advantageous to prevent the high interobserver variability among radiologists [18].
5. Conclusions
In summary, although current AI technology may not be able to replace the expertise and experience of physicians, AI could be used as an auxiliary resource. From the perspective of balancing human proactive errors and passive errors, it could be beneficial for the physicians to have a “second opinion” from the AI technology before making critical judgement calls on endometrial cancer. Our research is the first attempt to use AI technology to evaluate the invasion depth of myometrium in early stage endometrial cancers. There have not been any publications about AI applications in endometrial cancers. In the future, refinement of selection and establishment of a deep learning model with a larger image database are essential to improve the accuracy. We believe artificial intelligence has the potential to assist radiologists or serve as a reasonable alternative for pre-operative evaluation of the myometrial invasion depth of stage I endometrial cancers.
Acknowledgments
We appreciate QNAP (QNAP Systems, Inc.) team’s assistance in deploying a reliable AI server in this study.
Appendix A
Figure A1.
Performance and validation curves for all three architectures of the trained CNN models (VGG11, VGG16 and Resnet34) on MRI. The prediction (IoU) score of CNN models in reading the uterus on contrast-enhanced T1w (A) and T2w (B). The prediction (IoU) of CNN models in reading endometrium on contrast-enhanced T1w (C) and T2w (D).
Figure A2.
Graphs showing the relationship between pathologic and imaging findings regarding myometrial invasion. The correlation coefficient for contrast-enhanced T1w (A) and T2w (B) of a patient without uterine leiomyoma is 0.538 and 0.518, respectively.
Table A1.
Verification summary of the performance of three AI models on contrast-enhanced T1w using the validation dataset. The parameters of accuracy, sensitivity and specificity for both uterus and endometrium are shown.
| U-Net with VGG 11 | U-Net with VGG 16 | U-Net with ResNet34 | |
|---|---|---|---|
| Accuracy (Uterus) | 96.83% | 76.83% | 97.06% |
| Accuracy (Endometrium) | 85.96% | 73.13% | 84.31% |
| Mean loU (Uterus) | 94.20% | 75.30% | 93.92% |
| Mean loU (Endometrium) | 79.16% | 67.54% | 77.23% |
| Mean Dice (Uterus) | 96.94% | 84.01% | 96.78% |
| Mean Dice (Endometrium) | 87.62% | 78.22% | 86.24% |
| Mean Precision (Uterus) | 97.05% | 97.44% | 96.51% |
| Mean Precision (Endometrium) | 89.54% | 88.86% | 88.53% |
| Mean Recall (Uterus) | 96.83% | 76.83% | 97.06% |
| Mean Recall (Endometrium) | 85.96% | 73.13% | 84.31% |
| Mean Specificity (Uterus) | 96.83% | 76.83% | 97.06% |
| Mean Specificity (Endometrium) | 85.96% | 73.13% | 84.31% |
Table A2.
Verification summary of the performance of three AI models on T2w using the validation dataset. The parameters of accuracy, sensitivity and specificity for both uterus and endometrium are shown.
| U-Net with VGG 11 | U-Net with VGG 16 | U-Net with ResNet34 | |
|---|---|---|---|
| Accuracy (Uterus) | 95.80% | 95.49% | 96.86% |
| Accuracy (Endometrium) | 83.60% | 82.88% | 87.34% |
| Mean loU (Uterus) | 88.78% | 90.61% | 91.66% |
| Mean loU (Endometrium) | 73.18% | 73.63% | 79.31% |
| Mean Dice (Uterus) | 93.75% | 94.88% | 95.49% |
| Mean Dice (Endometrium) | 82.60% | 82.95% | 87.56% |
| Mean Precision (Uterus) | 91.90% | 94.28% | 94.20% |
| Mean Precision (Endometrium) | 81.67% | 83.02% | 87.78% |
| Mean Recall (Uterus) | 95.80% | 95.50% | 96.86% |
| Mean Recall (Endometrium) | 83.60% | 82.88% | 87.34% |
| Mean Specificity (Uterus) | 95.80% | 95.49% | 96.86% |
| Mean Specificity (Endometrium) | 83.60% | 82.88% | 87.34% |
Table A3.
Performance of each architecture of the trained CNN models on contrast-enhanced T1w and T2w. The parameters of learning rate, batch size, total epochs, epochs with the best value of IoU and mean IoU of both the uterus and endometrium are shown.
| Contrast-Enhanced T1w | T2w | |
|---|---|---|
| Type | Uterus / Endometrium | |
| Architecture | U-Net with VGG 11 | U-Net with ResNet34 |
| Optimizer | Adam | |
| Learning Rate | 1e-4 | |
| Batch size | 16 | |
| Total number of epochs run during training | 150 | |
| Epochs with the maximum value of loU (best model) | 89/95 | 137/77 |
| Mean loU of validation of Uterus/Endometrium (best model) | 92.64%/80.40% | 91.66%/79.31% |
Table A4.
Parameters and performance of all three architectures on “contrast-enhanced T1w” of the “uterus.”
| Loss Weight | Batch Size | Loss | Learning Rate | Architecture (U-Net with #) | Data | Augmentation | Best Epoch | Mean IoU (%) |
IoU 0 (%) |
IoU 1 (%) |
Mean Dice (%) |
Dice 0 (%) |
Dice 1 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 16 | dice | 0.0001 | VGG11 | T1WI | TRUE | 101 | 94.20 | 99.45 | 88.95 | 96.94 | 99.73 | 94.15 |
| 10 | 16 | Cross Entropy | 0.0001 | VGG11 | T1WI | TRUE | 98 | 94.10 | 99.44 | 88.76 | 96.88 | 99.72 | 94.04 |
| 10 | 16 | dice | 0.0001 | VGG16 | T1WI | TRUE | 150 | 75.30 | 97.70 | 52.89 | 84.01 | 98.83 | 69.19 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T1WI | TRUE | 131 | 93.92 | 99.42 | 88.42 | 96.78 | 99.71 | 93.86 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T1WI | FALSE | 133 | 92.06 | 99.24 | 84.87 | 95.72 | 99.62 | 91.82 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T1WI | FALSE | 115 | 91.62 | 99.18 | 84.06 | 95.46 | 99.59 | 91.34 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T1WI | TRUE | 140 | 93.66 | 99.40 | 87.93 | 96.64 | 99.70 | 93.57 |
| 10 | 32 | Cross Entropy | 0.0001 | ResNet34 | T1WI | TRUE | 104 | 91.17 | 99.26 | 83.08 | 95.19 | 99.63 | 90.76 |
Table A5.
Parameters and performance of all three architectures on “contrast-enhanced T1w” of the “endometrium.”.
| Loss Weight | Batch Size | Loss | Learning Rate | Architecture (U-Net with #) | Data | Augmentation | Best Epoch | Mean IoU (%) |
IoU 0 (%) |
IoU 1 (%) |
Mean Dice (%) |
Dice 0 (%) |
Dice 1 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 16 | dice | 0.0001 | VGG11 | T1WI | TRUE | 149 | 79.16 | 93.74 | 64.59 | 87.62 | 96.77 | 78.48 |
| 10 | 16 | dice | 0.0001 | VGG11 | T1WI | FALSE | 32 | 73.53 | 91.86 | 55.19 | 83.44 | 95.75 | 71.13 |
| 10 | 16 | dice | 0.0001 | VGG16 | T1WI | TRUE | 133 | 67.54 | 91.07 | 44.01 | 78.22 | 95.33 | 61.12 |
| 10 | 16 | dice | 0.0001 | VGG16 | T1WI | FALSE | 4 | 47.37 | 94.73 | 0.00 | 48.65 | 97.29 | 0.00 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T1WI | FALSE | 113 | 75.38 | 92.34 | 58.42 | 84.89 | 96.02 | 73.75 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T1WI | TRUE | 72 | 79.59 | 93.82 | 65.36 | 87.93 | 96.81 | 79.05 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T1WI | TRUE | 135 | 77.23 | 93.13 | 61.33 | 86.24 | 96.44 | 76.03 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T1WI | FALSE | 85 | 75.93 | 97.12 | 54.75 | 84.65 | 98.54 | 70.76 |
Table A6.
Parameters and performance of all three architectures on “T2w” of the “uterus.”
| Loss Weight | Batch Size | Loss | Learning Rate | Architecture (U-Net with #) | Data | Augmentation | Best Epoch | Mean IoU (%) |
IoU 0 (%) |
IoU 1 (%) |
Mean Dice (%) |
Dice 0 (%) |
Dice 1 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 16 | dice | 0.0001 | VGG11 | T2WI | TRUE | 135 | 88.78 | 98.92 | 78.64 | 93.75 | 99.46 | 88.04 |
| 10 | 16 | dice | 0.0001 | VGG11 | T2WI | FALSE | 59 | 85.59 | 98.55 | 72.64 | 91.71 | 99.27 | 84.15 |
| 10 | 16 | dice | 0.0001 | VGG16 | T2WI | TRUE | 141 | 90.61 | 98.92 | 82.30 | 94.88 | 99.46 | 90.29 |
| 10 | 16 | dice | 0.0001 | VGG16 | T2WI | FALSE | 115 | 87.56 | 98.70 | 76.41 | 92.99 | 99.34 | 86.63 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T2WI | FALSE | 148 | 85.73 | 98.52 | 72.94 | 91.80 | 99.25 | 84.35 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 137 | 91.66 | 99.19 | 84.14 | 95.49 | 99.59 | 91.38 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T2WI | FALSE | 149 | 78.67 | 97.53 | 59.82 | 86.80 | 98.75 | 74.86 |
| 10 | 32 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 124 | 89.25 | 98.92 | 79.58 | 94.04 | 99.46 | 88.63 |
| 5 | 64 | dice | 0.00005 | ResNet34 | T2WI | TRUE | 148 | 83.45 | 98.16 | 68.73 | 90.27 | 99.07 | 81.46 |
| 5 | 64 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 143 | 88.84 | 98.88 | 78.80 | 93.79 | 99.43 | 88.14 |
| 5 | 64 | dice | 0.0002 | ResNet34 | T2WI | TRUE | 136 | 90.90 | 99.12 | 82.68 | 95.04 | 99.56 | 90.52 |
| 10 | 64 | dice | 0.00005 | ResNet34 | T2WI | TRUE | 148 | 82.10 | 98.00 | 66.21 | 89.33 | 98.99 | 79.67 |
| 10 | 64 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 141 | 87.93 | 98.77 | 77.10 | 93.22 | 99.38 | 87.07 |
| 10 | 64 | dice | 0.0002 | ResNet34 | T2WI | TRUE | 127 | 90.30 | 99.02 | 81.58 | 94.68 | 99.51 | 89.85 |
| 20 | 64 | dice | 0.00005 | ResNet34 | T2WI | TRUE | 133 | 86.37 | 98.62 | 74.13 | 92.22 | 99.31 | 85.14 |
| 20 | 64 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 132 | 89.42 | 98.96 | 79.87 | 94.14 | 99.48 | 88.81 |
| 20 | 64 | dice | 0.0002 | ResNet34 | T2WI | TRUE | 135 | 91.33 | 99.16 | 83.49 | 95.29 | 99.58 | 91.00 |
| 10 | 16 | Cross Entropy | 0.0001 | ResNet34 | T2WI | TRUE | 134 | 91.50 | 99.19 | 83.80 | 95.39 | 99.59 | 91.19 |
| 10 | 72 | Cross Entropy | 0.0001 | ResNet34 | T2WI | TRUE | 124 | 88.57 | 98.85 | 78.29 | 93.62 | 99.42 | 87.82 |
| 10 | 72 | Cross Entropy | 0.0002 | ResNet34 | T2WI | TRUE | 131 | 90.22 | 99.03 | 81.40 | 94.63 | 99.51 | 89.74 |
| 10 | 72 | Cross Entropy | 0.0004 | ResNet34 | T2WI | TRUE | 80 | 90.07 | 99.04 | 81.11 | 94.54 | 99.52 | 89.57 |
Table A7.
Parameters and performance of all three architectures on “T2w” of the “endometrium.”
| Loss Weight | Batch Size | Loss | Learning Rate | Architecture (U-Net with #) | Data | Augmentation | Best Epoch | Mean IoU (%) |
IoU 0 (%) |
IoU 1 (%) |
Mean Dice (%) |
Dice 0 (%) |
Dice 1 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 16 | dice | 0.0001 | VGG11 | T2WI | FALSE | 18 | 77.88 | 94.63 | 61.12 | 86.56 | 97.24 | 75.87 |
| 10 | 16 | dice | 0.0001 | VGG11 | T2WI | TRUE | 43 | 79.07 | 95.23 | 62.90 | 87.39 | 97.56 | 77.23 |
| 10 | 16 | dice | 0.0001 | VGG16 | T2WI | FALSE | 69 | 73.63 | 95.56 | 51.70 | 82.95 | 97.73 | 68.16 |
| 10 | 16 | dice | 0.0001 | VGG16 | T2WI | TRUE | 3 | 46.67 | 93.33 | 0.00 | 48.28 | 96.55 | 0.00 |
| 10 | 16 | dice | 0.0001 | ResNet34 | T2WI | TRUE | 77 | 79.31 | 95.39 | 63.24 | 87.56 | 97.64 | 77.48 |
| 10 | 64 | dice | 0.00005 | ResNet34 | T2WI | TRUE | 136 | 77.87 | 95.17 | 60.57 | 86.48 | 97.52 | 75.44 |
| 20 | 64 | dice | 0.00005 | ResNet34 | T2WI | TRUE | 143 | 78.31 | 95.35 | 61.27 | 86.80 | 97.62 | 75.98 |
Author Contributions
Conceptualization—C.-C.C. and H.-C.D.; Methodology—H.-C.D., H.-K.D. and C.-C.C.; Data analysis and software—H.-K.D.; Writing-original draft preparation—H.-C.D. and H.-K.D.; Writing-review and editing—H.-C.D., H.-K.D. and Y.-H.L.; Supervision—M.-H.Y.; Project administration—H.-C.D., C.-C.C. and Y.-H.L.; Funding acquisition—H.-C.D., C.-C.C. and Y.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Tri-Service General Hospital, grant numbers TSGH-C108-115, TSGH-D109-106, TSGH-D109-107 and TSGH-E-109229, and the Ministry of Science and Technology, grant number MOST 107-2314-B-016-036.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design, data collection and analysis, writing of the manuscript, or decision to publish.
References
- 1.American Cancer Society . Global Cancer Facts & Figures. 4th ed. American Cancer Society; Atlanta, GA, USA: 2018. [Google Scholar]
- 2.Lortet-Tieulent J., Ferlay J., Bray F., Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 3.SGO Clinical Practice Endometrial Cancer Working Group. Burke W.M., Orr J., Leitao M., Salom E., Gehrig P., Olawaiye A.B., Brewer M., Boruta D., Herzog T.J., et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014;134:385–392. doi: 10.1016/j.ygyno.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 4.SGO Clinical Practice Endometrial Cancer Working Group. Burke W.M., Orr J., Leitao M., Salom E., Gehrig P., Olawaiye A.B., Brewer M., Boruta D., Herzog T.J., et al. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014;134:393–402. doi: 10.1016/j.ygyno.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N., Creutzberg C.L., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M., et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meissnitzer M., Forstner R. MRI of endometrium cancer—How we do it. Meissnitzer Forstner Cancer Imaging. 2016;16:11. doi: 10.1186/s40644-016-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson D.M., Connor G.P., Broste S.K., Krawisz B.R., Johnson K.K. Prognostic significance of gross myometrial invasion with endometrial cancer. Obstet. Gynecol. 1996;88:394–398. doi: 10.1016/0029-7844(96)00161-5. [DOI] [PubMed] [Google Scholar]
- 8.Mitamura T., Watari H., Todo Y., Kato T., Konno Y., Hosaka M., Sakuragi N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014;25:301–305. doi: 10.3802/jgo.2014.25.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcázar J.L., Gastón B., Navarro B., Salas R., Aranda J., Guerriero S. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2017;28:e86. doi: 10.3802/jgo.2017.28.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hricak H., Rubinstein L.V., Gherman G.M., Karstaedt N. MR imaging evaluation of endometrial carcinoma: Results of an NCI cooperative study. Radiology. 1991;179:829–832. doi: 10.1148/radiology.179.3.2028000. [DOI] [PubMed] [Google Scholar]
- 11.Choi H.-J., Lee S., Park B.K., Kim T.-J., Kim C.K., Park J.J., Choi C.H., Lee Y.-Y., Lee J.-W., Bae D.-S., et al. Long-term outcomes of magnetic resonance imaging-invisible endometrial cancer. J. Gynecol. Oncol. 2016;27:e38. doi: 10.3802/jgo.2016.27.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi W.L., Hosny A., Schabath M.B., Giger M.L., Birkbak N.J., Mehrtash A., Allison T., Arnaout O., Abbosh C., Dunn I.F., et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019;69:127–157. doi: 10.3322/caac.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidlich V., Weidlich G.A. Artificial intelligence in medicine and radiation oncology. Cureus. 2018;10:e2475. doi: 10.7759/cureus.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendelson E.B. Artificial intelligence in breast imaging—Potentials and limitations. AJR Am. J. Roentgenol. 2019;212:293–299. doi: 10.2214/AJR.18.20532. [DOI] [PubMed] [Google Scholar]
- 15.Hwang D.-K., Hsu C.-C., Chang K.-J., Chao D., Sun C.-H., Jheng Y.-C., Yarmishyn A.A., Wu J.-C., Tsai C.-Y., Wang M.-L., et al. Artificial intelligence-based decision-making for age-related macular degeneration. Theranostics. 2019;9:232–245. doi: 10.7150/thno.28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litjens G., Kooi T., Bejnordi B.E., Setio A.A.A., Ciompi F., Ghafoorian M., Van Der Laak J.A., Van Ginneken B., Sánchez C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Woo S., Kim S.Y., Cho J.Y., Kim S.H. Assessment of deep myometrial invasion of endometrial cancer on MRI: Added value of second-opinion interpretations by radiologists subspecialized in gynaecologic oncology. Eur. Radiol. 2017;27:1877–1882. doi: 10.1007/s00330-016-4582-1. [DOI] [PubMed] [Google Scholar]
- 19.Beddy P., Moyle P., Kataoka M., Yamamoto A.K., Joubert I., Lomas D.J., Crawford R., Sala E. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: Comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2012;262:530–537. doi: 10.1148/radiol.11110984. [DOI] [PubMed] [Google Scholar]
- 20.Sun H., Zeng X., Xu T., Peng G., Ma Y. Computer-aided diagnosis in histopathological images of the endometrium using a convolutional neural network and attention mechanisms. IEEE J. Biomed. Health Inform. 2019;24:1664–1676. doi: 10.1109/JBHI.2019.2944977. [DOI] [PubMed] [Google Scholar]
- 21.Yasaka K., Abe O. Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med. 2018;15:e1002707. doi: 10.1371/journal.pmed.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kermany D.S., Goldbaum M., Cai W., Valentim C.C., Liang H., Baxter S.L., McKeown A., Yang G., Wu X., Yan F., et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122–1131. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Ronneberger O., Fischer P., Brox T. U-Net: Convolutional networks for biomedical image segmentation. [(accessed on 17 August 2020)];arXiv. 2015 Available online: https://arxiv.org/abs/1505.04597.1505.04597 [Google Scholar]
- 24.Iglovikov V., Shvets A. TernausNet: U-Net with VGG11 encoder pre-trained on ImageNet for image segmentation. [(accessed on 17 August 2020)];arXiv. 2018 Available online: https://arxiv.org/abs/1801.05746.1801.05746v1 [Google Scholar]
- 25.Shvets A.A., Iglovikov V.I., Rakhlin A., Kalinin A.A. Angiodysplasia detection and localization using deep convolutional neural networks; Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications; Orlando, FL, USA. 17–20 December 2018. [Google Scholar]
- 26.He K., Zhang X., Ren S., Sun J. Deep residual learning for image recognition. [(accessed on 17 August 2020)];arXiv. 2015 Available online: https://arxiv.org/abs/1512.03385.1512.03385 [Google Scholar]
- 27.Iglovikov V., Mushinskiy S., Osin V. Satellite imagery feature detection using deep convolutional neural network: A Kaggle competition. [(accessed on 17 August 2020)];arXiv. 2017 Available online: https://arxiv.org/abs/1706.06169.1706.06169 [Google Scholar]
- 28.Iglovikov V., Rakhlin A., Kalinin A., Shvets A. Pediatric bone age assessment using deep convolutional neural networks. [(accessed on 17 August 2020)];arXiv. 2017 Available online: https://arxiv.org/abs/1712.05053.1712.05053 [Google Scholar]
- 29.Ching T., Himmelstein D.S., Beaulieu-Jones B.K., Kalinin A.A., Do B.T., Way G.P., Ferrero E., Agapow P.-M., Zietz M., Hoffman M.M., et al. Opportunities and obstacles for deep learning in biology and medicine. bioRxiv. 2017:142760. doi: 10.1098/rsif.2017.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G., Li W., Ourselin S., Vercauteren T. Automatic brain tumor segmentation using cascaded anisotropic convolutional neural networks. [(accessed on 17 August 2020)];arXiv. 2017 Available online: https://arxiv.org/pdf/1709.00382.pdf.1709.00382v2 [Google Scholar]
- 31.Paszke A., Chaurasia A., Kim S., Culurciello E. ENet: A deep neural network architecture for real-time semantic segmentation. [(accessed on 17 August 2020)];arXiv. 2016 Available online: https://arxiv.org/abs/1606.02147.1606.02147v1 [Google Scholar]
- 32.Arieno A., Chan A., Destounis S.V. A review of the role of augmented intelligence in breast imaging: From automated breast density assessment to risk stratification. AJR. 2019;212:259–270. doi: 10.2214/AJR.18.20391. [DOI] [PubMed] [Google Scholar]
- 33.Yuheng S., Hao Y. Image segmentation algorithms overview. [(accessed on 17 August 2020)];arXiv. 2017 Available online: https://arxiv.org/abs/1707.02051.1707.02051 [Google Scholar]
- 34.Vargas H.A., Akin O., Zheng J., Moskowitz C., Soslow R.A., Abu-Rustum N., Barakat R.R., Hricak H. The value of MR imaging when the site of uterine cancer origin is uncertain. Radiology. 2011;258:785–792. doi: 10.1148/radiol.10101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker J.L., Piedmonte M.R., Spirtos N.M., Eisenkop S.M., Schlaerth J.B., Mannel R.S., Barakat R., Pearl M.L., Sharma S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khadra G., Hannah D., Andrew B., Alberto D.L. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst. Rev. 2018;10:CD006655. doi: 10.1002/14651858.CD006655.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrea M., Maurice J.W., Gary L.K., Michael G.H., Giliola C., Karl C.P. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am. J. Obstet. Gynecol. 2000;182:1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 38.Ueno Y., Forghani B., Forghani R., Dohan A., Zeng X.Z., Chamming’S F., Arseneau J., Fu L., Gilbert L., Gallix B., et al. Endometrial Carcinoma: MR Imaging-based Texture Model for Preoperative Risk Stratification-A Preliminary Analysis. Radiology. 2017;284:748–757. doi: 10.1148/radiol.2017161950. [DOI] [PubMed] [Google Scholar]
- 39.Ytre-Hauge S., Dybvik J.A., Lundervold A., Salvesen Ø.O., Krakstad C., Fasmer K.E., Werner H.M., Ganeshan B., Høivik E., Bjørge L., et al. Preoperative tumor texture analysis on MRI predicts high-risk disease and reduced survival in endometrial cancer. J. Magn. Reson. Imaging. 2018;48:1637–1647. doi: 10.1002/jmri.26184. [DOI] [PubMed] [Google Scholar]