Abstract
Physical frailty and sarcopenia (PF&S) recapitulates all the hallmarks of aging and has become a focus in geroscience. Factors spanning muscle-specific processes (e.g., mitochondrial dysfunction in skeletal myocytes) to systemic changes (e.g., inflammation and amino acid dysmetabolism) have been pinpointed as possible contributors to PF&S pathophysiology. However, the search for PF&S biomarkers allowing the early identification and tracking of the condition over time is ongoing. This is mainly due to the phenotypic heterogeneity of PF&S, its unclear pathophysiology, and the frequent superimposition of other age-related conditions. Hence, presently, the identification of PF&S relies upon clinical, functional, and imaging parameters. The adoption of multi-marker approaches (combined with multivariate modeling) has shown great potential for addressing the complexity of PF&S pathophysiology and identifying candidate biological markers. Well-designed longitudinal studies are necessary for the incorporation of reliable biomarkers into clinical practice and for unveiling novel targets that are amenable to interventions.
Keywords: cytokines, extracellular vesicles, exosomes, geroscience, gut dysbiosis, inflammation, metabolomics, mitochondrial dysfunction, physical performance, skeletal muscle
1. Introduction
Sarcopenia is the progressive decline in muscle mass and strength that occurs during aging [1]. This condition is a hot topic in geriatric research and a public health priority [2], as it exposes older adults to increased risk of negative health-related events (such as disability, loss of independence, institutionalization, and death) [1]. However, its phenotypic heterogeneity, the unclear pathophysiology, and the frequent superimposition of other age-related conditions hamper the study of sarcopenia as a single phenomenon [3]. As a result, an univocal operational definition of sarcopenia is still missing, as are specific biomarkers that could be used, either in clinics or in research [4]. At the clinical level, sarcopenia overlaps with frailty and the age-related decline in physiologic reserve and homeostatic capacity, which predisposes older adults to a wide range of negative health-related events—including falls, morbidity, disability, hospitalization, institutionalization, and mortality [5]. In this scenario, the recognition of physical frailty and sarcopenia (PF&S) as a new entity, and its operationalization in the Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies (SPRINTT) project, have set a remarkable precedent for its clinical and regulatory recognition [6].
The decline in physical function is, indeed, the most evident change that occurs during sarcopenia, and a cardinal criterion for the identification of PF&S [7,8,9,10]. Physical function refers to a construct encompassing simple single-joint (e.g., handgrip strength) and multi-joint complex movements (e.g., walking speed), and may be assessed through a large array of tests [11,12]. Changes in physical performance occur slowly across years [11,13,14,15] and show great heterogeneity among people from different socioeconomic backgrounds [14]. This implies that age- and country-specific cut-off values might be needed in order to identify physical dysfunction [16]. At the same time, when considering physical dysfunction as the ultimate outcome of muscle failure [17], numerous cellular and molecular changes may occur alongside this phenomenon.
When exploring the pathways and processes involved in PF&S pathophysiology, several factors, from muscle-specific mitochondrial dysfunction to systemic changes (e.g., inflammation and amino acid dysmetabolism), have been pinpointed [9,10,18]. It is still unclear whether these processes share common roots and how cell-based alterations spread and are detected at the systemic level, contributing to the disabling cascade that characterizes PF&S. Low-grade inflammation and amino acid dysmetabolism have recently been associated with a specific pattern of small extracellular vesicles (sEVs) of mitochondrial origin, namely mitochondrial-derived vesicles (MDVs), which are proposed to function as shuttles that allow crosstalk between biological systems [7]. However, little is known about their complex regulatory network.
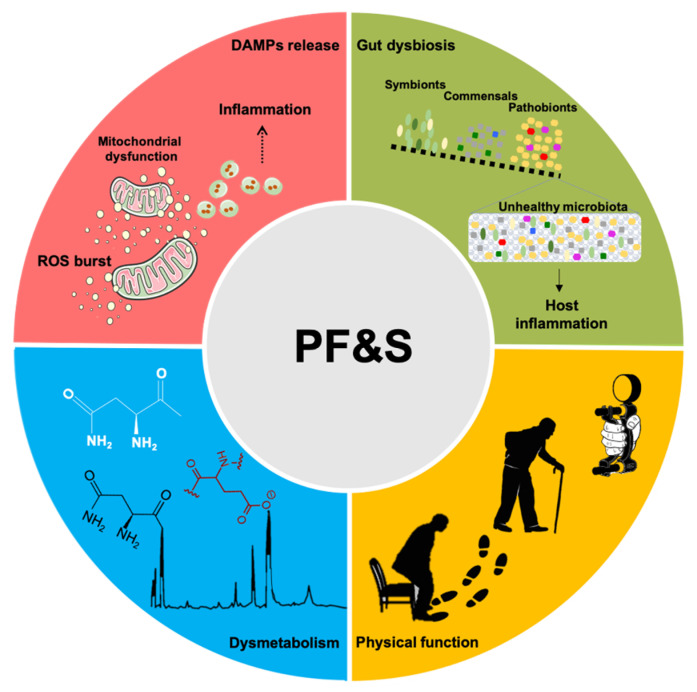
A conceptual framework has recently been agreed upon for the selection of blood-borne biomarkers to verify the existence of shared “hallmarks of aging” that can be targeted to extend the health span [19]. The pathophysiology of PF&S recapitulates all the hallmarks of aging [19,20,21], which makes it a prototypical geroscience condition [22]. In this scenario, multivariate analyses of biomediators, pertaining to different domains, may enable the identification of biomarkers for PF&S that capture its pathophysiological complexity (Figure 1) [7,8,9,10,23]. The identified pathways may, eventually, be used for drug development.
Figure 1.
Schematic representation of the main pathophysiological pathways contributing to physical frailty and sarcopenia (PF&S) (i.e., inflammation, gut dysbiosis, declines in physical function, and dysmetabolism), and related biomarkers. Abbreviations: DAMPs, damage-associated molecular patterns; ROS, reactive oxygen species.
Here, we will provide an overview of imaging, functional, and biological markers currently available for PF&S. We will also illustrate the prospect of exploiting specific biological processes to identify new biomarkers for the condition and to develop personalized interventions.
2. Imaging and Functional Markers
When Irwin Rosenberg prompted health professionals to pay serious attention to sarcopenia [24], he referred exclusively to age-related muscle atrophy, while changes in physical function and mobility were seen as possible secondary outcomes. Indeed, sarcopenia and muscle atrophy were synonyms until the 2000s [25,26,27], after which findings from observational studies indicated that losses in physical function occurred earlier, progressed more rapidly, and were better predictors of negative health-related events than a decline in muscle mass [13,28]. Indeed, the recognition of declining physical performance as a core feature of muscle aging has prompted the appraisal of sarcopenia as a composite condition that encompasses quantitative (mass) and qualitative (strength/function) muscular domains [29,30].
Body imaging techniques (e.g., dual energy x-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging (MRI)) are popular tools for the quantification of muscle or lean body mass [31]. However, all of them show notable pitfalls. Indeed, the results of DXA are heavily influenced by body thickness, hydration status, and extracellular fluid accumulation. Furthermore, DXA is unable to measure intramuscular adipose tissue [31]. On the other hand, the large-scale implementation of CT and MRI is hampered by high costs, technological complexity, and space requirements [31]. CT also exposes the test person to non-negligible doses of ionizing radiation.
The creatine (methyl-d3) dilution (D3-creatine) method is a recently developed approach that allows the accurate quantification of whole-body muscle mass [32]. The method relies on the irreversible conversion of creatine to creatinine, and its excretion in urine. The enrichment of urine D3-creatinine allows estimating the total body creatine pool size as a proxy for the whole-body skeletal muscle mass. This method involves the oral administration of stable isotope-labeled creatine (D3-creatine), followed by the collection of a single fasted urine sample 48–96 h later. The sample is assayed for creatine and creatinine (deuterated and unlabeled) via liquid chromatography mass spectrometry (LC-MS) [33,34,35]. By means of an algorithm, this method allows us to calculate the total body creatine pool size and the muscle mass from D3-creatinine enrichment in urine [33,34,35]. Estimates of the total body muscle mass, obtained by this method, show remarkable concordance with the whole-body MRI scans [33,34,35]. Differently from the DXA, the muscle mass quantified by the D3-creatine dilution method is strongly correlated with physical performance, and predicts incidents of falls and functional limitations [36,37,38,39].
Physical function starts declining around the third decade of life, with a steeper decrease beyond the age of 50, which suggests that the initial deflection of physical performance might be an early predictor of sarcopenia [11,13,14,15]. However, physical performance refers to a wide construct involving several components, including (but not limited to) muscle strength, power, and mobility. Therefore, several physical abilities are legitimate functional biomarkers of sarcopenia. The identification of specific components of physical function that, more so than others, predict sarcopenia is particularly challenging, especially because knowledge of this condition has grown considerably in the last two decades, as reflected by changes in its conceptual framework and operational definitions [30,40,41].
Muscle strength refers to the amount of force generated by a dynamic muscle contraction [42,43]. Isometric handgrip strength (IHG) and the five-time sit-to-stand test (5×STS) are two simple, inexpensive and quick tests used to assess upper and lower limb muscle strength, respectively, in different settings, including the community, hospitals, and nursing homes [4,29,30]. Cross-sectional [44,45,46,47,48] and prospective studies [44,47,49,50,51,52] have reported that low IHG is significantly associated with poor physical performance, mobility impairment, and disability. Notable findings by Rantanen et al. [52] reported that men with low IHG during midlife were at higher risk of developing physical dysfunction 25 years later [52]. Although several studies support the hypothesis that IHG might predict physical dysfunction (and, likely, also sarcopenia), there is also evidence indicating that IHG is not associated with lower extremity disability [53]. Therefore, the combination of upper and lower limb muscle strength testing might be necessary for adequately framing sarcopenia. The 5×STS is associated with many physical performance tests [54], progressive disability [53], and has been reported as a predictor of sarcopenia in older Brazilian women [55]. However, larger studies, also including older men, are needed to confirm and expand these initial findings.
Muscle power—the capacity to generate force in a short time interval—declines earlier in life and at a faster rate, while presenting a higher degree of association with some mobility tasks than muscle strength [13,56,57]. For instance, results from the InCHIANTI (Invecchiare in Chianti) study indicated that women aged between 50 and 60 years had a 20–30% reduction of lower limb muscle strength, while muscle power was reduced by ~50% [13]. Lower limb muscle power was also indicated as a better discriminator of poor mobility than upper and lower limb muscle strength [13,57].
In an attempt to empower the discriminatory capacity of physical function as a marker of PF&S, researchers have proposed combinations of physical tests that support the assessment of different physical abilities. The short physical performance battery (SPPB) provides a single score based on a person’s performance in balance tests, gait speed, and 5×STS [58]. In the seminal study by Guralnik et al. [58], the authors observed that people with low SPPB scores were almost five times more likely to develop disability in a short time interval. These findings were confirmed by a recent systematic review and meta-analysis [59] that indicated that lower SPPB was associated with worsening activities of daily living (ADL) and instrumental ADL.
Poor physical function is associated with chronic low-grade inflammation and antioxidant markers in older adults [60,61,62,63,64], thus suggesting that physical dysfunction is likely mediated by cellular mechanisms that, in combination with functional assessment, may be more informative than physical function alone.
3. Inflammation-Related Biomarkers
Dysregulation of the cytokine network is a major driver of aging and related conditions [65,66,67,68]. However, the inclusion of inflammatory markers in clinical practice, as a tool for identifying specific conditions, is far from being reached [19]. A core inflammatory profile with gender-specific signatures has been identified in the context of PF&S as composed by higher levels of C-reactive protein and lower concentrations of myeloperoxidase (MPO), interleukin (IL) 8, monocyte chemoattractant protein 1, and platelet-derived growth factor-BB (PDGF-BB). This set of mediators includes markers related to immunosenescence [69,70], micronutrient intake imbalance [71,72], and impaired muscle regeneration, in response to specific stimuli, such as oxidative stress [73,74,75,76,77]. Senescent cells face morphological and functional reshaping, manifested by a senescence-associated secretory phenotype (SASP) [78]. The SASP fingerprint is composed of ILs, chemokines, growth factors, proteases, and extracellular matrix components [78]. The release of these SASP factors induces perturbations in the local microenvironment through autocrine and paracrine signals, in order to prevent proliferation of damaged cells, as well as enabling the recruitment of immune cells and promoting tissue repair [79,80]. Age-related decline in cell quality control systems may induce insufficient clearance of senescent cells and support systemic inflammation, via the overproduction of SASP-related pro-inflammatory cytokines (e.g., IL1β, IL6, and IL8) [81].
A pro-disability effect has long been attributed to the chronic low-grade inflammation observed during aging, referred to as inflamm-aging [68,70,82]. Indeed, higher levels of pro-inflammatory cytokines have been associated with muscle wasting and reduced physical performance [70,83]. This inflammatory response, void of infectious agents and named “sterile inflammation”, is part of the innate immune response triggered by misplaced cellular components [84,85], which are rooted into the “danger theory” of inflammation [86]. According to this theory, the accumulation of damage-associated molecular patterns (DAMPs), and their release from injured cells into the circulation, triggers inflammation via caspase-1 activation and the release of proinflammatory cytokines [87]. Among DAMP molecules, those of mitochondrial origin, in light of their bacterial ancestry, are thought to contribute substantially to inflamm-aging by interacting with Toll-like receptors, NLR family pyrin domain containing 3 inflammasome activation, and cytosolic DNA sensing by the guanosine monophosphate–adenosine monophosphate (GMP–AMP) synthase–stimulator of interferon gene systems [88,89,90].
The mitochondrial involvement in the crosstalk between chronic inflammation and muscle wasting is not surprising given the central role of this organelle in skeletal myocyte viability. Mitochondria are highly interconnected organelles and form a dynamic network that operates via intra-mitochondrial contacts (as well as with the endoplasmic reticulum, lysosomes, and the actin cytoskeleton) to guarantee organelle homeostasis [91,92]. Recently, mitochondrial tubular protrusions, named mitochondrial nanotunnels, have also been identified as additional structures for mitochondrial interconnections, especially in mitochondria immobilized within post-mitotic tissues (e.g., skeletal muscle and cardiac tissue) [92]. These resident organelles are structurally limited in their fusion, and thus may communicate over long distances via nanotunnels [92]. Finally, Golgi-derived vesicles have been identified to participate in mitochondrial dynamics and are newly described contact sites involved in mitochondrial homeostasis [93]. However, the networking ability of mitochondria may be a double-edged sword. Indeed, it is unclear where the boundary lies between the protective role of the inflammation-derived circulating DAMPs and the detrimental effect of over-reactive inflammation. Severe mitochondrial damage and abnormal autophagosome formation were found in the skeletal muscle of IL10-null mice (IL10tm/tm), a rodent model of chronic inflammation and frailty [94]. Among mitochondrial DAMPs, circulating mitochondrial DNA (mtDNA) released from damaged organelles is a prominent candidate, linking inflammation with muscle decay [85]. Recent findings by our group support the hypothesis of mitochondrial impairment among the underlying pathogenetic mechanisms of sarcopenia [18]. In particular, as a result of failing quality control systems [91,95,96,97], the release of oxidized cell-free mtDNA and other mitochondrial components within MDVs have been associated with systemic inflammation in older adults with PF&S [7].
The characterization of the composition of exosomes/EVs released by senescent cells (eSASP) has revealed a specific EV SASP signature [98] from which it has been possible to track down their originating cells [99,100]. Therefore, EVs represent a unique tool for capturing the regulatory network of complex conditions and for the identification of cell- and stressor-specific biomarkers. The dissection of these pathways may provide relevant insights into the role played by inflammation in the disabling cascade of PF&S, allowing for the design of personalized treatment strategies.
4. Metabolic Markers
The analysis of the wide collection of endogenous metabolites in biological matrices, referred to as metabolomics, allows for the exploration of genotype–phenotype interactions under the impact of the environment. As such, metabolomics supports the analysis of the dynamic changes of organismal function and provides more informative data than gene or protein expression assays [101,102,103,104]. In the context of PF&S, the study of the dynamic metabolic responses to stressors and the characterization of the biochemical pathways involved are particularly relevant, as this condition is tightly associated with metabolic disorders [105,106].
Dietary protein and amino acids are building blocks for muscle protein synthesis and relevant factors for muscle plasticity and trophism [107]. Furthermore, these metabolites are at the crossroads of multiple biological processes, such as inflammation, insulin sensitivity, and redox homeostasis—all of which are candidates for age-related muscle atrophy and dysfunction [108,109]. Therefore, disarrangements in protein–amino acid metabolism may contribute substantially to the pathophysiology of sarcopenia [110,111].
Specific patterns of circulating amino acids have been associated with muscle mass [112] and quality [113] in functionally limited older adults. In particular, lower plasma concentrations of the branched-chain amino acids leucine and isoleucine have been found in sarcopenic, older Norwegian community dwellers [114]. Conversely, higher concentrations of proline characterized older Japanese people with sarcopenia [115]. Finally, it was found that frail older Japanese people had low levels of essential amino acids, compared with their non-frail peers [116]. On coupling the circulating-amino-acid profiling with the multivariate statistical modeling, the discriminatory power of the analytical approach enabled the exploration of the interrelationship between the protein–amino acid dyshomeostasis and PF&S [117]. Indeed, as observed in the BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons (BIOSPHERE) study, metabolomics coupled with a partial least squares–discriminant analysis (PLS-DA) allowed the distinct signatures of circulating amino acids and derivatives in older adults, with and without PF&S, to be identified [9,23]. In particular, participants with PF&S were characterized by higher serum levels of asparagine, aspartic acid, citrulline, ethanolamine, glutamic acid, sarcosine, and taurine, while higher concentrations of alpha amino butyric acid and methionine were found in non-PF&S controls [23]. Notably, the metabolic profile of people with PF&S was associated with a decrease in total energy, as well as the quality and quantity of dietary protein intake [23]. In particular, a link between a poor-quality protein diet or (selective) malabsorption and an impaired mitochondrial quality control mechanism was reported [23]. These findings are in keeping with results from the cross-sectional Maastricht Sarcopenia study (MaSS) in which selected nutritional biomarkers (e.g., essential amino acids, branched-chain amino acids, and eicosapentaenoic acid) were decreased in older, sarcopenic adults [118].
In a recent study by our group, multi-marker datasets (which include mediators pertaining to inflammation, metabolism, and mitochondrial dysfunction) were analyzed through an innovative strategy based on Sequential and Orthogonalized Covariance Selection (SO-CovSel) [22]. This approach allowed us to scale down the number of discriminant biomarkers for PF&S to only seven biomolecules (α-aminobutyric acid, asparagine, aspartic acid, citrulline, heat shock protein 72, MPO, and PDGF-BB) [22], which may speed up the implementation of cost-effective biomarkers in the clinical arena. These findings, albeit promising, need to be confirmed in larger-scale, longitudinal studies to integrate composite biochemical measurements into the routine assessment of PF&S.
5. Gut Microbiota
Past the age of 70, gut microbiota face substantial compositional and functional modifications [119,120] of which higher inter-individual variability, reduced biodiversity, and colonization by pathobionts are the main features (Figure 1) [119,120]. These changes, collectively referred to as gut dysbiosis, impact host physiology and expose older adults to a higher risk of infections [119,120]. Furthermore, gut dysbiosis is implicated in acute and chronic conditions, beyond the gastrointestinal system [120,121]. The cause–effect inferences between the gut dysbiosis and human diseases cannot yet be established [121]. However, growing evidence supports the existence of a relationship between changes in gut flora, chronic inflammation, and anabolic resistance in muscle wasting [122,123]. As such, gut dysbiosis has been proposed as a factor in the development of PF&S [124,125]. This view is supported by the observation that supplementation with specific Lactobacillus strains attenuates muscle wasting in a mouse model of acute leukemia [126], possibly via increased amino acid bioavailability. Indeed, the activity of host- and bacterium-derived proteases and peptidases along the gastrointestinal tract supports the hydrolysis of dietary proteins into peptides and amino acids [127,128]. These bioproducts, in turn, influence microbial growth and survival [129], and regulate energy and the protein homeostasis of the host [130,131]. Amino acids are also precursors of short chain fatty acids (SCFAs), particularly acetate, propionate and butyrate [132], of which acetate is mainly metabolized by muscle cells to produce energy [133].
The age-related decline in the barrier function of the gut mucosa is believed to play a major role in gut dysbiosis [134]. The establishment of a “leaky gut” during aging may favor the entry of gut microbes and/or related products into the circulation, where they trigger inflammation and contribute to immune system dysregulation [135,136]. In physiologic conditions, the gut flora balances pro- and anti-inflammatory responses [137]. Age-associated dysbiosis weakens the action of gut flora against adverse microbial colonization or metabolite removal [138]. In conjunction with this, the secretion of mucins by intestinal cells and the consequent reduction of SCFAs render the intestinal mucosa more permissive of pathogens entering [138,139]. Apart from being an energy source for colonic epithelial cells, SCFAs modulate the release of anti-inflammatory molecules, which are involved in host metabolism and immunity [140]. In particular, butyrate regulates the differentiation of CD4+ T cells into regulatory T cells, the induction of transforming growth factors β secretion by epithelial cells, and the production of IL10 and retinoic acid by dendritic cells and macrophages [140]. Therefore, local intestinal inflammation and its propagation, via the leakage of microbes and bacterium-derived inflammatory compounds into the blood, are blunted [140]. In further support of a link between gut dysbiosis and systemic inflammation are findings from a study where young mice were fed a high-fat diet—a known inducer of intestinal permeability [141]. In these rodents, systemic inflammation and leakage of lipopolysaccharide from the intestine into the circulation were described [141].
Chronic inflammation has been indicated as a trait d’union between gut dysbiosis and muscle structural and functional decline [124,125]. The determinants of this crosstalk are unclear, although studies in preclinical models have offered interesting clues. Germ-free mice are preserved from dietary-induced obesity via increased fatty acid metabolism [142]. This phenomenon occurs via activation of the 5′ adenosine monophosphate–activated protein kinase pathway, a gatekeeper of cellular energy status that activates the carnitine:palmitoyl transferase-1 in the muscle. As a result, mitochondria may be fueled by long chain fatty acylCoA and higher levels of the fasting-induced adipocyte factor, linked to the peroxisome proliferator-activated receptor, and gamma coactivator 1-alpha, the master regulator of mitochondrial biogenesis and oxidative metabolism [142]. The orchestration of these activities may assist in counteracting muscle atrophy.
Age-associated changes in the gut flora composition have been related to the progression of diseases and frailty in older adults. Van Tongeren et al. [143] were the first to report an association between the composition of gut microbiota and frailty. In particular, a reduction in the proportion of Lactobacilli, Bacterioides/Prevotella, and Faecalibacterium prausnitzii, and an increase in the proportion of Ruminococcus, Atopobium, and Enterobacteriacae were found in individuals showing high frailty scores [143]. The reduced abundance of butyrate-producing bacteria (e.g., Faecalibacterium prausnitzii) in frail older adults suggests a positive role for butyrate, namely in reinforcing the tight junctions of intestinal cells and preventing microbial spread into the circulation [144]. The reduced inflammation may, in turn, sustain muscle health [145]. Subsequent results from the ELDERMET study provided further confirmation of this hypothesis and linked butyrate-generating bacteria with functional capacity in older community-dwelling adults [146].
Finally, the application of a multi-marker analytical approach allowed identifying patterns of circulating mediators, which are composed of higher serum concentrations of aspartic acid, lower circulating levels of concentrations of threonine, and the macrophage inflammatory protein 1α (associated with increased abundance of Oscillospira and Ruminococcus microbial taxa, and decreased abundance of Barnesiellaceae and Christensenellaceae in older people with PF&S) [8]. The relationship between the abundance of specific intestinal bacteria, metabolic markers, and serum levels of specific inflammatory biomolecules suggests the existence of an additional pathway through which changes in gut microbiota may impinge on PF&S pathophysiology [8].
6. Conclusions
To date, the identification of PF&S relies upon clinical, functional, and imaging parameters. However, biological mediators pertaining to different domains (e.g., inflammation, amino acid metabolism, and gut microbiota) have been identified as candidate biomarkers for the condition (Table 1).
Table 1.
Biological markers associated with PF&S operationalized, according to the Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies (SPRINTT) project’s definition.
| Biological Domain | Biomarkers |
|---|---|
| Inflammation | CRP, HSP72, IL1β, IL6, IL8, MCP1, MIP1α, MPO, PDGF-BB |
| Amino acid metabolism | Asparagine, aspartic acid, citrulline, ethanolamine, glutamic acid, sarcosine, taurine, threonine |
| Gut microbiota | Barnesiellaceae, Christensenellaceae, Oscillospira, Ruminococcus |
Abbreviations: CRP, C-reactive protein; HSP72, heat shock protein 72; IL, interleukin; MCP1, monocyte chemoattractant protein 1; MIP1α, macrophage inflammatory protein 1α; MPO, myeloperoxidase; PDGF-BB, platelet-derived growth factor-BB.
Although a fairly large number of metabolic, microbial, and inflammatory biomolecules have been investigated for their association with PF&S, it cannot be excluded that other relevant markers might be obtained through the analysis of a larger set of biomediators. In light of this limitation (and the lack of a “gold standard” biomarker that can reliably predict functional impairment in older adults), the incorporation of these biological markers into clinical practice is yet to be achieved. The adoption of multi-marker approaches combined with multivariate modeling has shown great potential for addressing the complexity of PF&S pathophysiology and unveiling novel targets for interventions. Well-designed longitudinal studies are warranted to accomplish these ambitious tasks.
Abbreviations
| 5 × STS | Five-time sit-to-stand |
| ADL | Activities of daily living |
| BIOSPHERE | BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons |
| CRP | C-reactive protein |
| CT | Computed tomography |
| DAMPs | Damage-associated molecular patterns |
| DXA | Dual energy x-ray absorptiometry |
| HSP72 | HSP72, heat shock protein 72 |
| IHG | Isometric handgrip strength |
| IL | Interleukin |
| MaSS | Maastricht Sarcopenia study |
| MCP1 | Monocyte chemoattractant protein 1 |
| MDVs | Mitochondrial-derived vesicles |
| MIP1α | Macrophage inflammatory protein 1α |
| MPO | Myeloperoxidase |
| MRI | Magnetic resonance imaging |
| PDGF-BB | Platelet-derived growth factor-BB |
| PF&S | Physical frailty and sarcopenia |
| PLS-DA | Partial Least Squares–Discriminant Analysis |
| SASP | Senescence-associated secretory phenotype |
| SCFAs | Short chain fatty acids |
| SPRINTT | Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies |
| sEVs | Small extracellular vesicle |
| SO-CovSel | Sequential and Orthogonalized Covariance Selection |
| SPPB | Short physical performance battery |
Author Contributions
Conceptualization, A.P. and E.M.; writing—original draft preparation, A.P. and E.M.; writing—review and editing, H.J.C.-J., M.C., and R.C.; supervision, F.L. and R.B.; funding acquisition, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovative Medicines Initiative-Joint Undertaking (IMI-JU #115621), the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon”, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A., Collamati A., D’Angelo E., Pahor M., Bernabei R., et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017;29:11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 2.Beaudart C., Rizzoli R., Bruyère O., Reginster J., Biver E. Sarcopenia: Burden and challenges for public health. Arch. Public Health. 2014;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvani R., Picca A., Cesari M., Tosato M., Marini F., Manes-Gravina E., Bernabei R., Landi F., Marzetti E. Biomarkers for sarcopenia: Reductionism vs. complexity. Curr. Protein Pept. Sci. 2018;19:639–642. doi: 10.2174/1389203718666170516115422. [DOI] [PubMed] [Google Scholar]
- 4.Landi F., Calvani R., Cesari M., Tosato M., Martone A.M., Ortolani E., Savera G., Salini S., Sisto A., Picca A., et al. Sarcopenia: An overview on current definitions, diagnosis and treatment. Curr. Protein Pept. Sci. 2018;19:633–638. doi: 10.2174/1389203718666170607113459. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M., Calvani R., Marzetti E. Frailty in older persons. Clin. Geriatr. Med. 2017;33:293–303. doi: 10.1016/j.cger.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Cesari M., Landi F., Calvani R., Cherubini A., Di Bari M., Kortebein P., Del Signore S., Le Lain R., Vellas B., Pahor M., et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin. Exp. Res. 2017;29:81–88. doi: 10.1007/s40520-016-0716-1. [DOI] [PubMed] [Google Scholar]
- 7.Picca A., Beli R., Calvani R., Coelho-Júnior H.J., Landi F., Bernabei R., Bucci C., Guerra F., Marzetti E. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells. 2020;9:973. doi: 10.3390/cells9040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picca A., Ponziani F.R., Calvani R., Marini F., Biancolillo A., Coelho-Júnior H.J., Gervasoni J., Primiano A., Putignani L., Del Chierico F., et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: Results from the Biosphere study. Nutrients. 2019;12:65. doi: 10.3390/nu12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzetti E., Picca A., Marini F., Biancolillo A., Coelho-Junior H.J., Gervasoni J., Bossola M., Cesari M., Onder G., Landi F., et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Exp. Gerontol. 2019;122:129–138. doi: 10.1016/j.exger.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Calvani R., Picca A., Marini F., Biancolillo A., Gervasoni J., Persichilli S., Primiano A., Coelho-Júnior H.J., Bossola M., Urbani A., et al. A Distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: Results from the Biosphere study. Nutrients. 2018;10:1691. doi: 10.3390/nu10111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho-Junior H.J., Uchida M.C., Gonçalves I.O., Calvani R., Rodrigues B., Picca A., Onder G., Landi F., Bernabei R., Marzetti E. Age and gender-related changes in physical function in community-dwelling Brazilian adults aged 50 to 102 Years. J. Geriatr. Phys. Ther. 2019 doi: 10.1519/JPT.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 12.Dent E., Morley J.E., Cruz-Jentoft A.J., Woodhouse L., Rodríguez-Mañas L., Fried L.P., Woo J., Aprahamian I., Sanford A., Lundy J., et al. Physical frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Heal. Aging. 2019;23:771–787. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauretani F., Russo C.R., Bandinelli S., Bartali B., Cavazzini C., Di Iorio A., Corsi A.M., Rantanen T., Guralnik J.M., Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 14.Marzetti E., Hwang A.C., Tosato M., Peng L.-N., Calvani R., Picca A., Chen L.-K., Landi F. Age-related changes of skeletal muscle mass and strength among Italian and Taiwanese older people: Results from the Milan EXPO 2015 survey and the I-Lan longitudinal aging study. Exp. Gerontol. 2018;102:76–80. doi: 10.1016/j.exger.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Landi F., Calvani R., Tosato M., Martone A.M., Fusco D., Sisto A., Ortolani E., Savera G., Salini S., Marzetti E. Age-related variations of muscle mass, strength, and physical performance in community-dwellers: Results from the Milan EXPO Survey. J. Am. Med. Med Dir. Assoc. 2017;18:88.e17–88.e24. doi: 10.1016/j.jamda.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Coelho-Júnior H.J., Marzetti E., Picca A., Calvani R., Cesari M., Uchida M. Prevalence of prefrailty and frailty in South America: A systematic review of observational studies. J. Frailty Aging. 2020;36:1–17. doi: 10.14283/jfa.2020.22. [DOI] [PubMed] [Google Scholar]
- 17.Suetta C., Maier A.B. Is muscle failure a better term than sarcopenia? J. Cachex Sarcopenia Muscle. 2019;10:1146–1147. doi: 10.1002/jcsm.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzetti E., Calvani R., Lorenzi M., Tanganelli F., Picca A., Bossola M., Menghi A., Bernabei R., Landi F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2016;80:1–5. doi: 10.1016/j.exger.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Justice J.N., Ferrucci L., Newman A.B., Aroda V.R., Bahnson J.L., Divers J., Espeland M.A., Marcovina S., Pollak M.N., Kritchevsky S.B., et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. GeroScience. 2018;40:419–436. doi: 10.1007/s11357-018-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra F. The Emergence of Geroscience as an Interdisciplinary Approach to the enhancement of health span and life span. Cold Spring Harb. Perspect. Med. 2016;6:a025163. doi: 10.1101/cshperspect.a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvani R., Picca A., Marini F., Biancolillo A., Gervasoni J., Persichilli S., Primiano A., Coelho-Júnior H.J., Cesari M., Bossola M., et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: Results from the Biosphere study. GeroScience. 2020 doi: 10.1007/s11357-020-00197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvani R., Picca A., Marini F., Biancolillo A., Cesari M., Pesce V., Lezza A.M.S., Bossola M., Leeuwenburgh C., Bernabei R., et al. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (Biosphere) study: Rationale, design and methods. Eur. J. Intern. Med. 2018;56:19–25. doi: 10.1016/j.ejim.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 25.Fielding R.A. Effects of exercise training in the elderly: Impact of progressive-resistance training on skeletal muscle and whole-body protein metabolism. Proc. Nutr. Soc. 1995;54:665–675. doi: 10.1079/PNS19950066. [DOI] [PubMed] [Google Scholar]
- 26.Dutta C., Hadley E.C., Lexell J. Sarcopenia and physical performance in old age: Overview. Muscle Nerve. Suppl. 1997;5:S5–S9. doi: 10.1002/(SICI)1097-4598(1997)5+<5::AID-MUS2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R., Garry P.J., Lindeman R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 28.Häkkinen K., Häkkinen A. Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991;62:410–414. doi: 10.1007/BF00626612. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosato M., Marzetti E., Cesari M., Savera G., Miller R.R., Bernabei R., Landi F., Calvani R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017;29:19–27. doi: 10.1007/s40520-016-0717-0. [DOI] [PubMed] [Google Scholar]
- 32.Heymsfield S.B., Arteaga C., McManus C., Smith J., Moffitt S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 33.Clark R.V., Walker A.C., O’Connor-Semmes R.L., Leonard M.S., Miller R.R., Stimpson S.A., Turner S.M., Ravussin E., Cefalu W.T., Hellerstein M.K., et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans. J. Appl. Physiol. 2014;116:1605–1613. doi: 10.1152/japplphysiol.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark R.V., Walker A.C., Miller R.R., Semmes R.L.O., Ravussin E., Cefalu W.T. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: Accuracy and variability vs. MRI and DXA. J. Appl. Physiol. 2018;124:1–9. doi: 10.1152/japplphysiol.00455.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans W.J., Hellerstein M., Orwoll E., Cummings S., Cawthon P.M. D3 Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J. Cachex. Sarcopenia. Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cawthon P., Orwoll E.S., Peters K.E., Ensrud K.E., Cauley J.A., Kado D.M., Stefanick M.L., Shikany J.M., Strotmeyer E., Glynn N.W., et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018;74:844–852. doi: 10.1093/gerona/gly129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon P.M., Blackwell T., Cummings S.R., Orwoll E.S., Duchowny K.A., Kado D.M., Stone K.L., Ensrud K.E., Cauley J.A., Evans W.J., et al. Muscle mass assessed by D3-Creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020:glaa111. doi: 10.1093/gerona/glaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanker J., Patel S., Blackwell T., Duchowny K., Brennan-Olsen S., Cummings S.R., Evans W.J., Orwoll E.S., Scott D., Vogrin S., et al. Walking speed and muscle mass estimated by the D3-Creatine dilution method are important components of sarcopenia associated with incident mobility disability in older men: A classification and regression tree analysis. J. Am. Med. Dir. Assoc. 2020 doi: 10.1016/j.jamda.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orwoll E.S., Peters K.E., Hellerstein M., Cummings S.R., Evans W.J., Cawthon P. The Importance of muscle versus fat mass in sarcopenic obesity: A re-evaluation using D3-Creatine muscle mass versus DXA lean mass measurements. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020;75:1362–1368. doi: 10.1093/gerona/glaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 41.Chen L.-K., Lee W., Peng L.-N., Liu L.-K., Arai H., Akishita M. Recent ADVANCES IN SARCOPENIA RESEARCH in Asia: 2016 update from the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2016;17:767.e1–767.e7. doi: 10.1016/j.jamda.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Bergquist R., Weber M., Schwenk M., Ulseth S., Helbostad J.L., Vereijken B., Taraldsen K. Performance-based clinical tests of balance and muscle strength used in young seniors: A systematic literature review. BMC Geriatr. 2019;19:9. doi: 10.1186/s12877-018-1011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohannon R.W. Considerations and practical options for measuring muscle strength: A narrative review. BioMed Res. Int. 2019;2019:8194537. doi: 10.1155/2019/8194537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taekema D.G., Gussekloo J., Maier A.B., Westendorp R.G.J., De Craen A.J.M. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age. Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 45.Wiśniowska-Szurlej A., Ćwirlej-Sozańska A., Wołoszyn N., Sozańśki B., Wilmowska-Pietruszyńska A. Association between handgrip strength, mobility, leg strength, flexibility, and postural balance in older adults under long-term care facilities. Bio.Med. Res. Int. 2019;2019:1042834. doi: 10.1155/2019/1042834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H., Chen X., Dong R., Zhang W., Han P., Kang L., Ma Y., Jia L., Fu L., Hou L., et al. Clinical relevance of different handgrip strength indexes and cardiovascular disease risk factors: A cross-sectional study in suburb-dwelling elderly Chinese. J. Formos. Med. Assoc. 2019;118:1062–1072. doi: 10.1016/j.jfma.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Martien S., Delecluse C., Boen F., Seghers J., Pelssers J., Van Hoecke A.-S., Van Roie E. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch. Gerontol. Geriatr. 2015;60:252–258. doi: 10.1016/j.archger.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Stevens P.J., Syddall H.E., Patel H.P., Martin H.J., Cooper C., Sayer A. Is grip strength a good marker of physical performance among community-dwelling older people? J. Nutr. Heal. Aging. 2012;16:769–774. doi: 10.1007/s12603-012-0388-2. [DOI] [PubMed] [Google Scholar]
- 49.McLean R.R., Shardell M.D., Alley D.E., Cawthon P.M., Fragala M.S., Harris T.B., Kenny A.M., Peters K.W., Ferrucci L., Guralnik J.M., et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giampaoli S., Ferrucci L., Cecchi F., Noce C.L., Poce A., Dima F., Santaquilani A., Vescio M.F., Menotti A. Hand-grip strength predicts incident disability in non-disabled older men. Age. Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 51.Rantanen T., Avlund K., Suominen H., Schroll M., Frändin K., Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin. Exp. Res. 2002;14:10–15. [PubMed] [Google Scholar]
- 52.Rantanen T., Guralnik J.M., Foley D., Masaki K., Leveille S., Curb J.D., White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 53.Onder G., Penninx B.W.J.H., Ferrucci L., Fried L.P., Guralnik J.M., Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: Results from the women’s health and aging study. J. Gerontol. Ser. A Boil. Sci. Med.Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 54.Bohannon R.W. Reference values for the Five-Repetition Sit-to-Stand Test: A Descriptive meta-analysis of data from elders. Percept. Mot. Ski. 2006;103:215–222. doi: 10.2466/pms.103.1.215-222. [DOI] [PubMed] [Google Scholar]
- 55.Pinheiro P.A., Carneiro J., Coqueiro R., Pereira R., Fernandes M. “Chair stand test” as simple tool for sarcopenia screening in elderly women. J. Nutr. Heal. Aging. 2016;20:56–59. doi: 10.1007/s12603-016-0676-3. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T., Bean J.F., Fielding R.A. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J. Am. Geriatr. Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 57.Bean J.F., Leveille S., Kiely D.K., Bandinelli S., Guralnik J.M., Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003;58:M728–M733. doi: 10.1093/gerona/58.8.M728. [DOI] [PubMed] [Google Scholar]
- 58.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995;332:556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D.X., Yao J., Zirek Y., Reijnierse E.M., Maier A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachex. Sarcopenia. Muscle. 2019;11:3–25. doi: 10.1002/jcsm.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taaffe D.R., Harris T.B., Ferrucci L., Rowe J., Seeman T.E. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.M709. [DOI] [PubMed] [Google Scholar]
- 61.Cesari M., Penninx B.W.J.H., Pahor M., Lauretani F., Corsi A.M., Williams G.R., Guralnik J.M., Ferrucci L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004;59:M242–M248. doi: 10.1093/gerona/59.3.M242. [DOI] [PubMed] [Google Scholar]
- 62.Ferrucci L., Penninx B.W.J.H., Volpato S., Harris T.B., Bandeen-Roche K., Balfour J., Leveille S.G., Fried L.P., Guralnik J.M. Change in muscle strength explains accelerated decline of physical function in older women with high Interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 63.Cesari M., Pahor M., Bartali B., Cherubini A., Penninx B.W.J.H., Williams G.R., Atkinson H., Martin A., Guralnik J.M., Ferrucci L. Antioxidants and physical performance in elderly persons: The invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004;79:289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 64.Picca A., Calvani R., Leeuwenburgh C., Coelho-Júnior H.J., Bernabei R., Landi F., Marzetti E. Targeting mitochondrial quality control for treating sarcopenia: Lessons from physical exercise. Expert Opin. Ther. Targets. 2018;23:153–160. doi: 10.1080/14728222.2019.1559827. [DOI] [PubMed] [Google Scholar]
- 65.Calvani R., Brasili E., Praticò G., Capuani G., Tomassini A., Marini F., Sciubba F., Finamore A., Roselli M., Marzetti E., et al. Fecal and urinary NMR-based metabolomics unveil an aging signature in mice. Exp. Gerontol. 2014;49:5–11. doi: 10.1016/j.exger.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Varadhan R., Yao W., Matteini A., Beamer B.A., Xue Q.-L., Yang H., Manwani B., Reiner A., Jenny N., Parekh N., et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 69.Butcher S., Chahel H., Lord J.M. Ageing and the neutrophil: No appetite for killing? Immunology. 2000;100:411–416. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson D., Jackson T., Sapey E., Lord J. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Busti F., Campostrini N., Martinelli N., Girelli D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front. Pharmacol. 2014;5:83. doi: 10.3389/fphar.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Picca A., Mankowski R.T., Kamenov G.D., Anton S.D., Manini T.M., Buford T.W., Saini S.K., Calvani R., Landi F., Bernabei R., et al. advanced age is associated with iron dyshomeostasis and mitochondrial DNA damage in human skeletal muscle. Cells. 2019;8:1525. doi: 10.3390/cells8121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yahiaoui L., Gvozdic D., Danialou G., Mack M., Petrof B.J. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 2008;586:3991–4004. doi: 10.1113/jphysiol.2008.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez C.O., McHale M.J., Wells J.T., Ochoa O., Michalek J.E., McManus L.M., Shireman P.K. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Integr. Comp. Physiol. 2010;299:R832–R842. doi: 10.1152/ajpregu.00797.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J., Xiao Z., Qu C., Cui W., Wang X., Du J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1high macrophage infiltration. J. Immunol. 2014;193:5149–5160. doi: 10.4049/jimmunol.1303486. [DOI] [PubMed] [Google Scholar]
- 76.Scully D., Sfyri P., Verpoorten S., Papadopoulos P., Muñoz-Turrillas M.C., Mitchell R., Aburima A., Patel K., Gutiérrez L., Naseem K.M., et al. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol. 2018;225:e13207. doi: 10.1111/apha.13207. [DOI] [PubMed] [Google Scholar]
- 77.Liu B., Poon M., Taubman M.B. PDGF-BB enhances monocyte chemoattractant protein-1 mRNA stability in smooth muscle cells by downregulating ribonuclease activity. J. Mol. Cell. Cardiol. 2006;41:160–169. doi: 10.1016/j.yjmcc.2006.03.426. [DOI] [PubMed] [Google Scholar]
- 78.Coppe J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Boil. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.-W., Lasitschka F., Andrulis M., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheibye-Knudsen M., Fang E.F., Croteau D.L., Wilson D.M., Bohr V.A. Protecting the mitochondrial powerhouse. Trends. Cell. Boil. 2014;25:158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe S., Kawamoto S., Ohtani N., Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–569. doi: 10.1111/cas.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bano G., Trevisan C., Carraro S., Solmi M., Luchini C., Stubbs B., Manzato E., Sergi G., Veronese N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Soysal P., Stubbs B., Lucato P., Luchini C., Solmi M., Peluso R., Sergi G., Isik A.T., Manzato E., Maggi S., et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 84.López-Armada M.J., Riveiro-Naveira R.R., Vaamonde-García C., Valcarcel-Ares M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Landi F., Bernabei R., Marzetti E. Fueling inflamm-aging through mitochondrial dysfunction: Mechanisms and molecular targets. Int. J. Mol. Sci. 2017;18:933. doi: 10.3390/ijms18050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matzinger P. Tolerance, Danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 87.Krysko D., Agostinis P., Krysko O., Garg A., Bachert C., Lambrecht B.N., Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Collins L.V., Hajizadeh S., Holme E., Jonsson I.-M., Tarkowski A. Endogenously oxidized mitochondrial DNA induces In Vivo and In Vitro inflammatory responses. J. Leukoc. Boil. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 89.Cai X., Chiu Y.-H., Chen Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 90.Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Bossola M., Manes-Gravina E., Landi F., Bernabei R., Marzetti E. Circulating Mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018;21:350–359. doi: 10.1089/rej.2017.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Picca A., Calvani R., Coelho-Júnior H.J., Landi F., Bernabei R., Marzetti E. Inter-organelle membrane contact sites and mitochondrial quality control during aging: A geroscience view. Cells. 2020;9:598. doi: 10.3390/cells9030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vincent A.E., Turnbull D.M., Eisner V., Hajnóczky G., Picard M. Mitochondrial nanotunnels. Trends Cell Boil. 2017;27:787–799. doi: 10.1016/j.tcb.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rasmussen M.L., Robertson G.L., Gama V. Break on through: Golgi-derived vesicles aid in mitochondrial fission. Cell Metab. 2020;31:1047–1049. doi: 10.1016/j.cmet.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ko F., Abadir P.M., Marx R., Westbrook R., Cooke C., Yang H., Walston J. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp. Gerontol. 2016;73:23–27. doi: 10.1016/j.exger.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Picca A., Guerra F., Calvani R., Coelho-Júnior H.J., Bossola M., Landi F., Bernabei R., Bucci C., Marzetti E. Generation and release of mitochondrial-derived vesicles in health, aging and disease. J. Clin. Med. 2020;9:1440. doi: 10.3390/jcm9051440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Picca A., Guerra F., Calvani R., Bucci C., Monaco M.R.L., Bentivoglio A.R., Coelho-Júnior H.J., Landi F., Bernabei R., Marzetti E. Mitochondrial dysfunction and aging: Insights from the analysis of extracellular vesicles. Int. J. Mol. Sci. 2019;20:805. doi: 10.3390/ijms20040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picca A., Calvani R., Lorenzi M., Menghi A., Galli M., Vitiello R., Randisi F., Bernabei R., Landi F., Marzetti E. Mitochondrial dynamics signaling is shifted toward fusion in muscles of very old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2017;96:63–67. doi: 10.1016/j.exger.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Basisty N.B., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., Holtz A., Shah S., Sharma V., Ferrucci L., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS. Boil. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17:e12734. doi: 10.1111/acel.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Belov L., Hallal S., Best G., Matic K.J., Mulligan S.P., Christopherson R.I. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles. 2016;5:25355. doi: 10.3402/jev.v5.25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiehn O. Metabolomics—The link between genotypes and phenotypes. Plant. Mol. Biol. 2002;48:155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 102.Nicholson J.K., Wilson I.D. Understanding ’global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 103.Nicholson J.K., Lindon J.C. Metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 104.Holmes E., Loo R.L., Stamler J., Bictash M., Yap I.K.S., Chan Q., Ebbels T., De Iorio M., Brown I.J., Veselkov K., et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pérez-Tasigchana R.F., León-Muñoz L.M., Lopez-Garcia E., Gutierrez-Fisac J.L., Laclaustra M., Rodríguez-Artalejo F., Guallar-Castillon P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing. 2017;46:807–812. doi: 10.1093/ageing/afx023. [DOI] [PubMed] [Google Scholar]
- 106.Calvani R., Rodriguez-Mañas L., Picca A., Marini F., Biancolillo A., Laosa O., Pedraza L., Gervasoni J., Primiano A., Conta G., et al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: Results from the Metabofrail study. Nutrients. 2020;12:199. doi: 10.3390/nu12010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brook M.S., Wilkinson D.J., Phillips B.E., Pérez-Schindler J., Philp A., Smith K., Atherton P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta. Physiol. 2015;216:15–41. doi: 10.1111/apha.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhenyukh O., Civantos E., Ruiz-Ortega M., Sánchez M.S., Vázquez C., Peiró C., Egido J., Mas S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Boil. Med. 2017;104:165–177. doi: 10.1016/j.freeradbiomed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 109.Yoon M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8:405. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Landi F., Calvani R., Tosato M., Martone A.M., Ortolani E., Savera G., D’Angelo E., Sisto A., Marzetti E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients. 2016;8:295. doi: 10.3390/nu8050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pasini E., Corsetti G., Aquilani R., Romano C., Picca A., Calvani R., Dioguardi F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients. 2018;10:391. doi: 10.3390/nu10040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lustgarten M.S., Price L.L., Chale A., Phillips E.M., Fielding R.A. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013;69:717–724. doi: 10.1093/gerona/glt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moaddel R., Fabbri E., Khadeer M.A., Carlson O.D., González-Freire M., Zhang P., Semba R.D., Ferrucci L. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore longitudinal study of aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016;71:1266–1272. doi: 10.1093/gerona/glw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ottestad I., Ulven S.M., Øyri L.K.L., Sandvei K.S., Gjevestad G.O., Bye A., Sheikh N.A., Biong A.S., Andersen L.F., Holven K.B. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: A cross-sectional study. Br. J. Nutr. 2018;120:445–453. doi: 10.1017/S0007114518001307. [DOI] [PubMed] [Google Scholar]
- 115.Toyoshima K., Nakamura M., Adachi Y., Imaizumi A., Hakamada T., Abe Y., Kaneko E., Takahashi S., Shimokado K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE. 2017;12:e0185206. doi: 10.1371/journal.pone.0185206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adachi Y., Ono N., Imaizumi A., Muramatsu T., Andou T., Shimodaira Y., Nagao K., Kageyama Y., Mori M., Noguchi Y., et al. Plasma amino acid profile in severely frail elderly patients in Japan. Int. J. Gerontol. 2018;12:290–293. doi: 10.1016/j.ijge.2018.03.003. [DOI] [Google Scholar]
- 117.He Q. Metabonomics and its role in amino acid nutrition research. Front. Biosci. 2011;16:2451–2460. doi: 10.2741/3865. [DOI] [PubMed] [Google Scholar]
- 118.Ter Borg S., Luiking Y.C., Van Helvoort A., Boirie Y., Schols J.M.G.A., De Groot L.C.P.G.M. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community-dwelling older adults. J. Nutr. Heal. Aging. 2018;23:27–34. doi: 10.1007/s12603-018-1108-3. [DOI] [PubMed] [Google Scholar]
- 119.O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350:1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 120.O’Toole P.W., Jeffery I.B. Microbiome–health interactions in older people. Cell. Mol. Life Sci. 2017;75:119–128. doi: 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt T.S., Raes J., Bork P. The Human gut microbiome: From association to modulation. Cell. 2018;172:1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 122.Grosicki G.J., Fielding R.A., Lustgarten M.S. Gut Microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue. Int. 2017;102:433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ticinesi A., Lauretani F., Milani C., Nouvenne A., Tana C., Del Rio D., Maggio M., Ventura M., Meschi T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut–muscle axis? Nutrients. 2017;9:1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Picca A., Fanelli F., Calvani R., Mulè G., Pesce V., Sisto A., Pantanelli C., Bernabei R., Landi F., Marzetti E. Gut dysbiosis and muscle aging: Searching for novel targets against sarcopenia. Mediat. Inflamm. 2018;2018:1–15. doi: 10.1155/2018/7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ticinesi A., Nouvenne A., Cerundolo N., Catania P., Prati B., Tana C., Meschi T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients. 2019;11:1633. doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bindels L.B., Beck R., Schakman O., Martin J.C., De Backer F., Sohet F.M., Dewulf E.M., Pachikian B.D., Neyrinck A.M., Thissen J.-P., et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE. 2012;7:e37971. doi: 10.1371/journal.pone.0037971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Macfarlane G.T., Allison C., Gibson S.A.W., Cummings J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988;64:37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 128.Macfarlane G., Cummings J., Macfarlane S., Gibson G. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989;67:521–527. doi: 10.1111/j.1365-2672.1989.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 129.Morowitz M.J., Carlisle E.M., Alverdy J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically Ill. Surg. Clin. North. Am. 2011;91:771–785. doi: 10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Metges C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000;130:1857S–1864S. doi: 10.1093/jn/130.7.1857S. [DOI] [PubMed] [Google Scholar]
- 131.Bergen W.G., Wu G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009;139:821–825. doi: 10.3945/jn.109.104497. [DOI] [PubMed] [Google Scholar]
- 132.Besten G.D., Van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maruta H., Yoshimura Y., Araki A., Kimoto M., Takahashi Y., Yamashita H. Activation of AMP-activated protein kinase and stimulation of energy metabolism by acetic acid in L6 myotube cells. PLoS ONE. 2016;11:e0158055. doi: 10.1371/journal.pone.0158055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sovran B., Hugenholtz F., Elderman M., Van Beek A.A., Graversen K., Huijskes M., Boekschoten M.V., Savelkoul H.F.J., De Vos P., Dekker J., et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-018-35228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stecher B., Hardt W.-D. The role of microbiota in infectious disease. Trends. Microbiol. 2008;16:107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 136.MacPherson A.J., Geuking M.B., McCoy K.D. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkilä J., Monti D., Satokari R., Franceschi C., et al. through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schiffrin E.J., Morley J.E., Donnet-Hughes A., Guigoz Y. The inflammatory status of the elderly: The intestinal contribution. Mutat. Res. Mol. Mech. Mutagen. 2010;690:50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 140.Shapiro H., Thaiss C.A., Levy M., Elinav E. The cross talk between microbiota and the immune system: Metabolites take center stage. Curr. Opin. Immunol. 2014;30:54–62. doi: 10.1016/j.coi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 141.Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability In Vitro and In Vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD. Am. J. Pathol. 2012;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Tongeren S.P., Slaets J.P.J., Harmsen H.J.M., Welling G.W., Viterbo A., Harel M., Horwitz B.A., Chet I., Mukherjee P.K. Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 2005;71:6241–6246. doi: 10.1128/AEM.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peng L., Li Z.-R., Green R.S., Holzman I.R., Lin J. butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Londhe P., Guttridge D.C. Inflammation induced loss of skeletal muscle. Bone. 2015;80:131–142. doi: 10.1016/j.bone.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O’Connor E.M., Cusack S., Harris H.M.B., Coakley M., Lakshminarayanan B., O’Sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]