Abstract
The first antibiotic-producing actinomycete (Streptomyces antibioticus) was described by Waksman and Woodruff in 1940. This discovery initiated the “actinomycetes era”, in which several species were identified and demonstrated to be a great source of bioactive compounds. However, the remarkable group of microorganisms and their potential for the production of bioactive agents were only partially exploited. This is caused by the fact that the growth of many actinomycetes cannot be reproduced on artificial media at laboratory conditions. In addition, sequencing, genome mining and bioactivity screening disclosed that numerous biosynthetic gene clusters (BGCs), encoded in actinomycetes genomes are not expressed and thus, the respective potential products remain uncharacterized. Therefore, a lot of effort was put into the development of technologies that facilitate the access to actinomycetes genomes and activation of their biosynthetic pathways. In this review, we mainly focus on molecular tools and methods for genetic engineering of actinomycetes that have emerged in the field in the past five years (2015–2020). In addition, we highlight examples of successful application of the recently developed technologies in genetic engineering of actinomycetes for activation and/or improvement of the biosynthesis of secondary metabolites.
Keywords: actinomycetes, natural products, synthetic biology, genetic engineering, tools, improvement
1. Introduction
Actinomycetes are Gram-positive, mostly aerobic, filamentous bacteria of the phylum Actinobacteria [1,2,3,4]. They were initially regarded as organisms, which share morphological characteristics with both bacteria and fungi [5,6] and thus, they were considered as transitional forms between the two groups. This is reflected in the name “Actinomycetes” that was derived from Greek “atkis” or “aktin” (means ray) and “mykes” (means fungus) [7,8]. Actinomycetes have adapted to different ecological niches: terrestrial [9,10,11,12,13,14,15,16,17,18], aquatic [19,20,21,22,23,24,25] and artificial (“manmade”) [26,27]. In many cases, their biological function is indispensable. Some species are involved in decomposing complex mixtures of polymers in dead plants, animals and fungal materials contributing to humus formation and the recycling of biomaterials [28,29]. Actinomycetes can also provide a nitrogen-fixing function in symbiotic associations with plants [30,31] or take part in other complex, ecologically relevant interactions [32,33,34]. The most known example includes interactions with insects, where the antibiotics produced by actinomycetes protect the associated organism (e.g., termites, ants, beetles, wasps or they larvae) against detrimental microorganisms [35,36,37,38,39,40].
However, many researchers have drawn attention to actinomycetes mainly because of their biosynthetic potential for the production of secondary metabolites [41,42,43]. The discovery of the first actinomycete-derived antibiotic (actinomycin) [44] boosted the isolation of actinomycetes from diverse sources and the traditional screening involving the cultivation, extraction of metabolites and disk diffusion-based activity assays and/or chromatographic analysis [43,45,46,47]. Since the “Golden Age” of antibiotic discovery (1940s–1960s), several antimicrobial and other valuable compounds were discovered from actinomycetes. Many of these molecules have been developed to commercial products, including agrochemicals and pharmaceuticals [14,47,48,49,50,51]. Thus, actinomycetes are one of the most prolific and important sources of bioactive compounds. Since the whole genome sequencing of the first actinomycetes, which was the genome of Streptomyces coelicolor A3 [52], hundreds actinomycetes genomes were sequenced and annotated. These data revealed that the genomes encode several biosynthetic gene clusters (BGCs) for the production of diverse secondary metabolites. However, only a few of the BGCs are expressed at standard laboratory conditions and, therefore, the biosynthetic potential of many actinomycetes remains unexploited [53,54,55,56,57,58,59,60]. Numerous strategies were developed to induce the expression of the BGCs and access the corresponding products. While general concepts and methods of natural product discovery, including new isolation and strain cultivation methods, (meta)genomics-based approaches as well as modern metabolomics-inspired technologies were highlighted in other reviews [43,45,46,61,62,63,64,65,66,67,68], this review provides the reader with an overview on recent advances in molecular tools for the genetic engineering of actinomycetes.
2. Genetic Engineering of Actinomycetes
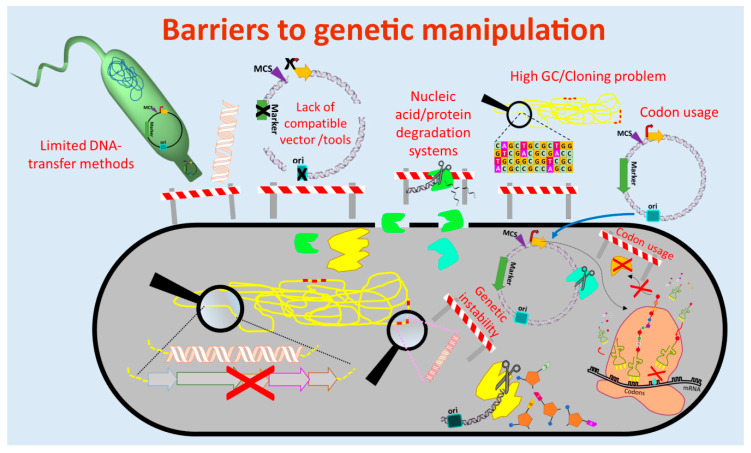
The currently used genetic engineering strategies for activation of BGC-expression and production of the respective compounds in actinomycetes include the expression of multiple copies of the whole BGC or factors that are limiting the production, expression of activator genes, deletion of genes encoding repressors of the BGC, refactoring of the BGC by substitution or modification of native regulatory elements (e.g., promoters) and/or expression of the BGC in optimized (e.g., genome-minimized, precursor-optimized), native or heterologous hosts [69,70,71,72,73] (Figure 1 and Figure 2). These in vivo engineering approaches require genetic manipulation that depends on molecular tools for assembling of genetic constructs and methods for their introduction into the host. In the past four decades, several barriers were identified that retarded the process of natural product discovery from actinomycetes. Lack of compatible molecular tools, limited cloning and DNA transfer methods, DNA degradation, genetic instability, high guanine-cytosine content (GC-content) of the genomes, and different codon usage of the newly introduced foreign DNA are barriers that often prevent the genetic manipulation of actinomycetes (Figure 1).
Figure 1.
Barriers to genetic manipulation of actinomycetes. Obstacles hindering the genetic manipulation and engineering of actinomycetes include the lack of strain-compatible tools, cloning methods for high guanine-cytosine content (GC-content) DNA sequences as well as transfer methods for introduction of the genetic constructs into the host. Furthermore, nucleic acid degradation systems present in actinomycetes and differences in codon usage may result in fragmentation of the genetic constructs and production of non-functional proteins, respectively. (Single elements of the figure (e.g., ribosome) were re-used from a previous publication [74] with permission from the Royal Society of Chemistry.)
Figure 2.
Breaking down the barriers to genetic manipulation and engineering of actinomycetes. Numerous methods and tools were developed to facilitate the genetic manipulation of actinomycetes. Suitable vectors and other vehicles (e.g., integrative and replicative systems) as well as fast and easy cloning strategies were developed to assemble genetic constructs, which are introduced into the host by using different transfer methods (e.g., conjugation and protoplast transformation). Protocols for avoiding DNA-degradation (e.g., treatment of the DNA with NaOH) and solutions for ensuring correct transcription/translation (e.g., codon usage optimization) were established. These innovations led to the generation of many gene deletions and (over)expression mutants (e.g., deletion of competitive biosynthetic pathways, overexpression of activators for activation of silent biosynthetic gene cluster (BGC)). This enormous progress facilitated the metabolic engineering of actinomycetes chassis for manufacturing bioactive natural products, including antibiotics. (Single elements of the figure (e.g., schematic representation of plasmid backbone) were re-used from a previous publication [74] with permission from the Royal Society of Chemistry.).
Therefore, enormous efforts have been undertaken to develop tools and methods to overcome these obstacles (Figure 2). Advances in sequencing technics [75] and bioinformatics tools for genome mining [63] facilitate cost-effective sequencing and a fast identification of BGCs and other potential targets for genetic engineering. Molecular biology enzymes [76,77,78], fast cloning strategies and synthetic biology which involves vectors and genetic parts (e.g., attachment sites, replicons, selection markers, promoters, terminators) [79,80,81,82,83,84] were optimized for introduction and maintenance of additional or new genetic material in actinomycetes strains. Methods for transfer of the generated construct into the cell (e.g., protoplast transformation, conjugation) were established [74,85].
During the past five years (2015–2020) a variety of technologies and protocols for engineering of actinomycetes genomes have been established. Therefore, we believe that a summary of the recent developments will be a valuable reference for the community focusing on actinomycetes and their natural products. To this end, we present an update on the molecular tools and methods for genetic engineering of actinomycetes. In the first part, details on new cloning strategies were described (Section 2.1). The second part contains an overview on recent advances in “genetic bricks” and molecular tools for genetic manipulation of actinomycetes (Section 2.2). As there is tremendous progress in the development of cloning strategies (Section 2.1) and CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR associated)-based tools (Section 2.2.4), these two sections have a “lexicon-like” structure. This should ensure an easy and time-saving search for the diverse tools and their explanation.
2.1. Assembly Strategies for Generation of Constructs for Genetic Engineering of Actinomycetes
DNA assembly and cloning strategies are indispensable techniques in the field of synthetic biology. Cloning of actinomycete sequences is especially challenging because of the high GC content genomes [48,86] and highly conserved and repetitive sequences (e.g., polyketide synthase (PKS) or nonribosomal peptide synthetase (NRPS) BGCs). Often, the BGC of interest spans more than 100 kb [87,88] and therefore efficient, fast and easy cloning tools are required. In the last five years (2015–2020), some new methods and updates of established protocols for cloning and assembling of genetic constructs were reported. Their successful application was demonstrated in bacterial strains including actinomycetes (Table 1).
Table 1.
Advantages and disadvantages of cloning strategies and example of successful application.
| Method | Advantages | Disadvantages | Example Organisms |
|---|---|---|---|
| iCatch [89] | + cloning of large fragments + complements other cloning methods (like TAR and CATCH) |
− previous strain editing (insertion of HE recognition sites) required | S. coelicolor [89] |
| Direct Pathway Cloning (DiPaC) [92] | + refactoring of BGCs is possible + cloning of full BGCs |
− PCR amplification of target sequence: risk of mutations − pure and high molecular weight gDNA required |
Saccharopolyspora erythreae [92] |
| Artificial gene operon assembly system (AGOS) [95] | + refactoring of BGCs is possible | − limited production of refactored BGCs | Streptomyces niveus [95] |
| Modified Gibson Assembly for cloning large high GC DNA fragments [99] | + reusable vector + increased assembly efficiency for high GC content DNA |
− PCR amplification of target sequence: risk of mutations | S. pristinaespiralis [99] |
2.1.1. iCatch
The iCatch [89] is an upgrade of the 2014 proposed iBrick principle [90] based on the BioBricks concept [91]. This tool was developed to facilitate “catching” of large regions (e.g., BGCs from actinomycetes). The BioBricks concept is based on the creation of DNA pieces flanked by XbaI and SpeI restriction sites, resulting in compatible cohesive ends after restriction enzyme digestion. This allows the easy assembly and ligation of any desired DNA sequence following the BioBricks standard. The concept is dependent on the specific restriction sites, which often appear in bacterial genomes, and, therefore, limits the insert length to the frequency of the restriction sites. The iBrick uses homing endonucleases (HEs, I-SceI and PI-PspI), enabling the cloning of big fragments since the HE recognition sites are longer (> 18 bp) and, therefore, rather rare in natural DNA sources. The iBrick strategy is suitable for assembling whole gene clusters in vectors using the BioBricks concept. As iBrick still has some limitations, such as low efficiencies and difficulties in capturing large BGCs from Actinomyces, iCatch was developed [89]. The target regions are released by genomic DNA (gDNA) digestions with HEs (as the target is flanked by I-SceI and PI-PspI sites) which are in vivo introduced via homologous recombination. The digestion takes place in an agarose gel plug to protect the DNA and the target fragments are cloned by self-ligation and transferred by electroporation into Escherichia coli. The iCatch is mainly an update on the precise “catching” of BGCs for further editing and assembly by applying the previously introduced iBrick method. Using this method, the actinorhodin (ACT) BGC from S. coelicolor was successfully obtained with an efficiency of 95.8% correct clones and heterologously expressed in a fast-growing thermophilic Streptomyces sp. strain. Furthermore, the obtained ACT cluster was further assembled into other iBrick components, which enabled their subsequent use for other studies [89].
2.1.2. Direct Pathway Cloning (DiPaC)
Direct Pathway cloning (DiPaC) [92] accomplishes the assembly of complete biosynthetic pathways by covering full BGCs with long-amplicon polymerase chain reaction (PCR), and the introduction of homologous nucleotide overhangs allows subsequent in vitro HiFi DNA assembly such as Gibson assembly. Instead of HiFi DNA assembly, sequence- and ligation-independent cloning (SLIC) [93] can also be used which is not as time consuming, because the terminal homologous sequences of the fragments anneal in vitro and the stitching of the gaps takes place in vivo after transformation, inside the E. coli cell [94]. DiPaC is particularly suitable for cloning of short to midsized BGCs with low- and high-GC DNA. Not just fast cloning, but also refactoring of natural product pathways, is possible with DiPaC. Using the DiPaC method, the successful transfer of the erythromycin BGC from Saccharopolyspora erythraea into a Streptomyces heterologous host was reported [92].
2.1.3. Artificial Gene Operon Assembly System (AGOS)
The artificial gene operon assembly system (AGOS) [95] is a “plug-and-play” method for the reconstruction and artificial assembly of gene operons of natural product pathways. The tool provides a set of entry plasmids (pAO-A → pAO-D, based on a pBluescript II SK (+) derivative), which are designed for the artificial gene operon manufacturing. Those plasmids contain an “entry cassette” which is comprised of two target sites, a controllable promoter (tcp830), a ribosome binding site (RBS) and specific restriction sites (XbaI and SpeI). Selection markers and specific intrinsic terminators are also incorporated, which delivers gene context-independency of each artificial gene operon. The target gene operon regions can be cloned into the entry plasmid by using the same restriction sites (XbaI and SpeI), which can either be done by de novo synthesis of the target region including the restriction sites, by introducing the sites during PCR amplification of the target regions, or via RED/ET-mediated recombination [96]. A derivative of the novBG01 cosmid [97], called mrsMR02, is the destination vector for the final assembly. It contains the genes needed for conjugational transfer and integration into the host genome as well as a multiple recombineering site for RED/ET-mediated recombineering [96]. The final assembly of the gene operons, present in the entry plasmids, takes place via the RED/ET-mediated recombination into the target sites on the destination vector. As proof of concept, Basitta et al. disassembled the well-studied 20 kb novobiocin BGC and successfully reorganized it again using AGOS [95].
2.1.4. Modified Gibson Assembly for Cloning Large High CG DNA Fragments
Gibson Assembly is a well-known and commonly used method in synthetic biology [98]. It enables the one-pot, seamless assembly of multiple large DNA fragments (up to 900 kb) into a linearized vector of choice, only requiring overlapping sequences of neighboring DNA molecules. The reaction mixture includes a T5 exonuclease, which produces terminal single-stranded DNA overhangs on the provided fragments, which are subsequently repaired by a DNA polymerase and sealed by a DNA ligase. This classical Gibson reaction turned out to be rather ineffective for the cloning of large high GC content DNA fragments. The high GC overlap-ends on the linearized vector self-ligated with a rate of 80% [99]. To solve this problem, two universal terminal single-stranded overhangs with high AT contents are added to the ends of the vector. This does not only decrease the level of self-ligation to 45%, but it also makes the modified vector suitable for repeated use in future assemblies. Additionally, the introduction of two restriction enzyme sites (NdeI/NheI) into the respective side of the overhangs facilitates the hierarchical and seamless assembly of large DNA molecules. With those improvements, the assembly efficiency of the PII (pristinamycin II) BGC from Streptomyces pristinaespiralis HCCB10218 was increased from 2.5% (using the classical protocol) to 20%–40% (using the modified version) [99].
2.2. Introduction of Genetic Constructs into the Host: Delivery and Engineering Tools
The introduction of constructs for genetic manipulation of actinomycetes requires robust vehicles and methods for effective transfer and intracellular maintenance of the introduced material. The most commonly used vehicles for genetic engineering of actinomycetes are replicative and integrative vectors. Less frequently, “jumping genetic elements”, such as transposons, are applied [85]. The main difference between the replicative and integrative plasmids is the fact that replicative plasmids contain origins of replication and are able to multiply in the host cell (low-, mid and high copy number plasmids), while integrative plasmids harbor integrases and sequences which facilitate their integration into the actinomycete′s chromosome (integrative element-based plasmids, sequence homology-based plasmids). In contrast to integrative plasmids which integrate into the chromosome at certain position (via att-sites, actinomycetes integrative and conjugative elements (AICE) [100] or via homologous fragments flanking the target region), transposons can “jump” into the chromosome at different sites. Therefore, transposons were often exploited for “random” mutagenesis [101]. Replicative and att-site-based integrative plasmids are frequently applied for gene (over)expression, while plasmids containing homology fragments serve for homologous recombination and are mostly used for targeted gene inactivation [74].
In addition to the origin of replication and the integration function, the molecular vehicles contain other genetic parts that support the cloning, ensure the functionality of the constructs and the genetic engineering purpose as well as the selection and identification of potential mutant clones (Figure 2). Those genetic parts include the multiple cloning site (MCS), constitutive or inducible promoters, RBSs, transcriptional terminators, selection markers and/or reporter systems [85]. In the past, usually single genetic parts were introduced or exchanged in plasmids or other vehicles to build new constructions. Using synthetic biology this concept was recently advanced in order to combine several genetic parts (also called modules) at once (“plug-and-play”) (Section 2.2.2) [80].
Most of the molecular vehicles were originally applied for genetic manipulation of streptomycetes and over time expanded to other actinomycetes [52,102]. The commonly used replicative and integrative vectors, transposons as well as the standard methods for their introduction into actinomycetes (transformation, conjugation) were described in numerous original publications and recent reviews [74,85,103,104,105,106,107,108,109]. However, in the past five years (2015–2020) several new or modified genetic parts (e.g., promoters, RBS and other regulatory elements), engineered replicative as well as integrative systems, and CRISPR/Cas-based devices were added to the “genetic toolbox” for refactoring the genomes of Actinomycetales strains.
2.2.1. Genetic Parts and Other Regulatory Elements for Engineering of Actinomycetes Genomes
To facilitate an efficient transcription/translation of sequences (e.g., gene (over)expression/protein production) in actinomycetes, diverse constitutive and inducible systems are applied. The most known and used constitutive systems are ermE*p [110], SF14*p [111], kasO*p [112]. Dynamic regulation of gene or pathway expression can be achieved by applying metabolite-responsive promoters such as the thiostrepton-(tipA*p) [113] or ε-caprolactam inducible promoter (nitA*p) [114], quorum sensing (operator/repressor) systems [115,116] (e.g., tetO–TetR [117], gylR and gylP1/P2 [85,118]), pleiotropic/pathway-specific regulators (e.g., SARP family, TetR family) [119,120,121,122], and protein/RNA-based biosensors (e.g., TetR-based biosensors) [123,124,125,126,127,128]. Some of these concepts were initially developed in E. coli and/or Saccharomyces cerevisiae and later adapted to actinomycetes. For details, interested readers are referred to recently published reviews [72,74,81,129,130,131,132].
In following, we provide the reader with numerous examples of new and updated systems for engineering of actinomycetes.
Newly Identified, Synthetic and Modified Promoters and Other Genetic Regulatory Elements for Construction of Expression Plasmids
The use of diverse technologies including omics (RNA-seq, Ribo-seq and TSSseq), reporter assays and other methods resulted in the development of workflows for screening and/or designing of transcription- and translation-relevant sequences (e.g., promoter-, Shine-Dalgarno- (or RBS), terminator regions) [133]. These may have the potential to be applied in engineering of a wide range of actinomycetes. In addition, the already established systems (e.g., Streptomyces lividans strains containing glucuronidase (GusA)) were utilized for diverse modifications (e.g., variation of the distance between the Shine-Dalgarno (SD) domain and the start codon [134]) resulting in refactored as well as new synthetic promoters [135,136,137,138,139]. Their features and examples of successful applications for engineering of actinomycetes genomes were presented in several publications [72,74,129,131,134,140,141,142,143].
Recently, Luo et al. used the data obtained from RNA-seq analysis and identified 32 promoters in Streptomyces albus J1074. The promoters were cloned and further characterized in a xylE-based reporter assay and qPCRs [144]. The analysis of the enzymatic activity and transcription levels demonstrated that in the pool of 32 promoters, there are 10 strong and 4 constitutive promoters including housekeeping and heat/cold shock gene promoters. For the 10 strong promoters a 2 to 10-fold increase of gene expression levels was observed [144], when compared to the expression driven by the ermE*p. Furthermore, the authors selected five out of the 10 strong promoters and used them for activation of the polycyclic tetramate macrolactam (PTM) gene cluster from Streptomyces griseus in heterologous systems (S. lividans 66, S. albus J1074, and S. coelicolor M1146). In contrast to the native strain S. griseus, heterologous hosts harboring the refactored gene cluster produced the PTM and the production yields were increased [144] compared to a previously described F60 construction [145]. These results suggest that the identified promoters may be widely applicable in synthetic biology platforms for activation of silent natural product biosynthetic pathways and characterization and/or optimization of already expressed BGCs in actinomycetes strains.
Similar study of genome wide screening and evaluation of promoters using transcriptome microarray data was conducted for S. coelicolor M145 [146]. A total of 8 out of 941 identified promoters were validated by a green fluorescent protein (GFP) reporter and real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR) in S. coelicolor, Streptomyces venezuelae, and S. albus and confirmed to drive a stable gene expression. Furthermore, four promoters were subjected to a “plug-and-play” platform to control the expression of the cryptic cluster of jadomycin B in S. venezuelae ISP5230. This led to different levels of the production of jadomycin B that corresponded to promoter strength [146]. Such microarray- and eGFP reporter assays-based approaches for identification of constitutive promoters were also successful for the industrial producer of natamycin S. chattanoogensis L10 [69,147]. The study resulted in the detection of the thlM4p promoter. The expression levels of genes under the thlM4p were sevenfold higher compared to the levels obtained with ermE*p. Application of the thlM4p promoter in an engineering experiment resulted in an increase of 30% of natamycin product yields compared to the same construction harboring ermE*p instead of thlM4p [147]. The same research group identified another strong promoter (groESp) in S. chattanoogensis L10 by using a proteomics-based approach [148].
The well-known GusA-reporter system was utilized for the same purpose of promoter identification in Actinoplanes sp. SE50/110, the natural producer of acarbose. RT-qPCR and mapping of the transcription starts allowed the identification of promoters with medium to strong expression [149].
An interesting approach was reported by Li et al. [150]. The authors used known inducible promoters for identification of the most optimal conditions for BGC expression in S. coelicolor (M145-OA) and S. venezuelae (Sv-Potr). Optimal induction conditions were determined by a response surface model, and, finally, inducible native promoters were identified by transcriptional analysis. Native promoters, which have shown a similar transcription profile to the inducible promoter under the optimal condition, were utilized to replace the inducible promoter following an elaborate replacement approach. With this strategy, it was possible to improve the titers of ACT and oxytetracycline (OTC).
The promoter kasO*p belongs to strong constitutive promoters. Bai et al. used the promoter region as a “template” and randomized the nucleotides downstream of the −10 sequences of kasO*p (library 1) as well as modified the spacer sequence between the −10 and −35 regions of kasO*p (library 2) [151]. Thereby, a set of 180 promoters was generated. Six (five from library 1 and one from library 2) synthetic promoters have shown a stronger activity than the original promoter (kasO*p). Furthermore, the activities of the synthetic promoters varied from 0.95% to 187.5%, and, therefore, the gradient strength of some of the promoters was tested in a proof-of-principle approach using the cryptic lycopene cluster. The functionality of the promoters could be confirmed in Streptomyces, as the lycopene BGC was activated and the compound was produced [151].
Zhao et al., used the heterologous σ70hrdB-dependent promoters, previously described as inefficient tools for gene expression in Streptomyces, and fused them with optimized 5′-untranslated regions (5′-UTRs) [152]. After modification of these systems (e.g., core promoter region of tac*p from E. coli was fused with the 5′-UTRR15 from kasOR15*p yielding tac*p), the σ70 -dependent promoters were efficiently recognized by Streptomyces housekeeping factor σhrdB. In addition, the 5′-UTRR15 was optimized by randomizing the RBS and rational design of artificial RBSs gaining tacRBS3*p, of which activity was tested for squalene expression. The tacRBS3*p promoter have shown to be 2.1, 3.6 and 17.6 times more active than tac*p, kasOR15*p, and tac*p, respectively [152].
Ji et al. constructed a library of highly randomized, synthetic regulatory sequences, which varied in the sequence of the constitutive promoter as well as in the RBS region [137]. A NRPS responsible for the production of the blue pigment indigoidine was used as rapid screening assay of a large pool of the regulatory sequences. Based on the outcome of this study, the strength of the regulatory sequences was classified into the strong, medium and weak. For example, such a pool of synthetic regulatory sequences with different strengths is of advantage whenever a fine-tuning of the gene expression is required in order to achieve the maximal production yields of a compound. The utility of the synthetic regulatory sequences for promoter engineering of natural product BGCs has been shown for instance for the actinorhodin BGC [137].
In contrast to constitutive systems, inducible systems enable a controlled gene or pathway expression. Recently, such synthetic inducible regulatory systems were optimized for modulation of secondary metabolite production in Streptomyces [138]. The authors of the study employed the modular design concept [83] to build a high performance synthetic inducible regulatory system. The inducible regulatory system consisted of three separate functional genetic modules: (1) the induction module, (2) the target expression module and (3) the repressor expression module. Already known and well-characterized repressor-based induction modules for streptomycetes, including anhydrotetracycline (TET, TetR/tetO), oxytetracycline (OTR, OtrR/otrO), cumate (CMT, CymR/cmtO) and resorcinol (ROL, RolR/rolO) [117,153,154], were selected for this approach [138]. Each of these induction modules were combined with the SF14RS repressor expression- and the kasO*RS target expression module. In total, four inducible regulatory cassettes (IRCs) were designed and stepwise optimized by testing their performance in heterologous expression of the indigoidine NRPS and the actinorhodin type II PKS genes using S. albus J1074. The cumate-based synthetic inducible regulatory system has shown a large dynamic range (66.4× fold increase). Such inducible systems or their parts can be inserted into various plasmids to enable tight control of metabolite production in actinomycetes. This was for example shown for the PnitA-NitR [114] construction that resulted in a set of inducible Streptomyces-E. coli shuttle vectors [155].
Riboswitches for Biosensors
Another way to regulate gene expression is to use natural or modified riboswitches [156,157]. Riboswitches are non-protein coding RNAs that regulate diverse cellular processes, including transcription and translation. These regulatory parts at the 5′-region of the mRNA control the expression through allosteric alterations of the structure. The detailed regulation via riboswitches is described in other reviews [157,158]. As riboswitches are selective and specific to different ligands, they were exploited for the development of biosensors and became an important tool of synthetic biology.
In general, biosensors are composed of three units: (I) a signal input module (transcription factors (TFs) or riboswitches), (II) a regulatory module (TF-dependent promoters) (III) and a signal output module (reporter genes) [81,159]. In 2015, a protocol for application of the synthetic theophylline-dependent riboswitch [160,161] in S. coelicolor was published. Only 85 nt are need to be inserted between the start site of transcription and the start codon of the target gene to successfully control gene expression [156]. Additionally, in 2016 a novel modular dual control system was developed for coupled gene regulation at transcriptional and translational levels at the same time [162]. This “innovation” is based on the theophylline riboswitch [161] and the resorcinol and cumate-inducible system [153]. The system is applied to facilitate highly inducible control and complete silencing in the absence of both inputs. The dual control system is specifically designed for Streptomyces and Actinobacteria and resembles the first example of completely tight systems reported for Actinobacteria [162].
More recently, an antibiotic-specific whole-cell biosensor was developed for screening and optimization of antibiotic producers [128]. The system was derived from the TetR transcriptional repressor and successfully used to improve production of the polyketide antibiotic pamamycin. The optimization of the biosensor by alterations of the promoter and operator of output module and the ligand affinity of transcription factor module resulted in promising outcomes. Possibly, this and additional biosensors will be applied for generation of new actinomycetes cell factories to enable a more efficient manufacturing of bioactive compounds.
2.2.2. Integrative and Replicative Expression Systems for Actinomycetes
Most of the integrative vectors which are used in genetic engineering of actinomycetes involve site-specific DNA recombination systems derived from phages (ΦC31 [163], ΦBT1 [164], VWB [165], TG1 [166], SV1 [167], R4 [168]) or from the integrative plasmid pSAM2 (λ-integrase) [169].
Vectors, which harbor the ΦBT1 site, integrate at the unique attB site that is localized in the SCO4848 gene (S. coelicolor genome) or into its orthologues in other streptomycetes [164]. The integration of a plasmid at this position into streptomycetes genomes obviously inactivates the gene SCO4849 which is co-transcribed with SCO4848. This may affect the cell differentiation and result in delayed spore germination that can be complemented by expression of SCO4849. To avoid this polar effect, Gonzalez-Quiñonez et al. modified the ΦBT1 integrative vector pMS82 [164] by introducing a copy of SCO4849 under the control of the promoter region of SCO4848. This resulted in the construction of the plasmids pNG1-4 (NG1 contains SCO4848*p + SCO4849, pNG2 contains SCO4848*p + SCO4849 and ermE*p + RBS + MCS + fd-ter, pNG3 contains SCO4848*p + SCO4849 and bla (resistance to ampicillin), and pNG4 contains SCO4848*p + SCO4849, ermE*p + RBS + MCS + fd-ter and bla). In two of these plasmids (pNG2 and pNG4), a copy of the ermE*p promoter was cloned for ensuring gene overexpression. As pNG3 and pNG4 contain the bla gene (ampicillin resistance), selection in E. coli is possible. The introduction of the system into mutants of S. griseus, S. lividans and S. clavuligerus carrying plasmid integration in SCO4849 or in its orthologues resulted in a restored phenotype. The advantage of using pNG1-4 plasmids is that the integrative vectors do not affect the cell differentiation and lead to a neutral phenotype in streptomycetes. The approach can be transferred to other integrative vectors which cause similar effects. Another solution for avoiding such an undesired polar effect due to integrations of plasmids at certain positions into the genome of actinomycetes, might be the use of alternative integration sites. In 2017, Fogg et al. described a new Streptomyces phage, ΦJoe [170]. The actinophage encodes a serine integrase and belongs to the largest Streptomyces phage cluster (R4-like). The int-attP locus of ΦJoe was cloned into pSET152 [108] by removing the ΦC31 int-attP part and inserting the sequence of the newly identified system (int-attP locus of ΦJoe). This resulted in the final plasmid pCMF92. The activity of the ΦJoe system was demonstrated in in vitro recombination assays and in vivo, in Streptomyces (e.g., S. venezuelae) and E. coli. The plasmid pCMF92 is now available for use in Streptomyces species and might be expanded to other actinomycetes in the future.
Similar to pCMF92, the E. coli–Streptomyces shuttle vector pDYN6902 was derived from pSET152 [108,171,172]. The integrative expression vector contains the original thiostrepton-inducible tipA*p and the E. coli low copy number replication control of the F (fertility) factor. The copy-number of this vector is 12 times lower (one or two copies per cell) compared to that of pIJ6902, which is of advantage for cloning and expression of toxic genes. The functionality of pDYN6902 was confirmed in Streptomyces ambofaciens (ATCC23877 and DSM40697) and in S. coelicolor A3(2).
Since 2015, several vectors were developed using the concept of modular construct assembly (also called “plug-and-play” strategy). For example, SEVA (Standard European Vector Architecture) is a web-based resource [173] which was developed for easier construction of optimal plasmids based on a fixed architecture [174,175]. The SEVA database allows for the generation of a non-ambiguous nomenclature and indexes for a repository of functional sequences and final constructs. Originally, the system was established for the deployment of complex prokaryotic phenotypes in Gram-negative bacteria, such as Escherichia or Pseudomonas. The SEVA users are provided with a protocol to design the desired plasmid by choosing three functional cassettes named modules: origins of replication, antibiotic selection markers and a variety of cargoes with different applications. The backbones of the vector and the modules were minimized and edited to remove nonessential sequences and at the same time retain their functionality. Furthermore, the modules are flanked by uncommon restriction sites that enable the users to interchange the cassette within the respective module for the assembly of the most optimal construct. Very recently, the SEVA principle was utilized for cloning of 23 novel shuttle vectors to expand the genetic toolbox for Streptomyces and other actinomycetes [176]. Because the ORI module of this collection of integrative (attP integration system), low-copy number (SCP2* replication origin) and medium-to-high-copy number (pIJ101 replication origin) vectors contains an origin of replication for Gram-negative bacteria and streptomycetes, the plasmids can be used in both groups of organisms. In addition, the maker module was varied to gain vectors with different resistance markers (apramycin, chloramphenicol, kanamycin and/or streptomycin). The functionality of these constructions was confirmed in heterologous systems by successful expression of GFP and the production of the plant flavonoid apigenin in S. albus J1074 [176].
Another example of modularly assembled, standardized vectors which were developed for refactoring of BGCs in actinomycetes, such as Streptomyces species, are the systems reported by Aubry et al. [80]. A set of twelve constructs (pOSV801-pOSV812) provides combinations of different resistance cassettes and four orthogonal integration sites. The inserted FLP (flippase) recombination (site-specific recombination) [177] target sites are enabling the recycling of the antibiotic resistance marker and thus, they reduce the risk of undesired homologous recombination in Streptomyces strains whenever additional vectors are used. Due to the modular “architecture” and the presence of compatible part, the modules can be easily exchanged. The integrative plasmids (pOSV801-pOSV812) contain 5 variable modules. Module 1 contains the E. coli p15A origin of replication and one of the FRT (flippase recognition target) sites [177] for the Flp recombinase mediated excision. The second module (module 2) represents the antibiotic resistance marker (hygromycin-, apramycin- or kanamycin resistance gene). Module 3 contains the second FRT site as well as the origin of transfer. In Module 4 the integration cassette (ϕBT1, ϕC31, pSAM2, VWB) is located. Finally, the last module (module 5) is the cloning module which enables an iterative assembly of genes (or sequences) using the BioBrick approach (Section 2.1) [82]. The functionality of the 12 vectors was verified by their integration into the chromosome of S. coelicolor M145, S. lividans TK23, and S. albus J1074. For example, using pOSV802 and the BioBrick method, the albonoursin gene cluster from Streptomyces noursei was cloned and introduced into S. coelicolor M145 by intergeneric conjugation. The obtained mutant was confirmed to produce albonoursin, which demonstrated the applicability of the system for engineering of actinomycetes.
Taken together, the modular concept (e.g., SEVA) and the recently assembled shuttle vectors as well as the public availability of sequences for exchanging the modules within the vector are of advantage for semi high-throughput generation of plasmids for engineering of actinomycetes.
Multiplex Integration Systems for Site-Specific Genome Engineering
In addition to the classical integrative vectors which involve one type of attachment/integration site, a couple of systems for multiplex integration into the host´s chromosome were established.
The multiplexed site-specific genome engineering strategy (MSGE) [178] is based on the “one integrase-multiple attB sites” concept. It enables a stable integration of multiple copies of target BGCs into one chromosome in a single step. Therefore, additional artificial attB sites have to be introduced into the genome of the host strain. The introduction of the additional attB sites can be accomplished by using CRISPR/Cas9 editing tools (e.g., CRISPR/Cas9 described by Huang et al. [179]) (Section 2.2.4). Such a modified strain is then used to introduce multiple copies the target BGCs via conjugation, which leads to multiplex integration of the BGC into the native and artificial attB sites. Using this strategy, a S. pristinaespiralis-expression strain with five additional copies of the pristinamycin II (PII) BGC was created. The fermentation of this strain resulted in the highest titres reported to that date [178]. In 2019 an updated “plug-and-play” toolkit, specifically designed for actinomycetes, was published (advanced MSGE (aMSGE)) [180]. It enables high-efficient, multi-locus integration of BGCs into heterologous expression hosts. The tool consists of 27 synthetic plasmids with either a single or multiple integration sites (up to four). The toolkit facilitates the integration of BGCs, shortening it to a single step by allowing the ligation of several integration modules into plasmids through Gibson cloning and simultaneously insertion using different native attB sites of the strain. The aMSGE was successfully applied in S. coelicolor and Streptomyces hygroscopicus [180].
Similar systems for multiplex integration of genetic constructs were presented by Phelan et al., [181]. The authors constructed three standardized, orthogonal integration vectors (pAV (apramycinR, attPVWB), pSC (spectinomycinR, attPΦC31) and pTB (thiostreptonR/beta lactamR, attPΦBT1) as a basis for simultaneous integration at all three attB sites present in the genome. Each of the vectors harbors an origin of replication (ori pMB1), selection marker (apramycin-, spectinomycin- or thiostrepton resistance gene), RP4 origin of transfer, an attP site, a gene encoding an integrase (VWB, ΦC31 or ΦBT1), identical 5′- and 3′-UTRs flanking the promoter with a RBS and the target gene for expression as well as unique restriction sites. The promoters included the ermE*p, gapdh(EL)*p and rpsL(RO)*p native promoters which were recently identified from S. albus [144], and synthetic promoters. In total, 45 derivatives were cloned using the basic vectors pAV, pSC and pTB. These expression systems were exploited for the characterization of heterologous promoters and various attB chromosomal integration sites for protein expression in S. venezuelae. This approach resulted in the identification of 15 promoters that possess relative strengths that vary over three orders of magnitude [181]. Moreover, the authors observed differences in protein expression depending on the site of chromosomal integration of the expression plasmid.
A similar strategy of multiplex integration to this, proposed by Phelan et al. [181], was reported by Ko et al. [182]. The integration functions of the two closely related actinophages, ΦOZJ and ΦWTR [182] were investigated. It was shown that plasmids containing the ΦOZJ attP-int integrated into genomes of a pool of selected actinomycetes, including biotechnologically relevant strains [182]. Based on this outcome, a complementary set of plasmids with compatible E.coli replicons, resistance determinants and orthogonal ΦC31, ΦBT1 or ΦOZJ integrating functions was constructed. The resulting plasmids pJMD5, pJMD13 and pJMD14, respectively, were transferred simultaneously into S. venezuelae, Streptomyces roseosporus and S. pristinaespiralis in a single conjugation experiment [182].
As the tools for multiplex integration simplify the introduction of multiple genes or operons into a host´s genome and seem to facilitate a more predictable product biosynthesis, their application may contribute to less-time consuming engineering of a broad range of streptomycetes and possibly other actinomycetes.
2.2.3. Transposon- and Homologous Recombination-Based Systems for Actinomycetes Engineering
In the past, transposons (e.g., Tn5) [183,184,185], homologous recombination [85,186,187,188,189,190] and site-specific recombination based on Cre/loxP, Dre/rox [101,191] as well as I-SceI [192,193] systems were extensively used to generate mutants in actinomycetes strains [41,101,194,195,196,197,198,199,200,201].
Transposon-based genome mutagenesis is mainly applied for systematic genetic studies of microorganisms [202,203]. However, the first Streptomyces transposons derived from Tn5, or IS493 were sub-optimal in terms of their transposition frequency and/or non-random mutations [85,129]. Later, a codon-optimized, hyperactive Tn5-based transposition system for in vivo random mutagenesis of Streptomyces genomes was developed [195,204,205]. It was demonstrated that the hyperactive transposase-based Tn5 transposition system integrates more randomly into the genome of S. coelicolor. This system was utilized to investigate the regulation of prodiginine in S. coelicolor [205].
The engineering of some of the non-Streptomyces species belonging to the order Actinomycetales (e.g., Planobispora sp., Kibdelosporangium sp., Amycolatopsis sp.) remains difficult as many of the established methods and tools fail in genetic manipulation of these strains (Figure 1). A new suicide vector p6SUI5ERPSL was constructed and optimized by Meyer et al. [206] to facilitate the deletion of genes in Amycolatopsis sp. The backbone of p6SUI5ERPSL includes an apramycin resistance gene (aacC), pBR origin of replication for E. coli (oriV) and the erythromycin promoter (ermE*p) upstream of the gene coding for 30S ribosomal subunit protein S12 from Saccharopolyspora erythraea (rpsL). RpsL confers sensitivity to streptomycin to clones which contain the p6SUI5ERPSL construct. This system can only be applied in hosts that are resistant to streptomycin due to mutations of the rpsL gene [207]. As Amycolatopsis sp. ATCC 39,116 is sensitive streptomycin, resistant mutants were generated, isolated and used to introduce constructs based on p6SUI5ERPSL, which contained flanking region for homologous recombination in Amycolatopsis ATCC 39116. With this strategy, three gene clusters (aldehyde oxidase (yagRST), vanillic acid decarboxylase (vdcBCD) and vanillate demethylase (vanAB)) were deleted in streptomycin resistant Amycolatopsis ATCC 39,116 derivatives. Therefore, the p6SUI5ERPSL system might serve as foundation for genetic modification of difficult to manipulate actinomycetes.
The I-SceI endonuclease [200] and the Cre recombinases [101,191] have been used to achieve large deletions in actinomycetes genomes.
I-SceI recognizes a rare 18-bp sequence (TAGGGATAACAGGGTAAT) and introduces double-strand breaks (DSBs) at this position. Such DSBs promote double-crossover recombination events. As double stranded breakage of the chromosome is lethal, only those cells of which genomes undergo homologous recombination can survive. So far, I-SceI recognition sites were not found in genomes of known and analyzed streptomycetes. Thus, I-SceI homing meganuclease and its recognition sequence can be exploited for genome editing in streptomycetes. Systems containing a codon-optimized I-SceI gene were developed for this purpose and successfully applied in S. coelicolor [200,208]. For instance, Fernández-Martínez and Bibb constructed the plasmid pIJ12738 plasmid which contained the recognition site for I-SceI and regions flanking the target sequence that was supposed to be deleted in the chromosome of S. coelicolor M1141. The plasmid was transferred into the strain, and the generated mutant clones were used as recipient for the delivery of the vectors pIJ12739 and pIJ12742, which harbor a codon optimized I-SceI meganuclease gene for expression in actinomycetes. Due to the activity of the I-SceI meganuclease and homologous recombination, the exconjugants revert to wild type or recombine their genomes to the engineered genotype. To demonstrate the functionality of the system, parts of the BGC of the undecylprodiginine complex of compounds were deleted in Streptomyces coelicolor M1141. Approximately half of the obtained clones possessed the desired marker-less genotype [208].
Recently also the Cre/loxP system was utilized for excision of a relatively large genomic region. In this case, 1.3-Mb and 0.7-Mb were deleted in the natamycin producer strain Streptomyces chattanoogensis L10 [69]. Two derivatives, S. chattanoogensis L320 and L321, were obtained and proposed for the developed of chassis for efficient polyketide production.
2.2.4. CRISPR/Cas–Based Editing Tools for Actinomycetes
Since its identification as clustered regularly short palindromic repeats (CRISPR) in 2002 [209] and the discovery of its defense functions against phages [210], the system has been used as a precise genetic manipulation tool in a multitude of organisms [211,212,213,214,215]. The CRISPR defense is based on “cooperation” of CRISPR arrays and CRISPR associated (Cas) genes [216,217], which can be split into three major stages: the adaptation, expression and interference stage. The whole CRISPR/Cas mechanism has been reviewed in detail previously [218,219,220]. For the application as editing tool, only the interference stage is utilized. Required for target cleavage are only the Cas9 nuclease (in most cases derived from the type II CRISPR/Cas system from Streptococcus pyogenes), a CRISPR RNA (crRNA) and a trans-activating crRNA (tracrRNA). The crRNA is a short RNA, which is encoded as spacer in the CRISPR array and matches a previously encountered foreign nucleic acid. The tracrRNA is also a short RNA which helps with the processing of the crRNA and its recruitment to Cas9. The Cas9/crRNA/tracrRNA complex can introduce DSBs at any sequence that matches the crRNA and contains a recognition motif, called protospacer adjacent motif (PAM). When applied as editing tool, the gene regions for crRNA and tracrRNA can be fused together to build one single synthetic guide RNA (sgRNA) [221]. For the cleavage of any site of choice, only a sgRNA targeting the desired locus must be designed. The specific target cleavage can either be applied in vivo or in vitro. After target cleavage in in vivo systems, the DBS are either repaired by homology directed repair (HDR) (in cases in which a homologous template DNA is available) or by non-homologous end joining (NHEJ) (in cases in which no template is present). By simultaneous introduction of a homologous template DNA, precise genome modifications can be achieved. The biggest advantage of the CRISPR/Cas technology is the fact that basically any site of interest can be targeted without laborious requirements. However, disadvantages like off-target effects [222] and Cas9 toxicity [223,224,225] must be considered.
CRISPR/Cas9-Mediated Genome Editing Using pCRISPomyces
The pCRISPomyces was designed for precise Streptomyces engineering, presenting the possibility of multiplex genome editing by chromosomal deletions [223]. The tool includes a codon optimized cas9 (from S. pyogenes) on a plasmid along with a tracrRNA and CRISPR array expression cassette in case of pCRISPomyces-1 or a sgRNA expression cassette for pCRISPomyces-2. Both versions of the plasmid contain several useful features such as a BbsI-flanked lacZ cassette for Golden Gate assembly of the spacer sequences, an XbaI site for addition of other elements such as editing templates for recombination-driven repair via Gibson assembly or traditional ligation, the colE1 origin for replication in E. coli and a RP4 origin for conjugational transfer as well as a temperature sensitive pSG5 rep region [226] for rapid clearance of the plasmid. Along with the target specific 15 bp protospacer sequence (3 bp PAM with 12 bp additional unique protospacer bases) a 2 kb editing template consisting of two homologous arms corresponding to the up- and downstream sequences of the target gene can be introduced into the plasmid. This feature prevents the tool from off-target effects and ensures precise deletion of the target sequence in the genome. Multiplex targeting is possible by addition of two different sgRNA cassettes. Using this method, an efficiency of 70%–100% for targeted chromosomal deletions between 20 bp and 30 kb in three different Streptomyces species was achieved. Furthermore, significantly higher editing efficiencies were observed using sgRNA targeting (pCRISPomyces-2) compared to tracrRNA/crRNA targeting (pCRISPomyces-1) [223]. There are several examples of application of the CRISPR/Cas9 tool in diverse strains, including Actinoplanes sp. [227] and a new Streptomyces species Streptomyces formicae [228].
CRISPR/Cas9-Mediated Genome Editing Using pKCcas9dO
pKCcas9dO is a tool for one-step CRISPR/Cas9-mediated genome editing. It was developed almost at the same time as the one published by Cobb et al., which was described above. Similar to the other toolkit, this high-efficiency editing system is also based on a pSG5 temperature sensitive plasmid harboring the thiostrepton-inducible promoter tipA and a codon optimized cas9 from S. pyogenes along with two homology-directed repair templates corresponding to the flanking regions of the target sequence and a target specific sgRNA [179]. The system was used by Huang et al. to create single gene deletions as well as whole BGC deletions of up to 82.8 kb in S. coelicolor with an efficiency of 60%–100%. Multiplex BGC deletion was carried out with an efficiency of 45%–54% and even single point mutations could be introduced with an efficiency of 64%. This expands the CRISPR/Cas9-mediated genome editing abilities to deletion of nearly 83 kb-BGCs as well as the precise introduction of single point mutations. Another successful example for application of this CRISPR/Cas9 system is the MSGE-based engineering of S. coelicolor and S. pristinaespiralis [178] (Section 2.2.2).
CRISPR/Cas9-Mediated Genome Editing Using pCRISPR-Cas9
Almost simultaneous to the two tools mentioned before, a third CRISPR/Cas9 toolkit was delivered in 2015 for precise and efficient genetic manipulation. Similar to pKCcas9dO [179], the pCRISPR-Cas9 vector [229], that is presented here, contains the tipA promoter and a codon-optimized cas9 accompanied by a designable sgRNA, which is inserted into NcoI and SnaBI restriction sites for easy exchange. An additional restriction site (StuI) is available for insertion of other elements such as homologous recombination templates. The vector is also based on a temperature-sensitive plasmid [230], which enables easy curing after the induction of Cas9 and the genetic engineering. This is another example of a CRISPR-Cas9 device that simplifies the genetic manipulation of high GC actinomycetes and provides an alternative to the time expensive introduction of single- and double cross over events in actinomycetes. The inactivation of two genes in S. coelicolor was possible without a homology-directed repair template, using the NHEJ pathway. However, low efficiencies ranging from 3% to 54%, depending on the used sgRNA, were observed. By optimization of the intrinsic NHEJ pathway via the co-expression of a DNA ligase, the generation of random-sized deletions around a precisely defined target position was achieved with an increased efficiency of 69%–77%. The addition of a homologous recombination template to the vector increased the deletion efficiency to nearly 100% [229]. Using this method, the sceliphrolactam BGC from a newly isolated Streptomyces sp. strain was identified [231]. Furthermore, the tool supported the elucidation of the dynemicin enediyne and anthraquinone pathway in Micromonospora chersina [232].
In addition, a system for reversible control of the gene expression of target genes was invented. This was named CRISPRi and stands for a CRISPR/dCas9-based interference system. As the name suggests, it is based on a catalytic dead version of Cas9 (dCas9) and uses a sgRNA targeting the promoter region. The dCas9 forms a roadblock on the DNA by binding to the promoter region without cutting the DNA, which can be easily reversed by inducing the loss of the pCRISPR-dCas9 plasmid [229].
CRISPR/dCas9-Mediated Multiplex Gene Repression
Further development on the CRISPR/dCas9 systems was performed by Zhao et al. to enable multiplex gene repression in the model strain S. coelicolor [233]. The integrative plasmid pSET152 with an encoded codon optimized dCas9 and sgRNA, both under the control of constitutive promotors (j23119 and ermE*p), was designed. After the integration of the plasmid into the genome, the dCas9 and sgRNA form a complex, bind the target site and stay there as roadblock unable to insert DSBs. This causes the repression of the gene expression. As the expression plasmid is integrated into the chromosome, this CRISPRi system is more stable compared to the temperature-sensitive pSG5-based system used before. The transformation efficiency with the pSET152-based plasmid backbone is also higher compared to pSG5-based plasmid transformations. By the insertion of up to four sgRNAs in the CRISPRi system, it ensures multiplex precise gene repression with the introduction of only one plasmid construct. This leads to a 2%–32% reduction of the target genes mRNA levels compared to the control. However, the repression efficiency of target genes becomes weaker when four genes are repressed at the same time compared to the repression efficiency of repressing just a single gene at a time. Using this method, functional gene screening in S. coelicolor was performed, which led to the identification of an orphan response regulator involved in bacterial growth [233].
CRISPR/Cas Base Editing System (CRISPR-BEST)
The CRISPR/Cas Base Editing SysTem (CRISPR-BEST) [234] comprises an adenosine (CRISPR-aBEST) and a cytidine (CRISPR-cBEST) deaminase-based base editor. CRISPR-aBEST can specifically convert an A:T base pair to a G:C base pair and the CRISPR-cBEST can convert a C:G base pair to a T:A within a target window of approximately 6–7 nucleotides. This technique is completely DBS-free and uses a Cas9 nickase (Cas9n) and sgRNA complex as delivery system for the deaminases to the target region. The base editing enables the introduction of stop-codons or loss-of-function mutations, without the formation of DSBs, which must be repaired in a certain way. Unbiased, genome-wide off-target evaluation showed a low level of off-target effects, which enables the application for broad genome editing studies. To expand the application of CRISPR-BEST, a Cys4-based sgRNA multiplexing system was also developed [234], which uses the type I-F Cas endoribonuclease Cas6 [235], also known as Cys4, instead of Cas9. Cys4 can self-process the sgRNA array and enables the expression of multiple sgRNAs with one single promotor and terminator, only separated by the Cys4-recognition sites. This is advantageous compared to approaches where each sgRNA needs an individual and independent transcription cassette for multiplex editing. CRISPR-BEST editing was successfully applied in S. coelicolor, Streptomyces griseofuscus and S. collinus [234]. Additionally, the CRISPy-web tool [236] was updated for identification and design of sgRNAs with regards to the application for CRISPR-BEST.
CRISPR/Cas9-CodA(sm) Combined System
In this approach, a CRISPR/Cas9 system is combined with a counter selective CodA(sm) [237] system. The D314A mutant of cytosine deaminase (CodA) converts supplemented 5′-flurocytosine (5FC) into 5′-flurouracil (5FU) which is highly toxic. This counter selective marker for Streptomyces and other bacteria was already reported in 2009 [237]. The delivery plasmid contains the codon-optimized cas9 (from S. pyogenes), a target-specific sgRNA, codA(sm) and aac(3)IV resistance cassette for selection as well as homologous sequences to the flanking regions of the target sequence. The plasmid is introduced in the actinobacteria through conjugation. Next, DSBs are created by the sgRNA-Cas9 co-activity. The genome is then repaired via homologous recombination using the provided sequences on the plasmid. The mutant strains which have spontaneously lost the delivery plasmid can then be selected using the CodA(sm) system, resulting in unmarked mutant strains. This allows the reuse of the same delivery vector for successive gene replacements. When the system was applied in S. coelicolor for editing the actinorhodin polyketide chain length factor gene, 99% of the tested plasmid-free S. coelicolor exconjugants were correct mutants [238]. This method was also applied in Streptomyces fradiae for manipulation of the neomycin BGC [239].
CRISPR/Cas9 Knock-In Strategy
In this approach, the strategic knock-in of promoters is conducted to activate silent BGCs in Streptomyces. This one-step strategy uses the pCRISPomyces-2 plasmid [223] to strategically replace the native promoter regions of main biosynthetic operons or pathway-specific activators with strong, constitutive promoters (kasO*p) which work in several Streptomyces species. Using this strategy, the activation of three previously uncharacterized BGCs in S. roseosporus, S. venezuelae and Streptomyces viridochromogenes was possible [240].
Generic CRISPR/Cas9 Approach Using the Same sgRNA for Editing
Editing of multicopy genes and mobile genetic elements, which have highly similar and repetitive DNA sequences might be very challenging in actinomycetes. A generic two-step approach using CRISPR/Cas9 was designed to specifically target one copy of a multicopy gene of interest [241]. Therefore, a non-replicative plasmid, containing “bait DNA” and flanking homologous regions of the target gene is integrated into the genome via recombination. Subsequently, the “bait DNA” is targeted using a generic sgRNA, resulting in a DSB near to the target gene. The repair of the DSBs leads to either reversion or gene copy-specific editing. Using this strategy, the deletion of the native copies of senogeneic silencers lsr2 paralogs in S. ambofaciens was achieved [241].
Fine-Tuning of Cas9 Expression
One of the biggest drawbacks while working with CRISPR/Cas9 genome editing tools is the toxicity of Cas9 expression for the cells [223,224,225]. The level of Cas9 expression, therefore, must be tightly controlled. When reducing the Cas9 level in the cell, transformation efficiency can be increased but it can also lead to insufficient Cas9 activity. To find the perfect balance, which is also dependent on the used expression strain, a plasmid toolkit was created. This allows for controlling the Cas9 expression during CRISR-Cas9-mediated recombination experiments. The expression is either translationally regulated using theophylline or under the control of a relatively weak constitutive promoter [225].
CRISPR/Cas9 TAR Cloning Approach
TAR (Transformation associated recombination)-Cloning [242] allows for isolation of large chromosomal regions without the effort of constructing a random clone library. The method is based on in vivo homologous recombination of a specific genome target and a linear TAR-Cloning vector containing anchor-like, specific terminal targeting sequences that are homolog to the sequence of interest. The method includes a co-transformation, in which the vector and genomic DNA are introduced into yeast followed by the homologous recombination of the flanking regions of the target sequences and the vector anchors. This results in circular Yeast Artificial Chromosomes (YAC) containing the target sequence. Using the standard TAR protocol, it is possible to clone DNA fragments up to 250 kb but the efficiency of obtaining clones with the correct construct is 1%–5%, which is very low. The efficiency can be increased to 30% by introducing DSBs into the chromosomal DNA near (>100 bp) the target region, typically performed by restriction enzymes. This leads to more efficient homologous recombination between the cloning vector anchor and the target genomic sequences, as they are embedded in shorter DNA fragments. In a newer approach the specificity of TAR cloning was increased by using CRISPR/Cas9 to introduce the DSBs [243]. This offers the advantage that nearly any sequence of interest can be cloned into a YAC vector, without the need of specific and rare restriction enzymes, which cut near the target sequence but not within. This method was applied for cloning parts of the pristinamycin BGC from S. pristinaespiralis [244] and/or the prodigiosin BGC from S. coelicolor [245].
Cas9-Assisted Targeting of Chromosome Segments (CATCH)
The Cas9-assisted targeting of chromosome segments (CATCH) [246] is a direct cloning approach, which uses Cas9-directed cleavage to excise the target fragments for cloning. Cell lysis and in vitro cleavage of the chromosomal DNA by sgRNA-guided Cas9 takes place in agarose gel plugs to protect the DNA from shearing. The digested DNA is then purified from the gel plugs and ligated into the bacterial artificial chromosome-vector (BAC) of choice by Gibson assembly. This allows one-step cloning of near-arbitrary, long bacterial genomic sequences up to 100 kb. Positive-rates of 90% were achieved when cloning the jadomycin gene cluster (36 kb) from S. venezuelae or the chlortetracycline gene cluster (32 kb) from Streptomyces aureofaciens. However, with the increasing fragment size, the efficiency decreased, showing only a 21% positive-rate when cloning 100 kb sequences [246].
Gibson Assembly Combined with CRISPR/Cas9
The classical Gibson assembly requires a linearized vector backbone into which the target fragments are ligated. Commonly, the vector is linearized either by restriction enzyme digestion or inverse PCR [98]. In this combined approach, the vector is cleaved using CRISPR/Cas9 and a specific designed sgRNA. It is a quick and convenient way to use any desired vector without the need of suitable restriction sites or appropriate length for PCR amplification. The target fragments are then assembled into the CRISPR/Cas9 digested vector following the standard Gibson protocol [247]. This was successfully applied for refactoring BGCs from S. pristinaespiralis and S. coelicolor [178].
In vitro Packaging Mediated One-Step Targeted Cloning
This approach combines the specific nicking, mediated by CRISPR/Cas9 and in vitro λ packaging for targeted cloning of natural product pathways [248]. The BGC of interest is released from the genome by in vitro cleavage with a target-specific sgRNA:CRISPR/Cas9 complex and subsequently ligated with the EcoRV-linearized pJTU2554 vector. Both the insert and vector have blunt-ends, which should lead to mostly correct ligation-products, as the remaining non-target gDNA is most likely to have none. Furthermore, the blunt-end ligation approach allows universal use of the EcoRV-linearized vector, as no overlapping regions to the target sequence are needed. The ligation mixture is in vitro packaged into λ phage particles, which are then used to infect E. coli cells. The cloning efficiency ranges from 18%–54%, depending on the insert size (preferably between 37.4 and 50.4 kb). This approach was successfully used to clone the pathways of Tü3010 (stu) from Streptomyces thiolactonus NRRL 15,439 and sisomicin (sis) from the genetically intractable Micromonospora inyoensis DSM 46,123 strain [248].
In the past five years (2015–2020) several CRISPR/Cas9-based tools were established and successfully applied to engineer streptomycetes strains and some species of other genera within Actinomycetales (Table 2). It is likely that those molecular devices will be further optimized and become even more “popular” than the traditional systems for genetic manipulation of actinomycetes.
Table 2.
Advantages and disadvantages of CRISPR/Cas-based genome editing and examples of successful application in actinomycetes.
| Method | Advantages | Disadvantages | Example Organisms |
|---|---|---|---|
| CRISPR/Cas9 engineering using pCRISPomyces [223] | + targeting any site of interest + multiplex targeting possible |
− problems in S. coelicolor [249] − time-consuming elimination of temperature sensitive plasmid − Cas9 toxicity possible − Cas9 off-target effects possible |
S. viridochromogenes [223] S. albus [223] S. lividans [223] Actinoplanes sp. [227] S. formicae [228] |
| CRISPR/Cas9 engineering using pKCas9dO [179] | + targeting any site of interest + multiplex targeting possible + inducible Cas9 expression |
− time-consuming elimination of temperature sensitive plasmid − Cas9 off-target effects possible |
S. coelicolor [178,179] S. pristinaespiralis [178] |
| CRISPR/Cas9 engineering using pCRISPR-Cas9 [229] | + targeting any site of interest + inducible Cas9 expression |
− time-consuming elimination of temperature sensitive plasmid − tipA in host genome required − Cas9 off-target effects possible |
S. coelicolor [229] Streptomyces sp. SD85 [231] Micromonospora chersina [232] |
| CRISPR/dCas9-mediated multiplex gene repression [233] | + targeting any site of interest + stable system through integrative plasmid -multiplex approach |
− Cas9 off-target effects possible − Cas9 toxicity possible − repression levels are lower when targeting multiple sites |
S. coelicolor [233] |
| CRISPR-BEST [234] | + targeting any site of interest + rescue approach when dCas9 was unsuccessful |
− tipA in host genome required - Cas9 off-target effects possible |
S. coelicolor [234]S. griseofuscus [234]S. collinus [234] |
| CRISPR/cas9-CodA(sm) [238] | + targeting any site of interest + unmarked mutants + reusable delivery vector + no off-target effects |
− Cas9 toxicity possible − Cas9 off-target effects possible |
S. coelicolor [238] S. fradiae [239] |
| CRISPR/Cas9 knock-in strategy [240] | + targeting any site of interest + activation of silent gene clusters |
− requires introduction of recombinant DNA for activation of BGC, may be challenging for some strains |
S. albus [240] S. lividans [240] Streptomyces rhodeosporus [240] S. venezuelae [240] S. viridochromogenes [240] |
| Generic CRISPR/Cas9 approach [241] | + no specific sgRNA design required + limited off-target effects |
− previous strain editing required | S. ambofaciens [241] |
| Fine-tuning Cas9 expression [225] | + reduced Cas9 toxicity | − non-toxic expression levels must be explored for each strain |
S. coelicolor [225] S. lividans [225] |
| CRISPR/Cas9 TAR cloning approach [243] | + targeting any site of interest + less-time intensive compared to traditional TAR cloning + no advanced experience in working with yeast required |
− working with yeast − Cas9 off-target effects |
S. pristinaespiralis [244] S. coelicolor [245] |
| Cas9-assisted targeting of chromosome segments (CATCH) [246] | + targeting any site of interest + one-step approach + cloning of large fragments + in gel cleavage protects gDNA from shearing |
− size limit of 150 kb − Cas9 off-target effects possible |
S. venezuelae [246] S. aureofaciens [246] |
| Gibson assembly and CRISPR/Cas9 [247] | + targeting any site of interest + no specific vectors or inverse PCR are required |
− Cas9 off-target effects possible |
S. pristinaespiralis [178] S. coelicolor [178] |
| In vitro packaging mediated one-step targeting cloning [248] | + refactoring possible + no strict requirements for gDNA preparation |
− fragment size limited to packaging capacity of 37.4–50.4 kb from λ phage |
S. thiolactonus [248] Micromonospora inyoensis [248] |
3. Summary and Conclusions
Molecular tools are essential for the genetic engineering of organisms, including actinomycetes-the important producers of bioactive compounds. In general, genetic engineering workflows involve the design and assembly of constructs, the introduction of these constructs into the host cell and intracellular processes that result in genetically modified strains and usually new phenotypes.
In the past, several methods and tools were developed to facilitate these steps. In most cases, established vehicles such as vectors (integrative or replicative systems) are used as backbone of the construction. These systems often already contain important genetic parts (origin of replication and/or integration functions, genes encoding CRISPR/Cas systems, selection markers and/or reporter systems, MCS, constitutive or inducible promoters, RBSs, transcriptional terminators). However, additional parts can be introduced into the construct. These parts can be amplified in a PCR reaction or excised from already existing sources. In case of actinomycetes DNA, the amplification and assembly of several fragments (e.g., cloning of BGCs) might be challenging because of the high GC-content of the DNA. This problem can be solved by using recently improved polymerases and optimized buffers in combination with fast assembly methods. For example, it was demonstrated that the implementation of iCatch, DiPaC, AGOS and modified Gibson Assembly resulted in functional constructs for actinomycete engineering. Instead of using the available genetic parts that might be inefficient in some actinomycetes, new or modified elements can be utilized. Recently, several engineering studies were published in which additional new or modified genetic parts (e.g., promoter-, RBS-engineering, components of biosensors) were identified and successfully utilized for fine-tuning and improvement of the production of actinomycete compounds.
After the desired genetic parts were assembled, the construct needs to be transferred into the actinomycete´s cell. This is usually accomplished using conjugation or protoplast transformation. Depending on which functions are provided on the construct, integration and/or replication take place. For instance, replicative and integrative plasmids are often applied for sequence/gene expression. Plasmids containing homologous fragments upstream and downstream of a target region are frequently used to facilitate the single- and double crossover for sequence/gene inactivation, including deletion. In particular, the traditional method for the generation of deletion mutants is very time-consuming. Recently, the revolutionary CRISPR/Cas system was adapted to actinomycetes for fast genome editing (e.g., insertion and expression, inactivation, deletion and repression of gene expression). In the meantime, several derivatives were reported (e.g., pCRISPomyces, pKCcas9dO, pCRISPR-Cas9, CRISPR/dCas9, CRISPR-BEST, CRISPR/Cas9-CodA(sm), pCRISPomyces-2, CATCH). Indeed, the implementation of the CRISPR/Cas systems resulted in the efficient genome engineering of several Streptomyces strains and some other actinomycetes.
In the past five years (2015–2020), terrific progress has been achieved in the field of molecular tools for genetic manipulation of actinomycetes. The newly developed tools provide new opportunities for natural product discovery and global metabolic engineering of actinomycetes. It is very likely that this progress will continue, and additional cell factories will be developed for manufacturing of various valuable products.
Abbreviations
| ACT | actinorhodin |
| AGOS | artificial gene operon assembly system |
| AICE | actinomycetes integrative and conjugative elements |
| aMSGE | advanced multiplexed site-specific genome engineering |
| BAC | bacterial artificial chromosome |
| BGC | biosynthetic gene cluster |
| CATCH | Cas9-assisted targeting of chromosome segments |
| CRISPR/Cas | clustered regularly interspaced short palindromic repeats/CRISPR associated |
| crRNA | CRISPR RNA |
| DiPaC | Direct pathway cloning |
| DSB | double-strand break |
| DNA | deoxyribonucleic acid |
| gDNA | genomic DNA |
| GFP | green fluorescent protein |
| GusA | glucuronidase |
| HDR | homology directed repair |
| HE | homing endonuclease |
| MCS | multiple cloning site |
| mRNA | messenger RNA |
| MSGE | multiplexed site-specific genome engineering |
| NHEJ | non-homologous end joining |
| NRPS | nonribosomal peptide synthetase |
| OTC | oxytetracycline |
| PCR | polymerase chain reaction |
| PKS | polyketide synthase |
| PTM | polycyclic tetramate macrolactam |
| qPCR | quantitative PCR |
| RBS | ribosome binding sites |
| Real-time RT-PCR | real-time reverse-transcription quantitative polymerase |
| RNA | ribonucleic acid |
| SD | Shine-Dalgarno |
| SEVA | standard European vector architecture |
| sgRNA | synthetic guide RNA |
| SLIC | sequence- and ligation-independent cloning |
| TAR | transformation associated recombination |
| tracrRNA | trans-activating CRISPR RNA |
| UTR | untranslated region |
| YAC | yeast artificial chromosome |
Author Contributions
The review was written and edited by the authors L.M., Y.T., and E.M.M.-K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors and work in their laboratory are supported by the Eberhard Karls Universität Tübingen, the Deutsche Forschungsgemeinschaft (DFG), the Bundesministeriums für Bildung und Forschung (BMBF) (FKZ 031L 0018A, ERASysApp), the German Center for Infection Research (DZIF) (TTU 09.912), and Biovet (Sofia, Bulgaria).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nouioui I., Carro L., Garcia-Lopez M., Meier-Kolthoff J.P., Woyke T., Kyrpides N.C., Pukall R., Klenk H.P., Goodfellow M., Goker M. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman W., Goodfellow M., Kämpfer P., Busse H.-J., Trujillo M., Ludwig W., Suzuki K.-I., Parte A. Bergey’s Manual of Systematic Bacteriology, “the Actinobacteria”. 2nd ed. Springer-Verlag; New York, NY, USA: 2012. pp. 1–2083. [Google Scholar]
- 3.Gao B., Gupta R.S. Phylogenetic Framework and Molecular Signatures for the Main Clades of the Phylum Actinobacteria. Microbiol. Mol. Biol. Rev. 2012;76:66–112. doi: 10.1128/MMBR.05011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma M., Lal D., Kaur J., Saxena A., Kaur J., Anand S., Lal R. Phylogenetic Analyses of Phylum Actinobacteria Based on Whole Genome Sequences. Res. Microbiol. 2013;164:718–728. doi: 10.1016/j.resmic.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Waksman S.A. Studies in the Metabolism of Actinomycetes: Part II. J. Bacteriol. 1919;4:307–330. doi: 10.1128/JB.4.4.307-330.1.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waksman S.A. Studies in the Metabolism of Actinomycetes: Part I. J. Bacteriol. 1919;4:189–216. doi: 10.1128/JB.4.3.189-216.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waksman S.A. On the Classification of Actinomycetes. J. Bacteriol. 1940;39:549–558. doi: 10.1128/JB.39.5.549-558.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barka E.A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Meier-Kolthoff J.P., Klenk H.P., Clement C., Ouhdouch Y., Van Wezel G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodfellow M., Nouioui I., Sanderson R., Xie F., Bull A.T. Rare Taxa and Dark Microbial Matter: Novel Bioactive Actinobacteria Abound in Atacama Desert Soils. Antonie Van Leeuwenhoek. 2018;111:1315–1332. doi: 10.1007/s10482-018-1088-7. [DOI] [PubMed] [Google Scholar]
- 10.Singh R., Dubey A.K. Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microbiol. 2018;9:1767. doi: 10.3389/fmicb.2018.01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X., Liu N., Li X., Ding Y., Shang F., Gao Y., Ruan J., Huang Y. Red Soils Harbor Diverse Culturable Actinomycetes That Are Promising Sources of Novel Secondary Metabolites. Appl. Environ. Microbiol. 2015;81:3086–3103. doi: 10.1128/AEM.03859-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson T.A. Fossil Actinomycetes in Middle Precambrian Glacial Varves. Science. 1967;155:1003–1005. doi: 10.1126/science.155.3765.1003. [DOI] [PubMed] [Google Scholar]
- 13.Poomthongdee N., Duangmal K., Pathom-aree W. Acidophilic Actinomycetes from Rhizosphere Soil: Diversity and Properties Beneficial to Plants. J. Antibiot. (Tokyo) 2015;68:106–114. doi: 10.1038/ja.2014.117. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto A., Takahashi Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. (Tokyo) 2017;70:514–519. doi: 10.1038/ja.2017.20. [DOI] [PubMed] [Google Scholar]
- 15.Chouvenc T., Elliott M.L., Sobotnik J., Efstathion C.A., Su N.Y. The Termite Fecal Nest: A Framework for the Opportunistic Acquisition of Beneficial Soil Streptomyces (Actinomycetales: Streptomycetaceae) Environ. Entomol. 2018;47:1431–1439. doi: 10.1093/ee/nvy152. [DOI] [PubMed] [Google Scholar]
- 16.Scott J.J., Oh D.C., Yuceer M.C., Klepzig K.D., Clardy J., Currie C.R. Bacterial Protection of Beetle-Fungus Mutualism. Science. 2008;322:63. doi: 10.1126/science.1160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaher F., Isenberg H.D., Rosenfeld M.H., Schatz A. The Distribution of Soil Actinomycetes Antagonistic to Protozoa. J. Parasitol. 1953;39:33–37. doi: 10.2307/3274056. [DOI] [PubMed] [Google Scholar]
- 18.Takasuka T.E., Book A.J., Lewin G.R., Currie C.R., Fox B.G. Aerobic Deconstruction of Cellulosic Biomass by an Insect-Associated Streptomyces. Sci. Rep. 2013;3:1030. doi: 10.1038/srep01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T., Yamada K., Zhou T., Harunari E., Igarashi Y., Terahara T., Kobayashi T., Imada C. Akazamicin, a Cytotoxic Aromatic Polyketide from Marine-Derived Nonomuraea Sp. J. Antibiot. (Tokyo) 2019;72:202–209. doi: 10.1038/s41429-018-0139-7. [DOI] [PubMed] [Google Scholar]
- 20.Iniyan A.M., Sudarman E., Wink J., Kannan R.R., Vincent S.G.P. Ala-Geninthiocin, a New Broad Spectrum Thiopeptide Antibiotic, Produced by a Marine Streptomyces Sp. Icn19. J. Antibiot. (Tokyo) 2019;72:99–105. doi: 10.1038/s41429-018-0115-2. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C., Xu L. Diversity of Aquatic Actinomycetes in Lakes of the Middle Plateau, Yunnan, China. Appl. Environ. Microbiol. 1996;62:249–253. doi: 10.1128/AEM.62.1.249-253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G.C., Kim Y.S., Kim M.J., Oh S.A., Choi I., Choi J., Park J.G., Chong C.K., Kim Y.Y., Lee K., et al. Presence, Molecular Characteristics and Geosmin Producing Ability of Actinomycetes Isolated from South Korean Terrestrial and Aquatic Environments. Water Sci. Technol. 2011;63:2745–2751. doi: 10.2166/wst.2011.610. [DOI] [PubMed] [Google Scholar]
- 23.Saadoun I., Hameed K.M., Moussauui A. Characterization and Analysis of Antibiotic Activity of Some Aquatic Actinomycetes. Microbios. 1999;99:173–179. [PubMed] [Google Scholar]
- 24.Ueda J.Y., Khan S.T., Takagi M., Shin-ya K. Jbir-58, A New Salicylamide Derivative, Isolated from a Marine Sponge-Derived Streptomyces Sp. Spd081030me-02. J. Antibiot. (Tokyo) 2010;63:267–269. doi: 10.1038/ja.2010.26. [DOI] [PubMed] [Google Scholar]
- 25.El-Hawary S.S., Sayed A.M., Mohammed R., Khanfar M.A., Rateb M.E., Mohammed T.A., Hajjar D., Hassan H.M., Gulder T.A.M., Abdelmohsen U.R. New Pim-1 Kinase Inhibitor from the Co-Culture of Two Sponge-Associated Actinomycetes. Front. Chem. 2018;6:538. doi: 10.3389/fchem.2018.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehbub M.F., Tanner J.E., Barnett S.J., Franco C.M., Zhang W. The Role of Sponge-Bacteria Interactions: The Sponge Aplysilla rosea Challenged by Its Associated Bacterium Streptomyces act-52a in a Controlled Aquarium System. Appl. Microbiol. Biotechnol. 2016;100:10609–10626. doi: 10.1007/s00253-016-7878-9. [DOI] [PubMed] [Google Scholar]
- 27.Minas W., Bailey J.E., Duetz W. Streptomycetes in Micro-Cultures: Growth, Production of Secondary Metabolites, and Storage and Retrieval in the 96-Well Format. Antonie Van Leeuwenhoek. 2000;78:297–305. doi: 10.1023/A:1010254013352. [DOI] [PubMed] [Google Scholar]
- 28.Bhatti A.A., Haq S., Bhat R.A. Actinomycetes Benefaction Role in Soil and Plant Health. Microb. Pathog. 2017;111:458–467. doi: 10.1016/j.micpath.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Saini A., Aggarwal N.K., Sharma A., Yadav A. Actinomycetes: A Source of Lignocellulolytic Enzymes. Enzyme Res. 2015;2015 doi: 10.1155/2015/279381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellstedt A., Richau K.H. Aspects of Nitrogen-Fixing Actinobacteria, in Particular Free-Living and Symbiotic Frankia. FEMS Microbiol. Lett. 2013;342:179–186. doi: 10.1111/1574-6968.12116. [DOI] [PubMed] [Google Scholar]
- 31.Dahal B., NandaKafle G., Perkins L., Brozel V.S. Diversity of Free-Living Nitrogen Fixing Streptomyces in Soils of the Badlands of South Dakota. Microbiol. Res. 2017;195:31–39. doi: 10.1016/j.micres.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Liu C., Jiang Y., Wang X., Chen D., Chen X., Wang L., Han L., Huang X., Jiang C. Diversity, Antimicrobial Activity, and Biosynthetic Potential of Cultivable Actinomycetes Associated with Lichen Symbiosis. Microb. Ecol. 2017;74:570–584. doi: 10.1007/s00248-017-0972-4. [DOI] [PubMed] [Google Scholar]
- 33.Li H., Sosa-Calvo J., Horn H.A., Pupo M.T., Clardy J., Rabeling C., Schultz T.R., Currie C.R. Convergent Evolution of Complex Structures for Ant-Bacterial Defensive Symbiosis in Fungus-Farming Ants. Proc. Natl. Acad. Sci. USA. 2018;115:10720–10725. doi: 10.1073/pnas.1809332115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Meij A., Willemse J., Schneijderberg M.A., Geurts R., Raaijmakers J.M., Van Wezel G.P. Inter- and Intracellular Colonization of Arabidopsis Roots by Endophytic Actinobacteria and the Impact of Plant Hormones on Their Antimicrobial Activity. Antonie Van Leeuwenhoek. 2018;111:679–690. doi: 10.1007/s10482-018-1014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaltenpoth M., Yildirim E., Gurbuz M.F., Herzner G., Strohm E. Refining the Roots of the Beewolf-Streptomyces Symbiosis: Antennal Symbionts in the Rare Genus Philanthinus (Hymenoptera, Crabronidae) Appl. Environ. Microbiol. 2012;78:822–827. doi: 10.1128/AEM.06809-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaltenpoth M. Actinobacteria as Mutualists: General Healthcare for Insects? Trends Microbiol. 2009;17:529–535. doi: 10.1016/j.tim.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Kurtboke D.I., French J.R., Hayes R.A., Quinn R.J. Eco-Taxonomic Insights into Actinomycete Symbionts of Termites for Discovery of Novel Bioactive Compounds. Adv. Biochem. Eng. Biotechnol. 2015;147:111–135. doi: 10.1007/10_2014_270. [DOI] [PubMed] [Google Scholar]
- 38.Visser A.A., Nobre T., Currie C.R., Aanen D.K., Poulsen M. Exploring the Potential for Actinobacteria as Defensive Symbionts in Fungus-Growing Termites. Microb. Ecol. 2012;63:975–985. doi: 10.1007/s00248-011-9987-4. [DOI] [PubMed] [Google Scholar]
- 39.Kim K.H., Ramadhar T.R., Beemelmanns C., Cao S., Poulsen M., Currie C.R., Clardy J. Natalamycin A, an Ansamycin from a Termite-Associated Streptomyces Sp. Chem. Sci. 2014;5:4333–4338. doi: 10.1039/C4SC01136H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beemelmanns C., Ramadhar T.R., Kim K.H., Klassen J.L., Cao S., Wyche T.P., Hou Y., Poulsen M., Bugni T.S., Currie C.R., et al. Macrotermycins A-D, Glycosylated Macrolactams from a Termite-Associated Amycolatopsis Sp. M39. Org. Lett. 2017;19:1000–1003. doi: 10.1021/acs.orglett.6b03831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertsen H.L., Musiol-Kroll E.M. Actinomycete-Derived Polyketides as a Source of Antibiotics and Lead Structures for the Development of New Antimicrobial Drugs. Antibiotics (Basel) 2019;8:157. doi: 10.3390/antibiotics8040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramani R., Sipkema D. Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products. Mar. Drugs. 2019;17:249. doi: 10.3390/md17050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genilloud O. Actinomycetes: Still a Source of Novel Antibiotics. Nat. Prod. Rep. 2017;34:1203–1232. doi: 10.1039/C7NP00026J. [DOI] [PubMed] [Google Scholar]
- 44.Waksman S.A., Woodruff H.B. Actinomyces antibioticus, a New Soil Organism Antagonistic to Pathogenic and Non-Pathogenic Bacteria. J. Bacteriol. 1941;42:231–249. doi: 10.1128/JB.42.2.231-249.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hug J.J., Bader C.D., Remskar M., Cirnski K., Muller R. Concepts and Methods to Access Novel Antibiotics from Actinomycetes. Antibiotics (Basel) 2018;7:44. doi: 10.3390/antibiotics7020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis K. The Science of Antibiotic Discovery. Cell. 2020;181:29–45. doi: 10.1016/j.cell.2020.02.056. [DOI] [PubMed] [Google Scholar]
- 47.Van der Meij A., Worsley S.F., Hutchings M.I., Van Wezel G.P. Chemical Ecology of Antibiotic Production by Actinomycetes. FEMS Microbiol. Rev. 2017;41:392–416. doi: 10.1093/femsre/fux005. [DOI] [PubMed] [Google Scholar]
- 48.Nett M., Ikeda H., Moore B.S. Genomic Basis for Natural Product Biosynthetic Diversity in the Actinomycetes. Nat. Prod. Rep. 2009;26:1362–1384. doi: 10.1039/b817069j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi Y., Nakashima T. Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics. Antibiotics (Basel) 2018;7:45. doi: 10.3390/antibiotics7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landwehr W., Wolf C., Wink J. Actinobacteria and Myxobacteria-Two of the Most Important Bacterial Resources for Novel Antibiotics. Curr. Top. Microbiol. Immunol. 2016;398:273–302. doi: 10.1007/82_2016_503. [DOI] [PubMed] [Google Scholar]
- 51.Dhakal D., Pokhrel A.R., Shrestha B., Sohng J.K. Marine Rare Actinobacteria: Isolation, Characterization, and Strategies for Harnessing Bioactive Compounds. Front. Microbiol. 2017;8:1106. doi: 10.3389/fmicb.2017.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentley S.D., Chater K.F., Cerdeno-Tarraga A.M., Challis G.L., Thomson N.R., James K.D., Harris D.E., Quail M.A., Kieser H., Harper D., et al. Complete Genome Sequence of the Model Actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 53.Baral B., Akhgari A., Metsa-Ketela M. Activation of Microbial Secondary Metabolic Pathways: Avenues and Challenges. Synth. Syst. Biotechnol. 2018;3:163–178. doi: 10.1016/j.synbio.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onaka H. Novel Antibiotic Screening Methods to Awaken Silent or Cryptic Secondary Metabolic Pathways in Actinomycetes. J. Antibiot. (Tokyo) 2017;70:865–870. doi: 10.1038/ja.2017.51. [DOI] [PubMed] [Google Scholar]
- 55.Ochi K., Hosaka T. New Strategies for Drug Discovery: Activation of Silent or Weakly Expressed Microbial Gene Clusters. Appl. Microbiol. Biotechnol. 2013;97:87–98. doi: 10.1007/s00253-012-4551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becerril A., Alvarez S., Brana A.F., Rico S., Diaz M., Santamaria R.I., Salas J.A., Mendez C. Uncovering Production of Specialized Metabolites by Streptomyces argillaceus: Activation of Cryptic Biosynthesis Gene Clusters Using Nutritional and Genetic Approaches. PLoS One. 2018;13:e0198145. doi: 10.1371/journal.pone.0198145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Wei W.P., Ye B.C. High GC Content Cas9-Mediated Genome-Editing and Biosynthetic Gene Cluster Activation in Saccharopolyspora erythraea. ACS Synth. Biol. 2018;7:1338–1348. doi: 10.1021/acssynbio.7b00448. [DOI] [PubMed] [Google Scholar]
- 58.Xu J., Zhang J., Zhuo J., Li Y., Tian Y., Tan H. Activation and Mechanism of a Cryptic Oviedomycin Gene Cluster Via the Disruption of a Global Regulatory Gene, adpA, in Streptomyces ansochromogenes. J. Biol. Chem. 2017;292:19708–19720. doi: 10.1074/jbc.M117.809145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thanapipatsiri A., Gomez-Escribano J.P., Song L., Bibb M.J., Al-Bassam M., Chandra G., Thamchaipenet A., Challis G.L., Bibb M.J. Discovery of Unusual Biaryl Polyketides by Activation of a Silent Streptomyces venezuelae Biosynthetic Gene Cluster. Chem. Biochem. 2016;17:2189–2198. doi: 10.1002/cbic.201600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochi K. Insights into Microbial Cryptic Gene Activation and Strain Improvement: Principle, Application and Technical Aspects. J. Antibiot. (Tokyo) 2017;70:25–40. doi: 10.1038/ja.2016.82. [DOI] [PubMed] [Google Scholar]
- 61.Monciardini P., Iorio M., Maffioli S., Sosio M., Donadio S. Discovering New Bioactive Molecules from Microbial Sources. Microb. Biotechnol. 2014;7:209–220. doi: 10.1111/1751-7915.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Bergeijk D.A., Terlouw B.R., Medema M.H., Van Wezel G.P. Ecology and Genomics of Actinobacteria: New Concepts for Natural Product Discovery. Nat. Rev. Microbiol. 2020 doi: 10.1038/s41579-020-0379-y. [DOI] [PubMed] [Google Scholar]
- 63.Baltz R.H. Gifted Microbes for Genome Mining and Natural Product Discovery. J. Ind. Microbiol. Biotechnol. 2017;44:573–588. doi: 10.1007/s10295-016-1815-x. [DOI] [PubMed] [Google Scholar]
- 64.Kealey C., Creaven C.A., Murphy C.D., Brady C.B. New Approaches to Antibiotic Discovery. Biotechnol. Lett. 2017;39:805–817. doi: 10.1007/s10529-017-2311-8. [DOI] [PubMed] [Google Scholar]
- 65.Genilloud O. Mining Actinomycetes for Novel Antibiotics in the Omics Era: Are We Ready to Exploit This New Paradigm? Antibiotics (Basel) 2018;7:85. doi: 10.3390/antibiotics7040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen C.T., Dhakal D., Pham V.T.T., Nguyen H.T., Sohng J.K. Recent Advances in Strategies for Activation and Discovery/Characterization of Cryptic Biosynthetic Gene Clusters in Streptomyces. Microorganisms. 2020;8:616. doi: 10.3390/microorganisms8040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S.Y., Medema M.H., Weber T. Antismash 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hug J.J., Krug D., Müller R. Bacteria as Genetically Programmable Producers of Bioactive Natural Products. Nat. Rev. Chem. 2020;4:172–193. doi: 10.1038/s41570-020-0176-1. [DOI] [PubMed] [Google Scholar]
- 69.Bu Q.-T., Yu P., Wang J., Li Z.-Y., Chen X.-A., Mao X.-M., Li Y.-Q.J.M.c.f. Rational Construction of Genome-Reduced and High-Efficient Industrial Streptomyces Chassis Based on Multiple Comparative Genomic Approaches. Microb. Cell. Fact. 2019;18:1–17. doi: 10.1186/s12934-019-1055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikeda H., Shin-ya K., Omura S.J. Genome Mining of the Streptomyces avermitilis Genome and Development of Genome-Minimized Hosts for Heterologous Expression of Biosynthetic Gene Clusters. J. Ind. Microbiol. Biotechnol. 2014;41:233–250. doi: 10.1007/s10295-013-1327-x. [DOI] [PubMed] [Google Scholar]
- 71.Komatsu M., Uchiyama T., Ōmura S., Cane D.E., Ikeda H. Genome-Minimized Streptomyces Host for the Heterologous Expression of Secondary Metabolism. Proc. Natl. Acad. Sci. USA. 2010;107:2646–2651. doi: 10.1073/pnas.0914833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Myronovskyi M., Luzhetskyy A. Heterologous Production of Small Molecules in the Optimized Streptomyces Hosts. Nat. Prod. Rep. 2019;36:1281–1294. doi: 10.1039/C9NP00023B. [DOI] [PubMed] [Google Scholar]
- 73.Myronovskyi M., Rosenkränzer B., Nadmid S., Pujic P., Normand P., Luzhetskyy A.J.M.E. Generation of a Cluster-Free Streptomyces albus Chassis Strains for Improved Heterologous Expression of Secondary Metabolite Clusters. Metab. Eng. 2018;49:316–324. doi: 10.1016/j.ymben.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Musiol-Kroll E.M., Tocchetti A., Sosio M., Stegmann E. Challenges and Advances in Genetic Manipulation of Filamentous Actinomycetes - the Remarkable Producers of Specialized Metabolites. Nat. Prod. Rep. 2019;36:1351–1369. doi: 10.1039/C9NP00029A. [DOI] [PubMed] [Google Scholar]
- 75.Loman N.J., Pallen M.J. Twenty Years of Bacterial Genome Sequencing. Nat. Rev. Microbiol. 2015;13:787–794. doi: 10.1038/nrmicro3565. [DOI] [PubMed] [Google Scholar]
- 76.F D.I.F., Micheli G., Camilloni G. Restriction Enzymes and Their Use in Molecular Biology: An Overview. J. Biosci. 2019;44:38. [PubMed] [Google Scholar]
- 77.Rittie L., Perbal B. Enzymes Used in Molecular Biology: A Useful Guide. J. Cell Commun. Signal. 2008;2:25–45. doi: 10.1007/s12079-008-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wohlleben W., Mast Y., Muth G., Rottgen M., Stegmann E., Weber T. Synthetic Biology of Secondary Metabolite Biosynthesis in Actinomycetes: Engineering Precursor Supply as a Way to Optimize Antibiotic Production. FEBS Lett. 2012;586:2171–2176. doi: 10.1016/j.febslet.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Baltz R.H. Synthetic Biology, Genome Mining, and Combinatorial Biosynthesis of NRPS-Derived Antibiotics: A Perspective. J. Ind. Microbiol. Biotechnol. 2018;45:635–649. doi: 10.1007/s10295-017-1999-8. [DOI] [PubMed] [Google Scholar]
- 80.Aubry C., Pernodet J.-L., Lautru S. Modular and Integrative Vectors for Synthetic Biology Applications in Streptomyces Spp. Appl. Environ. Microbiol. 2019;85:e00485–e00419. doi: 10.1128/AEM.00485-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Liu X., Jiang W., Lu Y. Recent Advances in Synthetic Biology Approaches to Optimize Production of Bioactive Natural Products in Actinobacteria. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shetty R.P., Endy D., Knight T.F., Jr. Engineering Biobrick Vectors from Biobrick Parts. J. Biol. Eng. 2008;2:5. doi: 10.1186/1754-1611-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Purnick P.E., Weiss R. The Second Wave of Synthetic Biology: From Modules to Systems. Nat. Rev. Mol. Cell Biol. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 84.Rebets Y., Brotz E., Tokovenko B., Luzhetskyy A. Actinomycetes Biosynthetic Potential: How to Bridge in Silico and in Vivo? J. Ind. Microbiol. Biotechnol. 2014;41:387–402. doi: 10.1007/s10295-013-1352-9. [DOI] [PubMed] [Google Scholar]
- 85.Kieser T., Bibb M.J., Buttner M.J., Chater K.F., Hopwood D.A. Practical Streptomyces Genetics. 1st ed. John Innes Foundation; Norwich, England: 2000. pp. 18–581. [Google Scholar]
- 86.Ventura M., Canchaya C., Tauch A., Chandra G., Fitzgerald G.F., Chater K.F., Van Sinderen D. Genomics of Actinobacteria: Tracing the Evolutionary History of an Ancient Phylum. Microbiol. Mol. Biol. Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinashi H., Shimaji M., Sakai A. Giant Linear Plasmids in Streptomyces Which Code for Antibiotic Biosynthesis Genes. Nature. 1987;328:454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- 88.Mast Y., Weber T., Gölz M., Ort-Winklbauer R., Gondran A., Wohlleben W., Schinko E. Characterization of the ‘Pristinamycin Supercluster’ of Streptomyces pristinaespiralis. Microb Biotechnol. 2011;4:192–206. doi: 10.1111/j.1751-7915.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J., Lu A., Liu J., Huang W., Wang J., Cai Z., Zhao G. Icatch: A New Strategy for Capturing Large DNA Fragments Using Homing Endonucleases. Acta Bioch. Bioph. Sin. 2018;51:97–103. doi: 10.1093/abbs/gmy139. [DOI] [PubMed] [Google Scholar]
- 90.Liu J.-K., Chen W.-H., Ren S.-X., Zhao G.-P., Wang J. Ibrick: A New Standard for Iterative Assembly of Biological Parts with Homing Endonucleases. PloS one. 2014;9:e110852. doi: 10.1371/journal.pone.0110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knight T. Idempotent Vector Design for Standard Assembly of Biobricks. [(accessed on 20 May 2020)]; Available online: http://hdl.handle.net/1721.1/21168.
- 92.Greunke C., Regina Duell E., D’Agostino P., Glöckle A., Lamm K., Am Gulder T. Direct Pathway Cloning (DiPaC) to Unlock Natural Product Biosynthetic Potential. Metab. Eng. 2018;47:334–345. doi: 10.1016/j.ymben.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Jeong J.-Y., Yim H.-S., Ryu J.-Y., Lee H.S., Lee J.-H., Seen D.-S., Kang S.G. One-Step Sequence-and Ligation-Independent Cloning as a Rapid and Versatile Cloning Method for Functional Genomics Studies. Appl. Environ. Microbiol. 2012;78:5440–5443. doi: 10.1128/AEM.00844-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Agostino P.M., Gulder T.A. Direct Pathway Cloning Combined with Sequence- and Ligation-Independent Cloning for Fast Biosynthetic Gene Cluster Refactoring and Heterologous Expression. ACS Synth. Biol. 2018;7:1702–1708. doi: 10.1021/acssynbio.8b00151. [DOI] [PubMed] [Google Scholar]
- 95.Basitta P., Westrich L., Rösch M., Kulik A., Gust B., Apel A.K. Agos: A Plug-and-Play Method for the Assembly of Artificial Gene Operons into Functional Biosynthetic Gene Clusters. ACS Synth. Biol. 2017;6:817–825. doi: 10.1021/acssynbio.6b00319. [DOI] [PubMed] [Google Scholar]
- 96.Gust B., Chandra G., Jakimowicz D., Yuqing T., Bruton C.J., Chater K.F. L Red-Mediated Genetic Manipulation of Antibiotic-Producing Streptomyces. Adv. Appl. Microbiol. 2004;54:107–128. doi: 10.1016/S0065-2164(04)54004-2. [DOI] [PubMed] [Google Scholar]
- 97.Eustáquio A.S., Gust B., Li S.-M., Pelzer S., Wohlleben W., Chater K.F., Heide L. Production of 8′-Halogenated and 8′-Unsubstituted Novobiocin Derivatives in Genetically Engineered Streptomyces coelicolor Strains. Chem. Biol. 2004;11:1561–1572. doi: 10.1016/j.chembiol.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Gibson D.G., Young L., Chuang R.-Y., Venter J.C., Hutchison C.A., Smith H.O. Enzymatic Assembly of DNA Molecules up to Several Hundred Kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 99.Li L., Zhao Y., Ruan L., Yang S., Ge M., Jiang W., Lu Y. A Stepwise Increase in Pristinamycin II Biosynthesis by Streptomyces pristinaespiralis through Combinatorial Metabolic Engineering. Metab. Eng. 2015;29:12–25. doi: 10.1016/j.ymben.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Te Poele E.M., Bolhuis H., Dijkhuizen L.J.A.V.L. Actinomycete Integrative and Conjugative Elements. Antonie Van Leeuwenhoek. 2008;94:127–143. doi: 10.1007/s10482-008-9255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegl T., Luzhetskyy A. Actinomycetes Genome Engineering Approaches. Antonie Van Leeuwenhoek. 2012;102:503–516. doi: 10.1007/s10482-012-9795-y. [DOI] [PubMed] [Google Scholar]
- 102.Jang M.S., Fujita A., Ikawa S., Hanawa K., Yamamura H., Tamura T., Hayakawa M., Tezuka T., Ohnishi Y. Isolation of a Novel Plasmid from Couchioplanes caeruleus and Construction of Two Plasmid Vectors for Gene Expression in Actinoplanes missouriensis. Plasmid. 2015;77:32–38. doi: 10.1016/j.plasmid.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 103.Kormanec J., Rezuchova B., Homerova D., Csolleiova D., Sevcikova B., Novakova R., Feckova L. Recent Achievements in the Generation of Stable Genome Alterations/Mutations in Species of the Genus Streptomyces. Appl. Microbiol. Biotechnol. 2019;103:5463–5482. doi: 10.1007/s00253-019-09901-0. [DOI] [PubMed] [Google Scholar]
- 104.Wohlleben W., Hartmann V., Hillemann D., Krey K., Muth G., Nussbaumer B., Pelzer S. Transfer and Establishment of DNA in Streptomyces (a Brief Review) Acta Microbiol. Immunol. Hung. 1994;41:381–389. [PubMed] [Google Scholar]
- 105.Deng Y., Zhang X., Zhang X. Recent Advances in Genetic Modification Systems for Actinobacteria. Appl. Microbiol. Biotechnol. 2017;101:2217–2226. doi: 10.1007/s00253-017-8156-1. [DOI] [PubMed] [Google Scholar]
- 106.Okanishi M., Suzuki K., Umezawa H. Formation and Reversion of Streptomycete Protoplasts: Cultural Condition and Morphological Study. J. Gen. Microbiol. 1974;80:389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- 107.Bibb M.J., Ward J.M., Hopwood D.A. Transformation of Plasmid DNA into Streptomyces at High Frequency. Nature. 1978;274:398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- 108.Bierman M., Logan R., O’Brien K., Seno E.T., Rao R.N., Schoner B.E. Plasmid Cloning Vectors for the Conjugal Transfer of DNA from Escherichia coli to Streptomyces Spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 109.Mazodier P., Petter R., Thompson C. Intergeneric Conjugation between Escherichia coli and Streptomyces Species. J. Bacteriol. 1989;171:3583–3585. doi: 10.1128/JB.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bibb M.J., Janssen G.R., Ward J.M. Cloning and Analysis of the Promoter Region of the Erythromycin Resistance Gene (ermE) of Streptomyces erythraeus. Gene. 1985;38:215–226. doi: 10.1016/0378-1119(85)90220-3. [DOI] [PubMed] [Google Scholar]
- 111.Labes G., Bibb M., Wohlleben W. Isolation and Characterization of a Strong Promoter Element from the Streptomyces ghanaensis Phage I19 Using the Gentamicin Resistance Gene (aacc1) of Tn1696 as Reporter. Microbiology. 1997;143:1503–1512. doi: 10.1099/00221287-143-5-1503. [DOI] [PubMed] [Google Scholar]
- 112.Xu G., Wang J., Wang L., Tian X., Yang H., Fan K., Yang K., Tan H. “Pseudo” Gamma-Butyrolactone Receptors Respond to Antibiotic Signals to Coordinate Antibiotic Biosynthesis. J. Biol. Chem. 2010;285:27440–27448. doi: 10.1074/jbc.M110.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murakami T., Holt T.G., Thompson C.J. Thiostrepton-Induced Gene Expression in Streptomyces lividans. J. Bacteriol. 1989;171:1459–1466. doi: 10.1128/JB.171.3.1459-1466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herai S., Hashimoto Y., Higashibata H., Maseda H., Ikeda H., Omura S., Kobayashi M. Hyper-Inducible Expression System for Streptomycetes. Proc. Natl. Acad. Sci. USA. 2004;101:14031–14035. doi: 10.1073/pnas.0406058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polkade A.V., Mantri S.S., Patwekar U.J., Jangid K. Quorum Sensing: An Under-Explored Phenomenon in the Phylum Actinobacteria. Front. Microbiol. 2016;7:131. doi: 10.3389/fmicb.2016.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhukya H., Bhujbalrao R., Bitra A., Anand R. Structural and Functional Basis of Transcriptional Regulation by TetR Family Protein CprB from S. coelicolor A3(2) Nucleic Acids Res. 2014;42:10122–10133. doi: 10.1093/nar/gku587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodriguez-Garcia A., Combes P., Perez-Redondo R., Smith M.C., Smith M.C. Natural and Synthetic Tetracycline-Inducible Promoters for Use in the Antibiotic-Producing Bacteria Streptomyces. Nucleic Acids Res. 2005;33:e87. doi: 10.1093/nar/gni086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hindle Z., Smith C.P. Substrate Induction and Catabolite Repression of the Streptomyces coelicolor Glycerol Operon Are Mediated through the GylR Protein. Mol. Microbiol. 1994;12:737–745. doi: 10.1111/j.1365-2958.1994.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 119.Wei J., He L., Niu G. Regulation of Antibiotic Biosynthesis in Actinomycetes: Perspectives and Challenges. Synth. Syst. Biotechnol. 2018;3:229–235. doi: 10.1016/j.synbio.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu G., Chater K.F., Chandra G., Niu G., Tan H. Molecular Regulation of Antibiotic Biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero-Rodriguez A., Robledo-Casados I., Sanchez S. An Overview on Transcriptional Regulators in Streptomyces. Biochim. Biophys. Acta. 2015;1849:1017–1039. doi: 10.1016/j.bbagrm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 122.Barreales E.G., Vicente C.M., De Pedro A., Santos-Aberturas J., Aparicio J.F. Promoter Engineering Reveals the Importance of Heptameric Direct Repeats for DNA Binding by Streptomyces Antibiotic Regulatory Protein-Large ATP-Binding Regulator of the LuxR Family (SARP-LAL) Regulators in Streptomyces natalensis. Appl. Environ. Microbiol. 2018;84:e00246-18. doi: 10.1128/AEM.00246-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J., Jensen M.K., Keasling J.D. Development of Biosensors and Their Application in Metabolic Engineering. Curr. Opin. Chem. Biol. 2015;28:1–8. doi: 10.1016/j.cbpa.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 124.Tan S.Z., Prather K.L. Dynamic Pathway Regulation: Recent Advances and Methods of Construction. Curr. Opin. Chem. Biol. 2017;41:28–35. doi: 10.1016/j.cbpa.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 125.Cheng F., Tang X.L., Kardashliev T. Transcription Factor-Based Biosensors in High-Throughput Screening: Advances and Applications. Biotechnol. J. 2018;13:e1700648. doi: 10.1002/biot.201700648. [DOI] [PubMed] [Google Scholar]
- 126.Kasey C.M., Zerrad M., Li Y., Cropp T.A., Williams G.J. Development of Transcription Factor-Based Designer Macrolide Biosensors for Metabolic Engineering and Synthetic Biology. ACS Synth. Biol. 2018;7:227–239. doi: 10.1021/acssynbio.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim N.M., Sinnott R.W., Sandoval N.R. Transcription Factor-Based Biosensors and Inducible Systems in Non-Model Bacteria: Current Progress and Future Directions. Curr. Opin. Biotechnol. 2019;64:39–46. doi: 10.1016/j.copbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Rebets Y., Schmelz S., Gromyko O., Tistechok S., Petzke L., Scrima A., Luzhetskyy A. Design, Development and Application of Whole-Cell Based Antibiotic-Specific Biosensor. Metab. Eng. 2018;47:263–270. doi: 10.1016/j.ymben.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 129.Baltz R.H. Genetic Manipulation of Secondary Metabolite Biosynthesis for Improved Production in Streptomyces and Other Actinomycetes. J. Ind. Microbiol. Biotechnol. 2016;43:343–370. doi: 10.1007/s10295-015-1682-x. [DOI] [PubMed] [Google Scholar]
- 130.Mehlhorn A., Rahimi P., Joseph Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors (Basel) 2018;8:54. doi: 10.3390/bios8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Myronovskyi M., Luzhetskyy A. Native and Engineered Promoters in Natural Product Discovery. Nat. Prod. Rep. 2016;33:1006–1019. doi: 10.1039/C6NP00002A. [DOI] [PubMed] [Google Scholar]
- 132.Palazzotto E., Tong Y., Lee S.Y., Weber T. Synthetic Biology and Metabolic Engineering of Actinomycetes for Natural Product Discovery. Biotechnol. Adv. 2019;37:107366. doi: 10.1016/j.biotechadv.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 133.Yi J.S., Kim M.W., Kim M., Jeong Y., Kim E.J., Cho B.K., Kim B.G. A Novel Approach for Gene Expression Optimization through Native Promoter and 5’ UTR Combinations Based on RNA-Seq, Ribo-Seq, and TSS-Seq of Streptomyces coelicolor. ACS Synth. Biol. 2017;6:555–565. doi: 10.1021/acssynbio.6b00263. [DOI] [PubMed] [Google Scholar]
- 134.Horbal L., Siegl T., Luzhetskyy A. A Set of Synthetic Versatile Genetic Control Elements for the Efficient Expression of Genes in Actinobacteria. Sci. Rep. 2018;8:491. doi: 10.1038/s41598-017-18846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Siegl T., Tokovenko B., Myronovskyi M., Luzhetskyy A. Design, Construction and Characterisation of a Synthetic Promoter Library for Fine-Tuned Gene Expression in Actinomycetes. Metab. Eng. 2013;19:98–106. doi: 10.1016/j.ymben.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 136.Seghezzi N., Amar P., Koebmann B., Jensen P.R., Virolle M.J. The Construction of a Library of Synthetic Promoters Revealed Some Specific Features of Strong Streptomyces Promoters. Appl. Microbiol. Biotechnol. 2011;90:615–623. doi: 10.1007/s00253-010-3018-0. [DOI] [PubMed] [Google Scholar]
- 137.Ji C.H., Kim J.P., Kang H.S. Library of Synthetic Streptomyces Regulatory Sequences for Use in Promoter Engineering of Natural Product Biosynthetic Gene Clusters. ACS Synth. Biol. 2018;7:1946–1955. doi: 10.1021/acssynbio.8b00175. [DOI] [PubMed] [Google Scholar]
- 138.Ji C.H., Kim H., Kang H.S. Synthetic Inducible Regulatory Systems Optimized for the Modulation of Secondary Metabolite Production in Streptomyces. ACS Synth. Biol. 2019;8:577–586. doi: 10.1021/acssynbio.9b00001. [DOI] [PubMed] [Google Scholar]
- 139.Guan C., Cui W., He X., Hu X., Xu J., Du G., Chen J., Zhou Z. Construction and Development of a Novel Expression System of Streptomyces. Protein Expr. Purif. 2015;113:17–22. doi: 10.1016/j.pep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 140.Wang W., Li X., Wang J., Xiang S., Feng X., Yang K. An Engineered Strong Promoter for Streptomycetes. Appl. Environ. Microbiol. 2013;79:4484–4492. doi: 10.1128/AEM.00985-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu N., Wei L., Liu J. Recent Advances in the Applications of Promoter Engineering for the Optimization of Metabolite Biosynthesis. World J. Microbiol. Biotechnol. 2019;35:33. doi: 10.1007/s11274-019-2606-0. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y., Ren C.Y., Wei W.P., You D., Yin B.C., Ye B.C. A CRISPR-Cas9 Strategy for Activating the Saccharopolyspora erythraea Erythromycin Biosynthetic Gene Cluster with Knock-In Bidirectional Promoters. ACS Synth. Biol. 2019;8:1134–1143. doi: 10.1021/acssynbio.9b00024. [DOI] [PubMed] [Google Scholar]
- 143.Xu X., Wang J., Bechthold A., Ma Z., Yu X. Selection of an Efficient Promoter and Its Application in Toyocamycin Production Improvement in Streptomyces diastatochromogenes 1628. World J. Microbiol. Biotechnol. 2017;33:30. doi: 10.1007/s11274-016-2194-1. [DOI] [PubMed] [Google Scholar]
- 144.Luo Y., Zhang L., Barton K.W., Zhao H. Systematic Identification of a Panel of Strong Constitutive Promoters from Streptomyces albus. ACS Synth. Biol. 2015;4:1001–1010. doi: 10.1021/acssynbio.5b00016. [DOI] [PubMed] [Google Scholar]
- 145.Luo Y., Huang H., Liang J., Wang M., Lu L., Shao Z., Cobb R.E., Zhao H. Activation and Characterization of a Cryptic Polycyclic Tetramate Macrolactam Biosynthetic Gene Cluster. Nat. Commun. 2013;4:2894. doi: 10.1038/ncomms3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li S., Wang J., Li X., Yin S., Wang W., Yang K. Genome-Wide Identification and Evaluation of Constitutive Promoters in Streptomycetes. Microb. Cell. Fact. 2015;14:172. doi: 10.1186/s12934-015-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang K., Liu X.F., Bu Q.T., Zheng Y., Chen X.A., Li Y.Q., Mao X.M. Transcriptome-Based Identification of a Strong Promoter for Hyper-Production of Natamycin in Streptomyces. Curr. Microbiol. 2019;76:95–99. doi: 10.1007/s00284-018-1589-7. [DOI] [PubMed] [Google Scholar]
- 148.Wang K., Chen X.A., Li Y.Q., Mao X.M. Identification of a Secondary Metabolism-Responsive Promoter by Proteomics for Over-Production of Natamycin in Streptomyces. Arch. Microbiol. 2019;201:1459–1464. doi: 10.1007/s00203-019-01710-3. [DOI] [PubMed] [Google Scholar]
- 149.Schaffert L., Marz C., Burkhardt L., Droste J., Brandt D., Busche T., Rosen W., Schneiker-Bekel S., Persicke M., Puhler A., et al. Evaluation of Vector Systems and Promoters for Overexpression of the Acarbose Biosynthesis Gene acbC in Actinoplanes Sp. Se50/110. Microb. Cell Fact. 2019;18:114. doi: 10.1186/s12934-019-1162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li S., Wang J., Xiang W., Yang K., Li Z., Wang W. An Autoregulated Fine-Tuning Strategy for Titer Improvement of Secondary Metabolites Using Native Promoters in Streptomyces. ACS Synth. Biol. 2018;7:522–530. doi: 10.1021/acssynbio.7b00318. [DOI] [PubMed] [Google Scholar]
- 151.Bai C., Zhang Y., Zhao X., Hu Y., Xiang S., Miao J., Lou C., Zhang L. Exploiting a Precise Design of Universal Synthetic Modular Regulatory Elements to Unlock the Microbial Natural Products in Streptomyces. Proc. Natl. Acad. Sci. USA. 2015;112:12181–12186. doi: 10.1073/pnas.1511027112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao M., Wang S.L., Tao X.Y., Zhao G.L., Ren Y.H., Wang F.Q., Wei D.Z. Engineering Diverse Eubacteria Promoters for Robust Gene Expression in Streptomyces lividans. J. Biotechnol. 2019;289:93–102. doi: 10.1016/j.jbiotec.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 153.Horbal L., Fedorenko V., Luzhetskyy A. Novel and Tightly Regulated Resorcinol and Cumate-Inducible Expression Systems for Streptomyces and Other Actinobacteria. Appl. Microbiol. Biotechnol. 2014;98:8641–8655. doi: 10.1007/s00253-014-5918-x. [DOI] [PubMed] [Google Scholar]
- 154.Wang W., Yang T., Li Y., Li S., Yin S., Styles K., Corre C., Yang K. Development of a Synthetic Oxytetracycline-Inducible Expression System for Streptomycetes Using De Novo Characterized Genetic Parts. ACS Synth. Biol. 2016;5:765–773. doi: 10.1021/acssynbio.6b00087. [DOI] [PubMed] [Google Scholar]
- 155.Matsumoto M., Hashimoto Y., Saitoh Y., Kumano T., Kobayashi M. Development of Nitrilase Promoter-Derived Inducible Vectors for Streptomyces. Biosci. Biotechnol. Biochem. 2016;80:1230–1237. doi: 10.1080/09168451.2016.1148577. [DOI] [PubMed] [Google Scholar]
- 156.Rudolph M.M., Vockenhuber M.P., Suess B. Conditional Control of Gene Expression by Synthetic Riboswitches in Streptomyces coelicolor. Methods Enzymol. 2015;550:283–299. doi: 10.1016/bs.mie.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 157.Mehdizadeh Aghdam E., Hejazi M.S., Barzegar A. Riboswitches: From Living Biosensors to Novel Targets of Antibiotics. Gene. 2016;592:244–259. doi: 10.1016/j.gene.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 158.Winkler W.C., Breaker R.R. Regulation of Bacterial Gene Expression by Riboswitches. Annu. Rev. Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 159.Findeiß S., Etzel M., Will S., Mörl M., Stadler P.F. Design of Artificial Riboswitches as Biosensors. Sensors. 2017;17:1990. doi: 10.3390/s17091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suess B., Fink B., Berens C., Stentz R., Hillen W. A Theophylline Responsive Riboswitch Based on Helix Slipping Controls Gene Expression in Vivo. Nucleic Acids Res. 2004;32:1610–1614. doi: 10.1093/nar/gkh321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rudolph M.M., Vockenhuber M.-P., Suess B. Synthetic Riboswitches for the Conditional Control of Gene Expression in Streptomyces coelicolor. Microbiology. 2013;159:1416–1422. doi: 10.1099/mic.0.067322-0. [DOI] [PubMed] [Google Scholar]
- 162.Horbal L., Luzhetskyy A. Dual Control System–a Novel Scaffolding Architecture of an Inducible Regulatory Device for the Precise Regulation of Gene Expression. Metab. Eng. 2016;37:11–23. doi: 10.1016/j.ymben.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Combes P., Till R., Bee S., Smith M.C. The Streptomyces Genome Contains Multiple Pseudo-attB Sites for the (Phi)C31-Encoded Site-Specific Recombination System. J. Bacteriol. 2002;184:5746–5752. doi: 10.1128/JB.184.20.5746-5752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gregory M.A., Till R., Smith M.C. Integration Site for Streptomyces Phage PhiBT1 and Development of Site-Specific Integrating Vectors. J. Bacteriol. 2003;185:5320–5323. doi: 10.1128/JB.185.17.5320-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Van Mellaert L., Mei L., Lammertyn E., Schacht S., Anne J. Site-Specific Integration of Bacteriophage VWB Genome into Streptomyces venezuelae and Construction of a VWB-Based Integrative Vector. (Pt 12)Microbiology. 1998;144:3351–3358. doi: 10.1099/00221287-144-12-3351. [DOI] [PubMed] [Google Scholar]
- 166.Morita K., Yamamoto T., Fusada N., Komatsu M., Ikeda H., Hirano N., Takahashi H. The Site-Specific Recombination System of Actinophage TG1. FEMS Microbiol. Lett. 2009;297:234–240. doi: 10.1111/j.1574-6968.2009.01683.x. [DOI] [PubMed] [Google Scholar]
- 167.Fayed B., Younger E., Taylor G., Smith M.C. A Novel Streptomyces Spp. Integration Vector Derived from the S. venezuelae Phage, SV1. BMC Biotechnol. 2014;14:51. doi: 10.1186/1472-6750-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miura T., Hosaka Y., Yan-Zhuo Y., Nishizawa T., Asayama M., Takahashi H., Shirai M. In Vivo and in Vitro Characterization of Site-Specific Recombination of Actinophage R4 Integrase. J. Gen. Appl. Microbiol. 2011;57:45–57. doi: 10.2323/jgam.57.45. [DOI] [PubMed] [Google Scholar]
- 169.Boccard F., Smokvina T., Pernodet J.L., Friedmann A., Guerineau M. The Integrated Conjugative Plasmid pSAM2 of Streptomyces ambofaciens Is Related to Temperate Bacteriophages. EMBO J. 1989;8:973–980. doi: 10.1002/j.1460-2075.1989.tb03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fogg P.C.M., Haley J.A., Stark W.M., Smith M.C.M. Genome Integration and Excision by a New Streptomyces Bacteriophage, PhiJoe. Appl. Environ. Microbiol. 2017;83:e02767-16. doi: 10.1128/AEM.02767-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thibessard A., Bertrand C., Hiblot J., Piotrowski E., Leblond P. Construction of pDYN6902, a New Streptomyces Integrative Expression Vector Designed for Cloning Sequences Interfering with Escherichia coli Viability. Plasmid. 2015;82:43–49. doi: 10.1016/j.plasmid.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 172.Huang J., Shi J., Molle V., Sohlberg B., Weaver D., Bibb M.J., Karoonuthaisiri N., Lih C.J., Kao C.M., Buttner M.J., et al. Cross-Regulation among Disparate Antibiotic Biosynthetic Pathways of Streptomyces coelicolor. Mol. Microbiol. 2005;58:1276–1287. doi: 10.1111/j.1365-2958.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- 173.Standard European Vector Architecture (Seva) [(accessed on 5 August 2020)]; Available online: http://seva.cnb.csic.es.
- 174.Durante-Rodriguez G., De Lorenzo V., Martinez-Garcia E. The Standard European Vector Architecture (Seva) Plasmid Toolkit. Methods Mol. Biol. 2014;1149:469–478. doi: 10.1007/978-1-4939-0473-0_36. [DOI] [PubMed] [Google Scholar]
- 175.Martinez-Garcia E., Goni-Moreno A., Bartley B., McLaughlin J., Sanchez-Sampedro L., Pascual Del Pozo H., Prieto Hernandez C., Marletta A.S., De Lucrezia D., Sanchez-Fernandez G., et al. Seva 3.0: An Update of the Standard European Vector Architecture for Enabling Portability of Genetic Constructs among Diverse Bacterial Hosts. Nucleic Acids Res. 2020;48:D1164–D1170. doi: 10.1093/nar/gkz1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garcia-Gutierrez C., Aparicio T., Torres-Sanchez L., Martinez-Garcia E., De Lorenzo V., Villar C.J., Lombo F. Multifunctional Seva Shuttle Vectors for Actinomycetes and Gram-Negative Bacteria. MicrobiologyOpen. 2020;9:1135–1149. doi: 10.1002/mbo3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schweizer H.P. Applications of the Saccharomyces cerevisiae Flp-FRT System in Bacterial Genetics. J. Mol. Microbiol. Biotechnol. 2003;5:67–77. doi: 10.1159/000069976. [DOI] [PubMed] [Google Scholar]
- 178.Li L., Zheng G., Chen J., Ge M., Jiang W., Lu Y. Multiplexed Site-Specific Genome Engineering for Overproducing Bioactive Secondary Metabolites in Actinomycetes. Metab. Eng. 2017;40:80–92. doi: 10.1016/j.ymben.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 179.Huang H., Zheng G., Jiang W., Hu H., Lu Y. One-Step High-Efficiency CRISPR/Cas9-Mediated Genome Editing in Streptomyces. Acta Bioch. Bioph. Sin. 2015;47:231–243. doi: 10.1093/abbs/gmv007. [DOI] [PubMed] [Google Scholar]
- 180.Li L., Wei K., Liu X., Wu Y., Zheng G., Chen S., Jiang W., Lu Y. aMSGE: Advanced Multiplex Site-Specific Genome Engineering with Orthogonal Modular Recombinases in Actinomycetes. Metab. Eng. 2019;52:153–167. doi: 10.1016/j.ymben.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 181.Phelan R.M., Sachs D., Petkiewicz S.J., Barajas J.F., Blake-Hedges J.M., Thompson M.G., Reider Apel A., Rasor B.J., Katz L., Keasling J.D. Development of Next Generation Synthetic Biology Tools for Use in Streptomyces venezuelae. ACS Synth. Biol. 2017;6:159–166. doi: 10.1021/acssynbio.6b00202. [DOI] [PubMed] [Google Scholar]
- 182.Ko B., D’Alessandro J., Douangkeomany L., Stumpf S., DeButts A., Blodgett J. Construction of a New Integrating Vector from Actinophage VarphiOZJ and Its Use in Multiplex Streptomyces Transformation. J. Ind. Microbiol. Biotechnol. 2020;47:73–81. doi: 10.1007/s10295-019-02246-7. [DOI] [PubMed] [Google Scholar]
- 183.Goryshin I.Y., Naumann T.A., Apodaca J., Reznikoff W.S. Chromosomal Deletion Formation System Based on Tn5 Double Transposition: Use for Making Minimal Genomes and Essential Gene Analysis. Genome Res. 2003;13:644–653. doi: 10.1101/gr.611403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reznikoff W.S. Tn5 as a Model for Understanding DNA Transposition. Mol. Microbiol. 2003;47:1199–1206. doi: 10.1046/j.1365-2958.2003.03382.x. [DOI] [PubMed] [Google Scholar]
- 185.Reznikoff W.S. Transposon Tn5. Annu. Rev. Genet. 2008;42:269–286. doi: 10.1146/annurev.genet.42.110807.091656. [DOI] [PubMed] [Google Scholar]
- 186.Kaniecki K., De Tullio L., Greene E.C. A Change of View: Homologous Recombination at Single-Molecule Resolution. Nat. Rev. Genet. 2018;19:191–207. doi: 10.1038/nrg.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weber T., Charusanti P., Musiol-Kroll E.M., Jiang X., Tong Y., Kim H.U., Lee S.Y. Metabolic Engineering of Antibiotic Factories: New Tools for Antibiotic Production in Actinomycetes. Trends Biotechnol. 2015;33:15–26. doi: 10.1016/j.tibtech.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 188.Doroghazi J.R., Buckley D.H. Widespread Homologous Recombination within and between Streptomyces Species. ISME J. 2010;4:1136–1143. doi: 10.1038/ismej.2010.45. [DOI] [PubMed] [Google Scholar]
- 189.Doroghazi J.R., Buckley D.H. A Model for the Effect of Homologous Recombination on Microbial Diversification. Genome Biol. Evol. 2011;3:1349–1356. doi: 10.1093/gbe/evr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wright W.D., Shah S.S., Heyer W.D. Homologous Recombination and the Repair of DNA Double-Strand Breaks. J. Biol. Chem. 2018;293:10524–10535. doi: 10.1074/jbc.TM118.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Herrmann S., Siegl T., Luzhetska M., Petzke L., Jilg C., Welle E., Erb A., Leadlay P.F., Bechthold A., Luzhetskyy A. Site-Specific Recombination Strategies for Engineering Actinomycete Genomes. Appl. Environ. Microbiol. 2012;78:1804–1812. doi: 10.1128/AEM.06054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jacquier A., Dujon B. An Intron-Encoded Protein Is Active in a Gene Conversion Process That Spreads an Intron into a Mitochondrial Gene. Cell. 1985;41:383–394. doi: 10.1016/S0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- 193.Jasin M. Genetic Manipulation of Genomes with Rare-Cutting Endonucleases. Trends Genet. 1996;12:224–228. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 194.Fernandez-Martinez L.T., Del Sol R., Evans M.C., Fielding S., Herron P.R., Chandra G., Dyson P.J. A Transposon Insertion Single-Gene Knockout Library and New Ordered Cosmid Library for the Model Organism Streptomyces coelicolor A3(2) Antonie Van Leeuwenhoek. 2011;99:515–522. doi: 10.1007/s10482-010-9518-1. [DOI] [PubMed] [Google Scholar]
- 195.Petzke L., Luzhetskyy A. In Vivo Tn5-Based Transposon Mutagenesis of Streptomycetes. Appl. Microbiol. Biotechnol. 2009;83:979–986. doi: 10.1007/s00253-009-2047-z. [DOI] [PubMed] [Google Scholar]
- 196.Bilyk B., Weber S., Myronovskyi M., Bilyk O., Petzke L., Luzhetskyy A. In Vivo Random Mutagenesis of Streptomycetes Using Mariner-Based Transposon Himar1. Appl. Microbiol. Biotechnol. 2013;97:351–359. doi: 10.1007/s00253-012-4550-x. [DOI] [PubMed] [Google Scholar]
- 197.Robertsen H.L., Musiol-Kroll E.M., Ding L., Laiple K.J., Hofeditz T., Wohlleben W., Lee S.Y., Grond S., Weber T. Filling the Gaps in the Kirromycin Biosynthesis: Deciphering the Role of Genes Involved in Ethylmalonyl-CoA Supply and Tailoring Reactions. Sci. Rep. 2018;8:3230. doi: 10.1038/s41598-018-21507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hoff G., Bertrand C., Piotrowski E., Thibessard A., Leblond P. Genome Plasticity Is Governed by Double Strand Break DNA Repair in Streptomyces. Sci. Rep. 2018;8:5272. doi: 10.1038/s41598-018-23622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Darbon E., Martel C., Nowacka A., Pegot S., Moreau P.L., Virolle M.-J. Transcriptional and Preliminary Functional Analysis of the Six Genes Located in Divergence of phoR/phoP in Streptomyces lividans. Appl. Microbiol. Biotechnol. 2012;95:1553–1566. doi: 10.1007/s00253-012-3995-2. [DOI] [PubMed] [Google Scholar]
- 200.Lu Z., Xie P., Qin Z. Promotion of Markerless Deletion of the Actinorhodin Biosynthetic Gene Cluster in Streptomyces coelicolor. Acta Biochim. Biophys. Sin. 2010;42:717–721. doi: 10.1093/abbs/gmq080. [DOI] [PubMed] [Google Scholar]
- 201.Siegl T., Petzke L., Welle E., Luzhetskyy A. I-SceI Endonuclease: A New Tool for DNA Repair Studies and Genetic Manipulations in Streptomycetes. Appl. Microbiol. Biotechnol. 2010;87:1525–1532. doi: 10.1007/s00253-010-2643-y. [DOI] [PubMed] [Google Scholar]
- 202.Luo S., Chen X.A., Mao X.M., Li Y.Q. Transposon-Based Identification of a Negative Regulator for the Antibiotic Hyper-Production in Streptomyces. Appl. Microbiol. Biotechnol. 2018;102:6581–6592. doi: 10.1007/s00253-018-9103-5. [DOI] [PubMed] [Google Scholar]
- 203.Baltz R.H., McHenney M.A., Cantwell C.A., Queener S.W., Solenberg P.J. Applications of Transposition Mutagenesis in Antibiotic Producing Streptomycetes. Antonie Van Leeuwenhoek. 1997;71:179–187. doi: 10.1023/A:1000177808686. [DOI] [PubMed] [Google Scholar]
- 204.Horbal L., Fedorenko V., Bechthold A., Luzhetskyy A. A Transposon-Based Strategy to Identify the Regulatory Gene Network Responsible for Landomycin E Biosynthesis. FEMS Microbiol. Lett. 2013;342:138–146. doi: 10.1111/1574-6968.12117. [DOI] [PubMed] [Google Scholar]
- 205.Xu Z., Wang Y., Chater K.F., Ou H.-Y., Xu H.H., Deng Z., Tao M. Large-Scale Transposition Mutagenesis of Streptomyces coelicolor Identifies Hundreds of Genes Influencing Antibiotic Biosynthesis. Appl. Environ. Microbiol. 2017;83:e02889–e02816. doi: 10.1128/AEM.02889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Meyer F., Pupkes H., Steinbuchel A. Development of an Improved System for the Generation of Knockout Mutants of Amycolatopsis Sp. Strain ATCC 39116. Appl. Environ. Microbiol. 2017;83:e02660-16. doi: 10.1128/AEM.02660-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lederberg J. Streptomycin Resistance; a Genetically Recessive Mutation. J. Bacteriol. 1951;61:549–550. doi: 10.1128/JB.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fernández-Martínez L.T., Bibb M.J. Use of the Meganuclease I-SceI of Saccharomyces cerevisiae to Select for Gene Deletions in Actinomycetes. Sci. Rep. 2014;4:7100. doi: 10.1038/srep07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jansen R., Embden J.D.A.v., Gaastra W., Schouls L.M. Identification of Genes That Are Associated with DNA Repeats in Prokaryotes. Mol. Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 210.Bolotin A., Quinquis B., Sorokin A., Ehrlich S.D. Clustered Regularly Interspaced Short Palindrome Repeats (CRISPRs) Have Spacers of Extrachromosomal Origin. Microbiology. 2005;151:2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 211.Ding Q., Regan S.N., Xia Y., Oostrom L.A., Cowan C.A., Musunuru K. Enhanced Efficiency of Human Pluripotent Stem Cell Genome Editing through Replacing Talens with CRISPRs. Cell Stem Cell. 2013;12:393. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Generoso W.C., Gottardi M., Oreb M., Boles E. Simplified CRISPR-Cas Genome Editing for Saccharomyces cerevisiae. J. Microbiol. Methods. 2016;127:203–205. doi: 10.1016/j.mimet.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 213.Jiang W., Bikard D., Cox D., Zhang F., Marraffini L.A. RNA-Guided Editing of Bacterial Genomes Using CRISPR-Cas Systems. Nat. Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shan Q., Wang Y., Li J., Zhang Y., Chen K., Liang Z., Zhang K., Liu J., Xi J.J., Qiu J.-L. Targeted Genome Modification of Crop Plants Using a CRISPR-Cas System. Nat. Biotechnol. 2013;31:686–688. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- 215.Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P. CRISPR Provides Acquired Resistance against Viruses in Prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 217.Van der Oost J., Jore M.M., Westra E.R., Lundgren M., Brouns S.J. CRISPR-Based Adaptive and Heritable Immunity in Prokaryotes. Trends Biochem. Sci. 2009;34:401–407. doi: 10.1016/j.tibs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 218.Amitai G., Sorek R. CRISPR–Cas Adaptation: Insights into the Mechanism of Action. Nat. Rev. Microbiol. 2016;14:67–76. doi: 10.1038/nrmicro.2015.14. [DOI] [PubMed] [Google Scholar]
- 219.Jackson S.A., McKenzie R.E., Fagerlund R.D., Kieper S.N., Fineran P.C., Brouns S.J. CRISPR-Cas: Adapting to Change. Science. 2017;356:eaal5056. doi: 10.1126/science.aal5056. [DOI] [PubMed] [Google Scholar]
- 220.Rath D., Amlinger L., Rath A., Lundgren M. The CRISPR-Cas Immune System: Biology, Mechanisms and Applications. Biochimie. 2015;117:119–128. doi: 10.1016/j.biochi.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 221.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang X.-H., Tee L.Y., Wang X.-G., Huang Q.-S., Yang S.-H. Off-Target Effects in CRISPR/Cas9-Mediated Genome Engineering. Mol. Ther-Nucl. Acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cobb R.E., Wang Y., Zhao H. High-Efficiency Multiplex Genome Editing of Streptomyces Species Using an Engineered CRISPR/Cas System. ACS Synth. Biol. 2015;4:723–728. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tong Y., Weber T., Lee S.Y. CRISPR/Cas-Based Genome Engineering in Natural Product Discovery. Nat. Prod. Rep. 2019;36:1262–1280. doi: 10.1039/C8NP00089A. [DOI] [PubMed] [Google Scholar]
- 225.Ye S., Enghiad B., Zhao H., Takano E. Fine-Tuning the Regulation of Cas9 Expression Levels for Efficient CRISPR-Cas9 Mediated Recombination in Streptomyces. J. Ind. Microbiol. Biotechnol. 2020:413–423. doi: 10.1007/s10295-020-02277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Muth G. The pSG5-Based Thermosensitive Vector Family for Genome Editing and Gene Expression in Actinomycetes. Appl. Microbiol. Biotechnol. 2018;102:9067–9080. doi: 10.1007/s00253-018-9334-5. [DOI] [PubMed] [Google Scholar]
- 227.Wolf T., Gren T., Thieme E., Wibberg D., Zemke T., Pühler A., Kalinowski J. Targeted Genome Editing in the Rare Actinomycete Actinoplanes Sp. Se50/110 by Using the CRISPR/Cas9 System. J. Biotechnol. 2016;231:122–128. doi: 10.1016/j.jbiotec.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 228.Qin Z., Munnoch J.T., Devine R., Holmes N.A., Seipke R.F., Wilkinson K.A., Wilkinson B., Hutchings M.I. Formicamycins, Antibacterial Polyketides Produced by Streptomyces formicae Isolated from African Tetraponera Plant-Ants. Chem. Sci. 2017;8:3218–3227. doi: 10.1039/C6SC04265A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tong Y., Charusanti P., Zhang L., Weber T., Lee S.Y. CRISPR-Cas9 Based Engineering of Actinomycetal Genomes. ACS Synth. Biol. 2015;4:1020–1029. doi: 10.1021/acssynbio.5b00038. [DOI] [PubMed] [Google Scholar]
- 230.Wohlleben W., Stegmann E., Süssmuth R.D. Molecular Genetic Approaches to Analyze Glycopeptide Biosynthesis. Methods Enzymol. 2009;458:459–486. doi: 10.1016/S0076-6879(09)04818-6. [DOI] [PubMed] [Google Scholar]
- 231.Low Z.J., Pang L.M., Ding Y., Cheang Q.W., Le Mai Hoang K., Thi Tran H., Li J., Liu X.-W., Kanagasundaram Y., Yang L., et al. Identification of a Biosynthetic Gene Cluster for the Polyene Macrolactam Sceliphrolactam in a Streptomyces Strain Isolated from Mangrove Sediment. Sci. Rep-UK. 2018;8:1594. doi: 10.1038/s41598-018-20018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cohen D.R., Townsend C.A. A Dual Role for a Polyketide Synthase in Dynemicin Enediyne and Anthraquinone Biosynthesis. Nat. Chem. 2018;10:231–236. doi: 10.1038/nchem.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhao Y., Li L., Zheng G., Jiang W., Deng Z., Wang Z., Lu Y. CRISPR/dCas9-Mediated Multiplex Gene Repression in Streptomyces. J. Biotechnol. 2018;13:e1800121. doi: 10.1002/biot.201800121. [DOI] [PubMed] [Google Scholar]
- 234.Tong Y., Whitford C.M., Robertsen H.L., Blin K., Jørgensen T.S., Klitgaard A.K., Gren T., Jiang X., Weber T., Lee S.Y. Highly Efficient DSB-Free Base Editing for Streptomycetes with CRSISPR-BEST. Proc. Natl. Acad. Sci. USA. 2019;116:20366–20375. doi: 10.1073/pnas.1913493116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Haurwitz R.E., Jinek M., Wiedenheft B., Zhou K., Doudna J.A. Sequence-and Structure-Specific RBA Processing by a CRISPR Endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Blin K., Pedersen L.E., Weber T., Lee S.Y. CRISPy-Web: An Online Resource to Design sgRNAs for CRISPR Applications. Synt. Syst. Biotechnol. 2016;1:118–121. doi: 10.1016/j.synbio.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dubeau M.-P., Ghinet M.G., Jacques P.-É., Clermont N., Beaulieu C., Brzezinski R. Cytosine Deaminase as a Negative Selection Marker for Gene Disruption and Replacement in the Genus Streptomyces and Other Actinobacteria. Appl. Environ. Microbiol. 2009;75:1211–1214. doi: 10.1128/AEM.02139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zeng H., Wen S., Xu W., He Z., Zhai G., Liu Y., Deng Z., Sun Y. Highly Efficient Editing of the Actinorhodin Polyketide Chain Length Factor Gene in Streptomyces coelicolor M145 Using CRISPR/Cas9-CodA (Sm) Combined System. Appl. Microbiol. Biotechnol. 2015;99:10575–10585. doi: 10.1007/s00253-015-6931-4. [DOI] [PubMed] [Google Scholar]
- 239.Zheng J., Li Y., Guan H., Zhang J., Tan H. Enhancement of Neomycin Production by Engineering the Entire Biosynthetic Gene Cluster and Feeding Key Precursors in Streptomyces fradiae Cgmcc 4.576. Appl. Microbiol. Biotechnol. 2019;103:2263–2275. doi: 10.1007/s00253-018-09597-8. [DOI] [PubMed] [Google Scholar]
- 240.Zhang M.M., Wong F.T., Wang Y., Luo S., Lim Y.H., Heng E., Yeo W.L., Cobb R.E., Enghiad B., Ang E.L. CRISPR–Cas9 Strategy for Activation of Silent Streptomyces Biosynthetic Gene Clusters. Nat. Chem. Biol. 2017;13:607–609. doi: 10.1038/nchembio.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Najah S., Saulnier C., Pernodet J.-L., Bury-Moné S. Design of a Generic CRISPR-Cas9 Approach Using the Same sgRNA to Perform Gene Editing at Distinct Loci. BMC Biotechnol. 2019;19:1–8. doi: 10.1186/s12896-019-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kouprina N., Larionov V. Tar Cloning: Insights into Gene Function, Long-Range Haplotypes and Genome Structure and Evolution. Nat. Rev. Genet. 2006;7:805–812. doi: 10.1038/nrg1943. [DOI] [PubMed] [Google Scholar]
- 243.Lee N.C., Larionov V., Kouprina N. Highly Efficient CRISPR/Cas9-Mediated TAR Cloning of Genes and Chromosomal Loci from Complex Genomes in Yeast. Nucleic Acids Res. 2015;43:e55–e55. doi: 10.1093/nar/gkv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Meng J., Feng R., Zheng G., Ge M., Mast Y., Wohlleben W., Gao J., Jiang W., Lu Y. Improvement of Pristinamycin I (PI) Production in Streptomyces pristinaespiralis by Metabolic Engineering Approaches. Synth. Syst. Biotechnol. 2017;2:130–136. doi: 10.1016/j.synbio.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liu P., Zhu H., Zheng G., Jiang W., Lu Y. Metabolic Engineering of Streptomyces coelicolor for Enhanced Prodigiosins (RED) Production. Sci. China Life Sci. 2017;60:948–957. doi: 10.1007/s11427-017-9117-x. [DOI] [PubMed] [Google Scholar]
- 246.Jiang W., Zhao X., Gabrieli T., Lou C., Ebenstein Y., Zhu T.F. Cas9-Assisted Targeting of Chromosome Segments CATCH Enables One-Step Targeted Cloning of Large Gene Clusters. Nat. Commun. 2015;6:8101. doi: 10.1038/ncomms9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang J.-W., Wang A., Li K., Wang B., Jin S., Reiser M., Lockey R.F. CRISPR/Cas9 Nuclease Cleavage Combined with Gibson Assembly for Seamless Cloning. BioTechniques. 2015;58:161–170. doi: 10.2144/000114261. [DOI] [PubMed] [Google Scholar]
- 248.Tao W., Chen L., Zhao C., Wu J., Yan D., Deng Z., Sun Y. In Vitro Packaging Mediated One-Step Targeted Cloning of Natural Product Pathway. ACS Synth. Biol. 2019;8:1991–1997. doi: 10.1021/acssynbio.9b00248. [DOI] [PubMed] [Google Scholar]
- 249.Alberti F., Corre C. Editing Streptomycete Genomes in the CRISPR/Cas9 Age. Nat Prod Rep. 2019;36:1237–1248. doi: 10.1039/C8NP00081F. [DOI] [PubMed] [Google Scholar]