Abstract
Limb girdle muscular dystrophy is a genetically inherited condition that primarily affects skeletal muscle leading to progressive, predominantly proximal muscle weakness at presentation. Autosomal dominant LGMD represent 10% of all LGMDs. HNRNPDL-related muscular dystrophy, LGMD1G/LGMD D3 (MIM#609115), is an extremely rare autosomal dominant adult onset myopathy described in a handful of families. Here we fully characterized the muscular and respiratory involvement of a 58 years old Italian woman presenting the previously reported pathogenic variant c.1132G > C p.(Asp378Asn) in the HNRNPDL gene.
Key words: Limb girdle muscular dystrophy, LGDM D3, HNRNPDL, respiratory muscle involvement
HNRNPDL-related muscular dystrophy (MIM#609115), Limb girdle muscular dystrophy D3 (LGMD D3), previously known as LGMD1G, is an extremely rare autosomal dominant adult onset myopathy thus far reported in fewer than 30 cases from one Chinese and four Latin-American families 1-5. All patients harbored mutations hitting the same residue located in the prion-like protein domain. They presented a limb girdle or combined proximo-distal muscular weakness without evident respiratory or cardiac involvement. Some had cataract.
We describe a 58-year-old Italian woman who presented in her forties difficulties in rising stairs followed by difficulties in arm elevation. She reported frequent and sudden falls on her knees. She also had frequent nocturnal awakenings and occasional headache in the morning when waking up. Her deceased mother, uncle, and grandfather presented a similar clinical history with proximal muscle weakness in their 40-50’s.
Neurologic examination at age 58 showed a waddling gait, asymmetric scapular winging (Fig. 1a), upper limb muscle weakness and atrophy (Fig. 1b) (4/5 MRC), finger flexor and extensor and interossei muscle weakness, proximal lower limb weakness (2/3 MRC), and foot flexors and extensor weakness and atrophy (4/5 MRC) predominant in the left side (Fig. 1c). Serum CK levels were normal.
Pulmonary workup revealed a FVC at 51% of predicted values, dropping at 45% in supine position, suggestive of diaphragmatic weakness. Chest X-Ray denoted right hemidiaphragm elevation (Fig. 1d). Pulse oximetry during sleeping hours showed an average 91% of SatO2, CO2 = 53mmHg, and an EGA showed a PCO2 = 49mmHg. Based on this, a non-invasive ventilation (NIV) during sleeping hours was set up. Cardiac workup including EKG and ultrasound was normal. Of note the patient developed bilateral cataract in her 40s.
Shoulder MRI showed mild hypotrophy and fat replacement of the deltoid muscle bilaterally, and marked hypotrophy and fat replacement of the pectoral, and flexor-extensor muscles of the elbow (Figs. 1e-f). MRI of the lower limbs revealed a specific pattern with adductor longus and rectus femoris sparing in the thigh, and relative sparing of the semimembranous and long head of biceps in the posterior compartment (Fig. 1g).
A vastus lateralis muscle biopsy performed elsewhere was not contributive as constituted by fibro-fatty connective tissue.
Massive and parallel sequencing of a gene panel containing over 200 genes associated with inherited muscle disorders identified the previously reported c.1132G > A p.(Asp378Asn) in HNRNPDL 3-4. The mutation was numbered according to the GenBank NM_031372.3 with+1 corresponding to the A of the ATG translation initiation codon and is not currently listed in gnomAD.
Common features of HNRNPDL-related muscular dystrophy were the typical clinical phenotype with late onset limb girdle weakness and distal upper limb and lower limb weakness. We also observed asymmetric scapular winging (Fig. 1a) and distal limb atrophy (Figs. 1b-c).
In our case, thigh MRI confirmed the presence of a common pattern with preservation of the rectus femoris and adductor longus after 18 years of disease activity (Fig. 1). In upper limbs, we observed a fat replacement of deltoid muscle bilaterally, and the pectoral, flexor-extensor muscles of the elbow associated with muscle bulk reduction (Figs. 1e-f). Whole body muscle MRI would be important to assess if a common muscular pattern might be identified in other muscular group such face or axial muscles.
The presence of frank respiratory muscle involvement with a diaphragmatic weakness leading to right hemidiaphragm elevation (Fig. 1d) is a novel finding in HNRNPDL-related myopathies, being previously described in a single case 5. We therefore suggest an accurate respiratory workup including FVC in sitting and supine position, EGA, and nocturnal oximetry to detect respiratory muscle dysfunction, and to adopt NVI whenever appropriate.
Our patient presented bilateral cataract, a finding repeatedly reported by others 3-5, and suggestive of a role for HNRNPDL in the molecular pathway related to opacity of the crystalline lens 6.
The same variant detected in the Brazilian and Chinese family described in 3 and 4 was found. The identification of the same mutation in different ethnic backgrounds leads us to speculate of a possible hotspot. However, the occurrence of a common Italian ancestor in the Brazilian family is also likely, due to the high rate of Italian immigration to Latin America in early 20th century. On the other hand, the identification of a different variant affecting the same codon (Asp378His) in a Uruguayan family 5, is a further argument suggesting a possible hotspot in that position.
Conclusions
Our report describes the first Italian HNRNPDL mutated patient presenting proximo-distal muscular weakness and respiratory muscle involvement needing non-invasive ventilation.
Figures and tables
Figure 1.
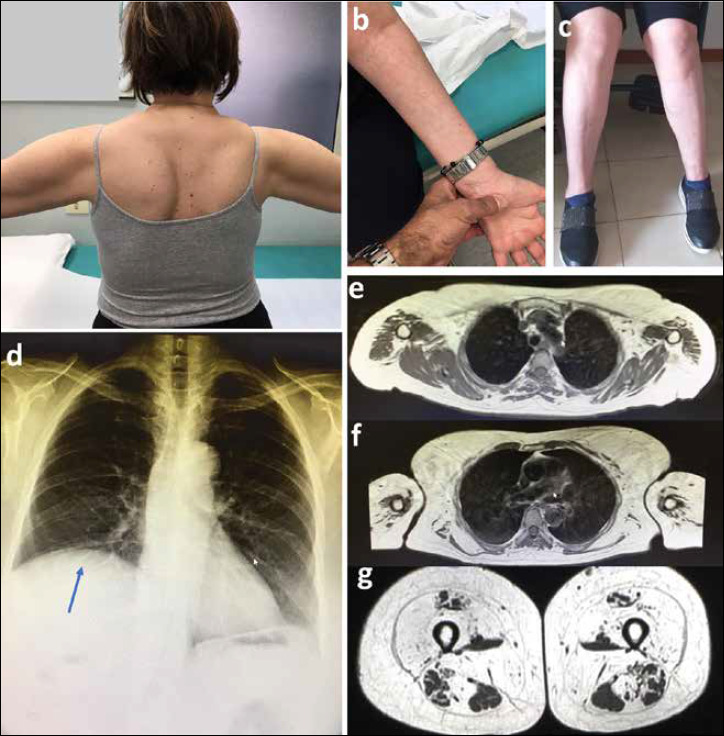
a. Asymmetric scapular winging; b. Distal arm atrophy; c. Asymmetric leg atrophy; d. Chest X-Ray showing elevation of the right hemidiaphragm muscle (indicated by an arrow); e-f. Upper limb T1 weighted MRI sections performed at 58 years. Severe fatty infiltration was noted on the deltoid muscles. At lower level there was a marked atrophy and fatty infiltration on pectoral, biceps, and triceps muscles; g. Thigh MRI, T1 weighted sections showing very relative muscle tissue sparing in rectus femoris and adductor longus muscles. Vastus lateralis, adductor magnus, and sartorius muscles are completely fatty infiltrated. In the posterior compartment only the semimembranous, semitendinous, and biceps femoris are relatively spared.
Acknowledgements
We thank our Patient for her compliance.
Footnotes
Ethical statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ethical standards
All procedures performed were in accordance with the ethical standards stated in the Declaration of Helsinki.
References
- 1.Straub V, Murphy A, Udd B, LGMD workshop study group (2018) 229th ENMC international workshop: limb girdle muscular dystrophies-nomenclature and reformed classification. Nardeen, the Netherlands, 17-19 March 2017. Neuromuscul Disord 28;8:702-10. [DOI] [PubMed] [Google Scholar]
- 2.Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. Lancet 2019;394:2025-38. [DOI] [PubMed] [Google Scholar]
- 3.Vieira NM, Naslavsky MS, Licinio L, et al. A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). Hum Mol Genet 2014;23:4103-10. https://doi.org/10.1093/hmg/ddu127 10.1093/hmg/ddu127 [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Chen H, Lu Y, et al. Limb girdle muscular dystrophy D3 HNRNPDL related in a Chinese family with distal muscle weakness caused by a mutation in the prion-like domain. J Neurol 2019;266:498-506. https://doi.org:10.1007:s00415-018-9165-4 10.1007/s00415-018-9165-4 [DOI] [PubMed] [Google Scholar]
- 5.Berardo A, Lornage X, Johari M, et al. HNRHNPDL-related muscular dystrophy: expanding the clinical morphological and MRI phenotypes. J Neurol 2019;266:2524-34. https://doi.org:10.1007:s00415-019-09437-3 10.1007/s00415-019-09437-3 [DOI] [PubMed] [Google Scholar]
- 6.Li RZ, Hou J, Wei Y, et al. hnRNPDL extensively regulates transcription and alternative splicing. Gene 2019;687:125-34. https://doi.org/10.1016/j.gene.2018.11.026 10.1016/j.gene.2018.11.026 [DOI] [PubMed] [Google Scholar]


