Abstract
O-1602 and O-1918 are atypical cannabinoid ligands for GPR55 and GPR18, which may be novel pharmaceuticals for the treatment of obesity by targeting energy homeostasis regulation in skeletal muscle. This study aimed to determine the effect of O-1602 or O-1918 on markers of oxidative capacity and fatty acid metabolism in the skeletal muscle. Diet-induced obese (DIO) male Sprague Dawley rats were administered a daily intraperitoneal injection of O-1602, O-1918 or vehicle for 6 weeks. C2C12 myotubes were treated with O-1602 or O-1918 and human primary myotubes were treated with O-1918. GPR18 mRNA was expressed in the skeletal muscle of DIO rats and was up-regulated in red gastrocnemius when compared with white gastrocnemius. O-1602 had no effect on mRNA expression on selected markers for oxidative capacity, fatty acid metabolism or adiponectin signalling in gastrocnemius from DIO rats or in C2C12 myotubes, while APPL2 mRNA was up-regulated in white gastrocnemius in DIO rats treated with O-1918. In C2C12 myotubes treated with O-1918, PGC1α, NFATc1 and PDK4 mRNA were up-regulated. There were no effects of O-1918 on mRNA expression in human primary myotubes derived from obese and obese T2DM individuals. In conclusion, O-1602 does not alter mRNA expression of key pathways important for skeletal muscle energy homeostasis in obesity. In contrast, O-1918 appears to alter markers of oxidative capacity and fatty acid metabolism in C2C12 myotubes only. GPR18 is expressed in DIO rat skeletal muscle and future work could focus on selectively modulating GPR18 in a tissue-specific manner, which may be beneficial for obesity-targeted therapies.
Keywords: Atypical Cannabinoids, O-1602, O-1918, GPR18, obesity and skeletal muscle
1. Introduction
Obesity rates and associated co-morbidities such as type two diabetes mellitus (T2DM) are increasing world-wide [1]. Pharmaceutically targeting these conditions may be beneficial in combination with a healthy diet and increased physical activity to help reduce the health-related costs and burdens for an individual, community and government.
Skeletal muscle is an important regulator of whole body energy expenditure and is a major determinant for resting energy expenditure in humans [2]. The skeletal muscle is a major site for glucose and fatty acid oxidation as well as insulin action and is an organ that is highly adaptable to environmental stressors such as obesity [3]. In obesity, there is an increased triglyceride content within skeletal muscle [4], which is associated with insulin resistance [5]. Pharmacologically modulating the skeletal muscle to alter signalling pathways and improve metabolic homeostasis in obesity and associated co-morbidities such as T2DM may therefore be beneficial. The skeletal muscle is heterogeneous in nature and is composed of different fibre phenotypes [6]; these fibre phenotypes can have either a more oxidative or glycolytic characteristic. The endocannabinoid system has previously been a pharmacological target for obesity [7]. As such, peripheral modulation of this system, particularly in the skeletal muscle, may be beneficial. Cannabinoid receptors (CB) CB1 and CB2 are expressed in the skeletal muscle [8], with CB1 influencing oxidative pathways [9]. Limited research has focused on atypical cannabinoid compounds and receptors within the skeletal muscle in obesity.
O-1602 and O-1918 are synthetic derivatives of cannabinoid compounds, which have an affinity/putative affinity to the putative cannabinoid receptors G Protein-Coupled Receptor (GPCR) 55 (GPR55) and GPR18 [10,11,12,13,14]. The putative cannabinoid receptor GPR55 [10] appears to have a role in regulating energy homeostasis [15]; GPR55 expression is up-regulated in the adipose tissue of obese humans when compared with non obese humans [16]. GPR55 deficiency in mice is associated with increased adiposity, reduced physical activity and energy expenditure as well as impaired insulin signalling in peripheral metabolic tissues [17]. GPR55 knockout mice have a slightly increased fasting plasma insulin compared with wild type mice, albeit not statistically significant [17]. GPR55 is expressed in the skeletal muscle obtained from a range of different species and cell lines including wild type mice (gastrocnemius), rat (soleus), L6 myotubes and human primary myotubes [17]; additionally, GPR55 expression appears to be increased when L6 cells are differentiated, as well as with pre-treatment with an endogenous ligand of GPR55, lysophosphatidylinositol (LPI) [17]. GPR18 expression has recently been verified in vascular smooth muscle obtained from human placenta [18] and previously has been shown to be expressed in cardiac tissue obtained from diabetic rats [19]. Currently, the expression of GPR18 in the skeletal muscle and thus its role, if any, in obesity is unknown.
Activation of GPR55 and GPR18 by O-1602 has previously been shown to enhance intracellular calcium mobilisation [13,20]. Recently, O-1602 enhanced intracellular calcium transients in mouse insulinoma (MIN6) pancreatic mouse β cell line, which led to an increase in insulin secretion; this study also showed that GPR55 protein was expressed in the MIN6 cell line [21]. GPR18 signalling, however, is complex and it has been found that either atypical cannabinoid ligand O-1602 or O-1918 enhance calcium-mediated mobilisation and MAPK activity, but the ligands did not influence β-arrestin translocation [13]. O-1918 has been suggested as a biased ligand for GPR18 with agonist activity [13], while others have found O-1918 to act as an antagonist for GPR18 [11]. In the skeletal muscle, increases in intracellular calcium are associated with muscle contraction positively influencing skeletal muscle homeostasis. Pharmacological modulation of GPR55 (unless otherwise stated) and GPR18 in the skeletal muscle could alter markers such as; nuclear factor of activated t-cells (NFAT), peroxisome proliferator-activated receptor-gamma coactivator alpha (PGC1α), pyruvate dehydrogenase kinase 4 (PDK4), adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1), which are influenced by calcium signalling [22,23,24,25] and may be a beneficial treatment for obesity.
Previous research indicates that atypical cannabinoid O-1602 has a role in energy homeostasis by increasing adiposity in rats [26]. The effect that O-1918 has on energy homeostasis is currently unclear, although cannabidiol (CBD), an analogue of O-1918, promotes a browning phenotype and lipolysis, while reducing thermogenesis and lipogenesis in 3T3-L1 adipocytes [27]. Abnormal cannabidiol (Abn-CBD), an analogue of O-1602, enhanced GPR18 expression in cardiac tissue obtained from diabetic rats; Abn-CBD restored both circulating and cardiac concentrations of adiponectin and nitric oxide, and diminished oxidative stress in diabetic rats, while O-1918 blunted these observed favourable effects in this model [28]. Our group has previously shown that chronic administration of atypical cannabinoid compounds do have an effect on whole body energy homeostasis in a rodent diet-induced obesity (DIO) model [29]. Specifically, in this DIO model, O-1602 reduced bodyweight, body fat and improved albuminuria, although it had adverse effects on the liver and kidney [29]. In the DIO model, O-1918 improved albuminuria, in the absence of an effect on body weight or total body composition [29]. Further, O-1918 treatment up-regulated a number of circulating pro-inflammatory cytokines and reduced the mass of brown fat pads, while having no effect on white fat pad mass [29]. While the atypical cannabinoids O-1602 and O-1918 appear, overall, not to have desirable effects in DIO systemically, understanding the effects that these compounds have on organs involved in the regulation of energy homeostasis, such as the skeletal muscle, may be beneficial.
This study aimed to determine whether GPR18 is expressed in the skeletal muscle of DIO rats and whether there is a variation in expression of GPR18 between red or white gastrocnemius skeletal muscle in either the presence or absence of O-1602 and O-1918. This study further aimed to determine the effect abnormal cannabinoid compounds O-1602 and/ or O-1918 have on the expression of markers involved in skeletal muscle homeostasis in a number of models including; C2C12 myotubes, whole muscle obtained from DIO rats, and human primary myotubes derived from obese and T2DM individuals.
2. Results
2.1. GPR18 Expression in Red and White Gastrocnemius in the Absence and Presence of Atypical Cannabinoids in DIO
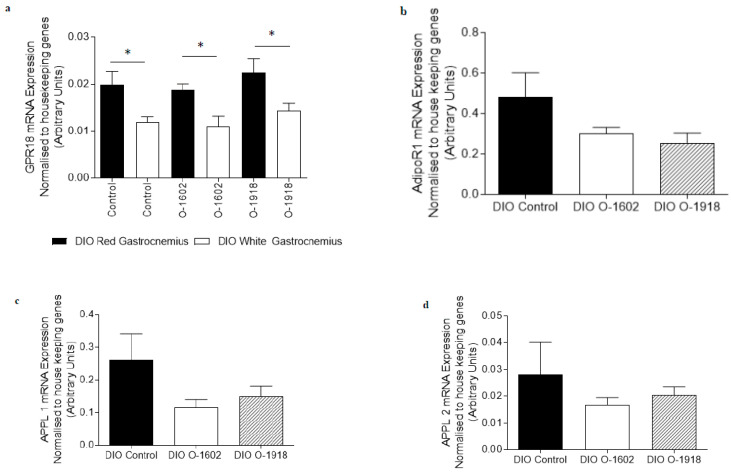
GPR18 mRNA was identified in both red and white gastrocnemius skeletal muscle obtained from DIO rats, both in the presence and the absence of atypical cannabinoid compounds O-1602 or O-1918 (Figure 1a). The abundance of GPR18 expression was increased in the red gastrocnemius skeletal muscle when compared with the white gastrocnemius skeletal muscle (p < 0.05); however, treatment with atypical cannabinoids did not significantly alter the expression of this receptor.
Figure 1.
The abundance of mRNA expressed for G Protein-Coupled Receptor 18 and markers involved in adiponectin signalling, fatty acid metabolism and oxidative capacity in red gastrocnemius skeletal muscle obtained from rats fed a high fat diet for 9 weeks to induce obesity. The diet induced obese (DIO) control rats, the DIO O-1602 rats and the DIO O-1918 rats were treated via intraperitoneal injection for a further 6 weeks. mRNA expression was normalised to the average of housekeeping genes cyclophilin and βActin and grouped data is reported as mean (arbitrary units) ± SEM. Figure 1a The red gastrocnemius treatment groups compared to the white gastrocnemius group (* significance p < 0.05). Figure 1b–i The DIO control group is compared to either the DIO O-1602 group or the DIO O-1918 group. (a) G Protein-Coupled Receptor 18 (includes both DIO red and white gastrocnemius); (b) Adiponectin Receptor 1 (AdipoR1); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (d) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2); (e) Peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α); (f) Forkhead box protein 01 (FOXO1); (g) Fatty Acid Translocase/Cluster of Differentiation 36 (FATCD/36); (h) beta-hydroxyacyl-CoA dehydrogenase (βHAD); (i) Pyruvate Dehydrogenase Kinase 4 (PDK4).
2.2. Atypical Cannabinoids Effect on mRNA Expression of Genes Involved in Skeletal Muscle Metabolism in Red and White Gastrocnemius in DIO
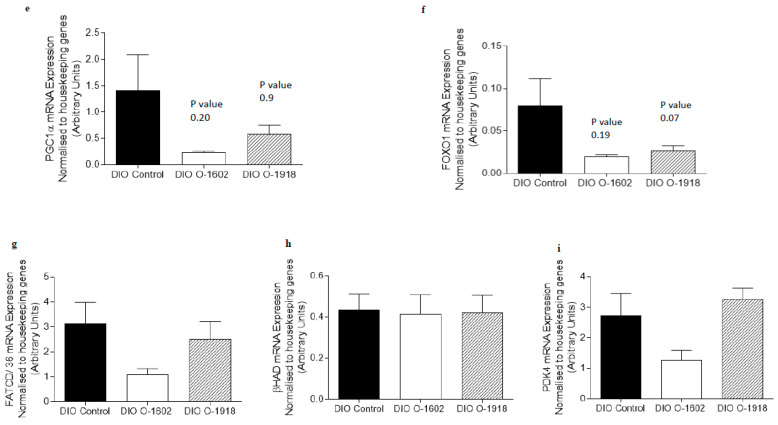
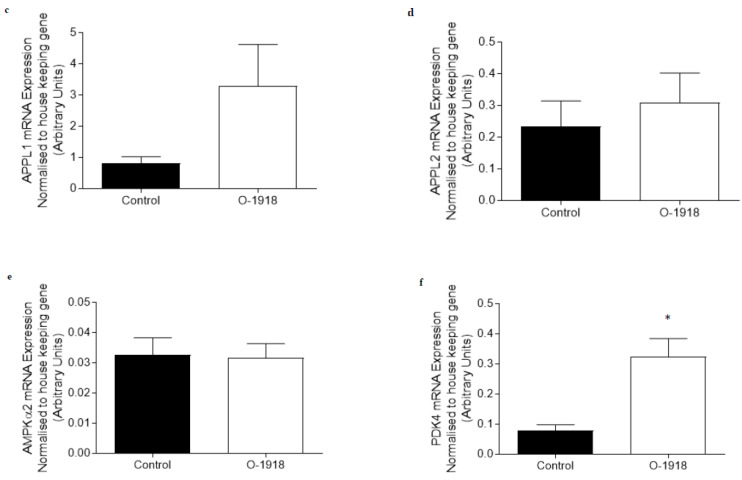
The mRNA expression of genes involved in adiponectin, fatty acid metabolism and oxidative capacity signaling pathways were not altered by treatment with O-1602 in red or white gastrocnemius skeletal muscle obtained from DIO rats (Figure 1b–i and Figure 2a–h) when compared with the DIO control group. APPL2 mRNA expression was increased (p < 0.05) in white gastrocnemius skeletal muscle (Figure 2c) following treatment with O-1918 compared to the DIO control group.
Figure 2.
The abundance of mRNA expressed for markers involved in adiponectin signalling, fatty acid metabolism and oxidative capacity in white gastrocnemius skeletal muscle obtained from rats fed a high fat diet for 9 weeks to induce obesity. The DIO control rats, DIO O-1602 rats and the DIO O-1918 rats were treated via intraperitoneal injection for a further 6 weeks. mRNA expression was normalised to the average of housekeeping genes cyclophilin and βActin and grouped data is reported as mean (arbitrary units) ± SEM. The DIO control group is compared to either the DIO O-1602 group (* significance p < 0.05) or the DIO O-1918 group (* significance p < 0.05). (a) Adiponectin Receptor 1 (AdipoR1); (b) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2); (d) Forkhead box protein 01 (FOXO1); (e) Peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α); (f) beta-hydroxyacyl-CoA dehydrogenase (βHAD); (g) Fatty Acid Translocase/Cluster of Differentiation 36 (FATCD/36); (h) Pyruvate Dehydrogenase Kinase 4 (PDK4).
The mRNA expression of all other genes measured involved in adiponectin, fatty acid metabolism and oxidative capacity signaling pathways were not altered in either red or white gastrocnemius skeletal muscle obtained from O-1918-treated DIO rats when compared with DIO control rats (Figure 1 and Figure 2).
2.3. Atypical Cannabinoids Effect on mRNA Expression of Genes Involved in Skeletal Muscle Metabolism in C2C12 Myotubes
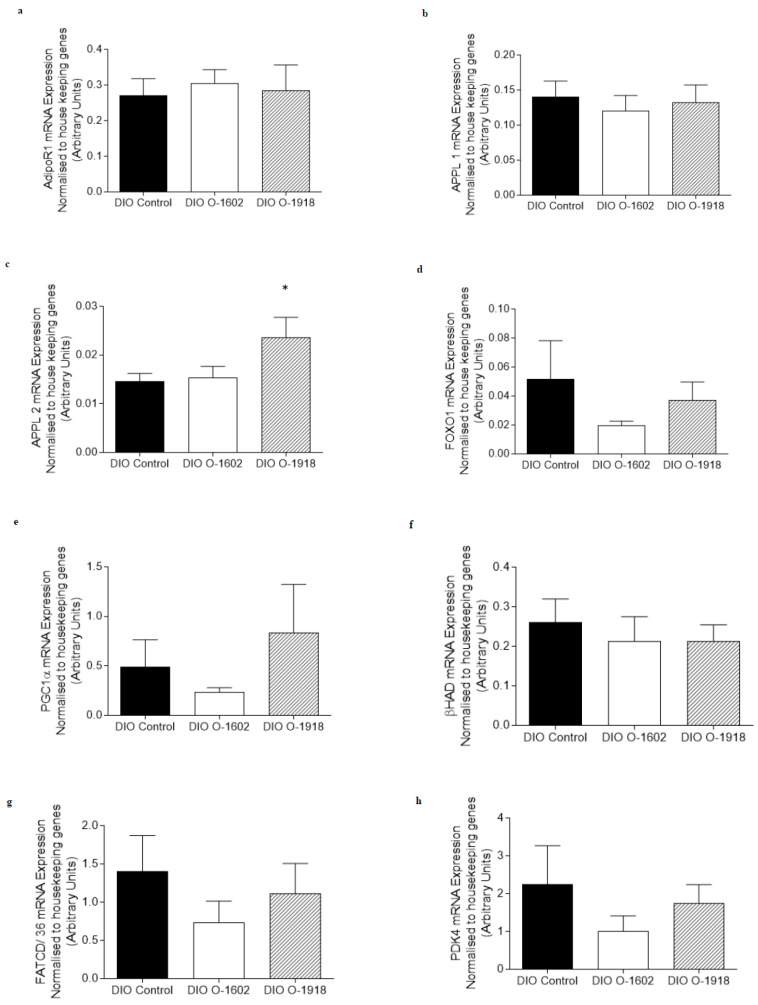
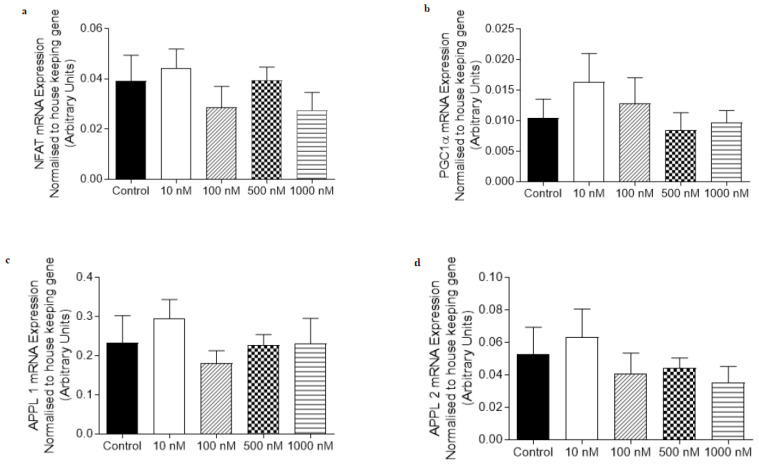
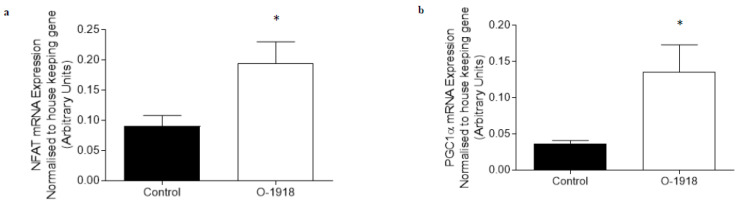
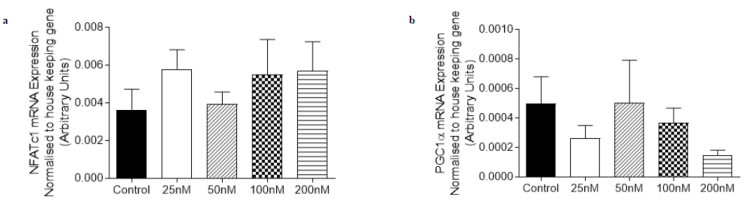
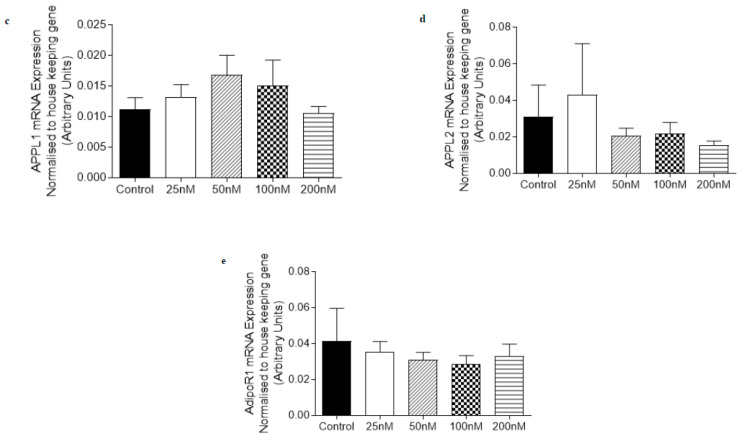
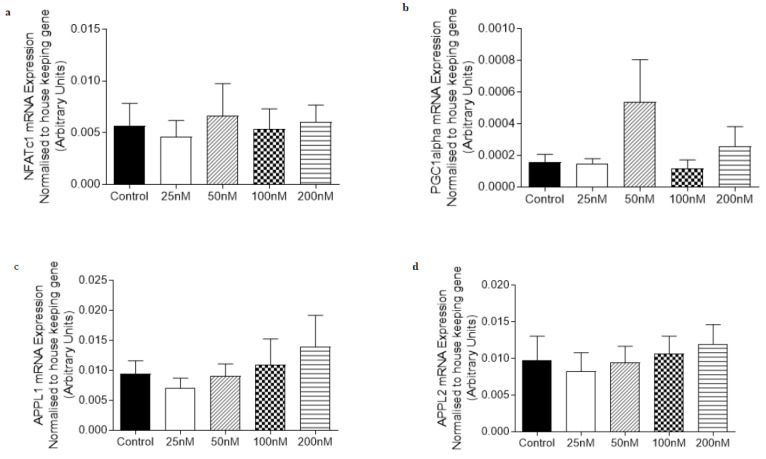
The mRNA expression of genes involved in adiponectin signaling (APPL1 and APPL2) and oxidative capacity (NFATc1 and PGC1α) were not altered by treatment with 10–1000 nM of O-1602 in C2C12 myotubes (Figure 3). Treatment with 100 nM of O-1918 on C2C12 myotubes caused an increase (p < 0.05) in the mRNA expression of NFATc1, PGC1α and PDK4 (Figure 4) when compared with the control group, while other markers including AMPKα2, APPL1 (p = 0.083) and APPL2 were not significantly altered when compared to the control group.
Figure 3.
The abundance of mRNA expressed for markers involved in adiponectin signalling, fatty acid oxidation and oxidative capacity in C2C12 myotubes treated for 24 h with O-1602 (10–1000 nM). mRNA expression was normalised to housekeeping gene Hypoxanthine Phosphoribosyltransferase (HPRT1) and grouped data is reported as mean (arbitrary units) ± SEM. (a) Nuclear Factor of Activated T-Cells calcineurin dependent 1 (NFATc1); (b) Peroxisome proliferator-activated receptor gamma co activator 1-alpha (PGC1α); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (d) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2).
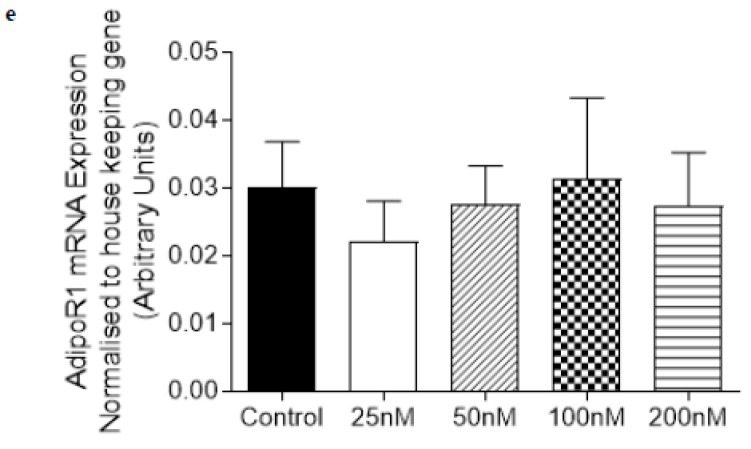
Figure 4.
The abundance of mRNA expressed for markers involved in adiponectin signalling, fatty acid oxidation and oxidative capacity in C2C12 myotubes treated for 24 h with O-1918 (100 nM). mRNA expression was normalised to housekeeping gene Hypoxanthine Phosphoribosyltransferase (HPRT1) and grouped data is reported as mean (arbitrary units) ± SEM (* significance p < 0.05). (a) Nuclear Factor of Activated T-Cells calcineurin dependent 1 (NFATc1); (b) Peroxisome proliferator-activated receptor gamma co activator 1-alpha (PGC1α); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (d) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2); (e) Adenosine Monophosphate Kinase alpha 2 (AMPKα2); (f) Pyruvate Dehydrogenase Kinase 4 (PDK4).
2.4. Effect of O-1918 on mRNA Expression of Oxidative Capacity and Adiponectin Signaling Genes in Human Primary Myotubes Obtained from Obese and Obese T2DM Individuals
Given our observations of O-1918 treatment on C2C12 myotubes and in the DIO rats, we then decided to determine the effect that O-1918 had on human primary myotubes derived from obese individuals and obese individuals with T2DM (Figure 5 and Figure 6). O-1918 did not have a significant effect on the mRNA expression of markers involved in oxidative capacity (NFAT or PGC1α) and adiponectin signaling (APPL1, APPL2 or AdipoR1) in either the obese or obese diabetic-derived myotubes.
Figure 5.
The abundance of mRNA expressed for markers involved in adiponectin signalling and oxidative capacity in human primary rectus abdominus-derived myotubes obtained from individuals that are obese treated for 24 h with O-1918 (25–200 nM). mRNA expression was normalised to housekeeping gene Cyclophilin and grouped data is reported as mean (arbitrary units) ± SEM. (a) Nuclear Factor of Activated T-Cells calcineurin dependent 1 (NFATc1); (b) Peroxisome proliferator-activated receptor gamma co activator 1-alpha (PGC1α); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (d) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2); (e) Adiponectin Receptor 1 (AdipoR1).
Figure 6.
The abundance of mRNA expressed for markers involved in adiponectin signalling and oxidative capacity in human primary rectus abdominus-derived myotubes obtained from individuals that are obese and have type two diabetes mellitus treated for 24 h with O-1918 (25–200 nM). mRNA expression was normalised to housekeeping gene Cyclophilin and grouped data is reported as mean (arbitrary units) ± SEM. (a) Nuclear Factor of Activated T-Cells calcineurin dependent 1 (NFATc1); (b) Peroxisome proliferator-activated receptor gamma co activator 1-alpha (PGC1α); (c) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 1 (APPL1); (d) Adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif 2 (APPL2); (e) Adiponectin Receptor 1 (AdipoR1).
3. Discussion
With the prevalence and incidence of obesity and related co-morbidities increasing world-wide [30], it is important to find different strategies to help address these health concerns. In addition to changes in dietary intake and physical activity, pharmacologically targeting the skeletal muscle to improve metabolic homeostasis [31] is a possible therapeutic strategy. We have recently shown that chronic administration of O-1602 or O-1918 has systemic effects in DIO rats [29]. Our previous study did not focus on the effects that these compounds had on the skeletal muscle, specifically, markers of fatty acid metabolism. Therefore, this study is the first to investigate atypical cannabinoid compounds O-1602 or O-1918 and their effect on skeletal muscle homeostasis in vitro in C2C12 myotubes and human primary myotubes, and in vivo in a DIO rat model.
Given that GPR55 is expressed in skeletal muscle obtained from rats [17] and appears to have a significant role in regulating insulin signalling [17], our study aimed to determine whether the other putative cannabinoid receptor, GPR18, was also expressed in skeletal muscle. The data included in this current study has shown for the first time that the putative cannabinoid receptor GPR18 is expressed in both red and white gastrocnemius skeletal muscle obtained from DIO rats. Our data suggests that GPR18 has a role in skeletal muscle metabolism, just like the traditional cannabinoid receptors CB1 and CB2, and the other putative cannabinoid receptor GPR55, all of which have previously been shown to be expressed in skeletal muscle [8,17] and all of which have a role in obesity [7,16,32].
Further, the results from this study showed that GPR18 mRNA expression was up-regulated in the red gastrocnemius when compared with the white gastrocnemius skeletal muscle, suggesting a variation in receptor expression between fibre types. Previous research shows that the CB1 receptor is down-regulated in soleus skeletal muscle obtained from obese Zucker rats when compared with lean Zucker rats [33]. In contrast, mice fed a high fat diet (HFD) for two months had up-regulated CB1 protein expression compared with standard chow-fed mice [34]. SR141716 treatment increased glucose uptake in the skeletal muscle of both standard chow- and high fat-fed mice [33]. CB1 receptors are mostly localised to the mitochondria in gastrocnemius and rectus abdominus skeletal muscle obtained from wild type mice, [35] although the effect that high fat feeding has on CB1 localisation within the mitochondria of skeletal muscle remains unclear. As GPR18 is up-regulated in red gastrocnemius in our study, this suggests that GPR18 may have a role in oxidative metabolism, however this finding is not reflective of cellular signalling and alterations that may occur as a result of receptor modulation, therefore further research into understanding the exact role of GPR18 in the skeletal muscle is required.
In addition to muscle type variation, systemic pharmacological treatment for six weeks with atypical cannabinoids O-1602 and O-1918 (compounds that have an affinity for the putative cannabinoid receptor GPR18 [11,13]), did not further alter the receptors’ mRNA expression in the DIO rats. Our results are similar to other cannabinoid research using AM251, an inverse agonist for the CB1 receptor, in which AM251 did not alter expression of the CB1 receptor in the skeletal muscle obtained from the abdominal wall of Wistar rats following two weeks of treatment, albeit these rats were fed a standard chow diet (SCD) and were not DIO [36]. In contrast, however, the same study did show an up-regulation of the other cannabinoid receptor CB2 with administration of the AM251 compound in SCD-fed Wistar rats [36]. In cardiac tissue obtained from streptozotocin (STZ)-induced male Wistar diabetic rats, a different atypical cannabinoid compound, abnormal cannabidiol (100 μg/kg), the analogue of O-1602, enhanced GPR18 protein expression (% control) following two weeks of treatment [28].
This study also aimed to determine whether the atypical cannabinoids O-1602 and O-1918 had an effect on skeletal muscle homeostasis in both C2C12 myotubes and human primary myotubes derived from obese or obese T2DM individuals (O-1918 only), as well as gastrocnemius skeletal muscle obtained from DIO rats. We have previously reported that rats fed a HFD for nine weeks have significantly greater body weight and body fat percentage when compared with rats fed a standard chow diet [37]. We have also reported that circulating concentrations of adiponectin were not altered by treatment with either O-1602 or O-1918 for six weeks in this DIO model [29]. Treatment for six weeks with O-1602, a biased agonist for GPR18 and an agonist for GPR55 [10,13] in DIO rats, caused no alterations in mRNA expression of markers involved in adiponectin signalling (AdipoR1, APPL1 and APPL2), fatty acid oxidation (FOXO1, βHAD, FATCD/36, PDK4) or oxidative capacity (PGC1α) in either the red or white gastrocnemius skeletal muscle. We have previously shown in this DIO model that O-1602 reduces total body fat and epididymal fat pad weight [29]. While we have demonstrated reduction in body fat in the DIO O-1602 rats as previously described [29], we did not observe any changes in the mRNA expression of any of the markers/pathways analysed in the skeletal muscle of these rats. In addition to the findings that O-1602 had on skeletal muscle in DIO, we also showed that O-1602 did not cause any alteration to markers of oxidative capacity (NFATc1 and PGC1α), or the positive regulator of adiponectin signalling APPL1 in C2C12 myotubes. Our study suggests that O-1602 does not appear to be effective in altering the skeletal muscle metabolism of these markers in the presence or absence of obesity.
Treatment with O-1918 in the red gastrocnemius did not alter markers of adiponectin signalling (AdipoR1, APPL1 and APPL2), markers of fatty acid metabolism (PDK4, FOXO1, βHAD, FATCD/36) or oxidative capacity (PGC1α). While in the glycolytic white gastrocnemius skeletal muscle, O-1918 up-regulated the mRNA expression of the negative regulator of adiponectin signalling, APPL2 [38]. However, in the white gastrocnemius skeletal muscle, O-1918 did not have any effect on other markers of adiponectin signalling (AdipoR1 or APPL1), fatty acid metabolism (FOXO1, βHAD, FAT/CD36 or PDK4) or oxidative capacity (PGC1α), while in the C2C12 myotubes, PGC1α, NFATc1, PDK4 and a trend for APPL1 were increased compared to control, which differed from the rat whole muscle tissue. The pharmacology for the atypical cannabinoid O-1918 is complex, as this compound acts as a putative antagonist for GPR55 and GPR18 [11], or as a biased agonist for GPR18 [13]. The fact that O-1918 enhances calcium mobilisation as a result of biased agonism at GPR18 [13] could help explain the up-regulation of mRNA for PGC1α [22] and NFAT, [23] and the trend for APPL 1 to be up-regulated [24] observed in our study. It has previously been reported that over expression of PGC1α in C2C12 myotubes resulted in activation of PDK4 mRNA and protein expression [25], and that over expression of PGC1α in C2C12 myotubes decreased the rate of glucose oxidation [25]. Therefore, the up-regulation of PGC1α mRNA expression observed in the C2C12 myotubes may also help to explain the up-regulation of PDK4 mRNA expression observed in our study.
4. Materials and Methods
4.1. Cell Culture
4.1.1. C2C12 Myotubes
C2C12 cells were a kind gift from Professor David Cameron-Smith (Deakin University, Melbourne, Australia). Mouse-derived C2C12 myoblasts were cultured in Dulbecco’s modified eagle high glucose growth medium (D-MEM) supplemented with 10% foetal bovine serum (v/v), 1% penicillin streptomycin (v/v), 0.5% amphotericin B (v/v) and incubated at 37°C, 5% CO2 in a humidity controlled environment as previously described [39]. C2C12 myoblasts were seeded into 6 well plates and differentiated into myotubes (within ~72 h) via supplementation with 2% horse serum (v/v), 1% penicillin streptomycin (v/v), 0.5% amphotericin B (v/v) [39]. C2C12 cells were serum starved in 0.1% BSA (w/v) and a D-MEM solution for six hours prior to treatment, then treated for 24 h with vehicle (0.1% ethanol; Sigma Aldrich, St Louise, MO, USA) (n = 8–9) or O-1602 (10 nM–1000 nM; Cayman Chemical, Ann Arbor, Michigan, USA) (n = 9) or O-1918 (100nM; Cayman Chemical, Ann Arbor, Michigan, USA) (n = 8) and all treatments were dissolved in 0.1% ethanol and suspended in a 0.1% BSA and a D-MEM solution. Following treatment, cells were washed with ice cold PBS; then lysed, on ice with TRIzol Reagent® (Invitrogen, Victoria, Australia) and stored at −80 °C for subsequent RNA extraction.
4.1.2. Human Primary Rectus Abdominus Myotubes
The approval for the collection of rectus abdominus skeletal muscle samples from obese individuals and obese individuals with T2DM was approved by the ethics committees at both Victoria University (St Albans, Victoria, Australia) and The Avenue Hospital (St Kilda, Victoria, Australia) approval number HRETH 08/158 (07/10/2008) and Trial 0100 (22/06/2008), respectively. A portion of rectus abdominus skeletal muscle was obtained during abdominal surgery to establish human primary myotube culture. Donor characteristics were obtained and are included in Table 1.
Table 1.
Characteristics for donors of rectus abdominus skeletal muscle.
| Characteristic | Group | |
|---|---|---|
| Obese (n = 8) | Obese Diabetic (n = 8) | |
| Sex | Female n = 5 Male n = 3 |
Female n = 5 Male n = 3 |
| Age (years) | 45.9 ± 4.9 | 48.6 ± 3.5 |
| Weight (kg) | 106.9 ± 6.8 | 114.2 ± 7.3 |
| Height (m) | 1.6 ± 0.0 | 1.7 ± 0.0 |
| BMI | 40.2 ± 1.8 | 39.5 ± 1.8 |
| Fasting Blood Glucose (mmol/L) | 5.3 ± 0.1 | 10.8 ± 1.5 * |
| Plasma Insulin (µU/L) | 9.5 ± 1.4 | 15.6 ± 2.6 |
| Hba1c % | 5.5 ± 0.1 | 8.8 ± 0.8 * |
| Cholesterol | 5.1 ± 0.5 | 4.9 ± 2.6 |
| Fasting Triglycerides | 1.4 ± 0.2 | 2.6 ± 0.6 |
| HDL-cholesterol | 1.4 ± 0.1 | 1.1 ± 0.1 * |
| LDL-cholesterol | 3.0 ± 0.4 | 3.2 ± 0.5 |
Values are expressed as means ± SEM. * Indicates a significant difference between obese and obese Diabetic groups (p < 0.05).
Human primary myotubes were established as previously described [40], once myoblasts reached passage 4 they were then differentiated into myotubes and incubated for 2 h in serum-free 0.1% BSA (w/v) alpha minimum essential media (αMEM). Following this, myotubes were treated for 24 h with αMEM, 0.1% BSA and either a dose range of 25–200 nM O-1918 or vehicle control (0.1% ethanol). Following treatment, cells were washed with ice cold PBS; then lysed, on ice with TRIzol Reagent® (Invitrogen, Carlsbad, California, USA) and stored at −80°C for subsequent RNA extraction.
4.2. Animal Care and High Fat Feeding
The approval of this study was obtained from the Animal Ethics Committee at the Howard Florey Institute (Parkville, Melbourne, Australia) (AEC 11-036). Seven week old male Sprague Dawley rats were purchased from the Animal Resource Centre (Canning Vale, Western Australia, Australia), then acclimatised to their new environment for at least seven days. Rats were singly housed for the duration of this study and fed a high fat diet HFD (21% fat diet by weight) [37,41] purchased from Specialty Feeds (Glen Forrest, Western Australia, Australia) and fed this diet for a total period of fifteen weeks. The first nine weeks of high fat feeding prior to treatment was to induce DIO [37].
O-1602 or O-1918 Pharmacological Intervention in DIO rats
The DIO rats continued the HFD for a subsequent six weeks following allocation into treatment groups, as previously described [29]. The treatment groups included; DIO Control (n = 11), DIO O-1602 (n = 6) and DIO O-1918 (n = 9). During the six weeks of pharmacological intervention, rats were administered a daily intraperitoneal (ip.) injection of either; a 0.75% Tween-80 saline solution (DIO Control), 5 mg/kg O-1602 (DIO O-1602) or 1 mg/kg O-1918 (DIO O-1918) dissolved in a 0.75% Tween-80 saline solution. O-1602 and O-1918 were sourced from Tocris Bioscience (Bristol, UK). The dose of O-1602 was selected due to the compound’s ability to reduce scores of colitis [42], while the dose of O-1918 was able to inhibit the hypotensive effects of abnormal cannabidiol [43].
Following the six week pharmacological treatment period, the rats were deeply anaesthetised using 3% isoflurane (Abbott, Botany, NSW, Australia), then red and white gastrocnemius skeletal muscles were surgically removed and a portion snap frozen in liquid nitrogen for subsequent analysis. Rats were then administered a lethal injection of 100 mg/kg sodium pentobarbitone (Virobac, Peakhurst, Australia) and euthanised via cardiac puncture.
4.3. RNA Extraction and cDNA Synthesis
Approximately 25–35 mg of red or white gastrocnemius skeletal muscle obtained from the pharmacologically treated DIO rats were utilised to extract RNA, as previously described [8,39]. RNA extracted from the rat tissue samples were treated with RQ1 RNAse-free DNAse kit (Promega Corporation, Madison, Wisconsin, USA) in accordance with manufacturer’s instructions. A total of 0.5 µg of RNA obtained from rat or cell culture was reverse transcribed into cDNA using the iScriptTM cDNA synthesis kit (BioRad Laboratories, Hercules, California, USA) in accordance with manufacturer’s instructions. In addition, 10 ng of cDNA was utilised for GPR18 expression and 2.5 ng of cDNA was utilised for analysis of all other genes involved in adiponectin signalling, fatty acid metabolism and oxidative capacity. The cDNA was stored at −20 °C.
Oligonucleotide primers were developed for selected genes using Oligoperfect Suite and then purchased from Geneworks Pty Ltd. (Adelaide, Australia). A BLAST search confirmed homologous binding for the target mRNA sequences. The forward and reverse oligonucleotide primer sequences for the genes of interest are detailed in Table 2.
Table 2.
Forward and Reverse Oligonucleotide Primer Sequences for ‘Real Time’ Polymerase Chain Reaction.
| Primer | Accession Number | Direction | Sequence |
|---|---|---|---|
| Rat Genes | |||
| Cyclophilin | NM_017101.1 | Forward (5′ 3′) | CTG ATG GCG AGC CCT TG |
| Reverse (5′ 3′) | TCT GCT GTC TTT GGA ACT TTG TC | ||
| β-Actin | NM_031144 | Forward (5′ 3′) | CTA AGG CCA ACC GTG AAA TGA |
| Reverse (5′ 3′) | CCA GAG GCA TAC AGG GAC AAC | ||
| GPR18 | NM_001079710.1 | Forward (5′ 3′) | GTG GGG GTC TGG ATA ATG AC |
| Reverse (5′ 3′) | CGC GTG AAG TTA AGC ACA TT | ||
| AdipoR1 | NM_207587.1 | Forward (5′ 3′) | TGA GGT ACC AGC CAG ATG TC |
| Reverse (5′ 3′) | CGT GTC CGC TTC TCT GTT AC | ||
| APPL1 | XM_008771023.1 | Forward (5′ 3′) | TCA CTC CTT CCC CAT CTT TC |
| Reverse (5′ 3′) | TAG AGA GAG GGC AGC CAA AT | ||
| APPL2 | NM_001108741.1 | Forward (5′ 3′) | TGC TCG GGC TAT TCA CAA |
| Reverse (5′ 3′) | AAA CAG GCC CGT GAC ACT | ||
| PGC1α | NM_031347.1 | Forward (5′ 3′) | ACC CAC AGG ATC AGA ACA AACC |
| Reverse (5′ 3′) | GAC AAA TGC TCT TTG CTT TAT TGC | ||
| FOXO1 | NM_001191846.2 | Forward (5′ 3′) | CTC GGC GGG CTG GAA |
| Reverse (5′ 3′) | TCA TTC TGT ACT CGA ATA AAC TTG | ||
| PDK4 | NM_053551.1 | Forward (5′ 3′) | GGG ATC TCG CCT GGC ACT TT |
| Reverse (5′ 3′) | CAC ACA TTC ACG AAG CAG CA | ||
| βHAD | AF095449.1 | Forward (5′ 3′) | TCG TGA CCA GGC AAT TCG T |
| Reverse (5′ 3′) | CCG ATG ACC GTC ACA TGC T | ||
| FAT/CD 36 | NM_031561.2 | Forward (5′ 3′) | GAC CAT CGG CGA TGA GAA A |
| Reverse (5′ 3′) | CCA GGC CCA GGA GCT TTA TT | ||
| Mouse Genes | |||
| HPRT1 | NM_013556.2 | Forward (5′ 3′) | GCAAACTTTGCTTTCCCTGG |
| Reverse (5′ 3′) | ACTTCGAGAGGTCCTTTTCAC | ||
| NFATc1 | NM_016791.3 | Forward (5′ 3′) | TCCAAAGTCATTTTCGTGGA |
| Reverse (5′ 3′) | GTTGCGGAAAGGTGGTATCT | ||
| PGC1α | NM_008904.1 | Forward (5′ 3′) | CACCCACAGGATCAGAACAA |
| Reverse (5′ 3′) | GGTCATCGTTTGTGGTCAGA | ||
| APPL1 | NM_145221.2 | Forward (5′ 3′) | ATCAGGCGGAAGAAGTGAGA |
| Reverse (5′ 3′) | TTTCTGATGCCCTACGATCC | ||
| APPL2 | NM_145220.2 | Forward (5′ 3′) | CCAAAAGTATGGACGGCTTC |
| Reverse (5′ 3′) | CTCAGCTTCCAGTTCCACCT | ||
| AMPKα2 | NM_178143.1 | Forward (5′ 3′) | GCCCAGATGAACGCTAAGAT |
| Reverse (5′ 3′) | TGCATACAGCCTTCCTGAGA | ||
| PDK4 | NM_013743.2 | Forward (5′ 3′) | GAGAAGAGCCCAGAAGACCA |
| Reverse (5′ 3′) | TCCACTGTGCAGGTGTCTTT | ||
| Human Genes | |||
| Cyclophilin | NM 021130.3 | Forward (5′ 3′) | CATCTGCACTGGCAAGACTGA |
| Reverse (5′ 3′) | TTCATGCCTTCTTTCACTTTGC | ||
| NFATc1 | NM_172390.1 | Forward (5′ 3′) | CCT CTC CAA CAC CAA AGTCC |
| Reverse (5′ 3′) | CGA TGT CCG TCT CTC CTT TC | ||
| PGC1α | NM_013261 | Forward (5′ 3′) | CAAGCCAAACCAACAACTTTATCTCT |
| Reverse (5′ 3′) | CACACTTAAGGTGCGTTCAATAGTC | ||
| AdipoR1 | NM_015999 | Forward (5′ 3′) | CGCCATGGAGAAGATGGAA |
| Reverse (5′ 3′) | TCATATGGGATGACCCTCC | ||
| APPL1 | NM_012096 | Forward (5′ 3′) | TCACTCCTTCCCCATCTTTC |
| Reverse (5′ 3′) | TAGAGAGAGGGCAGCCAAAT | ||
| APPL2 | NM_018171 | Forward (5′ 3′) | CACGCCCAATGGAAAATC |
| Reverse (5′ 3′) | CGACTGCCTCAGGGTTGT |
AdipoR1; Adiponectin Receptor 1, AMPKα2; 5′adenosine monophosphate-activated protein kinase α-2, APPL1; Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1, APPL2; Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 2, βActin; beta actin, βHAD; beta-hydroxyacyl-CoA dehydrogenase, FAT/CD36; Fatty Acid Translocase/ Cluster of Differentiation 36, FOXO1; Forkhead box protein O1, GPR18; G Protein-Coupled Receptor 18, HPRT1; Hypoxanthine-Guanine Phosphoribosyltransferase, NFATc1; nuclear factor of activated T-cells c1, PDK4; Pyruvate Dehydrogenase Kinase 4, PGC1α; Peroxisome proliferator-activated receptor gamma co-activator 1 alpha.
4.4. ‘Real Time’ Polymerase Chain Reaction (PCR)
To quantify mRNA expression of the genes of interest, ‘Real Time’ PCR, SYBR Green method [44] was utilised. SYBRTM Green (BioRad Laboratories, Hercules, California, USA) and the BioRad MY iQ® Real-Time PCR detection system were used. Forward and reverse oligonucleotide primer sequences for mouse, rat and human are included in Table 2. The samples were run for 40 or 50 cycles at 95 °C for 15 s and 60 °C for 60 s. Changes in mRNA expression were normalised to the average of housekeeping gene(s), cyclophilin and β actin in rat muscle, HPRT1 in C2C12 myotubes and cyclophilin in human primary myotubes and quantified using the validated 2−ΔΔct method. The data was reported in arbitrary units as previously described [45].
4.5. Statistical Analysis
Graph Pad Prism Software 8.1.2 was used to generate figures and perform statistical analysis, all grouped data is reported as mean ± SEM. The normality of the data was assessed using the Shapiro–Wilk Test. Normally distributed data were statistically analysed using an independent two tailed t-test and not normally distributed data were analysed using a Mann–Whitney two tailed test to determine the effect of treatment compared to control or each treatment group when comparing red gastrocnemius to white gastrocnemius skeletal muscle. A one way ANOVA and Tukey’s Multiple Comparisons Test was utilised to compare the treatment groups and control groups for the red or white gastrocnemius skeletal muscle GPR18 expression data as well as the human primary and C2C12 myotubes. Statistical significance for all data sets was accepted at p < 0.05.
5. Conclusions
Collectively, this is the first study to investigate the effect of two atypical cannabinoid compounds, O-1602 or O-1918, on the skeletal muscle homeostasis in a DIO model, metabolically stable C2C12 myotubes and human primary myotubes obtained from individuals that were obese or obese and had T2DM (O-1918 only). The results from this study suggest that O-1602 does not have an effect on the mRNA expression of a number of signalling pathways in the skeletal muscle under normal physiological conditions or in obesity. While O-1918 appears to have variable effects on skeletal muscle metabolism, the up-regulation of PDK4 in the in vitro model suggests a potential benefit for fatty acid metabolism. Oxidative metabolism markers PGC1α and NFATc1 were also up-regulated in the in vitro model; however, no alterations were observed in the in vivo DIO model. APPL2, the negative regulator of adiponectin signalling, was up-regulated in glycolytic white gastrocnemius skeletal muscle in the in vivo DIO model. The variation between findings could be due to a number of reasons such as species differences in receptor structure, function, activity and expression, and in vivo systemic changes affecting skeletal muscle function versus targeted in vitro administration of treatments. Therefore, in conclusion, O-1602 does not appear to be a suitable skeletal muscle target for obesity at the dosage and duration selected in the current study, while localised O-1918 treatment may be beneficial in targeting pathways of oxidative capacity and fatty acid metabolism in myotubes, albeit only in C2C12 myotubes. Further, based on our findings, future research focusing on mitochondrial count and membrane potentials, myogenesis markers (such as myostatin I, IIa and IIb, glycogen synthsis (GSK3b) and other markers of oxidative capacity (such as NRF2 and SOD1) may be of benefit.
Given that GPR18 is expressed in the skeletal muscle, selectively targeting this receptor in a tissue-specific manner, and understanding how its function may be altered in obesity through different associated mechanisms and the implications of different dietary interventions on receptor expression, would be beneficial in the search for targeted therapies for obesity and related co-morbidities.
Abbreviations
| GPR55 | G Protein Coupled Receptor 55 |
| GPR18 | G Protein Coupled Receptor 18 |
| DIO | Diet-Induced Obese |
| APPL2 | Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 2 |
| PGC1α | Peroxisome proliferator-activated receptor gamma co-activator 1 alpha |
| NFATc1 | Nuclear factor of activated T-cells c1 |
| PDK4 | Pyruvate Dehydrogenase Kinase 4 |
| T2DM | Type Two Diabetes Mellitus |
| CB1 | Cannabinoid Receptor 1 |
| CB2 | Cannabinoid Receptor 2 |
| GPCR | G Protein-Coupled Receptor |
| LPI | Lysophosphatidylinositol |
| MIN6 | Mouse insulinoma |
| APPL1 | AdipoR1; Adiponectin Receptor 1 |
| CBD | Cannabidiol |
| Abn-CBD | Abnormal Cannabidiol |
| SCD | Standard Chow Diet |
| STZ | Streptozotocin |
| AdipoR1 | Adiponectin Receptor 1 |
| FOXO1 | Forkhead box protein O1 |
| βHAD | Beta-hydroxyacyl-CoA dehydrogenase |
| FATCD/36 | Fatty Acid Translocase/Cluster of Differentiation 36 |
| D-MEM | Dulbecco’s modified eagle high glucose growth medium |
| α-MEM | Alpha minimum essential media |
| HFD | High Fat Diet |
| RNA | Ribonucleic Acid |
| cDNA | Complementary deoxyribonucleic acid |
Author Contributions
Conceptualization, A.C.S., K.A.J., L.O., M.L.M., D.H.H. and A.J.M.; methodology, A.C.S., L.O. and K.A.J. (conducted rat experiment), A.C.S. and L.M.C. (conducted human primary myotube experiment) and A.C.S., A.J.M., E.G. and L.O. conducted the C2C12 experiments, A.C.S., E.G. and A.J.M. conducted the RNA/PCR experiments; formal analysis, A.C.S. and A.J.M.; writing—original draft preparation, A.C.S.; writing—review and editing, A.C.S., K.A.J., L.O., e.g., L.M.C., M.L.M., D.H.H. and A.J.M.; supervision, A.J.M., D.H.H. and M.L.M.; project administration, A.C.S., L.O., K.A.J.; funding acquisition, D.H.H. and A.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Allen Foundation (D.H.H., A.J.M.). A.C.S. was supported by Australian Rotary Health and the Rotary Club of Ballarat South PhD scholarship and L.O. and K.A.J. were supported by an Australian Postgraduate Award for their PhD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organisation (WHO) Obesity and Overweight. [(accessed on 8 July 2018)]; Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Zurlo F., Larson K., Bogardus C., Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart C.E.H., Rittweger J. Adaptive processes in skeletal muscle: Molecular regulators and genetic influences. J. Musculoskelet Neuronal Interact. 2006;6:73–86. [PubMed] [Google Scholar]
- 4.Pagliassotti M.J., Pan D.A., Prach P.A., Koppenhafer T.A., Storlien L., Hill J.O. Tissue Oxidative Capacity, Fuel Stores and Skeletal Muscle Fatty Acid Composition In Obesity-Prone and Obesity-Resistant Rats. Obes. Res. 1995;3:459–464. doi: 10.1002/j.1550-8528.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 5.Pan D., Lillioja S., Kriketos A.D., Milner M.R., Baur L.A., Bogardus C., Jenkins A.B., Storlien L.H. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 6.Saltin B., Henriksson J., Nygaard E., Andersen P., Jansson E. FIBER TYPES AND METABOLIC POTENTIALS OF SKELETAL MUSCLES IN SEDENTARY MAN AND ENDURANCE RUNNERS. Ann. N. Y. Acad. Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- 7.Pagotto U., Vicennati V., Pasquali R. The endocannabinoid system and the treatment of obesity. Ann. Med. 2005;37:270–275. doi: 10.1080/07853890510037419. [DOI] [PubMed] [Google Scholar]
- 8.Cavuoto P., McAinch A.J., Hatzinikolas G., Janovská A., Game P., Wittert G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 2007;364:105–110. doi: 10.1016/j.bbrc.2007.09.099. [DOI] [PubMed] [Google Scholar]
- 9.Cavuoto P., McAinch A.J., Hatzinikolas G., Cameron-Smith D., Wittert G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007;267:63–69. doi: 10.1016/j.mce.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.-O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2009;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh D., Hu S.S.-J., Rimmerman N., Juknat A., Vogel Z., Walker J.M., Bradshaw H.B. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremshofer J., Siwetz M., Berghold V.M., Lang I., Huppertz B., Gauster M. A role for GPR55 in human placental venous endothelial cells. Histochem. Cell Boil. 2015;144:49–58. doi: 10.1007/s00418-015-1321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Console-Bram L., Brailoiu E., Brailoiu G.C., Sharir H., Abood M.E. Activation of GPR18 by cannabinoid compounds: A tale of biased agonism. Br. J. Pharmacol. 2014;171:3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henstridge C.M., Balenga N., Kargl J., Andradas C., Brown A.J., Irving A., Sanchez C., Waldhoer M. Minireview: Recent Developments in the Physiology and Pathology of the Lysophosphatidylinositol-Sensitive Receptor GPR55. Mol. Endocrinol. 2011;25:1835–1848. doi: 10.1210/me.2011-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simcocks A.C., O’Keefe L., Jenkin K.A., Mathai M.L., Hryciw D.H., McAinch A.J. A potential role for GPR55 in the regulation of energy homeostasis. Drug Discov. Today. 2014;19:1145–1151. doi: 10.1016/j.drudis.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Navarrete J.M., Catalán V., Whyte L., Díaz-Arteaga A., Vázquez-Martínez R., Rotellar F., Guzmán R., Gomez-Ambrosi J., Pulido M.R., Russell W., et al. The L- -Lysophosphatidylinositol/GPR55 System and Its Potential Role in Human Obesity. Diabetes. 2011;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipina C., Walsh S.K., Mitchell S.E., Speakman J.R., Wainwright C.L., Hundal H.S. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J. 2018;33:1299–1312. doi: 10.1096/fj.201800171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulu A., Sahoo P.K., Yuil-Valdes A., Mukherjee M., Van Ormer M., Muthuraj P.G., Thompson M., Anderson-Berry A., Hanson C., Natarajan S.K., et al. Omega-3 Fatty Acid-Derived Resolvin D2 Regulates Human Placental Vascular Smooth Muscle and Extravillous Trophoblast Activities. Int. J. Mol. Sci. 2019;20:4402. doi: 10.3390/ijms20184402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matouk A.I., Taye A., El-Moselhy M.A., Heeba G.H., Abdel-Rahman A.A. The Effect of Chronic Activation of the Novel Endocannabinoid Receptor GPR18 on Myocardial Function and Blood Pressure in Conscious Rats. J. Cardiovasc. Pharmacol. 2017;69:23–33. doi: 10.1097/FJC.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Zerbo S.Y., Rafacho A., Diaz-Arteaga A., Suárez J., Quesada I., Imbernon M.A., Ross R., Diéguez C., De Fonseca F.R., Nogueiras R., et al. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 2011;211:177–185. doi: 10.1530/JOE-11-0166. [DOI] [PubMed] [Google Scholar]
- 21.Vong C.T., Tseng H.H.L., Kwan Y.W., Lee S.M.-Y., Hoi M.P.M. G-protein coupled receptor 55 agonists increase insulin secretion through inositol trisphosphate-mediated calcium release in pancreatic β-cells. Eur. J. Pharmacol. 2019;854:372–379. doi: 10.1016/j.ejphar.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 24.Kido K., Ato S., Yokokawa T., Sato K., Fujita S. Resistance training recovers attenuated APPL1 expression and improves insulin-induced Akt signal activation in skeletal muscle of type 2 diabetic rats. Am. J. Physiol. Metab. 2018;314:E564–E571. doi: 10.1152/ajpendo.00362.2017. [DOI] [PubMed] [Google Scholar]
- 25.Wende A.R., Huss J.M., Schaeffer P.J., Giguère V., Kelly D.P. PGC-1α Coactivates PDK4 Gene Expression via the Orphan Nuclear Receptor ERRα: A Mechanism for Transcriptional Control of Muscle Glucose Metabolism. Mol. Cell. Boil. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz-Arteaga A., Vazquez M.J., Vazquez-Martinez R., Pulido M.R., Suárez J., Velasquez D.A., López M., Ross R.A., De Fonseca F.R., Bermúdez-Silva F.-J., et al. The atypical cannabinoid O-1602 stimulates food intake and adiposity in rats. Diabetes, Obes. Metab. 2011;14:234–243. doi: 10.1111/j.1463-1326.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- 27.Parray H.A., Yun J.W. Cannabidiol promotes browning in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2016;416:131–139. doi: 10.1007/s11010-016-2702-5. [DOI] [PubMed] [Google Scholar]
- 28.Matouk A.I., Taye A., El-Moselhy M.A., Heeba G.H., Abdel-Rahman A.A. Abnormal cannabidiol confers cardioprotection in diabetic rats independent of glycemic control. Eur. J. Pharmacol. 2017;820:256–264. doi: 10.1016/j.ejphar.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simcocks A.C., Jenkin K.A., O’Keefe L., Samuel C.S., Mathai M.L., McAinch A.J., Hryciw D.H. Atypical cannabinoid ligands O-1602 and O-1918 administered chronically in diet-induced obesity. Endocr. Connect. 2019;8:203–216. doi: 10.1530/EC-18-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organisation (WHO) Global Health Observary (Gho) Data. [(accessed on 17 July 2019)]; Available online: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/
- 31.Carey A.L., Kingwell B.A. Novel pharmacological approaches to combat obesity and insulin resistance: Targeting skeletal muscle with ‘exercise mimetics’. Diabetol. 2009;52:2015–2026. doi: 10.1007/s00125-009-1420-x. [DOI] [PubMed] [Google Scholar]
- 32.Rossi F., Bellini G., Luongo L., Manzo I., Tolone S., Tortora C., Bernardo M., Grandone A., Conforti A., Docimo L., et al. Cannabinoid receptor 2 as anti-obesity target: Inflammation, fat storage and browning modulation. J. Clin. Endocrinol. Metab. 2016;101:3469–3478. doi: 10.1210/jc.2015-4381. [DOI] [PubMed] [Google Scholar]
- 33.Lindborg K.A., Teachey M.K., Jacob S., Henriksen E.J. Effects of in vitro antagonism of endocannabinoid-1 receptors on the glucose transport system in normal and insulin-resistant rat skeletal muscle. Diabetes, Obes. Metab. 2010;12:722–730. doi: 10.1111/j.1463-1326.2010.01227.x. [DOI] [PubMed] [Google Scholar]
- 34.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 35.Mendizabal-Zubiaga J., Melser S., Bénard G., Ramos-Uriarte A., Reguero L., Arrabal S., Elezgarai I., Gerrikagoitia I., Suárez J., De Fonseca F.R., et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016;7:476. doi: 10.3389/fphys.2016.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crespillo A., Suárez J., Bermúdez-Silva F.-J., Rivera P., Vida M., Alonso M., Palomino A., Lucena M.A., Serrano A., Pérez-Martín M., et al. Expression of the cannabinoid system in muscle: Effects of a high-fat diet and CB1 receptor blockade. Biochem. J. 2010;433:175–185. doi: 10.1042/BJ20100751. [DOI] [PubMed] [Google Scholar]
- 37.Jenkin K.A., O’Keefe L., Simcocks A.C., Briffa J.F., Mathai M.L., McAinch A.J., Hryciw D.H. Renal effects of chronic pharmacological manipulation of CB 2 receptors in rats with diet-induced obesity. Br. J. Pharmacol. 2015;173:1128–1142. doi: 10.1111/bph.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C., Xin X., Xiang R., Ramos F.J., Liu M., Lee H.J., Chen H., Mao X., Kikani C.K., Liu F., et al. Yin-Yang Regulation of Adiponectin Signaling by APPL Isoforms in Muscle Cells. J. Boil. Chem. 2009;284:31608–31615. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornall L., Mathai M.L., Hryciw D., Simcocks A., O’Brien P.E., Wentworth J.M., McAinch A.J. GPR119 regulates genetic markers of fatty acid oxidation in cultured skeletal muscle myotubes. Mol. Cell. Endocrinol. 2013;365:108–118. doi: 10.1016/j.mce.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 40.McAinch A.J., Steinberg G., Mollica J., O’Brien P., Dixon J., Kemp B.E., Cameron-Smith D. Leptin stimulation of COXIV is impaired in obese skeletal muscle myotubes. Obes. Res. Clin. Pr. 2007;1:53–60. doi: 10.1016/j.orcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Cornall L.M., Mathai M.L., Hryciw D.H., McAinch A.J. Diet-induced Obesity Up-regulates the Abundance of GPR43 and GPR120 in a Tissue Specific Manner. Cell. Physiol. Biochem. 2011;28:949–958. doi: 10.1159/000335820. [DOI] [PubMed] [Google Scholar]
- 42.Schicho R., Bashashati M., Bawa M., McHugh D., Saur D., Hu H.-M., Zimmer A., Lutz B., Mackie K., Bradshaw H.B., et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm. Bowel Dis. 2011;17:1651–1664. doi: 10.1002/ibd.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Offertáler L., Mo F.-M., Batkai S., Liu J., Begg M., Razdan R.K., Martin B.R., Bukoski R.D., Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 44.Ponchel F., Toomes C., Bransfield K., Leong F.T., Douglas S.H., Field S.L., Bell S.M., Combaret V., Puisieux A., Mighell A.J., et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C.(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]