Abstract
Objectives
Spinal muscular atrophy is a monogenic disease characterized by progressive spinal and bulbar muscle weakness and atrophy. It is caused by the degeneration of alpha-motoneurons. The recent approval of the antisense oligonucleotide nusinersen highlights the need for reliable clinical tools to evaluate motor function in patients with neuromuscular disorders. Measurement of the bulbar neuromuscular function (e.g., bite force) could be an extension to existing motor scales, sensitive to more nuanced changes, especially in symptomatic patients with severely reduced functional abilities.
Materials and methods
Maximum bite force measurement was used to quantify changes of the masticatory function in adult monozygotic female twins with SMA type II. Using piezoelectric transducers, 550 observations were recorded for each patient during the first year of nusinersen therapy.
Results
During the application of four loading doses of nusinersen, bite force levels steadily increased and reached a statistically significantly higher level compared to the initial state in both patients. Subsequent maintenance doses coincided with smaller or no statistically significant changes in maximum bite force.
Conclusions
This pilot study indicates that the measurement of maximum bite force may be a useful tool to detect changes of the bulbar function in SMA patients. As such, it may supplement existing scales to identify treatment-related changes in motor function.
Key words: spinal muscular atrophy, motor neurons, masticatory force, antisense oligonucleotide
Introduction
Spinal muscular atrophy (SMA) is an orphan disease with an incidence of approximately 1:11,000 in Caucasians 1. Characterized by progressive neuromuscular degeneration, this autosomal-recessive disorder is caused by biallelic deletions and/or point mutations of the survival of motor neuron (SMN1) gene, leading to SMN protein deficiency 2,3. Depending on age of onset, achieved motor milestones and life expectancy, SMA is subcategorized into different types 4: infants with SMA type I show symptoms within the first 6 months of life, never achieve the ability to sit independently and have previously often died within the first two years of life. SMA type II patients who usually become symptomatic after 6 but before 18 months of life achieve independent sitting, but never gain the ability to walk. Life expectancy is reduced in these patients, but most of them live into adulthood. Patients with SMA type III learn to stand and walk but may lose these abilities during the course of the disease. Usually these patients have a normal life expectancy 5,6.
On 1 June 2017, the European Medicines Agency approved the antisense oligonucleotide drug nusinersen (Spinraza) for SMA therapy. Nusinersen alters the splicing of SMN2 pre-mRNA and thus increases the production of the full-length SMN protein. The drug has to be administered intrathecally. The initial treatment phase comprises four loading doses within two months. Maintenance doses are administered once every four months thereafter 7. Two multicenter, randomized, double-blind, sham-procedure controlled studies with infantile-onset SMA patients and later-onset SMA patients previously showed significant improvements in motor milestones under nusinersen medication 8,9.
With upcoming treatment options for SMA and other neuromuscular diseases 10, there is an urgent need for sensitive clinical tools to observe motor functions and thus drug efficacy, especially in symptomatic patients with a chronic course of disease and already limited motor abilities. The heterogeneity across different types and stages of SMA complicates the development of motor scales and reproducible outcome measures 11. Here, we developed a method to quantify changes of masticatory function, which is representative of patients’ bulbar neuromuscular function.
SMA usually first affects the spinal muscles and, later, to a lesser degree, the craniofacial and bulbar musculature 12. As such, patients in the advanced stages of SMA show reduced maximum masticatory muscle strength 13, often resulting in problems with mastication and swallowing 12,14. Common descriptive scales evaluating the motor function of SMA patients – such as the Hammersmith Functional Motor Scale Expanded (HFMSE) 15,16 or the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) 17,18 – do not account for limitations in the bulbar function and are inadequate to assess severely reduced motor function.
Assuming that nusinersen might also lead to an improvement in the bulbar function of patients with chronic SMA, we set out to test the usefulness and reliability of maximum bite force measurements in patients with chronic minimal neuromuscular function where well-established outcome scales, such as the HFMSE or CHOP INTEND, cannot pick up changes. Quantifying maximum bite force in the course of treatment might generate initial hypotheses about the impact of nusinersen in such patients.
The purpose of this pilot study was to assess the variation of maximum bite force over time, using piezoelectric transducers. Focusing on intraindividual, instead of interindividual variation, the study assessed maximum bite force in two subjects with SMA type II over the entire first year of nusinersen therapy. Eliminating as many confounding influences as possible, the study quantified the extent of relative change in maximum bite force, providing a first indication of the method’s feasibility in SMA patients.
Material and methods
Sample
Two adult monozygotic female twins with infantile onset SMA, later classified as SMA type II (patient 1 and 2) in view of their long-term survival, participated in this pilot study. Both have genetically confirmed SMA with a homozygous deletion of SMN1 and three copies of SMN2. At the time of the first bite force measurement, they were 49 years old. Their motor abilities were limited to minimal movements of single fingers and to a reduced facial mimic and bulbar function affecting chewing and swallowing. HFMSE scores remained at 0 (out of 66 possible points; a score of 66 points corresponds to the best motor function) in both patients before and after one year of nusinersen therapy. CHOP INTEND remained at 12 points in patient 1 and increased from 15 to 16 points in patient 2 (out of 64 possible points; a score of 64 points corresponds to the best motor function). Both patients had several teeth missing, a severe class II malocclusion and, in addition to limb contractures, bilateral temporomandibular contractures with limitation of mouth opening. Active maximum mouth opening, measured both at the outset and after one year, remained stable at 24.5 mm in patient 1 and 21 mm in patient 2, being about 50 % less than normal 19. Prior to their participation, the patients gave informed consent. The study was conducted in accordance with the Declaration of Helsinki. Measurements were taken during the first year of nusinersen therapy, starting 21 days before the first application.
Recording methods
Bite force was measured using calibrated piezoelectric transducers (Flexiforce A201-100, Tekscan Inc., South Boston, USA) in the form of thin plastic foil sheets (0.203 mm thickness, Ø 9.53 mm), with a maximum capacity of 445 N. Changes in the resistance of the sensor were detected by the multi-sensor measurement box CEBO-MSA64 (CESYS, Herzogenaurach, Germany) and digitally recorded.
The measurements were conducted unilaterally on the posterior teeth in a region of good intercuspidation. To improve contact to the sensor, the teeth were partially covered by intraorally fabricated occlusal bite blocks (Pattern resin LS, GC America Inc., Alsip, Illinois, USA). Self-adhesive, convex bumpers (Stabilit, Bahag AG, Mannheim, Germany, Ø 8.00 mm) were placed on the sensor, guiding it into a predefined concavity on the surface of the occlusal bite blocks. The intermaxillary distance was kept at a minimum.
Bite force was repeatedly measured over the course of one year at two different times of the day (9.00 am and 5.00 pm). The patients were asked to bite 10 times with maximum effort for a period of one second, each followed by one second of recovery. One measurement lasted 20 seconds. The parameter of interest, maximum bite force, was defined as the maximum value of each bite (in N), yielding 10 values per measurement point. All measurements took place at the home of the patients, keeping their head positions constant in a familiar lying position in order to minimize the influence of neck muscle strength. All measurements were conducted by a single trained examiner (TK).
Statistics
The outlined procedure resulted in two patient-specific datasets consisting of 550 observations clustered in 55 measurement points each. The data were descriptively analyzed using locally weighted scatterplot smoothing (LOESS) 20; the means of the 10 values of each measurement point are illustrated (Fig. 3). Inferential statistics relied on patient-specific models using ordinary least squares regression, regressing patients’ maximum bite force on the time points at which they were measured. Given that maximum bite force tended to decrease with each repetition, the position of each of the ten bites in a given measurement point was included as an additional control variable to account for systematic fatigue (values ranging between 1 and 10). Predictions of the quantities of interest are illustrated (Figs. 1-2). To account for the clustered data structure (i.e., observations nested in measurement points), all models relied on the clustered sandwich estimator 21. Differences in bite force over time were generally considered statistically significant at p < 0.05. Conducting multiple comparisons over time, the statistical tests were Bonferroni corrected 22. All statistical analyses were conducted using the statistical software R (version 3.5.1).
Figure 3.
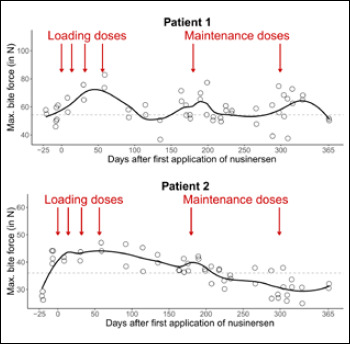
Maximum bite force in patient 1 and patient 2 over the first year of nusinersen therapy. Each circle indicates the mean value of 10 observations of maximum bite force per measurement point. Solid lines indicate the LOESS curve over time. Dashed lines indicate the mean maximum bite force before the first application of nusinersen.
Figure 1.
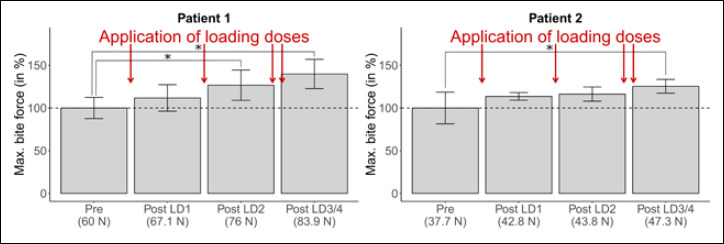
Changes in maximum bite force during the application of four loading doses of nusinersen (LD1/4, predicted values, in %). Pre entails seven measurement points before first application (=70 observations); each Post LD entails two measurement points within two weeks after application (=20 observations).
Figure 2.
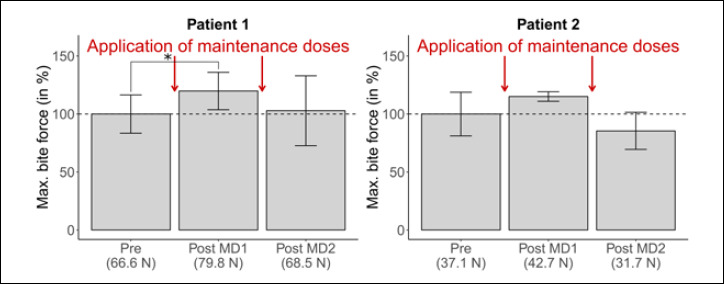
Changes in maximum bite force during the application of two maintenance doses of nusinersen (MD1/2, predicted values, in %). Pre entails seven measurement points before first application (=70 observations); Post MD1/2 entails two/four measurement points within two weeks after application (=20/40 observations).
Results
Over the period of one year, the two patients received six doses of nusinersen each. It was analysed how their application coincided with changes in maximum bite force, separately for the initial loading phase and for the subsequent maintenance phase.
Loading phase
During the application of four loading doses, maximum bite force steadily increases from baseline levels of 60 N / 37.7 N to 83.9 N / 47.3 N in patients 1 and 2 (Fig. 1). For patient 1, the increase is statistically significant after the second loading dose. The increase is weaker for patient 2, showing a statistically significant difference only after the fourth loading dose.
Maintenance phase
Following the loading doses, maintenance doses were applied every four months. After the application of the first maintenance dose, bite force levels are at 79.8 N / 42.7 N in patients 1 and 2, which is an increase relative to baseline levels (though weaker in size than after the fourth loading dose and statistically significant only for patient 1; see Figure 2). The application of the second maintenance dose is not accompanied by an increase in patient 1 over the subsequent two weeks (68.5 N). In the case of patient 2, the level of maximum bite force is even lower than before the first application of nusinersen, both before and after the second maintenance dose (31.7 N).
Changes in bite force over one year
Figure 3 descriptively summarizes all measurements over the complete period of observation. Maximum bite force decreases during the two observed four-month periods between drug administrations; temporary increases coincide with the application of the maintenance doses.
Inter- and intraindividual variation in bite force
Interindividually, patient 2 shows lower absolute values in maximum bite force than patient 1; intraindividually, values vary between measurement points. This variation between measurement points makes up ~50 % (patient 1) / ~90 % (patient 2) of the overall variation observed (as compared to variation across the 10 bites within measurement points). Intraindividually, variation between measurement points is also present before the application of nusinersen.
Discussion
The results of this pilot study, based on data from two SMA patients, generate two hypotheses that need confirmation in further patients: first, that changes in bite force over time coincide with the application of nusinersen; second, that they can be detected by isometric bite force measurements.
At initially 35.7 N and 63.4 N, the maximum bite forces of the two surveyed adult patients with SMA type II were substantially lower than normal (supplemental measurements in a healthy female adult yielded values at ~300 N). Despite the use of thin piezoelectric foil sheets – facilitating bite force measurements in SMA patients with a limited range of mandibular movement – jaw separation may have affected the absolute size of these values 23. Uninformative in absolute size, the respective values for the initial maximum bite forces underline the respective clinically observed limitations in the bulbar function of the two SMA patients and align with previous findings 13. Interindividual differences of pre-treatment levels of bite force are irrelevant for our serial measurements, given that our analyses relied on intraindividual changes over time.
Time-invariant measurement error may thus be a minor concern. Instead, greater attention should be paid to time-variant confounding. A number of potential time-variant confounders could be accounted for – either during measuring itself (e.g., patients’ head positions or time of the day) or ex-post statistically (e.g., fatigue due to repeated biting). However, unobservable factors, such as short-term fluctuations in physical fitness or individual motivation, remain more difficult to control. They may be the reason behind the unexplained variation between measurement points. In a similar vein, initial increases in bite force, as seen in patient 2, may be due to a learning effect 24. Similar patterns were also found in a healthy, untreated person (data not shown). A central task for future studies is thus to come up with ways to account for such potential influences of learning.
Notwithstanding these different sources of variation, the results indicate a systematic and statistically significant increase of maximum bite force that coincided with the application of nusinersen. Most notably, maximum bite force steadily increased with every additional application of the four loading doses in the first two months of treatment. The first maintenance dose coincided with a statistically significant increase in maximum bite force compared to baseline levels (though weaker in size than after the fourth loading dose). After the second maintenance dose, maximum bite force did not increase (in one patient, values were even below baseline level).
Overall, it could be speculated that maximum bite force changes with the application of nusinersen in adult SMA patients, especially at the onset of treatment, potentially indicating an altered bulbar function. Established scales did not capture any changes, potentially due to three reasons. First, neither HFMSE nor CHOP INTEND would capture changes that occur only in patients’ bulbar function. Second, CHOP INTEND has not been validated for SMA type II and HFMSE evaluates gross motor skills only. Third, in line with current practice, both scores have been assessed at much greater intervals.
This pilot study is the first to record bite force of SMA patients over a long time period during a causative treatment of SMA. It relies on a unique and so far, rare population: patients at an advanced state of SMA being treated with nusinersen (the patients here are among the first, hence the small sample size). The study builds on previous work that assessed bite force in patients with other neuromuscular disorders (e.g., Duchenne or Becker muscular dystrophy), either in terms of one-time measurements 25-27 or in terms of changes over time 28 – underlining the diagnostic potential of the approach.
The pilot study led to a number of insights that may be useful for further improvement of the method. First, measurement intervals should be chosen deliberately. This study covered the first year of nusinersen medication with 55 continuous measurement points. Being explorative in nature, the measurements took place at short, irregular intervals. Generally, future investigations may simplify the approach without risk by opting for fewer measurement points that focus on time periods close to the application of nusinersen. Also, to avoid any bias from learning effects, the number of measurement points before the first application of nusinersen should be sufficiently large. Second, a central challenge is the reduction of time-variant confounding between measurement points. The analyses showed that the largest share of unexplained variation existed between measurement time points – even so before the first application of nusinersen. In comparison, 10 repetitions per measurement seemed sufficient to capture existing variation within a measurement point. This finding suggests that the use of piezoelectric transducers provides reliable and reproducible results, aligning with previous studies 29-32. The continuous and ongoing development of piezoelectric sensors and their software may further simplify the process, especially so for measurements in larger populations 33. Concerning unexplained variation between measurement points, more knowledge is needed, including for healthy subjects. Finally, beside maximum bite force, future investigations may also look at changes in neuromuscular endurance. The present study focused on serial maximal levels of bite forces. Decreases in bite force over repetitions were treated merely as confounders and controlled for in the multiple regression analyses. Given that there is evidence for faster fatigue among SMA patients 13, it may be worthwhile focusing on muscle endurance as a potential outcome measure.
Conclusions
The quantitative measurement of maximum bite force could constitute a promising tool to evaluate the masticatory function as a readout for the bulbar function among patients with degenerative, neuromuscular diseases. It could supplement biomarkers such as electrophysiology or other recently suggested approaches 34 as a cost-efficient measurement to identify functional changes due to treatment. The above suggested improvement for its future application may help to realize the diagnostic potential of bite force quantification in patients with neuromuscular disorders.
Figures and tables
Acknowledgements
The authors thank Prof. Dr. Dr. Lapatki for his helpful suggestions concerning the bite force measurements.
Footnotes
Authors’ contributions
TK collected, analyzed, and interpreted the data. She wrote the first draft of the manuscript. RH initiated the study, contributed to patient recruitment, gave advice about the interpretation of the results, and revised the manuscript. BW gave advice about the interpretation of the results and revised the manuscript. JG provided support in the development of the method. CDW and ACL conducted the treatment of the patients with nusinersen and revised the manuscript. BB gave advice about the study design and revised the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 2018;28:103-15. https://doi.org/10.1016/j.nmd.2017.11.005 10.1016/j.nmd.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155-65. https://doi.org/10.1016/0092-8674(95)90460-3 10.1016/0092-8674(95)90460-3 [DOI] [PubMed] [Google Scholar]
- 3.Wirth B, Herz M, Wetter A, et al. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am J Hum Genet 1999;64:1340-56. https://doi.org/10.1086/302369 10.1086/302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munsat TL, Davies KE. International SMA consortium meeting. (26-28 June 1992, Bonn, Germany). Neuromuscul Disord 1992;2:423-8. https://doi.org/10.1016/S0960-8966(06)80015-5 10.1016/S0960-8966(06)80015-5 [DOI] [PubMed] [Google Scholar]
- 5.Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord 1995;5:3-5. https://doi.org/10.1016/0960-8966(94)00075-K 10.1016/0960-8966(94)00075-K [DOI] [PubMed] [Google Scholar]
- 6.Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy – A literature review. Orphanet J Rare Dis 2017;12:124 https://doi.org/10.1186/s13023-017-0671-8 10.1186/s13023-017-0671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SPIRANZA US Prescribing Information. https://www.spinraza.com/PI (Accessed 2 June 2017).
- 8.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017;377:1723-32. https://doi.org/10.1056/NEJMoa1702752 10.1056/NEJMoa1702752 [DOI] [PubMed] [Google Scholar]
- 9.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 2018;378:625-35. https://doi.org/10.1056/NEJMoa1710504 10.1056/NEJMoa1710504 [DOI] [PubMed] [Google Scholar]
- 10.Groen EJN, Talbot K, Gillingwater TH. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat Rev Neurol 2018;14:214-24. https://doi.org/10.1038/nrneurol.2018.4 10.1038/nrneurol.2018.4 [DOI] [PubMed] [Google Scholar]
- 11.Krosschell KJ, Maczulski JA, Scott C, et al. Reliability and validity of the TIMPSI for infants with spinal muscular atrophy type I. Pediatr Phys Ther 2013;25:140-8. discussion 9. https://doi.org/10.1097/PEP.0b013e31828a205f 10.1097/PEP.0b013e31828a205f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Bruggen HW, Wadman RI, Bronkhorst EM, et al. Mandibular dysfunction as a reflection of bulbar involvement in SMA type 2 and 3. Neurology 2016;86:552-9. https://doi.org/10.1212/WNL.0000000000002348 10.1212/WNL.0000000000002348 [DOI] [PubMed] [Google Scholar]
- 13.Granger MW, Buschang PH, Throckmorton GS, et al. Masticatory muscle function in patients with spinal muscular atrophy. Am J Orthod Dentofacial Orthop 1999;115:697-702. https://doi.org/10.1016/S0889-5406(99)70296-9 10.1016/S0889-5406(99)70296-9 [DOI] [PubMed] [Google Scholar]
- 14.Chen YS, Shih HH, Chen TH, et al. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr 2012;160:447-51. https://doi.org/10.1016/j.jpeds.2011.08.016 10.1016/j.jpeds.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 15.Main M, Kairon H, Mercuri E, et al. The Hammersmith Functional Motor Scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol 2003;7:155-9. https://doi.org/10.1016/S1090-3798(03)00060-6 10.1016/S1090-3798(03)00060-6 [DOI] [PubMed] [Google Scholar]
- 16.O’Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord 2007;17:693-7. https://doi.org/10.1016/j.nmd.2007.05.009 10.1016/j.nmd.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord 2010;20:155-61. https://doi.org/10.1016/j.nmd.2009.11.014 10.1016/j.nmd.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glanzman AM, McDermott MP, Montes J, et al. Validation of the Children’s Hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND). Pediatr Phys Ther 2011;23:322-6. https://doi.org/10.1097/PEP.0b013e3182351f04 10.1097/PEP.0b013e3182351f04 [DOI] [PubMed] [Google Scholar]
- 19.Gallagher C, Gallagher V, Whelton V, et al. The normal range of mouth opening in an Irish population. J Oral Rehabil 2004;31:110-6. https://doi.org/10.1046/j.0305-182X.2003.01209.x 10.1046/j.0305-182X.2003.01209.x [DOI] [PubMed] [Google Scholar]
- 20.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829-36. https://doi.org/10.1080/01621459.1979.10481038 10.1080/01621459.1979.10481038 [DOI] [Google Scholar]
- 21.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56:645-6. https://doi.org/10.1111/j.0006-341X.2000.00645.x 10.1111/j.0006-341X.2000.00645.x [DOI] [PubMed] [Google Scholar]
- 22.Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt 2014;34:502-8. https://doi.org/10.1111/opo.12131 10.1111/opo.12131 [DOI] [PubMed] [Google Scholar]
- 23.Arima T, Takeuchi T, Honda K, et al. Effects of interocclusal distance on bite force and masseter EMG in healthy participants. J Oral Rehabil 2013;40:900-8. https://doi.org/10.1111/joor.12097 10.1111/joor.12097 [DOI] [PubMed] [Google Scholar]
- 24.Thompson DJ, Throckmorton GS, Buschang PH. The effect of isometric exercise on maximum voluntary bite forces and jaw muscle strength and endurance. J Oral Rehabil 2001;28:909-17. https://doi.org/10.1111/j.1365-2842.2001.00772.x 10.1111/j.1365-2842.2001.00772.x [DOI] [PubMed] [Google Scholar]
- 25.Lagarde ML, van Alfen N, Geurts AC, et al. Orofacia muscles may be affected in early stages of Becker muscular dystrophy: a preliminary study. Muscle Nerve 2019;61:213-17. https://doi.org/10.1002/mus.26771 10.1002/mus.26771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimaraes AS, Carlsson GE, Marie SKN. Bite force and handgrip force in patients with molecular diagnosis of myotonic dystrophy. J Oral Rehabil 2007;34:195-200. https://doi.org/10.1111/j.1365-2842.2006.01665.x 10.1111/j.1365-2842.2006.01665.x [DOI] [PubMed] [Google Scholar]
- 27.Riera-Punet N, Martinez-Gomis J, Paipa A, et al. Alterations in the masticatory system in patients with amyotrophic lateral sclerosis. J Oral Facial Pain H 2018;3284-90. https://doi.org/10.11607/ofph.1882 10.11607/ofph.1882 [DOI] [PubMed] [Google Scholar]
- 28.Weijnen FG, van der Bilt A, Wokke JH, et al. Maximal bite force and surface EMG in patients with myasthenia gravis. Muscle Nerve 2000;23:1694-9. https://doi.org/10.1002/1097-4598(200011)23:11<1694::AID-MUS4>3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 29.Proffit WR, Fileds HW, Nixon WL. Occlusal forces in normal- and long-face adults. J Dent Res 1983;62:566-70. https://doi.org/10.1177/00220345830620051201 10.1177/00220345830620051201 [DOI] [PubMed] [Google Scholar]
- 30.Ferguson-Pell M, Hagisawa S, Bain D. Evaluation of a sensor for low pressure applications. Med Eng Phys 2000;22:657-63. https://doi.org/10.1016/S1350-4533(00)00080-1 10.1016/S1350-4533(00)00080-1 [DOI] [PubMed] [Google Scholar]
- 31.Fernandes CP, Glantz POJ, Svensson SA, et al. A novel sensor for bite force determinations. Dent Mater 2003;19:118-26. https://doi.org/10.1016/S0109-5641(02)00020-9 10.1016/S0109-5641(02)00020-9 [DOI] [PubMed] [Google Scholar]
- 32.Verma TP, Kumathalli KI, Jain V, et al. Bite force recording devices – A review. J Clin Diagn Res 2017;11:ZE01-ZE05. https://doi.org/10.7860/JCDR/2017/27379.10450 10.7860/JCDR/2017/27379.10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruse T, Lehmann HC, Braumann B, et al. The maximum bite force for treatment evaluation in severly affected adult SMA patients – Protocol for a longitudinal study. Front Neurol 2020;11:139 http://doi.org/10.3389/fneur.2020.00139 10.3389/fneur.2020.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashizume A, Banno H, Katsuno M, et al. Quantitative assessment of swallowing dysfunction in patients with spinal and bulbar muscular atrophy. Intern Med 2017;56:3159-65. https://doi.org/10.2169/internalmedicine.8799-16 10.2169/internalmedicine.8799-16 [DOI] [PMC free article] [PubMed] [Google Scholar]


