Abstract
Cardiac Sarcoidosis (CS) represents a unique diagnostic dilemma. Guidelines have been recently revised to reflect the established role of sophisticated imaging techniques. Trans-thoracic Echocardiography (TTE) is widely adopted for initial screening of CS. Contemporary TTE techniques could enhance detection of subclinical Left Ventricular (LV) dysfunction, particularly LV global longitudinal strain assessment which predicts event-free survival (meta-analysis of 5 studies, hazard ratio 1.28, 95% confidence interval 1.18-1.37, p < 0.0001). However, despite the wide availability of TTE, it has limited sensitivity and specificity for CS diagnosis. Cardiac Magnetic resonance Imaging (CMR) is a crucial diagnostic modality for suspected CS. Presence of late gadolinium enhancement signifies myocardial scar and enables risk stratification. Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) coupled with myocardial perfusion imaging can identify active CS and guide immunosuppressant therapy. Gallium scintigraphy may be considered although FDG-PET is often preferred. While CMR and FDG-PET provide complementary information in CS evaluation, current guidelines do not recommend which imaging modalities are essential in suspected CS and if so, which modality should be performed first. The utility of hybrid imaging combining both advanced imaging modalities in a single scan is currently being explored, although not yet widely available. In view of recent, significant advances in cardiac imaging techniques, this review aims to discuss changes in guidelines for CS diagnosis, the role of various cardiac imaging modalities and the future direction in CS.
Keywords: Cardiac sarcoidosis, trans-thoracic echocardiography, LV dysfunction, magnetic resonance imaging, immunosuppressant therapy, FDG-PET
1. Introduction
Sarcoidosis is a multi-system granulomatous disorder that can affect people of all ages and ethnic backgrounds [1]. Both females and males may be affected, with males being slightly younger at diagnosis (40-59 years) compared with females (50-69 years) [2]. While lung is the most commonly involved organ [2], Cardiac Sarcoidosis (CS) is more frequently diagnosed depending on the adopted technique and studied population. Clinical evidence of cardiac involvement is seen in 5% of individuals with systemic sarcoidosis, however, is reported in up to 27% of autopsy cases [3]. Cardiac involvement significantly impacts prognosis, with ventricular arrhythmias and Sudden Cardiac Death (SCD) not uncommon [4]. The annual incidence of suspected CS has risen significantly over the last two decades [5]. This is likely related to increased awareness and detection through more sophisticated cardiac imaging. Substantial progress in multi-modality imaging for CS has significant implications in how we diagnose, risk stratify, and treat CS, which is reflected in recently revised guidelines. In this review, we provide an overview of the state of CS diagnosis according to current guidelines, the landscape of imaging modalities in CS and their relevance from diagnosis to prognosis and future direction in the field.
2. Diagnosis of cardiac sarcoidosis according to guidelines
Cardiac sarcoidosis represents a unique diagnostic dilemma due to the lack of a gold standard diagnostic tool. Although histologic diagnosis from the endomyocardial biopsy is definitive, diagnostic yields can be as low as 25% given patchy myocardial infiltration pattern in CS [6]. The most commonly adopted clinical diagnostic criteria is the Heart Rhythm Society (HRS) and Japanese Ministry of Health and Welfare (JMHW) consensus which was revised in 2017 by the Japanese Circulation Society [7, 8] (Table 1).
Table 1.
Clinical criteria for cardiac involvement in sarcoidosis.
| Heart Rhythm Society (2014) | Japanese Circulation Society (2017) |
|---|---|
|
Histological diagnosis of extra-cardiac sarcoidosis AND ≥ 1 of the following: - Immunosuppressant responsive cardiomyopathy or HB - Unexplained LVEF < 40% - Unexplained sustained VT (spontaneous or induced) - Mobitz type II 2nd degree HB or 3rd degree HB - Patchy uptake on PET - Late gadolinium enhancement on CMR - Positive gallium uptake AND - Other causes of cardiac manifestation(s) have been reasonable excluded |
- ≥ 2 major criteria OR 1 major criteria and ≥ 2 minor criteria Major Criteria - High-grade AV block - Basal thinning of IV septum or abnormal ventricular wall anatomy - LVEF < 50% - 67Ga citrate scintigraphy or abnormal FDG-PET uptake - Late gadolinium enhancement on CMR Minor Criteria - Abnormal ECG findings - SPECT perfusion defects - EMB: interstitial fibrosis or monocyte infiltration over moderate grade |
Abbreviations: AV: Atrioventricular; CMR: Cardiac Magnetic Resonance Imaging; ECG: Electrocardiogram; EMB: Endomyocardial Biopsy; HB: Heart Block; IV: Interventricular; LVEF: Left Ventricular Ejection Fraction; PET: Positron Emission Tomography; SPECT: Myocardial Perfusion Scintigraphy.
Key changes in JMHW criteria reflect the increasingly recognised role of non-invasive, advanced cardiac imaging [8]. Major criteria for CS diagnosis have been revised to include abnormal uptake on Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) and Late Gadolinium Enhancement (LGE) on Cardiac Magnetic Resonance Imaging (CMR), which is now consistent with HRS criteria. A notable difference between guidelines is the requirement of histologic diagnosis of extra-cardiac sarcoidosis (i.e. biopsy-proven sarcoidosis outside of the heart if histologic CS is not obtained) in HRS criteria [7] which is not mandated in JMHW criteria. While isolated CS is uncommon with a small prospective study reporting an incidence of 1 out of 31 sarcoidosis patients (~3%) using FDG-PET [9], treatment may be altered in the clinical setting including early commencement of immunosuppression, heart failure treatment and even implantable cardioverter-defibrillator for primary prevention of SCD [10]. Thus patients with isolated CS should not be overlooked given early detection and treatment can allay progression.
3. Echocardiography
Patients with suspected CS will generally undergo certain screening tests during the initial evaluation. An Electrocardiogram (ECG), 12-lead ambulatory ECG monitoring, and Trans-Thoracic Echocardiography (TTE) are feasible for initial evaluation, particularly in individuals with known systemic disease. Trans-thoracic echocardiography is widely available, non-invasive, relatively reproducible and does not utilise radiation. While advanced imaging techniques are important in CS diagnosis, it is impractical to use such modalities for screening given cost and accessibility; these modalities will be discussed subsequently.
Various echocardiographic findings can be found in CS (Table 2; Figs. 1 and 2). The disease has a predilection for the basal interventricular septum and basal inferolateral wall but can also affect the valves, papillary muscles, and pericardium. Diastolic dysfunction occurs in early disease due to acute inflammation and tissue oedema with systolic dysfunction occurring later, particularly when accompanied by myocardial fibrosis. Echocardiography is superior to CMR in assessing significant mitral regurgitation, which may occur due to Left Ventricular (LV) dilatation, basal inferolateral aneurysm formation or granulomatous involvement of the papillary muscles. Classic features such as LV wall thinning and aneurysm formation usually manifest late and are non-specific. Presence of right heart dilatation and significant tricuspid regurgitation may represent Right Ventricular (RV) disease involvement or pulmonary hypertension secondary to pulmonary sarcoidosis [11].
Table 2.
Echocardiographic findings in cardiac sarcoidosis.
| Ventricles and Interventricular Septum | Wall thickening (non-coronary distribution) Interventricular thinning or aneurysm LV dilatation or aneurysm LV systolic dysfunction Increased ventricular wall echogenicity (esp. ventricular septum or LV free wall) RV dilatation / dysfunction (esp. basal septum) Ventricular dyssynchrony Global hypokinesis |
| Atria | Atrial wall hypertrophy (atrial lesions) |
| Valves | Mitral regurgitation Tricuspid regurgitation |
| Other findings | Pericardial effusion or tamponade Constrictive pericarditis Pulmonary hypertension |
Contemporary echocardiographic techniques may identify cardiac involvement in subclinical stages of disease. Speckle-tracking echocardiography is a tool that assesses regional and global myocardial deformation by tracking the motion of the endocardium and epicardium throughout the cardiac cycle in multiple LV segments [12]. While previously limited to research context, its clinical utility combined with visual assessment of wall motion is growing. In three recent studies, LV Global Longitudinal Strain (GLS) was consistently lower in CS patients compared with sarcoidosis patients without cardiac involvement, despite preserved LV Ejection Fraction (EF) [13-15]. Furthermore, LV GLS was also lower in sarcoidosis patients without diagnosed cardiac involvement compared with healthy controls [16, 17]. Thus LV GLS could detect subclinical LV dysfunction even before CS diagnosis is made according to current guidelines. Additionally, we found that LV GLS was an independent predictor of adverse cardiovascular outcomes in a meta-analysis of five studies with no inter-study heterogeneity (hazard ratio 1.28, 95% confidence interval 1.18-1.37, p < 0.0001; (Fig. 3) [13, 15-18]. The composite primary endpoint across studies was generally all-cause mortality, new ventricular arrhythmia, heart failure-related hospitalization or cardiac device implantation, with a median follow-up ranging from 8 to 57 months. Of note, these studies included only patients with preserved EF and in certain cases, patients had normal FDG-PET and absence of LGE-MR [15]. In addition, the assessment of segmental strain can identify areas of LGE on CMR. In a study by Orii and colleagues, the assessment of circumferential strain appeared more sensitive than the longitudinal strain in identifying segments of the LV with fibrosis, with an area under the curve of 0.96 [19].
Fig. (3).
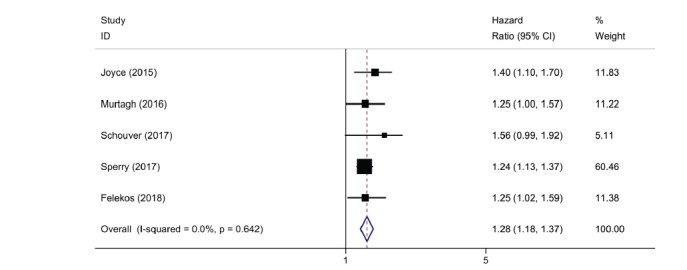
Left ventricular global longitudinal strain on echocardiography and risk of adverse cardiac events.
Despite the potential prognostic insight gained by LV GLS, there is currently no evidence that early therapy based on LV GLS alone, in the absence of LGE on CMR or enhancement on FDG-PET, improves outcomes. Furthermore, TTE has a low sensitivity for detecting CS compared with CMR [11]. Thus patients with suspected CS will likely undergo CMR, which has significantly higher resolution and prognostic insight. Nevertheless, LV GLS on TTE may assist in detecting early subclinical LV dysfunction and further research is needed to determine its clinical utility.
4. Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging is an important imaging modality in CS. It can detect acute inflammation through early gadolinium enhancement using T2-weighted signal and identify myocardial thinning and wall motion abnormalities. Presence of LGE, which reflects myocardial scar or fibrosis, is a critical CMR finding for CS. Epicardial distribution of LGE is a characteristic finding although other patterns are also seen [20]. Images of CMR findings in CS are shown in Fig. (4).
Fig. (4).
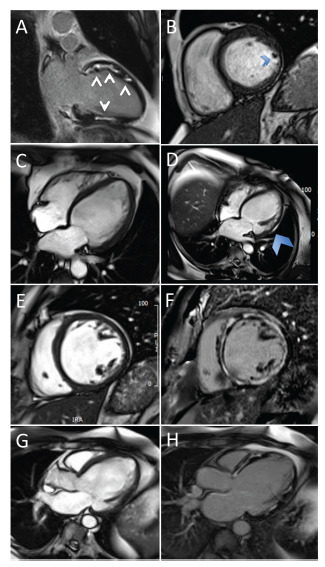
Cardiac magnetic resonance imaging findings in cardiac sarcoidosis. (A) Two-chamber view of LGE. (B) Four-chamber cine stack of lateral wall thinning. (C) Uniform wall on the first scan. (D) Four-chamber view of wall thinning. (E) and (F) SA stack of LGE. (G) and (H) Three-chamber view of LGE. LGE, late gadolinium enhancement. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
An early study highlighting the importance of LGE on CMR in CS showed that only one-fifth of biopsy-proven pulmonary sarcoidosis patients met criteria for CS diagnosis according to JMHW criteria at that time, despite almost three-quarters demonstrating LGE on CMR [21]. Another study used histopathologic examination to reveal consistency between CS diagnosis and LGE on CMR, while JMHW criteria missed the diagnosis in 2 out of 4 patients [22]. Moreover, LGE drastically affects prognosis with a 9-fold higher rate of major adverse cardiac events compared with sarcoidosis patients without LGE [22].
Late gadolinium enhancement on CMR is now recognized as a significant prognostic marker for mortality and ventricular arrhythmias in CS. In a meta-analysis of 10 studies and 760 sarcoidosis patients, presence of LGE was associated with an annualized event rate of 12% for the composite outcome of arrhythmogenic events (ventricular arrhythmia, Implantable Cardioverter-Defibrillator (ICD) shock, sudden cardiac death) compared with 1% in the absence of LGE [23]. Furthermore, the extent of LGE also confers incremental prognosis where a 1% increase in LGE burden corresponds to an 8% increase in the risk of death or ventricular arrhythmia [24]. Therefore, current guidelines suggest patients with LGE-CMR and reduced LVEF despite optimal medical therapy and a period of immunosuppression can be referred for diagnostic electrophysiology study to evaluate for inducible ventricular tachycardia and consideration of primary prevention ICD (Class IIa recommendation) [7]. Of note, LGE burden may also correspond to corticosteroid response with one study demonstrating that patients with a small-extent of LGE have an improvement in LV end-diastolic volume index and LVEF as opposed to patients with larger extent of LGE [25].
Cardiac magnetic resonance imaging should be performed in the context of suspected CS based on the presence of cardiac symptoms, abnormal electrocardiogram or Holter monitor or echocardiogram. However, certain circumstances may preclude the use of CMR. These include gadolinium-contrast toxicity and risk of nephrogenic systemic fibrosis in patients with advanced chronic kidney disease [26], and patients with MR incompatible implantable cardiac devices [27, 28]. In patients with a contraindication to CMR, FDG-PET should be utilized. Furthermore, CMR has limitations in distinguishing active inflammation from scar and FDG-PET may be more appropriate for monitoring disease development and treatment response.
5. FDG-PET and Myocardial Perfusion Imaging
Fluorodeoxyglucose positron emission tomography in combination with Myocardial Perfusion Imaging (MPI) is widely used to assess active inflammation in CS. FDG (18F-fluorodeoxyglucose) is a glucose analog that accumulates in macrophages and CD4 T-lymphocytes due to their increased metabolic activity. Accumulation in regions of ongoing inflammation enables visualization of active sarcoid granulomas compared with normal myocardium [29]. To achieve optimal imaging and differentiation, patients must adhere to a pre-imaging protocol that involves a carbohydrate-free diet for at least 12 hours prior to the scan and avoidance of activities associated with myocardial stress such as physical exercise. Both can lead to increased global metabolic uptake in the myocardium and obscure visualisation of focal regions of interest. An intravenous load of FDG is administered 60-90 minutes before image acquisition to allow adequate time for myocardial uptake. Presence of focal or focal-on-diffuse FDG uptake generally represents abnormality consistent with CS, and RV involvement portends a worse prognosis [30].
Myocardial perfusion imaging is often undertaken simultaneously with FDG-PET. While it identifies perfusion defects indicative of inflammation or scar, its low sensitivity and specificity precludes its use as a diagnostic imaging modality in isolation. Perfusion defects in CS are generally in a non-coronary distribution due to patchy granulomatous infiltration; however, coronary artery disease and other relevant diagnoses must still be excluded before accurate interpretation of results. Although difficult to quantify, the severity and extent of perfusion defects are associated with increasing fibrotic changes, particularly if FDG-uptake is negative [31].
In combination, FDG-PET with MPI can help identify patients with active CS and guide immunosuppressant initiation. Additionally, it can identify extra-cardiac regions of FDG-uptake more accessible to biopsy and can monitor treatment response. However, comparison with previous imaging can be difficult as display schemes normalise image intensity up to the most intense pixel and thus absolute intensity myocardial FDG uptake is relative to each individual scan. The role of FDG-PET with MPI in determining prognosis is unclear. The most convincing evidence arose from a study by Blankstein and colleagues who retrospectively evaluated 118 suspected CS patients referred for PET [30]. Over a median follow-up of 1.5 years, one-quarter suffered ventricular tachycardia or death and the presence of both abnormal perfusion and FDG-uptake was a predictor of adverse events when adjusted for JMWH criteria and LVEF. Of note, the individual presence of either abnormal FDG-uptake or perfusion defect was not a significant predictor alone, and the study did not adjust for the presence of LGE-CMR. Thus far, no further studies have confirmed these findings in a similar binary fashion [6, 32]. A recent study observed that the presence of any PET abnormality (FDG-uptake or perfusion) to be a significant predictor of adverse events on univariable analysis, but this was nullified when adjusted for LGE-CMR (HR 1.7, 95% CI 0.4-6.5) [33]. One explanation may be the importance of quantifying the extent and severity of perfusion-metabolism mismatch rather than a simplified binary measure of perfusion or metabolic uptake abnormality [34, 35]. While this supports the possible utility of PET as a prognostic tool, extensive quantification of FDG-uptake remains cumbersome given the many measurement metrics (e.g. total/mean/coefficient of variance of standardized uptake values), and its limited overall prognostic capability relative to LGE-CMR.
The primary purpose of FDG-PET in CS is to identify patients with active disease who might benefit from corticosteroid therapy and monitor treatment response. Positron emission tomography is an important imaging modality that uniquely complements CMR in the diagnostic evaluation of CS. It remains uncertain whether abnormal findings detected can consistently provide prognostic insight and further prospective data are needed to clarify the role of PET in this regard.
6. Gallium citrate scintigraphy
Gallium (67Ga) citrate scintigraphy has existed for several decades and is a nuclear medicine investigation that involves injection of radioactive isotope Ga67 and imaging acquisition of uptake around focal sites of infection, inflammation or rapid cell division (e.g. neoplasia). By approximately 24 hours post-injection, most of the isotope is bound to acute-phase proteins such as transferrin and lactoferrin which subsequently concentrate in areas of inflammation. While gallium uptake is included in current guidelines for CS diagnosis due to its high specificity and straightforward pre-imaging protocol [7, 8], it is less sensitive and associated with increased radiation exposure compared with FDG-PET [36]. Sensitivity may be improved in the setting of dual single-photon-emission CT (SPECT) scanning with perfusion imaging [37] although FDG-PET is generally preferred.
7. Sequence of Advanced Imaging Modalities and Role of Hybrid Imaging
Both CMR and FDG-PET offer complementary information in the evaluation of CS. Fig. (5) shows FDG-PET computed tomography compared with CMR for CS. Current guidelines do not recommend whether both advanced imaging modalities are essential in suspected CS, and which should be performed first. However recent data broadly support CMR as the initial imaging modality, followed by FDG-PET if required. In a retrospective analysis of 56 suspected CS patients who underwent both CMR and PET (median time between tests, 1.3 months), Bravo and colleagues identified 20 patients with LGE-CMR and abnormal-FDG uptake, and a further 16 patients with LGE-CMR but normal FDG uptake [33]. Of note, no patients were LGE-negative and FDG-positive. On multivariable analysis, the only independent predictor of the composite death and malignant ventricular arrhythmias was the presence of LGE-CMR. Given that 15 out of 16 events occurred in patients with LGE-CMR, these findings highlight the relative importance of CMR in the initial assessment of CS prognosis, and the need for PET can be considered based on whether CMR reveals LGE.
Fig. (5).
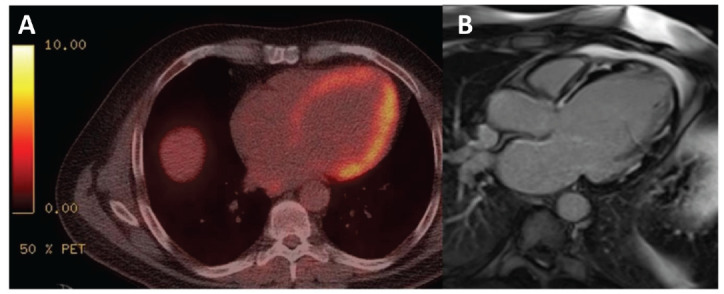
(A) Positron emission tomography-computed tomography compared with (B) cardiac magnetic resonance imaging. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In suspected CS patients with LGE-CMR, the subsequent role of FDG-PET is its ability to detect active myocardial inflammation and thus corticosteroid treatment response and monitoring. A retrospective study of 107 patients referred for CS evaluation showed that when PET data were added to CMR, almost one-half of patients had a re-classification of CS likelihood [38]. This re-classification also led to substantial changes in immunosuppressive therapy regimens in patients deemed highly probable of having CS. PET findings can influence treatment decisions and have a significant impact on patient outcomes. One example is in suspected CS patients with new complete heart block and who are PET and LGE-CMR positive. Such patients are more likely to have conduction recovery following corticosteroid therapy compared with patients who are LGE-CMR positive but FDG-PET negative [39], therefore potentially avoiding the need for permanent pacing. Thus FDG-PET is important in diagnosing and guiding therapy for CS, in particular for patients with LGE-CMR.
In centers where patients with suspected CS routinely undergo both CMR and FDG-PET, how to interpret abnormal-FDG PET uptake in the absence of LGE-CMR is uncertain. In the previously discussed study [38], eight out of 107 patients had abnormal FDG-PET uptake with negative CMR although only 2 were considered highly probable CS after reviewing the pattern and extent of FDG-uptake and available clinical information. While this clinical dilemma appears in approximately 8% of patients depending on referral bias and timing of referral [40, 41], concordance between PET and CMR may be increased if performed within 1 month of each other [42]. This clinical scenario could reflect failed suppression of background myocardial uptake but alternatively, early active CS without fibrotic changes and thus negative LGE-CMR. This is consistent with a retrospective study which found that in sarcoidosis patients with new onset AV block, PET is more often positive compared with CMR, suggesting a new active lesion and thus early inflammation which has yet to cause myocardial fibrosis/scar [43]. Given that suspected CS patients who receive early corticosteroid therapy are more likely to recover AV conduction and have less adverse LV remodeling [44, 45], a trial of immunosuppressants with follow-up FDG-PET appears reasonable.
Recent advancement in the evaluation of CS is hybrid imaging combining both CMR and FDG-PET in a single scan with the intention of improving diagnostic yield. In a prospective study of 25 patients with clinically suspected CS, co-registered hybrid PET and CMR imaging identified 8 patients with increased FDG-uptake co-localized in the same pattern of LGE [46]. Similarly, a European study which enrolled 51 suspected CS patients to undergo simultaneous PET-CMR reported an increased sensitivity with hybrid imaging (94% vs. 85% PET alone and 82% CMR alone) and that abnormal results on both PET and CMR was the strongest predictor of adverse cardiac events [32]. Although such technology is not widely available, it may have important prognostic implication in both baseline assessment and ongoing monitoring to better understand disease progression.
FUTURE DIRECTIONS
Insight into disease activity and prognosis has improved individualised care in CS. This includes tailored immunosuppressive regimens, monitoring of treatment response and identification of high-risk candidates who may benefit from closer follow-up or consideration of a primary prevention ICD. However, most evidence arises from retrospective observational data and prospective registries are needed to draw more conclusive results. Additionally, as the focus shifts towards non-invasive cardiac imaging for the detection of CS, there remains a lack of consensus on the optimal diagnostic algorithm. Some clinicians suggest immunomodulation should be commenced in any suspected CS patients who are FDG-PET avid or demonstrate LGE-CMR [47]. Others suggest patients without LGE-CMR regardless of FDG-PET activity have a good prognosis and may not require treatment initiation [11]. Randomised studies are required to understand how advanced cardiac imaging can direct treatment.
CONCLUSION
Despite significant advances in cardiac imaging, cardiac sarcoidosis remains a challenging diagnosis. TTE is an important screening modality and may provide prognostic insight through GLS measurement. CMR and FDG-PET provide complementary information for diagnosis, disease activity, and prognosis. Further prospective data are needed to assess the incremental value achieved with various cardiac imaging modalities and their role in guiding treatment and subsequent response.
Fig. (1).
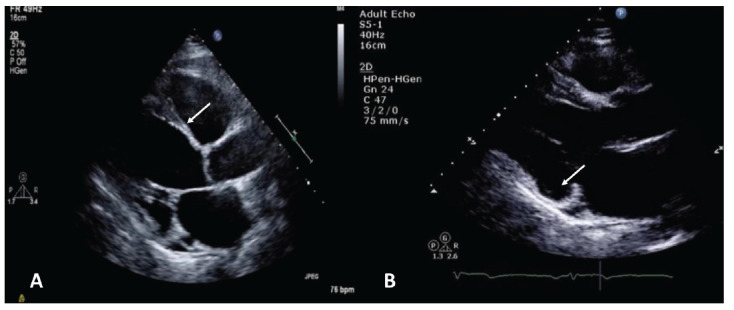
Classic echocardiographic appearances of cardiac sarcoid. (A) Basal interventricular septum thinning. (B) Thinning and aneurysmal dilatation of basal inferolateral wall. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
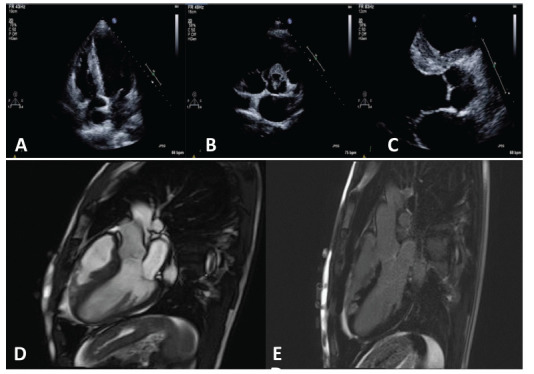
Echocardiographic images (upper panel) demonstrates evidence of echogenic areas of thickening along the right ventricular side of the interventricular septum (A and C) and in the peri-aortic region (B). Corresponding cardiac magnetic resonance imaging demonstrated myocardial oedema in the basal anteroseptum and mid inferoseptum, as well as in the right ventricular outflow tract and moderator band. Patchy and intense late gadolinium enhancement was seen corresponding to the regions of myocardial oedema (D and E). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N. Engl. J. Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Ungprasert P., Carmona E.M., Utz J.P., Ryu J.H., Crowson C.S., Matteson E.L. Epidemiology of sarcoidosis 1946-2013: A population-based study. Mayo Clin. Proc. 2016;91(2):183–188. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman K.J., Hutchins G.M., Bulkley B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204–1211. doi: 10.1161/01.CIR.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y., Iwai K., Tachibana T., et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann. N. Y. Acad. Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 5.Kandolin R., Lehtonen J., Airaksinen J., et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 6.Mehta D., Lubitz S.A., Frankel Z., et al. Cardiac involvement in patients with sarcoidosis: Diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 7.Birnie D.H., Sauer W.H., Bogun F., et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan annals of nuclear cardiology. 2017.
- 9.Juneau D., Nery P., Russo J., et al. How common is isolated cardiac sarcoidosis? Extra-cardiac and cardiac findings on clinical examination and whole-body 18F-fluorodeoxyglucose positron emission tomography. Int. J. Cardiol. 2018;253:189–193. doi: 10.1016/j.ijcard.2017.09.204. [DOI] [PubMed] [Google Scholar]
- 10.Steger A., Weichert W., Ibrahim T., Rischpler C. Isolated cardiac sarcoidosis: The crucial role of multimodal imaging with positron emission tomography/magnetic resonance imaging in diagnosis and therapy surveillance. Eur. Heart J. 2018;39(6):488. doi: 10.1093/eurheartj/ehx689. [DOI] [PubMed] [Google Scholar]
- 11.Kouranos V., Tzelepis G.E., Rapti A., et al. Complementary role of cmr to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc. Imaging. 2017;10(12):1437–1447. doi: 10.1016/j.jcmg.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Gorcsan J., III, Tanaka H. Echocardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011;58(14):1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Sperry B.W., Ibrahim A., Negishi K., et al. Incremental prognostic value of global longitudinal strain and 18f-fludeoxyglucose positron emission tomography in patients with systemic sarcoidosis. Am. J. Cardiol. 2017;119(10):1663–1669. doi: 10.1016/j.amjcard.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Pizarro C., Kluenker F., Hammerstingl C., Skowasch D. Diagnostic value of speckle-tracking echocardiography in confirmed cardiac sarcoidosis. Clin. Res. Cardiol. 2016;105(10):884–886. doi: 10.1007/s00392-016-1004-y. [DOI] [PubMed] [Google Scholar]
- 15.Murtagh G., Laffin L.J., Patel K.V., et al. Improved detection of myocardial damage in sarcoidosis using longitudinal strain in patients with preserved left ventricular ejection fraction. Echocardiography. 2016;33(9):1344–1352. doi: 10.1111/echo.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce E., Ninaber M.K., Katsanos S., et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur. J. Heart Fail. 2015;17(1):51–62. doi: 10.1002/ejhf.205. [DOI] [PubMed] [Google Scholar]
- 17.Schouver E.D., Moceri P., Doyen D., et al. Early detection of cardiac involvement in sarcoidosis with 2-dimensional speckle-tracking echocardiography. Int. J. Cardiol. 2017;227:711–716. doi: 10.1016/j.ijcard.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 18.Felekos I., Aggeli C., Gialafos E., et al. Global longitudinal strain and long-term outcomes in asymptomatic extracardiac sarcoid patients with no apparent cardiovascular disease. Echocardiography. 2018;35(6):804–808. doi: 10.1111/echo.13846. [DOI] [PubMed] [Google Scholar]
- 19.Orii M., Hirata K., Tanimoto T., et al. Myocardial damage detected by two-dimensional speckle-tracking echocardiography in patients with extracardiac sarcoidosis: Comparison with magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2015;28(6):683–691. doi: 10.1016/j.echo.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Satoh H., Sano M., Suwa K., et al. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J. Cardiol. 2014;6(7):585–601. doi: 10.4330/wjc.v6.i7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smedema J.P., Snoep G., van Kroonenburgh M.P., et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J. Am. Coll. Cardiol. 2005;45(10):1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Patel M.R., Cawley P.J., Heitner J.F., et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman G.C., Shaw P.W., Balfour P.C., Jr, et al. Prognostic value of myocardial scarring on cmr in patients with cardiac sarcoidosis. JACC Cardiovasc. Imaging. 2017;10(4):411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murtagh G., Laffin L.J., Beshai J.F., et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: Risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9(1):e003738. doi: 10.1161/CIRCIMAGING.115.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ise T., Hasegawa T., Morita Y., et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100(15):1165–1172. doi: 10.1136/heartjnl-2013-305187. [DOI] [PubMed] [Google Scholar]
- 26.Ramalho J., Semelka R.C., Ramalho M., Nunes R.H., AlObaidy M., Castillo M. Gadolinium-based contrast agent accumulation and toxicity: An update. AJNR Am. J. Neuroradiol. 2016;37(7):1192–1198. doi: 10.3174/ajnr.A4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinbane J.S., Colletti P.M., Shellock F.G. Magnetic resonance imaging in patients with cardiac pacemakers: Era of “MR Conditional” designs. J. Cardiovasc. Magn. Reson. 2011;13(1):63. doi: 10.1186/1532-429X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki T., Hansford R., Zviman M.M., et al. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circ Cardiovasc Imaging. 2011;4(6):662–670. doi: 10.1161/CIRCIMAGING.111.965764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Writing group
- 30.Blankstein R., Osborne M., Naya M., et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J. Am. Coll. Cardiol. 2014;63(4):329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skali H., Schulman A.R., Dorbala S. (18)F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr. Cardiol. Rep. 2013;15(5):352. doi: 10.1007/s11886-013-0370-6. [DOI] [PubMed] [Google Scholar]
- 32.Wicks E.C., Menezes L.J., Barnes A., et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur. Heart J. Cardiovasc. Imaging. 2018;19(7):757–767. doi: 10.1093/ehjci/jex340. [DOI] [PubMed] [Google Scholar]
- 33.Bravo P.E., Raghu G., Rosenthal D.G., et al. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int. J. Cardiol. 2017;241:457–462. doi: 10.1016/j.ijcard.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperry B.W., Tamarappoo B.K., Oldan J.D., et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc. Imaging. 2018;11(2 Pt 2):336–345. doi: 10.1016/j.jcmg.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Youssef G., Leung E., Mylonas I., et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012;53(2):241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 36.Jamar F., Buscombe J., Chiti A., et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J. Nucl. Med. 2013;54(4):647–658. doi: 10.2967/jnumed.112.112524. [DOI] [PubMed] [Google Scholar]
- 37.Malinowska E., Doboszyńska A., Śliwińska A., et al. The use of 67Ga scintigraphy in patients with sarcoidosis. Nucl. Med. Rev. Cent. East. Eur. 2018;21(1):59–65. doi: 10.5603/NMR.a2018.0007. [DOI] [PubMed] [Google Scholar]
- 38.Vita T., Okada D.R., Veillet-Chowdhury M., et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11(1):e007030. doi: 10.1161/CIRCIMAGING.117.007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orii M., Hirata K., Tanimoto T., et al. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complet heart block. Heart Rhythm: The Off J Heart. 2015;12(12):2477–2485. doi: 10.1016/j.hrthm.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Soussan M., Brillet P.Y., Nunes H., et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J. Nucl. Cardiol. 2013;20(1):120–127. doi: 10.1007/s12350-012-9653-3. [DOI] [PubMed] [Google Scholar]
- 41.Ohira H., Tsujino I., Ishimaru S., et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(5):933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 42.Ju Lee N., Lee B., Litt H. Cardiac MRI vs. myocardial 18F-FDG PET/CT in patients with clinical concern for cardiac sarcoid. J. Cardiovasc. Magn. Reson. 2015;17(Suppl. 1):O30. doi: 10.1186/1532-429X-17-S1-O30. [DOI] [Google Scholar]
- 43.Ohira H., Birnie D.H., Pena E., et al. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(2):259–269. doi: 10.1007/s00259-015-3181-8. [DOI] [PubMed] [Google Scholar]
- 44.Yodogawa K., Seino Y., Shiomura R., et al. Recovery of atrioventricular block following steroid therapy in patients with cardiac sarcoidosis. J. Cardiol. 2013;62(5):320–325. doi: 10.1016/j.jjcc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Chiu C.Z., Nakatani S., Zhang G., et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005;95(1):143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 46.Dweck M.R., Abgral R., Trivieri M.G., et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc. Imaging. 2018;11(1):94–107. doi: 10.1016/j.jcmg.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamzeh N.Y., Wamboldt F.S., Weinberger H.D. Management of cardiac sarcoidosis in the United States: A Delphi study. Chest. 2012;141(1):154–162. doi: 10.1378/chest.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]


