Abstract
The pathological consequences of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are multiple, with interstitial pneumonia and consecutive respiratory failure being the most dangerous clinical manifestations. Timely diagnosis and follow-up of pulmonary involvement need a comprehensive imaging strategy, which includes standard chest X-ray, chest computed tomography and lung ultrasound (LUS). In the last 10 years, LUS has become a useful, bedside and easily reproducible tool for lung examination. In the first part of this review, we present the pathophysiological background, technical principles and practical aspects of LUS in patients with SARS-CoV-2 infection. In the second part, the main echographic findings, their interpretation, and the clinical applications of LUS are overviewed. The review ends with the presentation of our work methodology, illustrated with images recorded from COVID-19 patients in our department.
Keywords: COVID-19, lung ultrasound, pneumonia
Introduction
COVID-19: Epidemiological and clinical context
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-coronavirus disease 2019 (COVID-19)-started at the end of 2019, affecting all the countries in the world. This was associated with serious medical, social, political, and economical consequences. Till date (08.06.2020), more than seven million persons are infected worldwide, with close to 500.000 casualties (1). The clinical picture is dominated by flu-like respiratory and general symptoms (such as fever, fatigue, loss of smell, cough, myalgia, diarrhea, and dyspnea). The disease is mild in the majority of cases. However, interstitial pneumonia and respiratory failure can occur in 15%–20% of patients. In the case of respiratory failure, dyspnea associated with hypoxemia is characteristic, and progression to more severe forms requiring mechanical ventilation could be rapid. The involvement of other organs, systems, and homeostatic mechanisms–liver, kidney, heart and circulatory system, nervous system, and coagulation system (hypercoagulability)–also occur frequently. The definitive diagnosis of active infection is provided by the detection of the virus in the patient’s nasopharyngeal secretions, using real-time reverse transcription polymerase chain reaction (rRT-PCR) assay. The clinical management of COVID-19 is still evolving and includes antiviral (mostly empirical and still investigational), and immunomodulatory therapies, as well as intensive care measures (such as mechanical ventilation) for those in critical conditions (2, 3).
Pathophysiological background of pulmonary involvement and respiratory failure in SARS-CoV-2 infection
Lung involvement is the main pathological feature of COVID-19, and is responsible for respiratory failure, the leading cause of death. The lung injury produced by SARS-CoV-2 starts with viral attachment to angiotensin converting enzyme 2 (ACE2) receptors, present on the apical surface of respiratory epithelial cells in the conductive airways. The infected respiratory epithelial cells are the source of the local and systemic (to distant organs) viral spread, a process which is facilitated by inflammation and alveolar-capillary damage (4). ACE2 is a membrane-associated aminopeptidase expressed in the pulmonary epithelium, vascular endothelia, renal and cardiovascular tissue, and the epithelia of small intestines and testes (5). Beyond replication in the epithelial cells, SARS-CoV-2 down-regulates the expression of ACE2 receptors, which results in increased angiotensin II levels and induction of further lung injury via angiotensin II receptor type 1 stimulation. Lung damage is also promoted by the so-called cytokine storm (hyperinflammatory response), a part of the patient’s immune reaction (4, 6).
On microscopy, lung injury in COVID-19 is characterized by alveolar hyaline membrane formation, fibrin exudates, epithelial damage, vascular congestion, and diffuse-type II pneumocyte hyperplasia. The pulmonary interstitium is infiltrated by monocytes, macrophages, and lymphocytes. In more advanced phases of the disease, thickening of the alveolar walls and interstitium can be observed, with intraalveolar organization caused by fibroblastic proliferation and extracellular matrix formation (7). An important and specific pathological finding in COVID-19 patients is the extensive microangiopathy of pulmonary vessels, associated with microthrombosis and neoangiogenesis (8).
The morphological lesions developed in the lungs have characteristic (although not specific) correspondents on chest computed tomography images: ground-glass opacities reflecting edema of the alveolar septa, hyperplasia of the interstitium, partial filling of airspaces, or their combination; crazy-paving patterns corresponding to hyperplasia of inter- and intra-lobular interstitial tissue; and consolidations, which correspond to advanced alveolar damage, and can appear in the center of ground-glass opacities or be patchy. The lesions are frequently localized in the lower lobes of both lungs, subpleurally. The global aspect in the early stages of lung injury corresponds to the diagnosis of viral pneumonia. In the case of progression, later stages of the disease can be accompanied by typical morphological and imaging pictures of acute respiratory distress syndrome (ARDS) (9).
Recent data support that vascular damage and dysfunction play an essential role in the development of respiratory failure and ARDS in COVID-19 patients. Insufficient hypoxic vasoconstriction response in poorly ventilated pulmonary areas causes hypoxemia by ventilation/perfusion mismatch. In the early phase of respiratory failure, the lung has low elastance, the ventilation-to-perfusion (VA/Q) ratio is low, and there is a low lung recruitability (type L phenotype). Progression of pulmonary lesions causes, in the later phase, the morpho-functional picture of a classical ARDS, with high pulmonary elastance, high right-to-left shunt and high lung recruitability (type H phenotype). These types of ARDS have to be considered when setting a mechanical ventilation strategy and parameters (tidal volume, positive end-expiratory pressure level, etc.) (10, 11).
Pulmonary involvement could be accompanied and aggravated by cardiac, liver, renal, and nervous system dysfunctions, as well as the prothrombotic state prone to cause thromboembolic complications.
Subsequently, we present the principal technical, practical and clinical data regarding the use of lung ultrasound (LUS)-an emerging bedside imaging tool-in evaluating pulmonary involvement in COVID-19 patients.
General principles of lung ultrasound examination
Despite the initial caveats due to the presence of air in the lungs, LUS has proven over time to be useful in the imaging of pulmonary structures (pleura, subpleural space, and parenchyma), gaining an important diagnostic and prognostic role in pulmonary medicine and cardiology. LUS came in handy in the recent COVID-19 era (12).
LUS is performed in most cases by using a convex ultrasound probe (with a variable frequency between 2 and 5 MHz). On the other hand, a linear probe (with a variable frequency between 4 and 12 MHz) allows a better definition of the pleural line and the proximal subpleural space. It is ideal when the machine has a lung preset, otherwise, an abdominal preset with a depth of 8–10 cm is used. This may differ depending on the patient’s body constitution. The gain and focus should be adjusted, and positioned to optimize the visualization of the pleural line and lung sliding. The mechanical index should be kept low, and the frame rate maximized (13, 14).
When scanning the lungs, a distinction should be made between healthy and pathological parenchyma. A-lines are horizontal artifacts, which can be seen in parallel with the thin, hyperechogenic pleural line that moves synchronously with respiratory movements. These artifacts are caused by the normally aerated lung. In the case of lungs with reduced air content (such as pulmonary congestion, acute interstitial lung injury, fibrosis), A-lines disappear, and B-lines appear. These are comet-tail-shaped, laser-beam like, reverberation artifacts, which begin at the pleural line and penetrate downwards to the bottom of the scanning sector, their motion being synchronous with breathing movements. B-lines are oriented vertically (when using a linear probe) or radially (when using a convex probe) and are hyperechoic. In the case of worsening congestion due to left heart failure, the number of B-lines increase, and pleural effusion can occur. If there is an inflammatory process in the lung interstitium, the number of B-lines increases, but because of the changes which can take place in the pleura and the adjacent pulmonary parenchyma, this could be accompanied by pleural line irregularity and disruption, and the appearance of a subpleural consolidation pattern. The latter appears on LUS as a “tissue-like” echogenic mass in the lung, arising from the pleural line in the absence of pleural effusion, and having a nearly identical echodensity with the liver (13-17).
The methodology of examination: Our practice
To avoid the nosocomial spread of the virus and minimize the risk of infection of healthcare workers, portable (hand-held) ultrasound devices are recommended for LUS examination in COVID-19 patients. They are easier to carry for bedside examinations, and can easily be disinfected and protected (by using a plastic foil for example) (18, 19).
For the LUS in our patients, General Electric V-Scan 2 (linear probe of 3.4–8.0 MHz) and Philips Lumify (convex probe of 2–5 MHz and linear probe of 4–12 MHz) hand-held ultrasound devices were used. The examination requisites, including the ultrasound device(s), disinfectant, ultrasound gel, disposable paper towel, a pack of gloves, and plastic foil were prepared on a pushable 4-wheel mobile table (Fig. 1). This preparation enabled the examination to be performed by one operator, at bedside. During the examination, the ultrasound recordings were saved for off-line analysis and further validation by two properly trained ultrasonographers.
Figure 1.

The worktable with the hand-held ultrasound device and the auxiliary tools
The LUS was performed with the patient in a sitting position, except for mechanically ventilated patients, who were examined in the supine position. The best examination protocol in COVID-19 patients is still subject to debate, however, we used a previously developed, standardized scanning protocol. Sixteen areas were scanned per patient, with a recording duration of 5–6 seconds per area, containing at least one complete respiratory cycle. The average time of LUS per patient was 7 minutes (13, 14, 20).
Between the two possible positions of the ultrasound probe in relation to the ribs, longitudinal (perpendicular) and transversal (parallel), the latter was our preferred approach. The chest wall was divided into 4 regions on both sides by 5 anatomical lines (parasternal, anterior and posterior axillary, scapular and paravertebral). Every region was further divided into an upper and a lower area (Fig. 2). Although the posterior chest wall (areas 7 and 8 on both sides) was not always accessible for examination as in the case of mechanically ventilated patients, we always tried to visualize this region (by tilting the patient on the side), because of the frequent occurrence of pulmonary lesions in this site, especially in the early phase of the disease. The sequence of examination was left to right, anterior to posterior and top-down (14, 17-19). The worksheet (score table) used in our department with the scanning areas is presented in Table 1.
Figure 2.

Example of the scanning areas (left side)
Table 1.
Scanning worksheet (score table) with the areas of LUS examination. The final results are introduced in the lower row
| Posterior | Postero-lateral | Antero-lateral | Anterior | Anterior | Antero-lateral | Postero-lateral | Posterior | |
|---|---|---|---|---|---|---|---|---|
| Upper | R7 | R5 | R3 | R1 | L1 | L3 | L5 | L7 |
| Lower | R8 | R6 | R4 | R2 | L2 | L4 | L6 | L8 |
| Total A-BBC score: | Nr. of pleural involvement - “p”: | Pleural effusion: | ||||||
Symbols: R - right; L- left; 1–8–the number of areas
Lung ultrasound patterns in COVID-19 patients
In the articles published so far, SARS-Cov-2 infection does not seem to produce characteristic or unique image patterns on LUS. The findings vary depending on the severity and nature of the inflammatory process in the lung. Thus, the spectrum of ultrasound changes ranges from normal-looking lung parenchyma and the image of simple interstitial involvement to consolidation (pneumonia) patterns. The type and characteristics of ultrasound findings correlate well with the pathological changes taking place in the lungs (19, 20).
The following patterns can be observed on LUS in COVID-19 patients (14, 19-21):
a) decrease/loss of air content: disappearance of A-lines;
b) presence of interstitial inflammatory infiltrates: B-lines (comet tails), which can be multiple and grouped; if they are confluent, the aspect is called the “waterfall” sign;
c) pleural involvement: thickening of the pleural line with irregularities (including “skip” lesions which represent the disruption of the pleural line) and reduced pleural sliding;
d) subpleural consolidations: ecogenic masses with multifocal, translobar and non-translobar distribution, sometimes associated with mobile bronchoaerograms;
e) pleural effusion (low incidence);
A special attention is required to differentiate B-lines from Z-lines. The latter are artifacts, which have a less defined starting point, do not move with lung sliding and do not influence A-lines. They are wider, less ecogenic than the pleural line, and do not extend to the bottom of the ultrasound image sector (12).
The A-BBC score (each letter represents a pattern) has been introduced recently for better characterization and quantification of pulmonary involvement by LUS. The components of this score are presented in Table 2. It is important to mention, that in the case of B1 and B2 severity classes, close attention must be paid to the pleural line. If pleural lesions are present, the letter “p” is precised, which means pleural involvement, a sign of disease severity (14, 20). The LUS patterns corresponding to the components of A-BBC score system are presented in Figure 3. The LUS images were obtained from COVID-19 patients hospitalized in our Department. Table 3 shows an example of a completed scanning worksheet (score table).
Table 2.
The A-BBC score system and its components
| Severity class | Score | Definition |
|---|---|---|
| A | 0 point | Normal pleural line and well-ventilated lung with the presence of a maximum of 3 B-lines. |
| B1 | 1 point | More than 3 B-lines, their confluence not exceeding more than 50% of the image sector, lack of clear subpleural involvement. |
| B2 | 2 points | Confluent B-lines that cover more than 50% of the image sector, lack of clear subpleural lesions. |
| C | 3 points | Presence of consolidation which can be associated with broncho-aerogram. |
Figure 3.
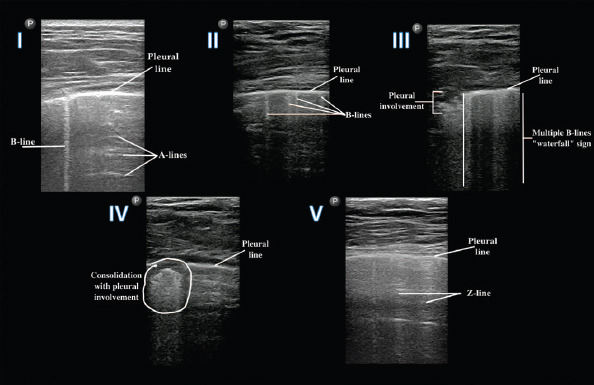
Images demonstrating the main changes on LUS in COVID-19 patients (the elements of A-BBC score). I–score 0 (class A): normal pleural line, 1 B-line, presence of A-lines; II–score 1 (class B1); normal pleural line, >3 B-lines; III - score 2 (class B2): multiple B-lines (“waterfall” sign), pleural involvement (“p”); IV - score 3 (class C): subpleural involvement (consolidation) and disruption of pleural line; V–Z-lines: vertical, wider lines, less ecogenic than the pleural line, without clinical significance
Table 3.
Example of a completed scanning worksheet (score table). The final results are in the lower row
| Posterior | Postero-lateral | Antero-lateral | Anterior | Anterior | Antero-lateral | Postero-lateral | Posterior | |
|---|---|---|---|---|---|---|---|---|
| Upper | 1 | 1p | 2p | 3 | 0 | 0 | 1 | 1 |
| Lower | 1 | 1 | 2 | 2p | 0 | 1 | 1p | 1 |
| Total A-BBC score: 18 | Nr. of pleural involvement - “p”: 4 | Pleural effusion: NO | ||||||
Clinical applications
There are three major clinical applications of LUS in the setting of COVID-19: (1) primary screening and diagnosis of lung involvement in patients presenting at the emergency room with a suspicion of COVID-19, (2) daily routine, or as occasion requires (such as deterioration of oxygen saturation), and monitoring of patients hospitalized in general wards, and (3) daily and regular monitoring of patients (with or without mechanical ventilation) in the intensive therapy wards. It is important to emphasize that LUS is frequently completed in clinical practice by point of care ultrasound, limited transthoracic echocardiographic (TTE) or critical care echo examinations (22, 23).
In the emergency setting, the application of LUS examination can reduce the contact time with patients, lowering the risk of disease transmission. In the case of a suggestive clinical picture, the presence of typical ultrasound findings can estabilish the diagnosis of COVID-19. In the case of positive LUS, even after a negative first rRT-PCR test, the patient has to be isolated and retested. Also, the severity of LUS lesions can be used as a basis for an initial risk stratification.
In hospitalized patients, sequential LUS provides an efficient tool for monitoring the progression or regression of pulmonary lesions. Variations in the number and aspect of B-lines and areas of alveolar congestion (consolidation) are most important in this regard. The reappearance of A-lines is a sign of parenchymal healing. Monitoring of pulmonary involvement by LUS also has an important role in the evaluation of the efficacy of different treatment modalities, and can help in or trigger therapeutic decisions. In critically ill patients, LUS can predict the chance of weaning from mechanical ventilation. Finally, it is important to mention that ultrasound findings always have to be interpreted while considering the clinical picture and blood oxygenation parameters (23-25).
Conclusion
In the last decade, LUS has gained a remarkable place in the bedside evaluation of cardiac and pulmonary patients. The main abilities of LUS rely on the visualization of pulmonary tissue (interstitium, parenchyma) and pleura in diverse pathological processes. In COVID-19, pulmonary lesions have a key role in determining the clinical course and prognosis. LUS as an easy imaging technique, could therefore be considered an important tool in the hands of clinicians for the diagnosis and follow-up of pulmonary involvement, and for making proper and timely therapeutic decisions.
Footnotes
Conflict of interest: None declared.
Peer-review: Internally peer-reviewed.
Authorship contributions: Concept – I.A.S., G.Á., A.F.; Design – I.A.S., A.F.; Supervision – G.Á., A.F.; Funding – Philips Medical Systems Romania provided the transducers used for the screening of COVID-19 patients; Materials – I.A.S., O.S.C., A.F.; Data collection and/or processing – I.A.S., O.S.C., A.F.; Analysis and/or interpretation – I.A.S., G.Á., A.V., O.S.C., A.F.; Literature search – I.A.S., G.Á., A.V., O.S.C., A.F.; Writing – I.A.S., G.Á., A.V., O.S.C., A.F.; Critical review – G.Á., A.V., O.S.C., A.F.
References
- 1. [accessed on 08 June 2020]. Available online: URL; https://www.worldometers.info/coronavirus/
- 2.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) Available online: URL; https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html .
- 3.National Institute of Health. COVID-19 treatment guidelines. Available online: URL; https://www.covid19treatmentguidelines.nih.gov/overview/
- 4.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–74. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19:Immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–14. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, et al. Imaging Features of Coronavirus disease 2019 (COVID-19):Evaluation on Thin-Section CT. Acad Radiol. 2020;27:609–13. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Ma X. Acute respiratory failure in COVID-19:is it “typical” ARDS? Crit Care. 2020;24:198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia:different respiratory treatments for different phenotypes?Version 2. Intensive Care Med. 2020;46:1099–102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargani L. Lung ultrasound:a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. doi: 10.1186/1476-7120-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargani L, Volpicelli G. How I do it:lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. doi: 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19:A Simple, Quantitative, Reproducible Method. J Ultrasound Med. 2020;39:1413–9. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picano E, Scali C. M, Ciampi Q, Lichtenstein D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc Imaging. 2018;11:1692–705. doi: 10.1016/j.jcmg.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein DA, Lascols N, Mezière G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30:276–81. doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 17.Gargani L, Soliman-Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic:enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa163. jeaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–51. doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith MJ, Hayward SA, Innes SM, Miller ASC. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020;75:1096–104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudea SM. Ultrasonography and SARS-CoV 2 infection:a review of what we know and do not yet know. Med Ultrason. 2020;22:129–32. doi: 10.11152/mu-2612. [DOI] [PubMed] [Google Scholar]
- 21.Peng QY, Wang XT, Zhang LN Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849–50. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkpatrick JN, Grimm R, Johri AM, Kimura BJ, Kort S, Labovitz AJ, et al. Recommendations for Echocardiography Laboratories Participating in Cardiac Point of Care Cardiac Ultrasound (POCUS) and Critical Care Echocardiography Training:Report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:409–22. doi: 10.1016/j.echo.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, Johri AM. Bedside Focused Cardiac Ultrasound in COVID-19 from the Wuhan Epicenter:The Role of Cardiac Point-of-Care Ultrasound, Limited Transthoracic Echocardiography, and Critical Care Echocardiography. J Am Soc Echocardiogr. 2020;33:676–82. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake DH, De Bonis M, Covella M, Agricola E, Zangrillo A, Zimmerman KG, et al. Echocardiography in Pandemic:Front-Line Perspective, Expanding Role of Ultrasound, and Ethics of Resource Allocation. J Am Soc Echocardiogr. 2020;33:683–9. doi: 10.1016/j.echo.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhemad B, Mongodi S, Via G, Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122:437–47. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]


