Abstract
Background: One of the many debated topics in inflammation research is whether this scenario is really an accelerated form of human wound healing and immunity-boosting or a push towards autoimmune diseases. The answer requires a better understanding of the normal inflammatory process, including the molecular pathology underlying the possible outcomes. Exciting recent investigations regarding severe human inflammatory disorders and autoimmune conditions have implicated molecular changes that are also linked to normal immunity, such as triggering factors, switching on and off, the influence of other diseases and faulty stem cell homeostasis, in disease progression and development.
Methods: We gathered around and collected recent online researches on immunity, inflammation, inflammatory disorders and AMPK. We basically searched PubMed, Scopus and Google Scholar to assemble the studies which were published since 2010.
Results: Our findings suggested that inflammation and related disorders are on the verge and interfere in the treatment of other diseases. AMPK serves as a key component that prevents various kinds of inflammatory signaling. In addition, our table and hypothetical figures may open a new door in inflammation research, which could be a greater therapeutic target for controlling diabetes, obesity, insulin resistance and preventing autoimmune diseases.
Conclusion: The relationship between immunity and inflammation becomes easily apparent. Yet, the essence of inflammation turns out to be so startling that the theory may not be instantly established and many possible arguments are raised for its clearance. However, this study might be able to reveal some possible approaches where AMPK can reduce or prevent inflammatory disorders.
Keywords: IL-1β, immunity, inflammation, NF-κB and AMPK, TNF-α, autoimmune diseases, inflammatory disorders
1. INTRODUCTION
World population is increasing at an alarming rate; therefore, new problems have been created in many areas, including jobs, shelter and, most importantly, foods. To fulfill the demand for food, the planting or cultivation of genetically modified foods is being encouraged, which may create some new health-related problems [1, 2]. Owing to break- throughs in technology, many people play and work at home nowadays. In addition, most of the corporate offices are filled with lifts and air conditioners, which make people engage in less physical exertion [3, 4]. Altogether, these are factors that contribute to several dysfunctions, including obesity, diabetes, vascular dysfunction, related metabolic syndrome and several inflammatory disorders [5].
Evidences suggest that inflammatory responses are highly correlated with many diseases. Over-activation of the immune cells and pro-inflam- matory cytokines can lead a subject to become diabetic. In addition, harmful cytokines aggravate the situation of an already diabetic subject and make treatment approach more complex [6]. In obesity, factors such as adipocytes, adiponectin and adipokines work as a gland to produce and secrete several soluble components that have direct and indirect connections with the inflamma- tory response. Inflammatory cascades may be invited, which then develop in the gathering of the foam cells and ultimately engage in the major role of complicating the scenario through processes such as coronary arterial calcification, atherosclerosis progression and heart failure [7]. Besides, every infection does some damage in the body and these damages invite immune cells and harmful inflammatory cytokines in the respective areas [8] On the other hand, aging has been blamed as a major phenomenon where the body tends to become less responsive to endoplasmic stress, apoptosis, oxidative stress and autophagy, making the body find it difficult to control hyperglycemia, visceral fats, metabolic syndromes and, most importantly, inflammation. Chronic inflammation depletes several necessary components such as Nuclear factor erythroid 2-related factor 2 (Nrf2)/ SKiNhead-1 (SKN1), Sirtuin (SIRT1), Unc-51 Like Autophagy Activating Kinase 1 (ULK1), Sestrins, p53, Heme oxygenase (HO-1), Forkhead box protein O (FoxO)/Aafachronic Acids (DAF) 16, Liver kinase (LK) B1, Calcium/calmodulin-dependent protein kinase (CaMKK) β and Transforming growth factor-β-activated kinase 1 (TAK1). On the contrary, the levels of harmful components and pathways, for instance, Protein phosphatase 2Cα (PP2Cα), Mammalian target of rapamycin (mTOR), CREB-regulated transcription coactivator 1 (CRTC1) and Nuclear Factor (NF)-κB are always activated [9]. Overproduction of inflammatory cells and pro-inflammatory cytokines are often reported in chronic illnesses such as Parkinson’s disease, stroke, thrombosis, hypertension, cirrhosis, Non-alcoholic fatty liver disease (NAFLD), skin disorders, edema, fibrosis, iron overload, Alzheimer’s and cancers [10, 11].
AMP-activated protein kinase (AMPK) is a prime energy sensor as a whole-body level, activated under conditions of glucose deprivation, heat shock, oxidative stress, and ischemia. It is also responsible for immune response, cell growth and polarity. Once activated, AMPK suppresses the necessary enzymes involved in ATP-consuming anabolic pathways and enhances cellular ATP supply [12, 13]. There are several bodily ways to trigger AMPK, such as regular and intense exercises, intermittent fasting and being stress-free, hence, the expressions of AMPK have been noticed in various cells, including hepatocytes, adipocytes, macrophages, smooth muscle cells, microglia cells, endothelial cells, glioma cells, cardiomyocytes and various cancer cells [14]. Resveratrol from either red wine or grape; Quercetin from many plants including fruits, vegetables, and grains; epigallocatechin-3-gallate from green tea and fatty fish like cod liver oil, salmon and trout fish are all-natural resource that, taken together, also stimulate AMPK [15]. Several anti-inflam- matory mechanisms of AMPK have been proposed so far, and it is mostly believed that AMPKα2 provides its anti-inflammatory effects through PARP-1 and Bcl-6 in the molecular levels [16]. On the other hand, exploring the appliance of genetic methods, mapping and a plethora of AMPK enhancers, rapid progress has demonstrated AMPK as an attractive therapeutic target for several human ailments, such as end-stage renal diseases, atherosclerosis, hepatitis, myocardial ischemia/re- perfusion injury, cancer and neurodegenerative disease [17]. Activation of AMPK can facilitate bacterial eradication in sepsis and related inflammatory conditions associated with the inhibition of neutrophil activation and chemotaxis [18]. Activation of AMPK also inhibits NF-κB signaling and inflammation although the mechanism is not direct; hence, it has a positive impact on health and lifespan [19]. Furthermore, inflammatory pathways are highly interconnected; therefore, inhibiting one cascade may be associated with blocking other inflammatory pathways, which makes the anti-inflammatory activity entirely dependent on other factors. In this study, we outlined the major molecular and biochemical pathways that contribute to the crosstalk between AMPK and inflammatory mediators. Our hypothetical figure legends and tables may correlate and establish interaction between AMPK and inflammation. This review outlines the principal mechanisms that govern the effects of inflammation, immunity and tumor development. It discusses attractive new targets for cancer therapy and prevention.
2. METHODOLOGIES
An interesting question that remains to be answered is whether or not AMPK can inhibit inflammatory pathways and responsible cytokines. The answer requires extended literature searching and establishes the various direct and indirect correlation between pathophysiology and AMPK interaction in human health and improving life-span. Therefore, our aim was to find molecular mechanisms responsible in inflammation research and to inhibit the pathways by AMPK inducers; therefore, the current study was designed depending on latest literatures which were published since January 2010 and of course with some exceptions. To collect the literatures, PubMed, Google Scholar, Science Direct and Scopus were basically searched and retrieved. A lot of short keywords like AMPK, neuroinflammation, oxidative stress, inflammatory cytokines, myocarditis, diabetes, nephritis, hepatitis, obesity, vasculitis and fat metabolizing genes were used to investigate the literatures. Both the natural and synthetic sources have been shown in Tables 1 and 2 to correlate the possible AMPK inducers or enhancers. Several possible biochemical pathways have been hypothesized to find out the possible pathophysiology and treatment approach between inflammatory disorders and applications of AMPK in various conditions. We included all qualitative and quantitative study types, including animal model and cell culture. Meta-analysis was not used as this review was primarily designed to provide a comprehensive overview of the field.
Table 1.
Natural sources of AMPK enhancers.
| Treatment Name | Applications/Outcomes of the Experiment | References |
|---|---|---|
|
Common Name: Yuja or Yuzu Scientific Name: Citrus junos Used parts: Peel extract |
-AMP-activated protein kinase (AMPK) activities were increased by Ethanol extract of yuja peel (YPEE) in a dose-dependent manner inside in both cell culture and mouse models. | [60] |
|
Name of the Plant: Korean red pepper Scientific Name: Capsicum annuum Used parts: An ethanol (70%) extract of Korean red pepper (Ekrp) |
-Ethanol (70%) extract of Korean red pepper enhances AMPK phosphorylationin 3T3-L1 adipocyte. | [61] |
|
Common Name: Hsiao Scientific Name: Astragalus membranaceus Bunge var. mongholicus Used parts: Astragalus polysaccharide extract |
-Astragalus polysaccharide interacted with AMPK activity in palmitate-induced RAW264.7 cells. | [62] |
|
Common Name: Thymoquinone Scientific Name: Nigella sativa Used parts: Seeds |
-Thymoquinone enhanced the phosphorylation adenosine monophosphate-activated protein kinase (AMPK) in male Kunming mice. | [63] |
|
Name of the Plant: Bitter melon Scientific Name: Momordica charantia Used parts: 5β,19-epoxy-25-methoxy-cucurbita-6,23-diene-3β,19-diol (EMCD) |
-EMCD was found to have activated AMP-activated protein kinase (AMPK) in TNF-α-induced inflammation in FL83B cells. | [64] |
|
Name of the Plant: Berberis and Coptis Scientific Name: Berberis aquifolium and Coptis chinensis Used parts: Raw Berberine |
-Berberine increased AMP-activated protein kinase (AMPK)/gluconeogenesis pathway in diabetic rat and HepG2 hepatocytes. | [65] |
|
Name of the Plant: Licochalcone Scientific Name: Glycyrrhiza inflata (G. inflata) Used parts: Licochalcone root |
-Licochalcone increased AMPK phosphorylation and Sirt1 expression in HepG2 cells. | [66] |
|
Name of the Plant: Sasa (bamboo) Scientific Name: Sasa borealis Used parts: Sasa borealis leaf extract |
-Sasa borealis leaf extract helped in the activation of the AMP-activated protein kinase in STZ-induced diabetic mice. | [67] |
|
Name of the Plant: Glabridin Scientific Name: Glycyrrhiza glabra Used parts: Raw Glabridin |
-AMPK activation with Glabridin was reported in (C57BL/6J strain) mice. | [68] |
|
Common Name: Tea Scientific Name: Cyclocaryapaliurus Used parts: Cyclocaric acid B and Cyclocarioside H |
-Cyclocaric acid B and Cyclocarioside H showed AMPK underlying mechanisms in 3T3-L1 adipocytes. | [69] |
|
Name of the Plant: Black ginseng Scientific Name: Panax ginseng C. A. Meyer Used parts: Black ginseng ethanol extract |
-Black ginseng extract improved AMPK protein activity in db/db mice. | [70] |
|
Name of the Plant: Katsura or Caramel Scientific Name: Cercidiphyllum japonicum Used parts: N/A |
-Dihydromyricetin improved AMPK signaling pathway in high fat diet-fed rats. | [71] |
|
Common Name: N/A Scientific Name: Boussingaultigracilis Miers var. pseudobaselloides Bailey Used parts: N/A |
-BEG increased phosphorylation of AMP-activated protein kinase (AMPK) in high-fat diet-induced obese rats. | [72] |
|
Name of the Plant: Wormwood Scientific Name: Artemisia scoparia and Artemisia santolinaefolia Used parts: Artemisia scoparia and Artemisia santolinaefolia extract |
-The extracts increased phosphorylation of AMPK α1 and AMPK activity significantly in DIO C57/B6J mice. | [73] |
|
Common Name: Exopolysaccharides Scientific Name: Enterobacter cloacae (E. cloacae) Z0206 Used parts: Selenium-enriched exopolysaccharides |
-Selenium-enriched Exopolysaccharides interacted with AMPK/SirT1 pathway in high-fat-diet induced diabetic KKAy mice. | [74] |
|
Name of the Plant: Compositae Scientific Name: Atractylodes Macrocephala Koidz Used parts: The dried rhizome of Baizhu |
-The compounds of Baizhu were associated with activation of AMP-activated protein kinase (AMPK) and PI3K/Akt pathways in mouse skeletal muscle C2C12 cells. | [75] |
|
Name of the Plant: Cucurbitaceae Scientific Name: Bryonia alba. L. Used parts: Cucurbitacin E |
-Cucurbitacin E induced autophagy via upregulating AMPK activity in ATG5-knocked down cells and DU145 cells. | [76] |
|
Name of the Plant: Figor Nopal or Prickly-pear cactus Scientific Name: Opuntia ficus-indica var. saboten Used parts: Fruit |
-Opuntia ficus-indica var. saboten improved peripheral glucose uptake through activation of AMPK/p38 MAPK pathway in db/db mice. | [77] |
|
Name of the Plant: Bitter Orange Scientific Name: Citrus aurantium Used parts: Polymethoxy flavonoids (Nobiletin and Tangeretin) |
-Citrus aurantium extract (Nobiletin and Tangeretin) enhanced phosphorylation of AMP‐activated protein kinase (AMPK) in male C57BL/6 mice. | [78] |
|
Name of the Plant: Ellis fruit Scientific Name: Gardenia jasminoides Ellis Used parts: Geniposide |
-Geniposide activated AMPK production in HepG2 Cells. | [79] |
|
Common Name: Chitin Source: Shrimps, Crabs and Insects Used parts: Chitosan oligosaccharide |
-Chitosan oligosaccharide prevented the development of aberrant crypt foci in a mouse model of colitis-associated colorectal cancer via a mechanism involving AMPK activation. | [80] |
|
Name of the Plant: Sophocarpine Source: Foxtail-like sophora herb and seed Used parts: Seeds |
-Sophocarpine alleviated hepatocyte steatosis through activating AMPK signaling pathway in pathogen-free male SD rats. | [81] |
|
Name of the Plant: Wild Hop Scientific Name: Humulus japonicus Used parts: Humulus japonicus extract |
-Humulus japonicus activated AMPK-SIRT1 pathway in Yeast and human fibroblast cells | [82] |
|
Name of the Plant: Rosemary Scientific Name: Rosmarinus officinalis L. Used parts: Rosmarinus officinalis L. extract |
-Rosemary extract triggered activating AMPK and PPAR pathways in HepG2 cells. | [83] |
|
Name of the Plant: Bitter Melon Scientific Name: Momordica charantia Used parts: Bitter Melon Triterpenoids |
-Activation of AMPK was noticed by Bitter Melon Triterpenoids in L6 myoblasts. | [84] |
|
Common Name: Quercetin Source: Onions, Apples, Tea, Berries, Cauliflower, and Red wine Used parts: Quercetin-3-O-glucuronide |
-Quercetin was involved in a manner dependent on AMPKα1/2 pathway activation in high fat diet mice. | [85] |
|
Common Name: Naringin Source: Citrus fruits Used parts: Fruit’s Albedo, membrane and pith |
-Naringin was associated via Interacting with AMPK activation in osteoblast-like UMR-106 cells | [86] |
Table 2.
Synthetic molecules which enhance AMPK activation.
| Used Components | Applications/Outcomes of the Experiment | References |
|---|---|---|
| Name of the Chemical: A-769662 | -AMPK-α1 was activated. | [56] |
| Name of the Chemical: AA005 | -AMPK activation was noticed causing by 2-deoxyglucose regulation. |
[112] |
| Name of the Chemical: AICAR | -The treatment resulted in significant induction AMPK inside melanoma cells. | [113] |
| Name of the Chemical: TSC2-pS1387 | -AMPK is induced upon insulin induction in Raptor knockdown cells. | [114] |
| Name of the Chemical: Rb2 and Rd | -The phosphorylation of AMPK was increased by Rb2 in HepG2 cells. | [115] |
| Name of the Chemical: Baicalin | -Baicalin activated CaMKKβ/AMPK/ACC pathway in a dose-response pattern in high fat diet-induced obese animal. | [116] |
| Name of the Chemical: Omega-3 polyunsaturated fatty acids | -Omega-3 polyunsaturated fatty acids activation of AMPK/SIRT1 pathway was noticed in Macrophage. | [117] |
| Name of the Chemical: Tangeretin | -Regulation of AMPK signaling pathways in C2C12 myotubes was reported in high-fat diet-induced obese mice. | [118] |
| Name of the Chemical: Oleuropein | - Oleuropein activated AMPK in C2C12 muscle cells. | [119] |
| Name of the Chemical: Glucagon-like peptide-1 (GLP-1) analog Liraglutide | -Glucagon-like peptide-1 (GLP-1) analog Liraglutide induced AMPK dependent mechanism Endothelial cell. | [120] |
| Name of the Chemical: Sildenafil | - Sildenafil enhanced mechanism in a demyelination model. | [121] |
| Name of the Chemical: Curcumin | -Curcumin induced AMPK–SREBP signaling pathway in Streptozotocin-induced type 1 diabetic rats. | [122] |
| Name of the Chemical: Metformin and Telmisartan | -Activation of the AMPK-PARP1 cascade was observed by Metformin and Telmisartan. | [123] |
| Name of the Chemical: Lipoic acid | -Lipoic acid triggered AMPK production in adipose tissue of low- and high-fat-fed rats. | [124] |
| Name of the Chemical: Mangiferin | -Activation of AMPK was reported by Mangiferin in hyperlipidemic rats. | [125] |
| Name of the Chemical: Capsaicin | -Capsaicin interacted with reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. | [126] |
| Name of the Chemical: ETC-1002 | -ETC-1002 responded LKB1-dependent activation of macrophage AMPK. | [127] |
|
Name of the Chemical: CTRP1 Protein |
-CTRP1 protein enhanced AMP-activated protein kinase (AMPK) activation in diet-induced weight gain in mice. | [128] |
| Name of the Chemical: Compound K | -Compound K interacted with AMPK/JNK pathway in type 2 diabetic mice and in MIN6 β-cells. | [129] |
| Name of the Chemical: Ramipril | -Ramipril interacted with CaMKKβ/AMPK and Heme Oxygenase-1 activation in cultured human aortic endothelial cells (HAECs) and a type-2 diabetic animal model. | [130] |
| Name of the Chemical: Compounds designated as 4a, 4b, 4h, 4j, 4k, 4z, 4aa, 4bb | -Compounds potently activated AMPK against colorectal cancer stem cells. | [131] |
| Name of the Chemical: Imiquimod | -Imiquimod activated AMPK in skin cancer cells. | [132] |
| Name of the Chemical: Tris (1, 3-dichloro-2-propyl) phosphate (TDCIPP) | -TDCIPP promoted AMPK/mTOR/ULK1 pathways in SH-SY5Y cells. | [133] |
| Name of the Chemical: Fibroblast growth factor 21 (FGF21) | -FGF21 activates AMPK signaling in hepatic cells. | [134] |
| Name of the Chemical: Pterostilbene | -Activation of AMPK was found by Pterostilbene in human prostate cancer cells. | [135] |
| Name of the Chemical: Thalidezine | -Thalidezine acted as AMPK activator in apoptosis-resistant cells. | [136] |
| Name of the Chemical: Rutaecarpine and its novel analogues (R17 and R18) | -Rutaecarpine analogues helped in AMPK activation in 3T3-L1 adipocytes. | [137] |
| Name of the Chemical: Nitroxoline | -Nitroxoline showed a crucial role on AMPK/mTOR signaling pathway in both hormone-sensitive (LNCaP) and hormone-refractory prostate cancer cells (PC-3 and DU-145). | [138] |
| Name of the Chemical: Fidarestat | -Fidarestat regulated mitochondrial Nrf2/HO-1/AMPK pathway in colon cancer cells. | [139] |
| Name of the Chemical: Methylisoindigo | -Meisoindigo activated AMPK cascade in primary PDAC cell line. | [140] |
| Name of the Chemical: (E)-3-(4-chlorophenyl)-N-(7-hydroxy-6-methoxy-2-oxo-2H-chromen-3-yl) acrylamide (SC-III3) | -SC-III3 activated AMPK/mTOR signaling pathway by acting on mitochondria in human hepatoma HepG2 cells. | [141] |
| Name of the Chemical: Betulinic acid | -AMPK siRNA was observed in the microglial polarization. | [142] |
3. IMMUNITY, INFLAMMATION AND AUTOIMMUNE DISEASES
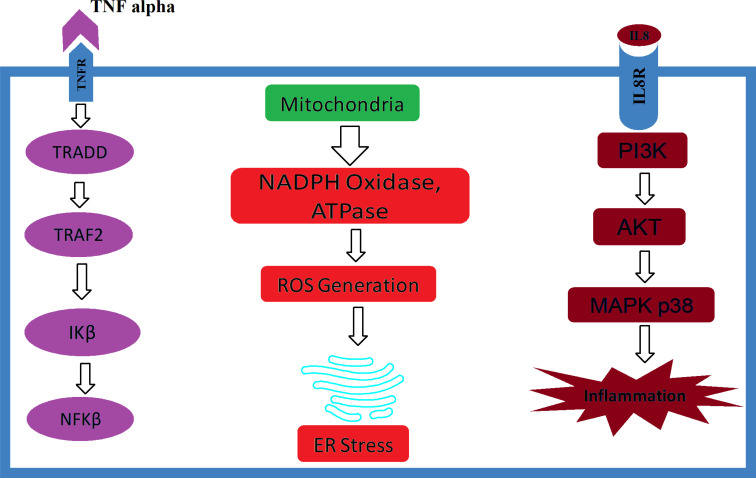
Having any kind of inflammatory response in a biological system explains the ultimate preventive and protective mechanisms that keep the body safe and active in response to harmful stimuli. Inflammatory defenses can be initiated either against live organisms or dead components [20, 21]. However, a disease may be initiated when an inflammatory cascade system persists for a longer time in a particular tissue or an organ. The acute scenario generally ends with necrosis of the tissue; conversely, chronic inflammation may eventually be responsible for end-stage organ dysfunctions [6, 22]. Followed by systemic exposure, cardiovascular inflammation is the most common reason for cardiac failure. Vascular inflammatory responses often initiate several pathological conditions such as myocardial infarction, systemic hypertension, atherosclerosis, cardiomyopathy, myocarditis and, in extreme cases, heart failure. Biological investigations show increased cardiac markers, for instance, uric acid, creatine kinase (CK)-MB, α-troponin, Xanthine oxidase (XO), inducible NOS, Lactase dehydrogenase (LDH), Myoglobin and Protein kinase C [23, 24]. Inflammatory components like macrophages, monocytes, T-lymphocytes, natural killer cells, mast cells, helper cells and dendritic cells are reported in end-stage renal diseases. Harmful cytokines such as activator protein-1, transforming growth factors-β, interferon-γ, nuclear factor-κB, tumor necrosis factor-α, and various clusters of differentiation (Fig. 1) are evaluated in the kidney dysfunction subjects [6]. Elevated markers such as Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Alanine Aminotransferase (ALT), Bilirubin and Inducible Nitric Oxide Synthase (iNOS) have been associated with Nonalcoholic Steatohepatitis (NASH) and NAFLD subjects. Besides, Toll-Like Receptor (TLR), Interleukin (IL), Transforming Growth Factor (TGF), Tumor Necrosis Factor (TNF) and Activator Protein (AP) also have been reported in liver damage models [25]. Cytotoxic T-cells and natural killer cells have been linked with pancreatic damage, making the subject to become diabetic [26]. Brain inflammation, on the other hand, is very specific and has been associated with cognitive impairment and Alzheimer’s disease, as well as Parkinson’s disease when excess inflammatory cytokines are reported in the central nervous system [27]. Inside the bone, overexpression of harmful immune cytokines such as uric acid, Xanthine oxidase TNF-α, Macrophage Inflammatory Protein (MIP) and IL-6 has been associated with osteoarthritis and rheumatoid arthritis, which lead to osteoporosis [28]. In the alimentary tract, stimulations like Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), foods and microorganisms may be involved in inducing several health-related issues, for instance, gastritis, inflammatory bowel syndrome, ulcers and esophagitis [29]. Chronic inflammation may turn into cancers in the longer run. Unwanted immune cells and cytokines are seen in the tumor’s surroundings and are more likely to accord with tumor growth progression, and immune-suppression than they are to mount an effective host anti-tumor response. Around the tumor environment, inflammatory cells serve as “fuel that feeds the flames,” which helps the tumor cells to be resistant and survive. Cancer severity and susceptibility may be linked with fundamental polymorphisms of inflammatory cytokine mRNA or genes, and inhibition or deletion of inflammatory cytokines prevents the development of experimental cancer [30]. Furthermore, inflammatory cells have also been reported to reject an organ immediately after the transplant or in the long run [31].
Fig. (1).
Role of inflammatory cytokines on various cellular components.
On the other hand, overexpression of inflammatory cells and cytokines can also get worse in other diseases, which can make other therapies less effective. Investigations suggested that immune cells are highly migratory, causing excess cells and cytokines to move around and interfere with other systems and thereby start a new problem in the migratory area. Besides, antibodies can be life-threatening for the host when they fight against their own host. Both innate and adaptive immunities are responsible for developing autoimmune diseases [32]. There are hundreds of autoimmune diseases that have been reported so far and have emerged as a serious headache for contemporary healthcare practitioners. Chronic inflammation is the most common factor and might accelerate in the progression of various autoimmune diseases, including psoriasis [33], lupus erythematosus [34], Addison’s disease [35], Graves' disease [36], Hashimoto's thyroiditis [37], autoimmune hepatitis [38], type-1 diabetes [39], rheumatoid arthritis [40], celiac disease [41], Sjögren syndrome [42], polymyositis [43], Chronic obstructive pulmonary disease (COPD) and asthma [44] and many more.
4. IS INFLAMMATION GOOD OR BAD?
Immunity, inflammation and inflammatory diseases have been recognized since the beginning of recorded medical knowledge and practice. Inflammation is a process of complex biological representation to cellular damage caused either by necrosis or infection or lysis, in which the feedback system by the immune system endeavors to neutralize or eliminate injurious stimuli and starts wound healing and regenerative processes [21, 25]. For instance, interleukin-6, a key tumor-pro- moting inflammatory marker generated by innate immunity, signals, at least, three regeneration-promoting transcription factors such as Notch protein, yes-associated protein 1 (YAP), and Signal transducer and activator of transcription 3 (STAT3), which are also involved in stem cell activation in both autocrine and paracrine systems. However, it has been found that inflammation is more evolutionarily ancient and is triggered by foreign invaders such as viral structures, known as pathogen-associated molecular patterns (PAMPs), or normal cellular constituents secreted upon injury and cell death, known as damage-associated molecular patterns (DAMPs). Both DAMPs and PAMPs are identified by pattern-recognition receptors (PRRs); most of these belong to the toll-like receptor family [45, 46]. To initiate a wound-healing mechanism in the injured area, the recruitment of immune cells and related soluble factors must be accumulated. This process may be accelerated with the involvement of cell cytokines and related markers. Along with leukocyte migration at almost any site of inflammation, monocyte recruitment around the wounds involves the chronological steps of trans-migration, endothelial cell activation and cell-to-cell interaction through the endothelium into the extra-vascular space although sometimes monocytes became a resident cell in tissues and not suffer migration. As monocytes represent only about three percent of circulating leukocytes, the rate of monocyte influx around wounds is far from stochiometric. Initially, monocytes like antigen-presenting cells and neutrophils may be recruited by factors produced rapidly once the injury occurred. From the wound, many soluble factors from the coagulation cascade, markers released from platelet degranulation, and related activated complement components. Another significant group of chemoattractants that are generated from the wound is the chemokines, a group of related small and soluble proteins that display highly conserved cysteine amino acid residues. Chemokines can be released by many cell types and individual chemokines may preferentially recruit particular populations [47, 48]. All these cells have the ability to invade the injury site by squeezing their sizes. Vascular permeability is increased due to heat generation in the area. Similarly, fluid retention may be more as more cells are recruited. Owing to increasing heat or fever, the antibody might be produced faster and transported into the injured area. Wound healing may be possible due to the onset of the accumulation of immune cells and the initiation of inflammation; hence, sometimes the situation can be deleterious if the production and recruitment are not switched off after the healing of the injury (Fig. 1). Abnormal and uncontrolled immune cells may be responsible for enhancing autoimmune diseases [49].
5. AN INTRODUCTION OF AMPK
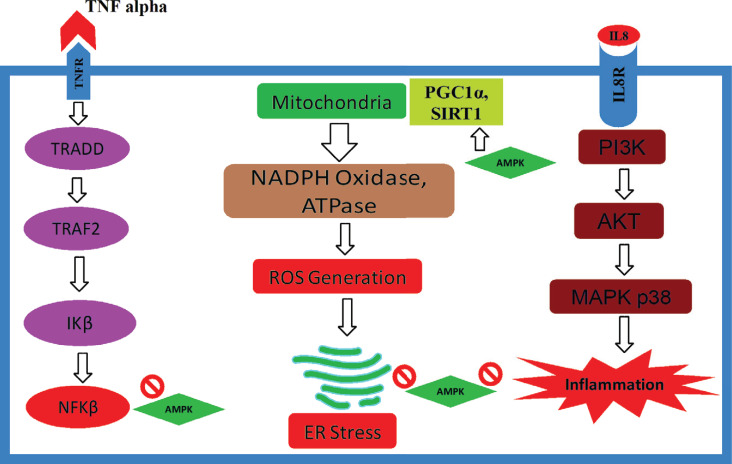
AMP-activated protein kinase (AMPK) is also known as a cellular and biochemical energy sensor and is activated when intracellular energy is reported to be insufficient or low. It has also drawn numerous attentions for organismal metabolism and central regulator key source that promotes ATP generation by inducing the expression of many proteins that are involved in the catabolism process during preserving energy while utilizing biosynthetic pathways. Along with regulating metabolic energy balance, AMPK plays a prominent role in maintaining growth and reprogramming metabolism, and recent reports have suggested new connections to biochemical processes like those involving cell polarity and autophagy, which opens new doors in unexplored cellular mechanisms [14, 50]. Among the many beneficial properties of AMPK, free fatty acid, which is synthesized by fatty acid synthase (FAS), is also significantly regulated by AMP-activated protein kinase [51, 52]. It is also known to regulate the energy metabolism counting lipid and glucose metabolism by providing an intracellular energy sensor. This master regulator is basically a heterotrimer that consists of a catalytic subunit α (Alpha) and two regulatory subunits β (Beta) and γ (Gamma) functions [14, 50]. The process is activated by the phosphorylation of Thr172 within α subunit, which is catalyzed by several kinase, for instance, Ca2+/calmodulin-dependent protein kinase-kinase β (CaMKKβ) and liver kinase B1 (LKB1/STK11) [53]. Sterol regulatory element-binding protein-1c (SREBP-1c) has been identified as a coordinator of fatty acid synthase (FAS) and can be suppressed by the AMPK. SREBP-1c is being considered as a good target owing to have controlling expression of several lipogenic enzymes, that persuade the suppression of FFA synthesis to manage obesity [51, 54]. AMPK signaling has been involved in enhanced stress resistance, energy metabolic homeostasis, and qualified cellular housekeeping, which are the hallmarks of upgraded health and extended lifespan. In addition, AMPK-mediated stimulations such as SIRT1, FoxO/DAF-16 and Nrf2/SKN-1 signaling pathways also improve cellular stress resistance during cellular survival and prevent aging. Moreover, they also control autophagy via mTOR and ULK1 signaling, which improves the quality of cellular housekeeping genes. Furthermore, studies suggest that Protein kinase B (AKT), a prime protein kinase in insulin/IGF pathway, can inhibit AMPK signaling, which demonstrated that AKT increased the phosphorylation of AMPKα1 at Ser485/Ser491, which subsequently prevented the phosphorylation of AMPK at Thr172 by LKB1 and thus suppressed the activation of AMPK [9, 55]. In spite of being the main energy sensor, activation of AMPK enhances heme oxygenase-1 gene expression, which shows the anti-apoptotic effect of AMPK, and this might provide an important adaptive response during survival [56]. The antioxidant effect of AMPK was noticed in several experiments, which may often have the potential of helping the cellular components against free radical-mediated oxidative stress (Tables 1 and 2). AMP-activated protein kinase also significantly reduced free radical components and enhanced the expression of the antioxidant enzymes such as manganese (Mn) superoxide dismutase (SOD), selenium, glutathione and catalase in phosphatase and tensin homolog deleted from chromosome 10 (PTEN) pathways [57]. Involvement of AMKP is also connected with retinal vasodilator by enhancing eNOS and NO-mediated pathway [58]. Taken together, different subunits of AMPK have also been reported to be localized at lysosomes to promote autophagy; activate many upstream kinases such as LKB1, STRAD and CAMKKβ; inhibit several anabolic pathways like the acetyl-CoA carboxylases (ACC1 and ACC2), glycogen synthases (GYS1 and GYS2) and HMGCR; stimulate various catabolic pathways, for instance, TXNIP, PFKFB3 and TBC1D1; promote mitochondrial biogenesis by improving PGC1 family. The peroxisome proliferator-activated receptors (PPARs) family, TFEB and ULK1 complex; regulate mitochondrial dynamics by interacting with Mitochondrial fission factor (MFF), ETC and dynamin-related protein 1 (Drp1); monitor autophagy and mitophagy by interfering with mTORC1, ATG14L, Beclin1 and AMBRA1; and regulate several other related protein and enzymes (Fig. 2) [59].
Fig. (2).
. Preventive role of AMPK against various cytokines.
6. REGULATION AND ACTIVATION OF AMPK
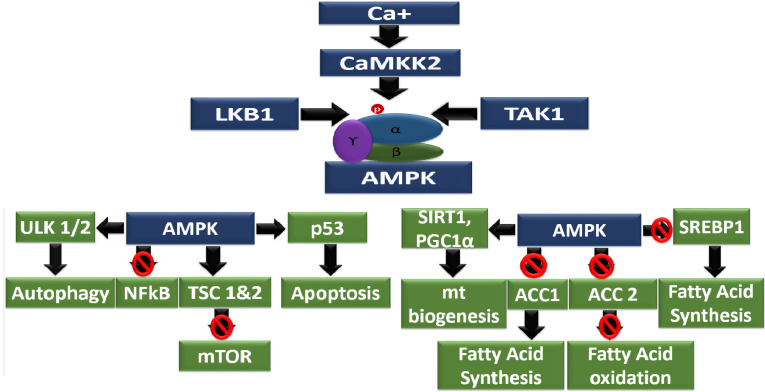
In all eukaryotes, AMPK serves the essential function of conserving cellular energy levels and catalyzing several key elements and their production. Since the last couple of decades, the focus-on AMPK has been thoroughly implemented, as it is considered a new potential target for various diseases, including obesity, diabetes, non-alcoholic steatohepatitis (NASH) and diabetic nephropathy. Many investigations and advances have been made in the arena of AMPK structural biology, and they are beginning to provide detailed molecular understanding into the overall topology of these intriguing enzymes and how the binding of small components elicit subtle conformational changes leading to their opening and protection from dephos- phorylation. However, AMPK structure and function on specific molecular interactions are yet to be properly disclosed while applying direct natural or synthetic AMPK activators of enhancers [50, 87].
The processes of regulating mammalian AMPK exists as a heterotrimeric complex consisting of 3 subunits that transpire as multiple isoforms. The catalytic α-subunits (α1/ α2) and the structurally crucial β- subunits (β1/β2) and γ-subunits (γ1/γ2/γ3) are encoded by 7 different genes. The catalytic subunit preserves a highly conserved Ser/Thr kinase domain close to the N-terminus in the activation ring. These isoforms are encoded by diverse genes that are separately expressed and have exclusive tissue-specific expression outlines, creating the prospect of producing 12 distinct heterotrimer amalgamations. Phosphorylation of Thr-172 within this circle is critical for enzyme activity [88]. Phosphorylation of Thr-172 within this loop is critical for enzyme activity. In addition to regulation through phosphorylation, AMPK- subunit activity has been found to be self-regulated by a region identified as C-terminal to the kinase domain (Fig. 2) [89, 90]. This auto-inhibitory sequence (AIS) is similar to ubiquitin connected domains, which are mostly conserved sequences inside the AMPK sub-family of kinases. Investigations performed using AIS deletion constructs demonstrate a larger than 10-fold enlargement in kinase property as compared with constructs containing the AIS [91, 92]. The AMPK-subunits normally do not display catalytic activity, but they have emerged to be complex for AMPK α-β-γ complex assemblage and glycogen sensing in the cell [93]. Phosphorylation of AMPK α-subunit helps in the phosphorylation of MKP1, ULK1 and mTORC1 and regulates lipolysis, insulin resistance and Mitophagy [94]. Domains located close to the C-terminus of the -subunit have been revealed to interrelate with regions on the α- and γ-subunits, which recommends that the β-subunit, in part, may participate as a scaffold to sustain AMPK heterotrimeric complex assemblage. The β-subunits also restrain glycogen-binding domains (GBD) that arbitrate AMPK’s connection with glycogen and could assist the colocalization of AMPK with its component glycogen synthase (GS). This phenomenon may permit the cell to coordinate the maintenance of glycogen production with glycogen concentration and energy accessibility [95, 96]. In addition to these, a potential role for β-subunit myristoylation has been incorporated to be important for suitable AMPK cell membrane activation and localization [97]. Activation of β-subunit helps in the phosphorylation of MFF, which is responsible for mitochondrial fission [89]. The AMPK γ-subunits control enzyme activity by sensing comparative intracellular AMP, ADP and ATP concentrations. This is achieved by the complex interactions of adenine nucleotide with 4 frequent cystathionone-β-synthase (CBS) motifs occurring in pairs known as Bateman domains [98, 99]. Activation of γ-subunit helps in the phosphorylation of PGC1α, which further regulates mitochondrial biogenesis [89]. It is now hypothesized that the CBS motifs assemble in an approach that results in the formation of 4 adenine binding sites. In the binding sites, site 4 has a comparatively very high affinity for AMP and generally does not readily exchange for ADP or ATP, whereas sites 1 and 3 bind AMP, ADP, and ATP competitively [100]. Site 2 seems to be mostly unoccupied, and its role in maintaining AMPK action has not been properly elucidated [101, 102]. In the beginning, AMPK inauguration was believed to be mediated mainly by AMP binding; however, new research has shown that both ADP and AMP binding result in conformational changes that trigger AMPK in 2 ways: by promoting Thr-172 phosphorylation by upstream kinases; and by antagonizing its dephos- phorylation with protein phosphatase(s) [103, 104]. Conversely, only AMP has been demonstrated to, in a straight line, increase phosphorylated AMPK (Thr172) property via a mainly allosteric mechanism. Research on kinetic enzyme assays has shown that allosteric commencement by AMP results in a greater than 10-fold enhancement in activity, while the activation resulting from Thr-172 phosphorylation of the α-subunit is bigger than 100-fold. In combination, these 2 triggering mechanisms produce a greater than 1000-fold increase in activity [105].
Several AMPK kinases have been implicated in mediating AMPK Thr-172 phosphorylation in vitro; hence, only 2 have been reported to be physiologically appropriate in in vivo studies. The major AMPK kinase is the ubiquitously produced tumor suppressor liver kinase B1 (LKB1) [106, 107]. Even though LKB1 activity is not reliant on the phosphorylation of its activation ring, significant enhancement requires binding of the scaffold protein MO25 and stabilization by Ste20-mediated adaptor (STRAD) protein interaction [108]. Taken together, these 3 proteins form an inherently dynamic trimeric LKB1-STRAD-MO25 complex that has been shown to mediate-AMPK Thr-172 phosphorylation in different mammalian physiology. Since the discovery of LKB1 as an AMPK kinase, with the interaction of Ca2+ /calmodulin-dependent kinase (CaMKK), which phosphorylates Thr-172 in response to induced cytosolic Ca2+ levels autonomous of changes in adenine nucleotides [109]. It is hypothesized that this interaction may couple ATP-requiring developments, which is frequently activated through enhancement in cytosolic Ca2+, with AMPK-dependent restoration of energy change before a noteworthy reduction in ATP concentrations take place. The physiological significance of another important AMPK kinase, TAK1, continues to be established [110, 111].
7. OXIDATIVE STRESS AND INFLAMMATION
The term oxidative stress is often described as oxidant-mediated cell damage, for example, by free radicals (reactive oxygen species and reactive nitrogen species). In the environment, several oxidants are present, including water containing arsenic, which is responsible for many life-threatening diseases like hepatic damage, kidney failure, heart dysfunctions and neurodegeneration [143]. Like environmental oxidants, fried or over-cooked food sometimes generates many oxidants like advanced glycation end products (AGEs) that may lead to diabetes and cardiovascular complications [26, 144]. On the other hand, cellular metabolism and reproduction sometimes produce unnecessary and unwanted byproducts that can act as oxidants and furthermore help in cellular necrosis and apoptosis. In addition, dying cells or cancer cells might secrete several oxidants which further interfere in many biochemical pathways, leading to the progression of pathophysiology [145, 146]. Free radicals are mostly generated during energy production in mitochondria. As the liver is the main source of mitochondrial generation, it suffers more from free radicals. In patients with Nonalcoholic Steatohepatitis (NASH), the hepatic mitochondria exhibit ultrastructural lesions and reduced activity of the respiratory chain complexes [147]. This phenomenon decreases the activity of the respiratory chain, resulting in the accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that oxidize fat accumulations to form lipid peroxidation products, which in turn, cause necrosis, steatohepatitis, hepatitis, fibrosis and cirrhosis. Overproduction of mitochondrial ROS formation in cirrhotic and steatohepatitis could directly damage mitochondrial DNA and related respiratory chain polypeptides, trigger NF-κB activation and the hepatic synthesis of TNF-α and ILs (Fig. 3) [148]. Glomerulonephritis has also been connected in animal models when levels of free radicals, such as malondialdehyde, advanced oxidation protein products, thiobarbiturates, 15-f2t-isoprostane and hydrogen peroxide, are exceeded due to chemical treatment [149]. Similarly, Xanthine oxidase (XO), α-troponin, creatine kinase-MB, uric acid and hydroxyl ion have been correlated with myocarditis, vasculitis, cardiac hypertrophy and fibrosis [6, 23]. Neurodegeneration and neurodermatitis have also been associated when free radical-mediated oxidative stress; this was induced in rats [150]. Natural oxidants such as arsenic and aflatoxins often induce oxidative stress and have been associated with skin inflammation [143]. Besides, having little or no antioxidants such as superoxide dismutase, catalase, α-tocopherol, β-carotene, ascorbate and glutathione in the body can lead to inflammation (Fig. 1) [20, 151].
Fig. (3).
Anti-inflammatory property of AMPK on inflammatory cytokines.
8. DIABETES AND INFLAMMATION
Both type-1 and type-2 diabetes mellitus are the leading causes of morbidity and mortality nowadays. Prevention of diabetes mellitus and its associated risk factors, mainly cardiovascular morbidity and mortality, have become major health concerns throughout the world. Along with obesity, the pathophysiology of so-called “diabesity”, that is, diabetes mellitus in the milieu of obesity has recently gained significant awareness [152]. The exact cause of diabetes is yet to be explored; however, the relationship between type-2 diabetes and inflammation is more complex than what has been reported so far by the animal and human studies. First, inflammation possibly underlies also the development of insulin resistance and not only of pancreas dysfunction but also responsible with adipose tissue, brain, liver and kidney that eventually resulting of obesity and a critical contributing factor to the development of metabolic disease [153, 154]. Other studies have also suggested that infection from cytomegalovirus (CMV) is also responsible for damaging the pancreas [26]. In addition, excess glucose accelerates the formation and production of advanced glycation end-products (AGEs), interacting with its receptor named RAGE, which further generates reactive oxygen species and initiates inflammatory signaling cascades. Consequently, AGEs have significant roles in the pathogenesis of diabetic complications. It is reported that RAGE is also expressed on monocytes T-lymphocytes and macrophages, which complicates the phenomenon [155]. Similar theories have also been proposed by other investigations, where the authors showed that cytokines released by both activated macrophages and T-lymphocytes, especially interleukin-1 (IL-1), have been compromised as immunological effector molecules that both prevent insulin release from the pancreatic β-cell and accelerate β-cell destruction that can lead to hyperglycemia [156]. Moreover, nitric oxide (NO) produced by iNOS directly encourages the activities of both the inducible and constitutive isoforms of COX, further augmenting the overactivation of these pro-inflammatory markers, NO and prostaglandins, which may be necessary in maintaining or initiating the inflammatory signaling and knocking down the β cell related with autoimmune diabetes [157]. Furthermore, insulin resistance keeps the excess sugar in the blood, which signals diacylglycerol (DAG) that interacts with mitogen-activated protein kinase (MAPK) and Inositol triphosphate (IP3). This situation results in harmful immune cytokine transcription factors being transcribed in the nucleus, which worsens the diabetes and starts attracting other intact organs [158]. It has also been reported that during the progression of diabetes a number of biochemical pathways and mechanical factors come together in the endothelium, leading to endothelial damages, vascular inflammation and macrovascular diseases [159].
9. OBESITY AND INFLAMMATION
Obesity has been a sober concern as it is becoming an epidemic with its vast health consequences. Not only are physicians worried but nutritionists and physiotherapists are also facing difficulties managing the situation, especially in the Western world and urban areas. Molecules to treat obesity are very few in the market and pipeline. Medicinal chemists are also trying to discover newer components, as obesity contains several other complicated factors in its pathophysiology. Obesity is often conveyed by excess fat storage in tissues other than adipose tissues, including the skeletal muscle and liver, which may initiate to local insulin resistance and could trigger inflammation, as in steatohepatitis [160]. Similarly, in the vascular system, excess fat deposition may trigger vasculitis, which further accumulates foam cells, and the patient might ultimately end-up with atherosclerosis [7]. In addition, studies also found that patients with central obesity are at risk for chronic kidney disease such as obesity-related glomerulopathy, which may turn into end-stage renal diseases [161]. High-fat and high-sugar diet have also been associated with myocarditis and cardiac hypertrophy by AGE-RAGE interaction [162]. On the other hand, the immune system’s prime role is maintaining or regaining homeostasis upon external or internal threats. Inflammation is an evolutionary perpetuated danger control program that steers from host defense against stimuli as inflammation can prevent pathogens from entering and spreading at disrupted barriers [163]. Recent studies suggest that adipocytes are now considered as a gland since they secrete various factors such as vaspin, adiponectin, hepcidin, omentin, chemerin, visfatin, apelin and many inflammatory and pro-inflammatory cytokines. Following the discovery of tumor necrosis factor-α and leptin as soluble/secretory products of adipose tissues in the early 1990s, consequently, obesity research has focused on the new functional role of adipocytes, as an active endocrine gland/organ. Many more inflammatory peptides have been linked to adiposity, with obesity ultimately characterized as a state of low-grade metaflammation or systemic inflammation, which may connect obesity to its co-morbidities [164]. Obesity also aggravates several other phenomenons such as thrombosis, nephritis, atherosclerosis, stroke, diabetes, vasculitis, systemic hypertension, allergies, osteoarthritis, NAFLDs, alzheimer’s disease, insulin resistance, rheumatic carditis and fibrosis; therefore, controlling and managing obesity can play a pivotal role in preventing non-communicable diseases and other co-morbid situations [165].
10. INFLAMMATION AND CARDIOVASCULAR DISEASES
The human heart is the most important organ that pumps blood throughout the body passing through the circulatory system, supplying necessary oxygen and nutrients to the tissues and removing excess carbon dioxide and other waste materials. In spite of several advancements in medical science, cardiologists recommend that hepatic subjects visit hepatologists and vice versa as both the pathophysiologies interfere with each other [7, 166]. There are several factors that have been considered for cardiovascular diseases and one of them is cardiovascular inflammation, for example, mechanical stress, smoke exposure, hypercholesterolemia, hyperhomocysteinemia and chronic infection leading to atherothrombotic cardiovascular disease (CVD), coronary arterial disease (CAD), stroke, and peripheral arterial disease [7]. Among all the common risk factors, obesity is a highly prevalent condition and is heterogeneous, which has the highest impact on developing cardiovascular disease (CVD)s and related complications. Laboratory investigations and epidemiological reports have consistently demonstrated that the assembling of excess visceral fat is related to the huge risk of CVD as well as several metabolic and inflammatory perturbations. Since the beginning of the 2000’s, reports from multiple investigations have served to emphasize that arterial calcification and atherosclerosis have several correlations between inflammatory components and vascular dysfunctions that may serve as a vital element to discover pathophysiological processes and to unveil a new therapeutic approach [167]. Clinical evidence has also highlighted the finding that the expanded visceral fat is infiltrated by macrophages, which are then enhanced in the process of producing foam cells. These cells are responsible for conducting “cross-talk” with adipose tissue through numerous significant mechanisms [168]. In recent times, cytokines such as interleukin-6 and tumor necrosis factor-α soluble, as well as adhesion molecules, for example, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, have been considered with both risk factors and diseases and may serve as potential therapeutic targets for the nonspecific “anti-inflammatory” treatment of arterial disease [8, 169]. There have been a series of cases where heart failure subjects were diagnosed with extreme concentration of inflammatory markers [170]. On the other hand, Reg3β, a cardiac marker, has been responsible for the acute inflammatory response, often found higher in serum concentration in patients with acute coronary syndrome and also found connected in myocardial infarction [171]. Prior investigations suggested that the serum- and glucocorticoid-inducible kinase (SGK1) play a key role in inflammation and cardiac remodeling. A different correlation has also been established between SGK1 and cardiac angiotensin, which often potentiates the damaging property of the heart [172]. Dipeptidyl peptidase 4 (DPP)-4, on the other hand, known as a potent cardiac inflammatory marker has also been found in cardiac damage subjects [6]. Besides, xanthine oxidase (XO), α-troponins and creatine kinase-MB have also been blamed and reported in various heart research works as inflammatory cytokines [23].
11. INFLAMMATION AND HEPATIC DYSFUNCTIONS
Hepatic damages can be initiated with a viral infection, smoking, chronic alcoholism, substance abuse and poly-pharmacy, which can be worsened by diabetes, obesity, aging, chronic diseases and sedentary lifestyle. Inflammations are the pathogenic outcomes of various types of acute and chronic liver insults and stimulations, and these facilitate the progression of liver diseases like hepatitis, steatosis, cirrhosis and fibrosis [173, 174]. Components of the innate immune system instigate and maintain hepatic inflammation through mediator generation, resulting in the activation of their pathogen-derived products perceived by pattern recognition receptors on the cell surface [174]. Dendritic cells, which are part-innate immune cells, play a prime role in sensing pathogens and initiating adaptive immune responses by the activation and regulation of T-lymphocytes [175]. Unfortunately, liver inflammation is the most complicated phenomenon as no particular therapy is recommended other than taking a rest and eating healthy [176]. Both non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD) are mediated through various inflammatory stimulations and have recently been epidemic, especially in Western countries, because they have very few effective drug treatments, as the molecular and cellular mechanisms underlying their hepato-protective functions remain largely uncharacterized. Concurrently, owing to the presence of obesity, is quite difficult to achieve weight reduction or a reduction of central obesity or visceral fat with the treatment of NAFLD in the perspective of ‘gold standard’. Early diagnosis and management of the underlying condition remain the mainstay of treatment to avoid chronic inflammation [177, 178]. In many investigations of NAFLD, the risks of progressing metabolic and cardiovascular morbidities are much higher than the risks of developing hepatic disorders. In fact, NAFLD is connected as the liver manifestation of the metabolic syndrome, which passes on to a cluster of cardiovascular risk factors linked with insulin resistance, including visceral or central obesity, hypertension, dyslipidemia and type-2 diabetes mellitus [179-181].
12. INFLAMMATION AND RENAL DAMAGES
In a human body, the kidneys play a major role in producing lifesaving hormones, regulating blood pressure, monitoring fluid and electrolyte balance and excreting body wastes [149]. Inflammation in the kidney is more common in females than males since males benefit from the advantages of the activity of testosterone [182, 183]. Hypertension, urinary infection, diabetes, proteinuria and renin-angiotensin system (RAS) have been primarily identified as risk factors for renal inflammation. Similarly, cardiovascular dysfunctions and hepatic damages also play a significant role in the progression and development of kidney inflammation. The involvement of reactive oxygen spices is equally responsible for the inflammatory pathways [6]. Apoptosis and inflammation are prime causes of organ damage in the course of renal injury. General aspects of renal inflammation can be unwanted apoptosis, kidney morphology, injury induction, and activity of the glomerulus function. Activated caspases, the typical effector enzymes of apoptosis, are able to initiate not only apoptosis but also inflammation after renal injury of ischemia-reperfusion in experimental models [184]. Along with the secretion of IL-1β, secretion of IL-18 and a programmed form of cell death, toll-like receptor (TLR)-driven danger signaling in kidney disease, evolving reports now suggest a similar involvement in various inflammasome in renal inflammation subjects [185]. Since the last two decades, basic science has brought up a new perceptive of how pathogens prompt inflammation, mainly by the discovery of RIG-like receptors, NOD-like receptors (NLRs), toll-like receptors (TLRs), and C-type lectin receptors (CLRs). Especially, the toll-like receptors (TLRs) have spurred interest in kidney inflammation outside infectious disease research. It was a groundbreaking discovery that is not only pathogen-associated but also demonstrates that sterile damage-asso- ciated molecular patterns (PAMPs and DAMPs) stimulate TLRs to signal pro-inflammatory cytokines and chemokines that further trigger inflammation. The latest entry into this concept is the inflammasomes in renal inflammation, which increases research interest in the nephrologists [186, 187]. Inflammation in the kidney may reduce the activity of tubular secretion, fluid and electrolytes re-absorption, and waste elimination, causing the subject to be dependent on regular dialysis. Besides, inflammatory cells damage and reduce cell wall integrity, enhance endoplasmic reticulum (ER) stress, prevent mitochondrial biogenesis and interfere with the normal DNA duplication, which pushes the subject toward serious health issues [23, 149].
13. MOLECULAR MECHANISMS OF AMPK ON INFLAMMATION
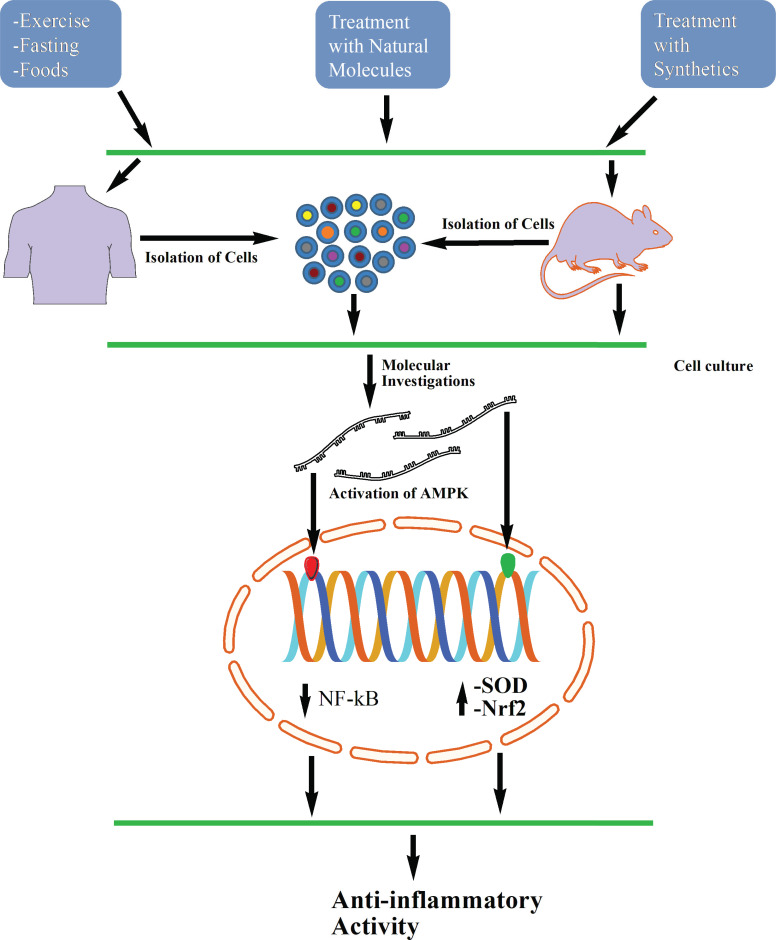
The etiology of inflammation inside the body is a mystery and the exact mechanisms are yet to be explored. Besides, inflammatory pathways trigger many incidents of biochemical signaling; therefore, other treatment strategies may be less effective to achieve their proper goals. In addition, immunity will show differently with individual subjects as inflammatory responses vary from each other. Moreover, no one can predict how the body will react with foods, weather, medications and foreign invaders [169, 181]. Both the naturally derived and synthetic components (Fig. 4) have been proven effective by activating AMPK to minimize inflammation in both animals and cell cultures (Tables 1 and 2).
Fig. (4).
Proposed hypothetical figure where AMPK can participate to inhibit various diseases.
14. EFFECT OF AMPK ON VARIOUS KINASES
AMPK shows a potential capacity to reduce the inflammatory stages but direct preventive mechanisms are yet to be seen. However, recent investigations show several anti-inflammatory activities of AMPK in in-vitro and in-vivo models where researchers found that pharmacological activation of AMPK instantly inhibited the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway in various cells, including in cultured vascular endothelial cells and fibroblasts. Relatively very few studies have investigated how the JAK-STAT pathway is controlled by AMPK and exerts anti-inflammatory property as this is a predominantly important concern to address given the pathophysiological roles of enhanced JAK-STAT signaling in multiple chronic inflammatory circumstances, for instance, hepatitis, nephritis, atherosclerosis, colitis and rheumatoid arthritis, as well as various cancers and hematological disorders driven by mutational activation of JAK isoforms. In vitro kinase assays showed that AMPK directly phosphorylated 2 residues (Ser515 and Ser518) within the Src homology 2 domain of JAK1, and activation of AMPK enhanced the interaction between JAK1 and 14-3-3 proteins; therefore, suppression of inflammatory signaling may be achieved [188]. Furthermore, activation of macrophages and increased cytokine infiltration have been experimented in adipose tissue from wild-type mice transplanted with bone marrow from AMPKβ1-/- mice, and taken as a whole, it has been proven clearly that AMPK can limit inflammatory cell infiltration and activation in multiple settings (Table 3) [189].
Table 3.
Role of AMPK enhancers/inducers on inflammation and related disorders.
| AMPK Enhancers | Experimental Design | Applications/Outcomes of the Experiment | References |
|---|---|---|---|
|
Name of the Component: Xanthohumol Dose: 10 or 50 mg/kg |
Model: Wild-type (WT) and Nrf2−/− (knockout) C57BL/6 mice | -Induction of AMPK reduced ROS and cytokine secretion. -Txnip/NLRP3 inflammasome and NF-κB signaling pathways were also found to be inhibited. |
[206] |
|
Name of the Component: Metformin Dose: 200 mg/kg |
Model: Six months older male Wister rats | -The treatment reduced cellular levels of nuclear factor-κB, Tumor Necrosis Factor-α and Cyclooxygenase-2 through AMPK pathway. | [203] |
|
Name of the Component: AICAR Dose: 11 pM |
Model: Endothelial cells | -Induction of AMPK demonstrated an anti-inflammatory pathway linking with PARP-1, and Bcl-6 pathways. | [16] |
|
Name of the Component: Retinoic acid Dose: 10 µM |
Model: BALB/c mice | -The treatment inhibited tissue factor and HMGB1 via modulation of AMPK activity in TNF-α activated endothelial cells and LPS-injected mice. | [194] |
|
Name of the Component: Cilostazol Dose: 0.1% Cilostazol with chow food |
Model: Sprague Dawley rats |
-Induction of AMPK inhibited NF-κB activation, as well as the induction of iNOS mRNA and protein expression, within the aortas of LPS-treated rats. | [227] |
|
Name of the Component: 2,3,4’,5-tetrahydroxystilbene-2-O-β-d-glucoside (THSG) Dose: 10, 20, 40, 80, and 100 µM |
Model: Mouse primary microglia and cell culture | -LPS-induced NF-κB activation and neuro-inflammatory response (TNF-α, IL-6, and PGE2) activating AMPK/Nrf2 pathways. | [215] |
| Name of the Component: Ginseng Saponin Metabolite Rh3 | Model: N/A | -Rh3 exerts an anti-inflammatory effect in microglia by modulating AMPK and its downstream signaling pathways such as phosphatidylinositol 3-kinase (PI3K)/Akt and JAK1/STAT1. | [228] |
|
Name of the Component: Quercetin, Luteolin and EGCG Dose: 10 µmol/l each |
Model: EA.hy-926 cells | -The treatment inhibited ER stress-associated TXNIP and NLRP3 inflammasome activation, and therefore protected endothelial cells from inflammation and apoptosis. | [207] |
|
Name of the Component: Luteolin Dose: 100 µM |
Model: HepG2 hepatocarcinoma cells | -AMPK activity was found to be critical for the inhibition of intracellular ROS in turn mediate NF-κB signaling. | [216] |
|
Name of the Component: Methotrexate and A769662 Dose: N/A |
Model: Human monocytes and bone marrow-derived macrophages | -Methotrexate-induced AMPK activation was associated with a reduction in the production of pro-inflammatory cytokines (IL-6, IL-1 β, and TNF-α) in response to LPS and TNF stimulation. | [198] |
|
Name of the Component: Metformin Dose: 5 µM |
Model: Wild type C57/Bl6 mice | -Metformin specifically inhibited LPS-induced IL-1β production and boosts IL-10 induction via AMKPα1- and or AMPKβ1 pathways. | [199] |
|
Name of the Component: LY294002 and or CP-690550 Dose: 20 µM |
Model: C57BL/6J mice | - AMPKα1 is required for IL-10 activation of the PI3K/Akt/mTORC1 and STAT3-mediated anti-inflammatory pathways. | [200] |
|
Name of the Component: Lindenenyl acetate Dose: 20 µM |
Model: The immortalized HPDL cell line | -Inducible nitric oxide synthase (iNOS) derived nitric oxide (NO) and cyclooxygenase-2 (COX-2) derived prostaglandin E2 (PGE2) production were found to be downregulated with the association with AMPK induction. | [202] |
|
Name of the Component: Emodin Dose: 0-80 µM |
Model: Mouse primary microglia and cell culture | -The approach effectively inhibited the production of pro-inflammatory cytokines, TNF-α and IL-6, and reduced the level of IκBα phosphorylation with the involvement of AMPK/Nrf2 Activation. | [229] |
|
Name of the Component: Monascin and Ankaflavin Dose: 10 µM |
Model: Mouse liver cell FL83B | -AMPK suppressed the production of inflammatory cytokines, including IL-6, TNF-α, and TGF-β. | [195] |
|
Name of the Component: Flufenamic Acid Dose: 50 µM |
Model: NRK-52E cells | -The strategy significantly suppressed nuclear factor- activity and inducible nitric-oxide synthase expression triggered by interleukin-1 and tumor necrosis factor- α. | [230] |
|
Name of the Component: Xanthohumol Dose: 5 µM |
Model: CHO-ARE-LUC cells | -Inhibition of GSK3β or mTOR is not involved in the observed AMPK boost, and -AMPK also enhanced the activity of the Nrf2/HO-1 signaling pathway. | [209] |
|
Name of the Component: Genetically induced or Ghrelin Dose: 100 nM |
Model: GHS-R knockout mice | -Ghrelin induction activated AMPK in primary endothelial cells showed potential activity in atherosclerosis by reducing inflammatory and proinflammatory cytokines. | [226] |
|
Name of the Component:AMP mimetic ZMP Dose: 100 µM |
Model: Vascular endothelial cells | -AMPK-mediated JAK-STAT signaling inhibition suppressed either by the IL-6Rα or in IL-6. | [231] |
|
Name of the Component: Berberine and SB203580 Dose: 10 µM and 10 µM |
Model: Sprague-Dawley rats | -The therapy reduced cleaved caspase 3, iNOS, Collagen-II, chondrocyte apoptosis and ameliorated cartilage degeneration via activating AMPK signaling and suppressing p38 MAPK activity. | [204] |
|
Name of the Component: trans-caryophyllene (TC) Dose: 50 µM |
Model: Rat cortical neurons/glia | -TC reduced cerebral ischemic injury via activation of the AMPK-CREB pathway. | [232] |
|
Name of the Component: Mangiferin Dose: 0.1, 1, 10 μmol/L |
Model: EA.hy926 cells | -The treatment ameliorated endothelial dysfunction by inhibition of ER stress-associated TXNIP/NLRP3 inflammasome activation and increased IL-1β secretion. in the endothelial cells | [208] |
|
Name of the Component: Wogonin Dose: 10–100 μM |
Model: Human glioma cell lines |
-The strategy blocked cell cycle progression at the G1 phase and induced apoptosis by inducing p53 expression and further upregulating p21 expression. | [233] |
|
Name of the Component: Ilexgenin A Dose: 80 mg/kg |
Model: Sprague-Dawley rats and ICR male mice | -The treatment prevented NLRP3 inflammasome activation by down-regulation of NLRP3 and cleaved caspase-1 induction, and -reduced IL-1β secretion was also noticed associate with AMPK activation. |
[234] |
|
Name of the Component: CRPE55IB Dose: 40 µM |
Model: Isolated primary microglia cultures | -CRPE55IB inhibited LPS-induced NF-κB activation and neuro-inflammatory response in microglia upregulating AMPK/Nrf2 pathways. | [210] |
|
Name of the Component: Resveratrol Dose: 0.1 or 10 µM |
Model: RAW 264.7 macrophage cells | -Enhancement of AMPK suppressed LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macrophage cells. | [12] |
|
Name of the Component: RSVA314 and RSVA405 Dose: 3 μM each |
Model:C57BL/6 females cell cultures | -Enhancers of AMPK potently inhibited mTOR signaling, activated autophagy, and triggered Aβ clearance by the lysosomal system. | [235] |
|
Name of the Component: Ursolic Acid Dose: 2.5 mM to 10 mM dose-dependently |
Model:3T3-L1 pre-adipocytes | -Ursolic Acid inhibited CEBPα and β-actin in the fat cells via LKB1/AMPK pathways. | [51] |
|
Name of the Component: Nitric oxide (NO) Dose: 100 mmol/L |
Model: Human endothelial cells | - An inhibition of the IKK kinase and decreased NF-κB activation and TNF-α expression were observed with AMPKα2 phosphorylation. | [236] |
15. EFFECT OF AMPK ON TUMOR NECROSIS FACTORS
One of the major inflammatory cytokines is tumor necrosis factor (TNF), which has been associated with many disease conditions like hepatitis that leads to changes in the normal liver architecture, which could lead to liver failure [190]. TNF-α is also responsible for excess bleeding in the kidney, hampers homeostasis, leading to anemia, and finally, triggers renal failure [190, 191]. The presence of TNF-α is often evaluated in heart failure subjects as well [192]. This protein is found highly responsible for pancreatic β-cell damage, which ultimately triggers insulin resistance and type-I diabetes mellitus [193]. Taken together, inhibiting TNF from releasing macrophages can be a very effective approach to minimizing several diseases. To investigate the role of TNF-α on an endothelial cell (EC), mice were performed on LPS-injected subjects, and Retinoic acid (0, 0.01, 0.1, 1 and 10 mM) was applied on EC. It was observed that the introduction of Retinoic acid reduced cellular damages by activating the AMPK pathway [194]. Another investigation noticed that providing high-fat diet C57BL/6 to mice may lead to an inflammatory marker such as TNF-α, which ultimately causes nonalcoholic steatohepatitis. When AMPK enhancers, i.e. Monascin and Ankaflavin were given to mice, AMPK inducers promoted AMPK phosphorylation and inhibited the steatosis-related mRNA expression and inflammatory cytokines secretion like tumor necrosis factor-α and, thereby, improved nonalcoholic steatohepatitis (Table 3) [195].
16. EFFECT OF AMPK ON INTERLEUKINS
Interleukins (ILs) not only play a role in hereditary auto-inflammatory diseases but also strongly participate in atherosclerosis, chronic kidney disease and advanced metastatic colorectal cancer. The IL family consists of 11 different cytokines and 10 receptors although these are alienated into three subfamilies. The major sources of IL are macrophages, β-cells, monocytes, dendritic cells, Th2 cells, just activated naive CD4+ cell, memory CD4+ cells, mast cells and many more which generally bind with CD121α/IL1R1, CD121β/IL1R2, CD25/IL2RA, CD122/IL2RB, CD132/IL2RG, CD126/IL6RA, CD130/IR6RB and many others that mainly target activated T cells, NK cells, macrophages, oligodendrocytes, hematopoietic stem cells, T helper cells and β-cells to trigger their responsible cascade systems. It is also established that blocking IL and TNF can be a very good solution to controlling and minimizing inflammatory and autoimmune diseases, of course, with some exceptions such as gout and uric acid and Xanthine oxidase-mediated phenomenon [196, 197]. Human monocyte-derived macrophages (MDM) and mou- se bone marrow-derived macrophages (BMDM) along with AICAR and A769662 were investigated to observe the role of inflammation and AMPK interactions and it was noticed that both AMPK-enhancers were able to reduce IL-6 and IL-1 β levels in response to LPS and TNF stimulation; thereby, the authors strongly suggested that these agents can be a good target for the development of new anti-inflammatory drugs [198]. Another study showed that Metformin prevented the production of ROS from NADH: Ubiquinone oxidoreductase to limit induction of IL-1β in LPS-activated macrophages. The study further proved that Metformin was able to boost IL-10 to enhance anti-inflam- matory activity since the activation of AMPK in the cell culture of wild-type C57/Bl6 mice; thereby, this molecule can be a good target for reducing diabetes-associated inflammations [199]. AMPK is a conserved serine/threonine kinase with a decisive function in playing an additional role as a regulator of inflammatory activity, especially in leukocytes. IL-10-induced gene expression and the anti-inflammatory function of AMPK phosphorylated both Tyr705 and Ser727 residues of STAT3 in an AMPKα1-dependent manner, and this phosphorylation signaling was blocked by the reticence of Ca2+/calmodulin-dependent protein kinase β, an upstream enhancer of AMPK, and by the mTORC1 blocker rapamycin, respectively. The impaired STAT3 phosphorylation in response to IL-10 observed in AMPKα1-deficient macrophages was accompanied by a decreased suppressor of inflammatory signaling 3 expression and the insufficiency of IL-10 to suppress LPS-induced pro-inflammatory cytokine production (Table 3) [200].
17. EFFECT OF AMPK ON CYCLOOXYGENASES FAMILY MEMBERS
Eicosanoids and endocannabinoids, the metabolites of arachidonic acid, have miscellaneous functions in the regulation of immunity and inflammation. Arachidonic acid (AA) is one of the major polyunsaturated ω-6 fatty acids. AA is metabolized by three groups of oxygenases, namely lipoxygenases (LOX), cyclooxygenases (COX), and cytochrome P450s (CYP) to a number of biologically active eicosanoids, which lead to the productions of several inflammatory mRNA, including iNOS, leukotrienes, COX-1, COX-2, COX-3 and many more, which are responsible for several inflammatory diseases [201]. Inducible nitric oxide synthase (iNOS) derived nitric oxide (NO) and cyclooxygenase-2 (COX-2) often play a major role in initiating inflammatory cascade by the activation of prostaglandin E2 (PGE2) production, which is often connected with several diseases; therefore, blocking this pathway may be effective in preventing inflammation. When Lindenenyl acetate was given in the immortalized HPDL cell line, it blocked LPS-induced inflammatory NO, PGE2, IL-1β, TNF-α, IL-6 and IL-12 production via heme oxygenase-1 and AMPK-dependent pathways [202]. A study has shown that when Metformin (200 mg/kg) was applied to ischemia subjects, it diminished inflammatory cascade by reducing COX-2/β-actin signaling through the induction of AMPK-independent activation [203]. Osteoarthritis subjects often suffer more as the pathophysiology is really complex and the treatment approach is really difficult. When Berberine was applied on nitric oxide-induced rat chondrocyte, it down-regulated expressions of inducible nitric oxide synthase (iNOS), Type II collagen (Col II) at protein levels and caspase-3, which were accompanied by improved adenosine monophosphate-activated protein kinase (AMPK) phosphorylation and reduced phosphorylation of p38 mitogen-activated protein kinase (MAPK) (Table 3) [204].
18. EFFECT OF AMPK ON INFLAMMASOME
The inflammasome is a complex of proteins paradox that through the activity of caspase-1 and the downstream substrates gasdermin D, IL-18 and IL-1β execute an inflammatory form of cell death termed pyroptosis. Activation of this complex phenomenon sometimes involves the adaptation (Inflammasome Adaptor Protein Apoptosis-Associated Speck-Like Protein Containing CARD) and up-regulation of sensors, including NLRP3, NLRC4, NLRP1, AIM2, and pyrin, which are triggered by different stimulating factors such as infectious agents, xenobiotics and changes in cell homeostasis [205]. Cytokine secretion and the inhibition of inflammasomes can play a major role in preventing inflammatory cascades. Xanthohumol inhibited lipopolysaccharide (LPS)-induced acute lung injury by preventing the reduction of Txnip/NLRP3 inflammasome by up-regulating AMPK/GSK3β-Nrf2 signal axis [206]. Another experiment evaluated that quercetin, luteolin and epigallocatechin gallate (10mmol/L each) were found to be anti-inflammatory when applied on endothelial cells. These molecules reduced TXNIP and NLRP3-mediated inflammation and apoptosis with the regulation of AMPK [207]. Endoplasmic reticulum stress has been associated with various inflammatory markers, which further initiate apoptosis in normal cells; therefore, unwanted and immature cell death occurred. To prevent the endoplasmic reticulum (ER) stress and associated inflammation, endothelial cells were treated with high glucose (25 mmol/L) exposure, which resulted in higher levels of IL-1β and IL-6 production, which ultimately triggered TXNIP and NLRP3-mediated inflammasome. However, when Mangiferin (0.1, 1, 10 μmol/L) was applied on EA.hy926 cells, it prevented TXNIP and NLRP3 inflammasome activation via regulation of AMPK (Table 3) [208].
19. EFFECT OF AMPK ON OXIDATIVE STRESS
Free radicals, including reactive oxygen species and reactive nitrogen species, sometimes interact with the cell membrane, cell cytoplasm, mitochondria and genetic codes, leading to cell damage where local inflammatory cells are attracted and invited, which aggravates the situation. Antioxidant genes, on the contrary, play a vital role in protecting the cellular components and help in cell survival; therefore, these genes can fight against inflammatory cytokines either directly or indirectly. Induction of heme oxygenase, superoxide desmutase, catalase, Nrf-2 and glutathione can be a very innovative approach to preventing inflammatory diseases. A study suggested that Xanthohumol leads to AMPK activation via transcription of HO-1 mRNA in an LKB1-dependent manner and thus observed subsequent AMPK-mediated enhancement of the Nrf2/HO-1 response in MEF and CHO-ARE-Luc were cultured cells [209]. [209]. When a novel compound from Polygonum multiflorum (CRPE55IB) was applied in the culture of primary microglia of mice, it was found that CRPE55IB activated Nrf2-mediated anti-neuroinflammation and also influenced AMPK pathway in microglia (Table 3) [210].
20. EFFECT OF AMPK ON NUCLEAR FACTOR- κB
NF-κB is a prototypical transcription factor that regulates and processes several harmful inflammatory and pro-inflammatory signaling largely based on the function of NF-κB in the expression of pro-inflammatory genes including chemokines, cytokines, and adhesion molecules. Signaling involved with NF-κB is activated with either LPS or ILR or TNFR around the cell surface and further interacts with cytoplasmic Iκκ and finally triggers DNA prototypic components such as iNOS, IL, COX, TNF, INF, Caspase and many more. It has, therefore, been associated with several human diseases and end-stage organ failure, making it the possible “holy grail” as a target for new anti-inflammatory drugs and the prevention of chronic diseases [211]. NF-κB expression and related pathways are observed throughout the body. In the lungs, it triggers CXCL1 in response to LPS and TNF-α, which plays a central role in chronic obstructive pulmonary disease [212]. In the brain, it enhanced pro-inflammatory transcription factor NF-kB (p50/p65) complex and was significantly increased in NF-κB-sensitive messenger RNA (mRNA) and microRNA (miRNA)-linked signaling circuits. Therefore, a deficiency was noticed in the gene expression in the CNS, which was highly connected with inflammatory neuro-degeneration, i.e. altered innate-immune responses, amyloidogenesis, deficits in neurotrophic signaling and synaptogenesis, as well as the inability to clear self-aggregating waste material from the neuronal cell cytoplasm and parenchyma [213]. In the liver, this pathway is interrelated with Ang-II and MAPK, which involves hepatitis, hepatic fibrosis, cirrhosis and cancer in both alcoholic and non-alcoholic patients [20, 173]. In the kidney, it is associated with renal ischemia/reperfusion injury by mediated NLRP3 inflammasome and triggered by the NF-κB signaling [214]. A study suggested that 2,3,4',5-Tetrahydroxystilbene-2-O-β-d-glucoside (THSG), when applied (10, 20, 40, 80, and 100 µM) on LPS-induced microglia, ameliorated iNOS, COX-2, TNF-α, and IL-6 activation in microglia by inhibiting NF-κB and activating AMPK/Nrf2 pathways. The authors also demonstrated that the anti-inflammatory activities of THSG were also reversed by the application of an AMPK inhibitor [215]. Another study suggested that Resveratrol acts as AMPK activator by interacting with nuclear factor κ-B (NF-κB)-dependent cyclooxygenase (COX)-2 activation in LPS-treated RWA 264.7 macrophages; therefore, the inflammatory markers such as tumor necrosis factor (TNF)-α and TNF receptor 1 were down-regulated (Fig. 3) [12]. In cancer, inflammatory cells are floating around the tumor cells and tumor cells have the tendency to move where there is chronic inflammation. HepG2 hepatocarcinoma cells were treated with Luteolin (100µ/mol), and nuclear factor-κB modulation was observed due to the activation of the AMPK pathway (Table 3) [216]. However, frustrating results may sometimes show from a potent investigation. A study found that cultured human ASM cells were treated with Troglitazone and Rosiglitazone, which reduced several inflammatory cytokines such as IL-1β-induced release of IL-6, vascular endothelial growth factor (VEGF), TNF-α-induced release of eotaxin and regulated on activation, normal T expressed and released (RANTES), and the IL-4-induced release of eotaxin. However, the study also confirmed that the anti-inflammatory activity of Troglitazone was not achieved by PPARγ or AMPK although it was equally effective to treat Asthma when compared with Rosiglitazone (Tables 2 and 3) [217].
21. EFFECT OF AMPK ON DIABETESASSOCIATED INFLAMMATION
Several investigations have reported that diabetes is highly associated with inflammation. In the pancreas, T-cells and NK cells attack β-cells, which lead to loss of insulin production and the subjects permanently depend on outside insulin. In addition, excess sugar in the system triggers JNK and LDL, which further prompts inflammatory cascade, leading to insulin resistance. Further- more, free sugar induces reactive oxygen species, which is solely responsible for diabetic nephro- pathy; therefore, subjects have to depend on dialysis [26, 181]. Moreover, chronic diabetes is connected with diabetic neuropathy, diabetic car- diomyopathy, diabetic nephropathy and diabetic myopathy. It is also proved that all the destructive outcomes are highly associated with inflammatory cytokines in the diabetic environment; therefore, controlling diabetes can be an alternative approach to reducing inflammation. Reports have also evaluated that the introduction of AMPK in the system may control inflammatory markers, resulting in the improvement in the β-cells towards the secretion functioning insulin. Studies have suggested that Chlorogenic acid, an AMPK enhancer, significantly stimulated glucose uptake in skeletal muscle in Leprdb/db mice and Hepatoma HepG2 cell line. The treatment also inhibited hep- atic G6Pase expression and activity, diminished hepatic steatosis, and enhanced lipid profiles and skeletal muscle glucose uptake, which in turn, improved fasting glucose level, glucose tolerance, insulin sensitivity and dyslipidemia [218]. Anti- diabetic activity of Nigella sativa has been observed through the activation of the AMPK Pathway. In this investigation, N. sativa (2 g eq plant/kg) was given as an extract on Meriones shawi by daily intragastric gavage for 4 weeks and improved plasma lipid profile, insulin, leptin, and adiponectin levels were found through AMPK pathway (Tables 1 and 3) [219]. Metfor- min has been investigated in many studies and found as an AMPK activator. However, Metfor- min activates AMPK only at high, supra-pharmacological doses in vitro, while the effect in vivo could be indirectly mediated by other secondary mediators, such as metabolites derived from gut flora. A study correlated that Metformin could be indirectly mediated by its effect on gut flora which influences AMPK, Complex I and glycerophosphate dehydrogenase to inhibit hepatic gluconeogenesis, thereby, prevent ageing process and improve better life-span [220]. Besides, potent molecules such as quercetin and other flavonoids are poorly absorbed with their original form, hence, are extensively metabolized by the help of gut flora, which may explain their AMKP activa- ting properties forth worth [221].
22. EFFECT OF AMPK ON OBESITY ASSOCIATED INFLAMMATION
On the other hand, obesity is also associated with low-grade chronic inflammation. Reports have also indicated that adipose tissue in obesity is in a heightened state of inflammation. Macro- phages and T-cells can infiltrate adipose tissue and are found responsible for the mainstream of inflammatory cytokine production. Besides, an introduction of Foam cell by macrophage can aggravate the process; therefore, it has been extremely important to manage obesity as well as obesity-mediated inflammation [7, 222]. When C57BL/6 mice were fed with a high-fat diet, they were found with excess pro-inflammatory cyto- kines and macrophage infiltrations in adipose tissues which resulted in hyperlipidemia, decrea- sed insulin sensitivity and severe glucose into- lerance. Corosolic acid, an AMPK enhancer, was once applied on obese mice; inhibited IKKβ phosphorylation and down-regulation of the gene expressions of pro-inflammatory cytokines were observed. Along with histological evidence, pro- tein investigations showed an improved insulin signaling transduction by modification of Ser/Thr phosphorylation of IRS-1 and downstream Akt, which explains the enhanced insulin sensitivity in high fat diet-fed mice via AMPK pathways [223]. Adipocyte culture from animal or human subjects may be used as mimic in the laboratory to introduce anti-obesity drugs. To investigate the anti-inflammatory activity of Ursolic acid (UA) on 3T3-L1 adipocytes, 2.5 to 20 mM UA for 6 days was applied on the cultured cells and significant changes were observed. It was reported that the treatment suppressed preadipocyte differentiation and adipogenesis through LKB1/AMPK pathway. UA was also reversed by the AMPK siRNA, thereby improving PPARγ, C/EBP members and SREBP-1c (Table 3) [51].
23. EFFECT OF AMPK ON CARDIOVASCULAR- ASSOCIATED INFLAMMATION
Nowadays, chronic vascular disease has been pointed out as the main cause of coronary heart disease and cerebrovascular disease; therefore, preventing this scenario is vital. In the vascular system, excess accumulation and production of very-low-density lipoprotein, iNOS, xanthine oxidase, uric acid, creatine kinase-MB, α-troponin, B-type natriuretic peptide and free radicals often damage the cardiovascular system. Besides, mac- rophase aggregation and Foam cell accumulation trigger atherosclerosis, thrombosis, sudden heart attack and stroke. Inflammation in the cardiovas- cular system has been correlated with hyperten- sion, myocardial infarction and rheumatic myocar- ditis [23, 24]. Metformin and compound C (AICAR), as well as AMPK inducers, attenuated LPS-induced myocardial inflammation and heart injury when they were applied on mice. The investigation found reduced cardiac expression of pro-inflammatory cytokines including MPO, CK-MB, BNP, tumor necrosis factor alpha-α, interleu- kin-1β and interleukin-6 in endotoxin-challenged mice through AMPK involvement [224]. Another investigation suggested that AMPK activation could contribute to the regulation of immune res- ponses in the isolated heart through the inhibition of TLR4 activity, independent of circulatory immunity [225]. A study suggested that AMPK plays protective roles in atherosclerosis by reducing the inflammatory response as well as unwanted immune cell proliferation. When knockout mice were treated with Ghrelin (100 nM), it activated AMPK in primary endothelial cells which showed potential activity in atherosclerosis by reducing inflammatory and pro-inflammatory cytokines [226]. Endothelial dysfu- nction is strongly correlated with cardiovascular complications, especially in middle-aged and older subjects. To investigate the role of AMPK on the vascular system, Mangiferin (0.1, 1, 10 μmol/L) was applied on endothelial cells, which were treated with high glucose (25 mmol/L) exposure to induce ER stress and promoted ROS production. The investigation found that Mangiferin not only restored the loss of the mitochondrial membrane potential and inhibited caspase-3 activity but also inhibited ET-1 secretion and improved NO pro- duction when cells were exposed to high glucose through AMPK interaction (Table 3) [208].
24. LIMITATIONS WITH AMPK ENHANCERS
Every study has some drawbacks, whether it is related to methods or possible outcomes; therefore, no investigation can satisfy the reader’s hunger as groundbreaking reports are always expected. Besides, plant-derived direct phytonutrients or extracts are often blamed as they do not have an exact dose, have poor pharmacokinetic properties and show adverse events in higher doses. Similarly, synthetic AMPK inducers, such as AICAR or Resveratrol, have unfavorable pharmacokinetic properties (i.e., poor bioavailability, shorter half-life and highly effective concentration) and harsh metabolic complications, for instance, lactic acidosis and massive uric acid production [111, 237]. In addition, AICAR also interferes with other vital proteins and enzymes, such as S-adenosylhomo- cysteine hydrolase and glycogen phosphorylase. To counter this problem, the imidazopyridine derivative S27847, which is 7 times more potent than AICAR, has been introduced while considering AMPK activation. However, research is still not sufficiently developed to properly investigate both pharmacokinetic properties and pharmacodynamic interaction [238, 239]. Recent focus has been on Metformin as it controls both diabetes and obesity-associated inflammation; hence, a recent investigation links Metformin-induced activation of AMPK to induced biogenesis of Alzheimer’s amyloid in mouse brain. On the contrary, it is suspected that perhaps unpredictability in the efficiency of hepatic uptake by the OCT1 transporter may interact with amyloid protein family [240, 241]. Although small molecules help in the activation of AMPK quite effectively, they also suffer from poor bioavailability and overall inauspicious pharmacokinetic properties. A-769662 is the first selective molecule for the activation of AMPK and showed reasonable EC50 and the in-vivo EC50 inhibition, thus showing no advantage was reported in different AMPK activators. Besides, the safety profile of this molecule remains unknown. The discovery that A769662 triggers AMPK complexes and that utterlyen closes the 1-subunit can be a significant drawback for the potential therapeutic use of this compound of protein expressing the ubiquitous 1-subunit but not in tissue-like skeletal muscle with AMPK 2 complexes. Indeed, unlike Metformin, A769662 was not capable of stimulating glucose uptake in skeletal muscle [242, 243]. Many other promising components have performed effectively in different animal models and cell lines consistent with effects expected from an AMPK inducer and shown to be safe and efficacious in clinical studies involving obese subjects and diabetes patients. These might offer tremendous promise as a new novel treatment to counter chronic illnesses and inflammatory diseases.
CONCLUSION AND FUTURE DIRECTIONS
Our findings suggested that people are experiencing a more and more sedentary lifestyle, which causes child and adult obesity. Overweight subjects also suffer from osteoporosis, infertility, early aging, arthritis, hypertension, stroke and finally, premature death. As the prevalence of obesity is increasing with every passing moment, preventive measures should be taken. AMPK can be an alternative therapeutic approach to preventing and managing obesity. Our literature search also showed that blood glucose might be controlled by AMPK activation; therefore, AMPK inducers or enhancers can participate in fighting against uncontrolled sugar and insulin resistance. We have shown several biochemical pathways to understand the relationship between inflammation and AMPK. Low-grade inflammation is a relevant component in the etiology of many western, highly prevalent diseases such as obesity, diabetes and related metabolic syndrome thus any approach targeting this biological phenomenon could eventually represent an additional, valuable tool. Given the emerging role of AMPK in inflammatory pathways, interventions aimed to activate this kinase may help to ameliorate the harmful effect of low-grade inflammation in the development of type-2 diabetes mellitus, obesity and cardiovascular dysfunctions (Fig. 4). There has been noteworthy progress over the last few decades in identifying potential molecule direct AMPK activators, and we now clearly understand the mechanisms by which many selective drugs can work to activate the kinase. However, a key challenge for the future is to translate findings from the animal models and cell line-based investigations through to human physiology/pharmacology/pathology in order to show the best approaches for maximizing the therapeutic potential of targeting AMPK. It is suggested that along with AMPK enhancers, a little bit of exercise and controlling food habit may be beneficial to treat inflammatory disorders. Natural resources are encouraged over synthetics when AMPK enhancers are recommended.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ACE
Angiotensin-Converting Enzyme
- AMPK
AMP-Activated Protein Kinase
- AngII
Angiotensin II
- AOPP
Advanced Oxidation Protein Products
- AP
Activator Protein
- AT1R
Angiotensin II Receptor Type 1
- BW
Body Weight
- CHD
Coronary Heart Disease
- CK
Creatine Kinase
- COX
Cyclooxygenase
- CREB
cAMP Response Element Binding Protein
- CVDs
Cardiovascular Diseases
- DAG
Diacylglycerol Protein Kinase C
- ERK1/2
Extracellular Signal-Regulated Protein Kinases ½
- GLUT
Glucose Transporter Type
- HDL
High Density Lipoprotein
- HIF
Hypoxia Inducible Factor
- IL
Interleukin
- INF
Interferon
- iNOS
inducible Nitric Oxide Synthase
- LDL
Low Density Lipoprotein
- LFA
Lymphocyte Function-Associated Antigen
- LPS
Lipopolysaccharide
- MCP
Monocyte Chemoattractant Protein
- MMP
Matrix Metaloprotenase
- MPO
Myeloperoxidase
- NAC
N-Acetyl Cysteine
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NF
Nuclear Factor
- NOX
NADPH Oxidase
- PAI
Plasminogen Activator Protein
- PI3K
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase
- PKC
Protein Kinase C
- PPAR
Peroxisome Proliferator-Activated Receptor
- ROS
Reactive Oxygen Species
- STAT
Signal Transducer and Activator of Transcription
- TLR
Toll-Like Receptor
- TNF
Tumor Necrosis Factor
- TRAP
Thrombin Receptor-Activating Peptide
- UA
Uric Acid
- VSMC
Vascular Smooth Muscle Cells
- XO
Xanthine Oxidase
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bohanec M., Boshkoska B.M., Prins T.W., Kok E.J. SIGMO: a decision support system for identifica-tion of genetically modified food or feed products. Food Control. 2017;71:168–177. doi: 10.1016/j.foodcont.2016.06.032. [DOI] [Google Scholar]
- 2.Tosun J., Schaub S. Mobilization in the European Public Sphere: The struggle over genetically modified organisms. Rev. Policy Res. 2017;34(3):310–330. doi: 10.1111/ropr.12235. [DOI] [Google Scholar]
- 3.Baars J., Dannefer D., Phillipson C., Walker A. Aging, globalization and inequality: The new critical gerontology. 1st ed. CRC Press; 2016. [Google Scholar]
- 4.Mohib M.M., Hasan I., Chowdhury W.K., Chow-dhury N.U., Mohiuddin S., Sagor M.A.T., Reza H.M., Alam M.A. Role of angiotensin II in hepatic inflammation through MAPK pathway: A review. Hepatitis. 2016;2:2. [Google Scholar]
- 5.Hotamisligil G.S. Inflammation and metabolic disor-ders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Alam M.A., Chowdhury M.R.H., Jain P., Sagor M.A.T., Reza H.M. DPP-4 inhibitor sitagliptin pre-vents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol. Metab. Syndr. 2015;7(1):107. doi: 10.1186/s13098-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam P., Raka M.A., Khan S., Sarker J., Ahmed N., Nath P.D., Hasan N., Mohib M.M., Tisha A., Taher S.M.A. A clinical review of the effectiveness of tomato (Solanum lycopersicum) against cardiovascu-lar dysfunction and related metabolic syndrome. J. Herb. Med. 2018;16:100235. doi: 10.1016/j.hermed.2018.09.006. [DOI] [Google Scholar]
- 8.Lowe G.D. The relationship between infection, in-flammation, and cardiovascular disease: an overview. Ann. Periodontol. 2001;6(1):1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an in-tegrated signaling network. Ageing Res. Rev. 2012;11(2):230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Handschin C., Spiegelman B.M. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunnumakkara A.B., Sailo B.L., Banik K., Harsha C., Prasad S., Gupta S.C., Bharti A.C., Aggarwal B.B. Chronic diseases, inflammation, and spices: how are they linked? J. Transl. Med. 2018;16(1):14. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi C-O., Jeon B.T., Shin H.J., Jeong E.A., Chang K.C., Lee J.E., Lee D.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Resveratrol acti-vates AMPK and suppresses LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macro-phage cells. Anat. Cell Biol. 2011;44(3):194–203. doi: 10.5115/acb.2011.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie D.G., Schaffer B.E., Brunet A. AMPK: an energy-sensing pathway with multiple inputs and out-puts. Trends Cell Biol. 2016;26(3):190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy ho-meostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovelace E.S., Polyak S.J. Natural products as tools for defining how cellular metabolism influences cel-lular immune and inflammatory function during chronic infection. Viruses. 2015;7(12):6218–6232. doi: 10.3390/v7122933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gongol B., Marin T., Peng I-C., Woo B., Martin M., King S., Sun W., Johnson D.A., Chien S., Shyy J.Y-J. AMPKα2 exerts its anti-inflammatory effects through PARP-1 and Bcl-6. Proc. Natl. Acad. Sci. USA. 2013;110(8):3161–3166. doi: 10.1073/pnas.1222051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Zhong L., Wang F., Zhu H. Dissecting the role of AMP-activated protein kinase in human dis-eases. Acta Pharm. Sin. B. 2017;7(3):249–259. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park D.W., Jiang S., Tadie J-M., Stigler W.S., Gao Y., Deshane J., Abraham E., Zmijewski J.W. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol. Med. 2013;19(1):387–398. doi: 10.2119/molmed.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salminen A., Hyttinen J.M.T., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signal-ing and inflammation: impact on healthspan and lifespan. J. Mol. Med. (Berl.) 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taher S.M.A., Mohib M.M., Azam M.S., Rahman A., Tanmoy F.T., Chowdhury W.K., Chowdhury N.U., Reza H.M., Alam M.A. Angiotensin-II, a po-tent peptide, participates in the development of hepat-ic dysfunctions. Curr. Med. Chem. Immunol. Endocr. Metab. Agents. 2016;16(3):161–177. [Google Scholar]
- 21.Mohib M.M., Afnan K., Paran T.Z., Khan S., Sarker J., Hasan N., Hasan I., Sagor M.A.T. Bene-ficial role of citrus fruit polyphenols against hepatic dysfunctions: a review. J. Diet. Suppl. 2017;15(2):223–250. doi: 10.1080/19390211.2017.1330301. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury N.U., Farooq T., Abdullah S., Mahadi A.S., Hasan M.M., Paran T.Z., Hasan N., Mohib M.M., Sagor M.A.T., Alam M.A. Matrix metalloproteinases (MMP), a major responsible downstream signaling molecule for cellular damage-a review. Mol. Enz. Drug Tar. 2016;2:3. doi: 10.21767/2572-5475.10019. [DOI] [Google Scholar]
- 23.Sagor M.A.T., Tabassum N., Potol M.A., Alam M.A. Xanthine oxidase inhibitor, allopurinol, pre-vented oxidative stress, fibrosis, and myocardial dam-age in isoproterenol induced aged rats. Oxid. Med. Cell. Longev. 2015;2015: 478039. doi: 10.1155/2015/478039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagor M.A.T., Mohib M., Tabassum N., Ahmed I., Reza H. Fresh seed supplementation of Syzygium cumini attenuated oxidative stress, inflammation, fibrosis, iron overload, hepatic dysfunction and renal injury in acetaminophen induced rats. J. Drug Metab. Toxicol. 2016;7(2):1–10. [Google Scholar]
- 25.Alam M.A., Sagor A.T., Tabassum N., Ulla A., Shill M.C., Rahman G.M.S., Hossain H., Reza H.M. Caffeic acid rich Citrus macroptera peel powder supplementation prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. Clinical Phytoscience. 2018;4(1):14. doi: 10.1186/s40816-018-0074-y. [DOI] [Google Scholar]
- 26.Mohib M.M., Rabby S.F., Paran T.Z., Hasan M.M., Ahmed I., Hasan N., Sagor M.A.T., Mohiuddin S. Protective role of green tea on diabetic nephropathy- A review. Cogent Biol. 2016;2(1): 1248166 [Google Scholar]
- 27.Al-Amin M.M., Sultana R., Sultana S., Rahman M.M., Reza H.M. Astaxanthin ameliorates prenatal LPS-exposed behavioral deficits and oxidative stress in adult offspring. BMC Neurosci. 2016;17(1):11. doi: 10.1186/s12868-016-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush K.A., Farmer K.M., Walker J.S., Kirkham B.W. Reduction of joint inflammation and bone ero-sion in rat adjuvant arthritis by treatment with inter-leukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46(3):802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 29.Anderson L.A., Johnston B.T., Watson R.G., Mur-phy S.J., Ferguson H.R., Comber H., McGuigan J., Reynolds J.V., Murray L.J. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66(9):4975–4982. doi: 10.1158/0008-5472.CAN-05-4253. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 31.Braza M.S., van Leent M.M., Lameijer M., Sanchez-Gaytan B.L., Arts R.J., Pérez-Medina C., Conde P., Garcia M.R., Gonzalez-Perez M., Brahmachary M. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity. 2018;49(5):819–828. doi: 10.1016/j.immuni.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacotte S., Brun S., Muller S., Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2009;1173(1):310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 33.Hamminga E.A., van der Lely A-J., Neumann H.A., Thio H.B. Chronic inflammation in psoriasis and obesity: implications for therapy. Med. Hypotheses. 2006;67(4):768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Wong C.K., Ho C.Y., Li E.K., Lam C.W. Eleva-tion of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9(8):589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 35.Saevik Å.B., Åkerman A.K., Grønning K., Nermoen I., Valland S.F., Finnes T.E., Isaksson M., Dahlqvist P., Bergthorsdottir R., Ekwall O., Skov J., Nedrebø B.G., Hulting A.L., Wahlberg J., Svartberg J., Höybye C., Bleskestad I.H., Jørgen-sen A.P., Kämpe O., Øksnes M., Bensing S., Husebye E.S. Clues for early detection of autoim-mune Addison’s disease - myths and realities. J. Intern. Med. 2018;283(2):190–199. doi: 10.1111/joim.12699. [DOI] [PubMed] [Google Scholar]
- 36.Uslu-Beşli L., Kabasakal L., Sağer S., Cicik E., Asa S., Sönmezoğlu K. Orbital flourine-18-fluorodeoxyglucose positron emission tomography in patients with Graves’ disease for evaluation of active inflammation. Nucl. Med. Commun. 2017;38(11):964–970. doi: 10.1097/MNM.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 37.Aktas G., Sit M., Dikbas O., Erkol H., Altinordu R., Erkus E., Savli H. Elevated neutrophil-tolymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Rev Assoc Med Bras (1992) 2017;63(12):1065–1068. doi: 10.1590/1806-9282.63.12.1065. [DOI] [PubMed] [Google Scholar]
- 38.Christen U. Animal models of autoimmune hepatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1865(5):970–981. doi: 10.1016/j.bbadis.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Needell J.C., Brown M.N., Zipris D. Involvement of adipose tissue inflammation and dysfunction in vi-rus-induced type 1 diabetes. J. Endocrinol. 2018;238(1):61–75. doi: 10.1530/JOE-18-0131. [DOI] [PubMed] [Google Scholar]
- 40.George M.D., Østergaard M., Conaghan P.G., Em-ery P., Baker D.G., Baker J.F. Obesity and rates of clinical remission and low MRI inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2017;76(10):1743–1746. doi: 10.1136/annrheumdis-2017-211569. [DOI] [PubMed] [Google Scholar]
- 41.Shah A., Walker M., Burger D., Martin N., Ko-loski N., Jones M., Talley N. Link between celiac disease and inflammatory bowel disease. J. Clin. Gastroenterol. 2018;53(7):514–522. doi: 10.1097/MCG.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 42.Nocturne G., Mariette X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018;14(3):133–145. doi: 10.1038/nrrheum.2018.1. [DOI] [PubMed] [Google Scholar]
- 43.Clark K.E.N., Isenberg D.A. A review of inflamma-tory idiopathic myopathy focusing on polymyositis. Eur. J. Neurol. 2018;25(1):13–23. doi: 10.1111/ene.13357. [DOI] [PubMed] [Google Scholar]
- 44.Juergens L.J., Racké K., Tuleta I., Stoeber M., Juergens U.R. Anti-inflammatory effects of 1, 8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy. 2017;5:1–8. doi: 10.1016/j.synres.2017.08.001. [DOI] [Google Scholar]
- 45.Shalapour S., Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 2015;125(9):3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi K., Karin M. NF-κB, inflammation, im-munity and cancer: coming of age. Nat. Rev. Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 47.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 2011;13: e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo S., Dipietro L.A. Factors affecting wound heal-ing. J. Dent. Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X., Gao Y., Liu C., Ni H., Mulvihill M. Sub-stituted nicotinamide inhibitors of BTK and their preparation and use in the treatment of cancer, in-flammation and autoimmune disease. Google Patents, WO2015048662A3. 2018.
- 50.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and me-tabolism. Nat. Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y., Li Y., Zhao T., Wang Y., Sun C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS One. 2013;8(7): e70135. doi: 10.1371/journal.pone.0070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woods A., Azzout-Marniche D., Foretz M., Stein S.C., Lemarchand P., Ferré P., Foufelle F., Carling D. Characterization of the role of AMP-activated pro-tein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 2000;20(18):6704–6711. doi: 10.1128/MCB.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon S. -.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48(7): e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motoshima H., Goldstein B.J., Igata M., Araki E. AMPK and cell proliferation- AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 2006;574(Pt 1):63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., Schlattner U., Walli-mann T., Carling D., Hue L., Rider M.H. Insulin antagonizes ischemia-induced Thr172 phosphoryla-tion of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J. Biol. Chem. 2006;281(9):5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 56.Liu X.M., Peyton K.J., Shebib A.R., Wang H., Korthuis R.J., Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am. J. Physiol. Heart Circ. Physiol. 2011;300(1):H84–H93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park C.E., Yun H., Lee E-B., Min B-I., Bae H., Choe W., Kang I., Kim S-S., Ha J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induc-tion in prostate cancer cells. J. Med. Food. 2010;13(4):815–820. doi: 10.1089/jmf.2009.1359. [DOI] [PubMed] [Google Scholar]
- 58.Mori A., Ishikawa E., Amano T., Sakamoto K., Nakahara T. Anti-diabetic drug metformin dilates retinal blood vessels through activation of AMP-activated protein kinase in rats. Eur. J. Pharmacol. 2017;798:66–71. doi: 10.1016/j.ejphar.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Herzig S., Shaw R.J. AMPK: guardian of metabo-lism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S.H., Hur H.J., Yang H.J., Kim H.J., Kim M.J., Park J.H., Sung M.J., Kim M.S., Kwon D.Y., Hwang J-T. Citrus junos tanaka peel extract exerts antidia-betic effects via AMPK and PPAR-both in vitro and in vivo in mice fed a high-fat diet. Evid. Based Complement. Alternat. Med. 2013;2013: 921012. doi: 10.1155/2013/921012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H.J., Jang D-J., Hwang J-T. Anti-diabetic effects of Korean red pepper via AMPK and PPAR-γ activation in C2C12 myotubes. J. Funct. Foods. 2012;4(2):552–558. doi: 10.1016/j.jff.2012.02.016. [DOI] [Google Scholar]
- 62.Lu J., Chen X., Zhang Y., Xu J., Zhang L., Li Z., Liu W., Ouyang J., Han S., He X. Astragalus poly-saccharide induces anti-inflammatory effects depend-ent on AMPK activity in palmitate-treated RAW264.7 cells. Int. J. Mol. Med. 2013;31(6):1463–1470. doi: 10.3892/ijmm.2013.1335. [DOI] [PubMed] [Google Scholar]
- 63.Bai T., Yang Y., Wu Y-L., Jiang S., Lee J.J., Lian L-H., Nan J-X. Thymoquinone alleviates thioacetam-ide-induced hepatic fibrosis and inflammation by ac-tivating LKB1-AMPK signaling pathway in mice. Int. Immunopharmacol. 2014;19(2):351–357. doi: 10.1016/j.intimp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Cheng H-L., Kuo C-Y., Liao Y-W., Lin C-C. EMCD, a hypoglycemic triterpene isolated from Momordica charantia wild variant, attenuates TNF-α-induced inflammation in FL83B cells in an AMP-activated protein kinase-independent manner. Eur. J. Pharmacol. 2012;689(1-3):241–248. doi: 10.1016/j.ejphar.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M., Lv X., Li J., Meng Z., Wang Q., Chang W., Li W., Chen L., Liu Y. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol. Cell. Endocrinol. 2012;363(1-2):122–130. doi: 10.1016/j.mce.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han J.Y., Park S.H., Yang J.H., Kim M.G., Cho S.S., Yoon G., Cheon S.H., Ki S.H. Licochalcone suppresses LXRα-induced hepatic lipogenic gene ex-pression through AMPK/Sirt1 pathway activation. Toxicol. Res. 2014;30(1):19–25. doi: 10.5487/TR.2014.30.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam J.S., Chung H.J., Jang M.K., Jung I.A., Park S.H., Cho S.I., Jung M.H. Sasa borealis extract ex-erts an antidiabetic effect via activation of the AMP-activated protein kinase. Nutr. Res. Pract. 2013;7(1):15–21. doi: 10.4162/nrp.2013.7.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Lee J-W., Choe S.S., Jang H., Kim J., Jeong H.W., Jo H., Jeong K-H., Tadi S., Park M.G., Kwak T.H., Man Kim J., Hyun D.H., Kim J.B. AMPK activation with glabridin ameliorates adiposi-ty and lipid dysregulation in obesity. J. Lipid Res. 2012;53(7):1277–1286. doi: 10.1194/jlr.M022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu K.N., Jiang C.H., Tian Y.S., Xiao N., Wu Z.F., Ma Y.L., Lin Z., Fang S.Z., Shang X.L., Liu K., Zhang J., Liu B.L., Yin Z.Q. Two triterpeniods from Cyclocarya paliurus (Batal) Iljinsk (Juglan-daceae) promote glucose uptake in 3T3-L1 adipo-cytes: The relationship to AMPK activation. Phytomedicine. 2015;22(9):837–846. doi: 10.1016/j.phymed.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 70.Kang O-H., Shon M-Y., Kong R., Seo Y-S., Zhou T., Kim D-Y., Kim Y-S., Kwon D-Y. Anti-diabetic effect of black ginseng extract by augmenta-tion of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017;17(1):341. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi L., Zhang T., Liang X., Hu Q., Huang J., Zhou Y., Chen M., Zhang Q., Zhu J., Mi M. Di-hydromyricetin improves skeletal muscle insulin re-sistance by inducing autophagy via the AMPK signal-ing pathway. Mol. Cell. Endocrinol. 2015;409:92–102. doi: 10.1016/j.mce.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Kim H., Choung S-Y. Anti-obesity effects of Bous-singaulti gracilis Miers var. pseudobaselloides Bailey via activation of AMP-activated protein kinase in 3T3-L1 cells. J. Med. Food. 2012;15(9):811–817. doi: 10.1089/jmf.2011.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z.Q., Zhang X.H., Yu Y., Tipton R.C., Raskin I., Ribnicky D., Johnson W., Cefalu W.T. Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by en-hancing hepatic insulin and AMPK signaling inde-pendently of FGF21 pathway. Metabolism. 2013;62(9):1239–1249. doi: 10.1016/j.metabol.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X., Wang F., Yang H., Chen J., Ren Y., Yu-an Z., Wang X., Wang Y. Selenium enriched ex-opolysaccharides produced by Enterobacter cloacae Z0206 alleviate adipose inflammation in diabetic KKAy mice through the AMPK/SirT1 pathway. Mol. Med. Rep. 2014;9(2):683–688. doi: 10.3892/mmr.2013.1859. [DOI] [PubMed] [Google Scholar]
- 75.Chao C-L., Huang H-C., Lin H-C., Chang T-C., Chang W-L. Sesquiterpenes from Baizhu stimulate glucose uptake by activating AMPK and PI3K. Am. J. Chin. Med. 2016;44(5):963–979. doi: 10.1142/S0192415X16500531. [DOI] [PubMed] [Google Scholar]
- 76.Zha Q-B., Zhang X-Y., Lin Q-R., Xu L-H., Zhao G-X., Pan H., Zhou D., Ouyang D-Y., Liu Z-H., He X-H. Cucurbitacin E induces autophagy via downregulating mTORC1 signaling and upregu-lating AMPK activity. PLoS One. 2015;10(5): e0124355. doi: 10.1371/journal.pone.0124355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leem K-H., Kim M-G., Hahm Y-T., Kim H.K. Hypoglycemic effect of Opuntia ficus-indica var. saboten is due to enhanced peripheral glucose uptake through activation of AMPK/p38 MAPK pathway. Nutrients. 2016;8(12):800. doi: 10.3390/nu8120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi B.K., Kim T.W., Lee D.R., Jung W.H., Lim J.H., Jung J.Y., Yang S.H., Suh J.W. A polymeth-oxy flavonoids-rich Citrus aurantium extract amelio-rates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. Phytother. Res. 2015;29(10):1577–1584. doi: 10.1002/ptr.5415. [DOI] [PubMed] [Google Scholar]
- 79.Guo L., Zheng X., Liu J., Yin Z. Geniposide sup-presses hepatic glucose production via AMPK in HepG2 cells. Biol. Pharm. Bull. 2016;39(4):484–491. doi: 10.1248/bpb.b15-00591. [DOI] [PubMed] [Google Scholar]
- 80.Muanprasat C., Wongkrasant P., Satitsri S., Moon-wiriyakit A., Pongkorpsakol P., Mattaveewong T., Pichyangkura R., Chatsudthipong V. Activation of AMPK by chitosan oligosaccharide in intestinal epi-thelial cells: Mechanism of action and potential appli-cations in intestinal disorders. Biochem. Pharmacol. 2015;96(3):225–236. doi: 10.1016/j.bcp.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Song C-Y., Shi J., Zeng X., Zhang Y., Xie W-F., Chen Y-X. Sophocarpine alleviates hepatocyte stea-tosis through activating AMPK signaling pathway. Toxicol. In Vitro. 2013;27(3):1065–1071. doi: 10.1016/j.tiv.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 82.Sung B., Chung J.W., Bae H.R., Choi J.S., Kim C.M., Kim N.D. Humulus japonicus extract exhibits antioxidative and anti-aging effects via modulation of the AMPK-SIRT1 pathway. Exp. Ther. Med. 2015;9(5):1819–1826. doi: 10.3892/etm.2015.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tu Z., Moss-Pierce T., Ford P., Jiang T.A. Rose-mary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J. Agric. Food Chem. 2013;61(11):2803–2810. doi: 10.1021/jf400298c. [DOI] [PubMed] [Google Scholar]
- 84.Iseli T.J., Turner N., Zeng X-Y., Cooney G.J., Kraegen E.W., Yao S., Ye Y., James D.E., Ye J-M. Activation of AMPK by bitter melon triterpenoids involves CaMKKβ. PLoS One. 2013;8(4): e62309. doi: 10.1371/journal.pone.0062309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J., Deng X., Liu N., Li M., Liu B., Fu Q., Qu R., Ma S. Quercetin attenuates tau hyperphos-phorylation and improves cognitive disorder via sup-pression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods. 2016;22:463–476. doi: 10.1016/j.jff.2016.01.036. [DOI] [Google Scholar]
- 86.Wang D., Ma W., Wang F., Dong J., Wang D., Sun B., Wang B. Stimulation of Wnt/β-Catenin sig-naling to improve bone development by naringin via interacting with AMPK and Akt. Cell. Physiol. Biochem. 2015;36(4):1563–1576. doi: 10.1159/000430319. [DOI] [PubMed] [Google Scholar]
- 87.Zhang C., Hawley S., Zong Y., Hawley S.A., Zong Y., Li M., Wang Z., Gray A., Ma T., Cui J., Feng J.W., Zhu M., Wu Y-Q., Li T.Y., Ye Z., Lin S-Y., Yin H., Piao H-L., Hardie D.G., Lin S-C. Fructose-1, 6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawley S.A., Davison M., Woods A., Davies S.P., Beri R.K., Carling D., Hardie D.G. Characterization of the AMP-activated protein kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein ki-nase. J. Biol. Chem. 1996;271(44):27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 89.Day E.A., Ford R.J., Steinberg G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017;28(8):545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Hwang J-T., Kwon D.Y., Yoon S.H. AMP-activated protein kinase: a potential target for the dis-eases prevention by natural occurring polyphenols. N. Biotechnol. 2009;26(1-2):17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Crute B.E., Seefeld K., Gamble J., Kemp B.E., Witters L.A. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J. Biol. Chem. 1998;273(52):35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 92.Bendayan M., Londono I., Kemp B.E., Hardie G.D., Ruderman N., Prentki M. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron mi-croscopy. J. Histochem. Cytochem. 2009;57(10):963–971. doi: 10.1369/jhc.2009.954016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hudson E.R., Pan D.A., James J., Lucocq J.M., Hawley S.A., Green K.A., Baba O., Terashima T., Hardie D.G. A novel domain in AMP-activated pro-tein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr. Biol. 2003;13(10):861–866. doi: 10.1016/S0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 94.Peixoto C.A., Oliveira W.H., Araújo S.M.D.R., Nunes A.K.S. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol. 2017;298(Pt A):31–41. doi: 10.1016/j.expneurol.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Polekhina G., Gupta A., Michell B.J., van Denderen B., Murthy S., Feil S.C., Jennings I.G., Campbell D.J., Witters L.A., Parker M.W., Kemp B.E., Stapleton D. AMPK β subunit targets metabol-ic stress sensing to glycogen. Curr. Biol. 2003;13(10):867–871. doi: 10.1016/S0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 96.McBride A., Ghilagaber S., Nikolaev A., Hardie D.G. The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9(1):23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oakhill J.S., Chen Z-P., Scott J.W., Steel R., Cas-telli L.A., Ling N., Macaulay S.L., Kemp B.E. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl. Acad. Sci. USA. 2010;107(45):19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease pro-tein. Trends Biochem. Sci. 1997;22(1):12–13. doi: 10.1016/S0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 99.Carling D., Sanders M.J., Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 2008;32:S55–S59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 100.Kemp B.E., Oakhill J.S., Scott J.W. AMPK struc-ture and regulation from three angles. Structure. 2007;15(10):1161–1163. doi: 10.1016/j.str.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Bonen A., Han X-X., Habets D.D., Febbraio M., Glatz J.F., Luiken J.J. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007;292(6):E1740–E1749. doi: 10.1152/ajpendo.00579.2006. [DOI] [PubMed] [Google Scholar]
- 102.Morrison A., Yan X., Tong C., Li J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2011;301(3):H895–H902. doi: 10.1152/ajpheart.00137.2011. [DOI] [PubMed] [Google Scholar]
- 103.Sanders M.J., Grondin P.O., Hegarty B.D., Snowden M.A., Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davies S.P., Helps N.R., Cohen P.T., Hardie D.G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C α and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377(3):421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 105.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006;281(43):32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 106.Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 107.Brajenovic M., Joberty G., Küster B., Bouwmeester T., Drewes G. Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. J. Biol. Chem. 2004;279(13):12804–12811. doi: 10.1074/jbc.M312171200. [DOI] [PubMed] [Google Scholar]
- 108.Baas A.F., Boudeau J., Sapkota G.P., Smit L., Medema R., Morrice N.A., Alessi D.R., Clevers H.C. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22(12):3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hawley S.A., Pan D.A., Mustard K.J., Ross L., Bain J., Edelman A.M., Frenguelli B.G., Hardie D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 110.Momcilovic M., Hong S-P., Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006;281(35):25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 111.Srivastava R.A.K., Pinkosky S.L., Filippov S., Hanselman J.C., Cramer C.T., Newton R.S. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012;53(12):2490–2514. doi: 10.1194/jlr.R025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y-Q., Cheng X., Guo L-X., Mao C., Chen Y-J., Liu H-X., Xiao Q-C., Jiang S., Yao Z-J., Zhou G-B. Identification of an annonaceous acetogenin mimetic, AA005, as an AMPK activator and autophagy inducer in colon cancer cells. PLoS One. 2012;7(10): e47049. doi: 10.1371/journal.pone.0047049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Woodard J., Platanias L.C. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem. Biophys. Res. Commun. 2010;398(1):135–139. doi: 10.1016/j.bbrc.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sonntag A.G., Dalle Pezze P., Shanley D.P., Thedieck K. A modelling-experimental approach reveals insulin receptor substrate (IRS)-dependent regulation of adenosine monosphosphate-dependent kinase (AMPK) by insulin. FEBS J. 2012;279(18):3314–3328. doi: 10.1111/j.1742-4658.2012.08582.x. [DOI] [PubMed] [Google Scholar]
- 115.Teng H., Chen L., Fang T., Yuan B., Lin Q. Rb2 inhibits α-glucosidase and regulates glucose metabolism by activating AMPK pathways in HepG2 cells. J. Funct. Foods. 2017;28:306–313. doi: 10.1016/j.jff.2016.10.033. [DOI] [Google Scholar]
- 116.Xi Y., Wu M., Li H., Dong S., Luo E., Gu M., Shen X., Jiang Y., Liu Y., Liu H. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell. Physiol. Biochem. 2015;35(6):2349–2359. doi: 10.1159/000374037. [DOI] [PubMed] [Google Scholar]
- 117.Xue B., Yang Z., Wang X., Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7(10): e45990. doi: 10.1371/journal.pone.0045990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim M.S., Hur H.J., Kwon D.Y., Hwang J-T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012;358(1):127–134. doi: 10.1016/j.mce.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Hadrich F., Garcia M., Maalej A., Moldes M., Isoda H., Feve B., Sayadi S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016;151:167–173. doi: 10.1016/j.lfs.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 120.Krasner N.M., Ido Y., Ruderman N.B., Cacicedo J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. 2014;9(5): e97554. doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nunes A.K.S., Rapôso C., Rocha S.W.S., Barbosa K.P., Luna R.L., da Cruz-Höfling M.A., Peixoto C.A. Involvement of AMPK, IKβα-NFκB and eNOS in the sildenafil anti-inflammatory mechanism in a demyelination model. Brain Res. 2015;1627:119–133. doi: 10.1016/j.brainres.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 122.Soetikno V., Sari F.R., Sukumaran V., Lakshmanan A.P., Harima M., Suzuki K., Kawachi H., Watanabe K. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J. Nutr. Biochem. 2013;24(5):796–802. doi: 10.1016/j.jnutbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 123.Shang F., Zhang J., Li Z., Zhang J., Yin Y., Wang Y., Marin T.L., Gongol B., Xiao H., Zhang Y.Y., Chen Z., Shyy J.Y., Lei T. Cardiovascular protective effect of metformin and telmisartan: reduction of PARP1 activity via the AMPK-PARP1 cascade. PLoS One. 2016;11(3): e0151845. doi: 10.1371/journal.pone.0151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prieto-Hontoria P.L., Pérez-Matute P., Fernández-Galilea M., Alfredo Martínez J., Moreno-Aliaga M.J. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. Eur. J. Nutr. 2013;52(2):779–787. doi: 10.1007/s00394-012-0384-7. [DOI] [PubMed] [Google Scholar]
- 125.Niu Y., Li S., Na L., Feng R., Liu L., Li Y., Sun C. Mangiferin decreases plasma free fatty acids through promoting its catabolism in liver by activation of AMPK. PLoS One. 2012;7(1):e30782. doi: 10.1371/journal.pone.0030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S-H., Hwang J-T., Park H.S., Kwon D.Y., Kim M-S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013;439(1):66–70. doi: 10.1016/j.bbrc.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 127.Filippov S., Pinkosky S.L., Lister R.J., Pawloski C., Hanselman J.C., Cramer C.T., Srivastava R.A.K., Hurley T.R., Bradshaw C.D., Spahr M.A., Newton R.S. ETC-1002 regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMPK. J. Lipid Res. 2013;54(8):2095–2108. doi: 10.1194/jlr.M035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peterson J.M., Aja S., Wei Z., Wong G.W. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J. Biol. Chem. 2012;287(2):1576–1587. doi: 10.1074/jbc.M111.278333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guan F.Y., Gu J., Li W., Zhang M., Ji Y., Li J., Chen L., Hatch G.M. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 β-cells. Life Sci. 2014;107(1-2):42–49. doi: 10.1016/j.lfs.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 130.Tian S., Ge X., Wu K., Yang H., Liu Y. Ramipril protects the endothelium from high glucose-induced dysfunction through CaMKKβ/AMPK and heme oxygenase-1 activation. J. Pharmacol. Exp. Ther. 2014;350(1):5–13. doi: 10.1124/jpet.114.212928. [DOI] [PubMed] [Google Scholar]
- 131.Kenlan D., Rychahou P.G., Sviripa V., Watt D., Evers B.M. New potent AMPK activators against colorectal cancer stem cells. Proceedings: AACR 106th Annual Meeting, Philadelphia, PA, USA, April 18-22; 2015. [Google Scholar]
- 132.Wang S-T., Huang S-W., Kao J-K., Liang S-M., Chen Y-J., Chen Y-Y., Wu C-Y., Shieh J-J. Imiquimod-induced AMPK activation causes translation attenuation and apoptosis but not autophagy. J. Dermatol. Sci. 2015;78(2):108–116. doi: 10.1016/j.jdermsci.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 133.Li R., Zhou P., Guo Y., Lee J-S., Zhou B. Tris (1, 3-dichloro-2-propyl) phosphate induces apoptosis and autophagy in SH-SY5Y cells: involvement of ROS-mediated AMPK/mTOR/ULK1 pathways. Food Chem. Toxicol. 2017;100:183–196. doi: 10.1016/j.fct.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 134.Salminen A., Kauppinen A., Kaarniranta K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. J. Mol. Med. (Berl.) 2017;95(2):123–131. doi: 10.1007/s00109-016-1477-1. [DOI] [PubMed] [Google Scholar]
- 135.Lin V.C-H., Tsai Y-C., Lin J-N., Fan L-L., Pan M-H., Ho C-T., Wu J-Y., Way T-D. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J. Agric. Food Chem. 2012;60(25):6399–6407. doi: 10.1021/jf301499e. [DOI] [PubMed] [Google Scholar]
- 136.Law B.Y.K., Gordillo-Martínez F., Qu Y.Q., Zhang N., Xu S.W., Coghi P.S., Mok S.W.F., Guo J., Zhang W., Leung E.L.H., Fan X.X., Wu A.G., Chan W.K., Yao X.J., Wang J.R., Liu L., Wong V.K.W. Thalidezine, a novel AMPK activator, eliminates apoptosis-resistant cancer cells through energy-mediated autophagic cell death. Oncotarget. 2017;8(18):30077–30091. doi: 10.18632/oncotarget.15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen Y-C., Zeng X-Y., He Y., Liu H., Wang B., Zhou H., Chen J-W., Liu P-Q., Gu L-Q., Ye J-M., Huang Z.S. Rutaecarpine analogues reduce lipid accumulation in adipocytes via inhibiting adipogenesis/lipogenesis with AMPK activation and UPR suppression. ACS Chem. Biol. 2013;8(10):2301–2311. doi: 10.1021/cb4003893. [DOI] [PubMed] [Google Scholar]
- 138.Chang W-L., Hsu L-C., Leu W-J., Chen C-S., Guh J-H. Repurposing of nitroxoline as a potential anticancer agent against human prostate cancer: a crucial role on AMPK/mTOR signaling pathway and the interplay with Chk2 activation. Oncotarget. 2015;6(37):39806–39820. doi: 10.18632/oncotarget.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shukla K., Sonowal H., Saxena A., Ramana K.V., Srivastava S.K. Aldose reductase inhibitor, fidarestat regulates mitochondrial biogenesis via Nrf2/HO-1/AMPK pathway in colon cancer cells. Cancer Lett. 2017;411:57–63. doi: 10.1016/j.canlet.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng X., Kim J.Y., Ghafoory S., Duvaci T., Rafiee R., Theobald J., Alborzinia H., Holenya P., Fredebohm J., Merz K-H., Mehrabi A., Hafezi M., Saffari A., Eisenbrand G., Hoheisel J.D., Wölfl S. Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Mol. Oncol. 2016;10(6):806–824. doi: 10.1016/j.molonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao P., Dou Y., Chen L., Li L., Wei Z., Yu J., Wu X., Dai Y., Xia Y. SC-III3, a novel scopoletin derivative, induces autophagy of human hepatoma HepG2 cells through AMPK/mTOR signaling pathway by acting on mitochondria. Fitoterapia. 2015;104:31–40. doi: 10.1016/j.fitote.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 142.Li C., Zhang C., Zhou H., Feng Y., Tang F., Hoi M.P.M., He C., Ma D., Zhao C., Lee S.M.Y. Inhibitory effects of betulinic acid on LPS-induced neuroinflammation involve M2 microglial polarization via CaMKKβ-dependent AMPK activation. Front. Mol. Neurosci. 2018;11:98. doi: 10.3389/fnmol.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chowdhury W., Tisha A., Akter S., Zahur S., Hasan N. The role of arsenic on skin diseases, hair fall and inflammation: an immunological review and case studies. J. Clin. Exp. Dermatol. Res. 2017;8(384):2. doi: 10.4172/2155-9554.1000384. [DOI] [Google Scholar]
- 144.Sagor M.A.T., Reza H.M., Tabassum N., Sikder B., Ulla A., Subhan N., Hemayet H.M., Ashraful A.M. Supplementation of rosemary leaves (Rosmarinus officinalis) powder attenuates oxidative stress, inflammation and fibrosis in carbon tetrachloride (CCl4) treated rats. Curr. Nutr. Food Sci. 2016;12(4):288–295. doi: 10.2174/1573401312666160816154610. [DOI] [Google Scholar]
- 145.Toyokuni S., Okamoto K., Yodoi J., Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358(1):1–3. doi: 10.1016/0014-5793(94)01368-B. [DOI] [PubMed] [Google Scholar]
- 146.McCord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108(8):652–659. doi: 10.1016/S0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 147.Ren L-P., Chan S.M., Zeng X-Y., Laybutt D.R., Iseli T.J., Sun R-Q., Kraegen E.W., Cooney G.J., Turner N., Ye J-M. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS One. 2012;7(2): e30816. doi: 10.1371/journal.pone.0030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2007;22(Suppl. 1):S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 149.Sagor M.A.T., Reza H.M., Tabassum N., Rahman M.M., Alam M.A. Fresh bitter melon fruit (Momordica charantia) attenuated oxidative stress, fibrosis and renal injury in carbon tetrachloride treated rats. Dhaka Uni. J. Pharm. Sci. 2018;16(2):205–214. doi: 10.3329/dujps.v16i2.35258. [DOI] [Google Scholar]
- 150.Al-Amin M.M., Reza H.M., Saadi H.M., Mahmud W., Ibrahim A.A., Alam M.M., Kabir N., Saifullah A.R., Tropa S.T., Quddus A.H. Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur. J. Pharmacol. 2016;777:60–69. doi: 10.1016/j.ejphar.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 151.Chowdhury W., Arbee S., Debnath S., Bin Zahur S., Akter S. Potent role of antioxidant molecules in prevention and management of skin cancer. J. Clin. Exp. Dermatol. Res. 2017;8(3):1–7. [Google Scholar]
- 152.Duncan B.B., Schmidt M.I., Pankow J.S., Ballantyne C.M., Couper D., Vigo A., Hoogeveen R., Folsom A.R., Heiss G. Atherosclerosis risk in communities study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 153.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 154.Prattichizzo F., De Nigris V., Spiga R., Mancuso E., La Sala L., Antonicelli R., Testa R., Procopio A.D., Olivieri F., Ceriello A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018;41:1–17. doi: 10.1016/j.arr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 155.Yan S.F., Ramasamy R., Schmidt A.M. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008;4(5):285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 156.Prattichizzo F., De Nigris V., Mancuso E., Spiga R., Giuliani A., Matacchione G., Lazzarini R., Marcheselli F., Recchioni R., Testa R., La Sala L., Rippo M.R., Procopio A.D., Olivieri F., Ceriello A. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–181. doi: 10.1016/j.redox.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McDaniel M.L., Kwon G., Hill J.R., Marshall C.A., Corbett J.A. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 1996;211(1):24–32. doi: 10.3181/00379727-211-43950D. [DOI] [PubMed] [Google Scholar]
- 158.Biondi-Zoccai G.G., Abbate A., Liuzzo G., Biasucci L.M. Atherothrombosis, inflammation, and diabetes. J. Am. Coll. Cardiol. 2003;41(7):1071–1077. doi: 10.1016/S0735-1097(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 159.Hartge M.M., Unger T., Kintscher U. The endothelium and vascular inflammation in diabetes. Diab. Vasc. Dis. Res. 2007;4(2):84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 160.Stienstra R., Duval C., Müller M., Kersten S. PPARs, obesity, and inflammation. PPAR Research. 2007;2007:1–10. doi: 10.1155/2007/95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salvatore S.P., Chevalier J.M., Kuo S.F., Audia P.F., Seshan S.V. Kidney disease in patients with obesity: It is not always obesity-related glomerulopathy alone. Obes. Res. Clin. Pract. 2017;11(5):597–606. doi: 10.1016/j.orcp.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 162.Tikellis C., Thomas M.C., Harcourt B.E., Coughlan M.T., Pete J., Bialkowski K., Tan A., Bierhaus A., Cooper M.E., Forbes J.M. Cardiac inflammation associated with a Western diet is mediated via activation of RAGE by AGEs. Am. J. Physiol. Endocrinol. Metab. 2008;295(2):E323–E330. doi: 10.1152/ajpendo.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taniguchi Y., Yoshioka N., Nakata K., Nishizawa T., Inagawa H., Kohchi C., Soma G. Mechanism for maintaining homeostasis in the immune system of the intestine. Anticancer Res. 2009;29(11):4855–4860. [PubMed] [Google Scholar]
- 164.Forsythe L.K., Wallace J.M., Livingstone M.B.E. Obesity and inflammation: the effects of weight loss. Nutr. Res. Rev. 2008;21(2):117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 165.Dixon J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010;316(2):104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 166.Sagor M.A.T., Rabbi M.G., Rahman M.M., Islam M., Rahman M.T., Mohib M.M., Khan M.M.R., Alam M.A. Chronic kidney disease might lead to hepatic dysfunction on a chronic hypertensive rat model study. World J Pharm Res. 2015;4(11):1939–1956. [Google Scholar]
- 167.Mehta A., Patel J., Al Rifai M., Ayers C.R., Neeland I.J., Kanaya A.M., Kandula N., Blaha M.J., Nasir K., Blumenthal R.S., Joshi P.H. Inflammation and coronary artery calcification in South Asians: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Atherosclerosis. 2018;270:49–56. doi: 10.1016/j.atherosclerosis.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mathieu P., Lemieux I., Després J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 2010;87(4):407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 169.Chowdhury N.U., Tisha A., Sarker J., Nath P.D., Ahmed N., Abdullah S., Farooq T., Mahmud W., Mohib M.M., Sagor M.A.T. Targeting inducible Nitric Oxide Synthase (iNOS) in the prevention of vascular damage and cardiac inflammation. J. Angiotherapy. 2018;2(1):067–077. [Google Scholar]
- 170.Biddle M., Moser D., Song E.K., Heo S., Payne-Emerson H., Dunbar S.B., Pressler S., Lennie T. Higher dietary lycopene intake is associated with longer cardiac event-free survival in patients with heart failure. Eur. J. Cardiovasc. Nurs. 2013;12(4):377–384. doi: 10.1177/1474515112459601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lörchner H., Widera C., Hou Y., Elsässer A., Warnecke H., Giannitsis E., Hulot J-S., Braun T., Wollert K.C., Pöling J. Reg3β is associated with cardiac inflammation and provides prognostic information in patients with acute coronary syndrome. Int. J. Cardiol. 2018;258:7–13. doi: 10.1016/j.ijcard.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 172.Gan W., Ren J., Li T., Lv S., Li C., Liu Z., Yang M. The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(1):1–10. doi: 10.1016/j.bbadis.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 173.Reza H.M., Tabassum N., Sagor M.A.T., Chowdhury M.R.H., Rahman M., Jain P., Alam M.A. Angiotensin-converting enzyme inhibitor prevents oxidative stress, inflammation, and fibrosis in carbon tetrachloride-treated rat liver. Toxicol. Mech. Methods. 2016;26(1):46–53. doi: 10.3109/15376516.2015.1124956. [DOI] [PubMed] [Google Scholar]
- 174.Reza H.M., Sagor M.A.T., Alam M.A. Iron deposition causes oxidative stress, inflammation and fibrosis in carbon tetrachloride-induced liver dysfunction in rats. Bangladesh J. Pharmacol. 2015;10(1):152–159. doi: 10.3329/bjp.v10i1.21711. [DOI] [Google Scholar]
- 175.Szabo G., Mandrekar P., Dolganiuc A. Seminars in liver disease: 2007. Thieme Medical Publishers; 2007. Innate immune response and hepatic inflammation. pp. 339–350. [DOI] [PubMed] [Google Scholar]
- 176.Marzeea A.R., Abida T., Salma K., Tasfiq Z.P., Nowshin A., Mohammad M.M., Md A.T.S., Sarif M. Inhibitory role of resveratrol in the development of profibrogenesis and fibrosis mechanisms. Immunol. Endocr. Metab. Agents Med. Chem. 2018;18(1):80–104. doi: 10.2174/1871522218666180523102923. [DOI] [Google Scholar]
- 177.Sagor A.T., Chowdhury M.R.H., Tabassum N., Hossain H., Rahman M.M., Alam M.A. Supplementation of fresh ucche (Momordica charantia L. var. muricata Wild) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complement. Altern. Med. 2015;15(1):115. doi: 10.1186/s12906-015-0636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chowdhury M.R.H., Sagor M.A.T., Tabassum N., Potol M.A., Hossain H., Alam M.A. Supplementation of Citrus maxima peel powder prevented oxidative stress, fibrosis, and hepatic damage in carbon tetrachloride (CCl4) treated rats. Evid. Based Complement. Alternat. Med. 2015;2015: 598179. doi: 10.1155/2015/598179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Edens M.A., Kuipers F., Stolk R.P. Non‐alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obesity. Rev. 2009;10(4):412–419. doi: 10.1111/j.1467-789X.2009.00594.x. [DOI] [PubMed] [Google Scholar]
- 180.Tsochatzis E.A., Papatheodoridis G.V. Is there any progress in the treatment of non-alcoholic fatty liver disease? World J. Gastrointest. Pharmacol. Ther. 2011;2(1):1–5. doi: 10.4292/wjgpt.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mahmud L.S.M., Tisha A., Sagor A.T. A compre-hensive review on effective role of Apple polyphenols in the treatment of obesity, diabetes, and liver dys-functions with some possible molecular mechanisms. 2018.
- 182.Park K.M., Kim J.I., Ahn Y., Bonventre A.J., Bonventre J.V. Testosterone is responsible for en-hanced susceptibility of males to ischemic renal inju-ry. J. Biol. Chem. 2004;279(50):52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 183.Stenvinkel P., Wanner C., Metzger T., Heimbürger O., Mallamaci F., Tripepi G., Malatino L., Zoccali C. Inflammation and outcome in end-stage renal fail-ure: does female gender constitute a survival ad-vantage? Kidney Int. 2002;62(5):1791–1798. doi: 10.1046/j.1523-1755.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 184.Daemen M.A., de Vries B., Buurman W.A. Apop-tosis and inflammation in renal reperfusion injury. Transplantation. 2002;73(11):1693–1700. doi: 10.1097/00007890-200206150-00001. [DOI] [PubMed] [Google Scholar]
- 185.Lorenz G., Darisipudi M.N., Anders H-J. Canoni-cal and non-canonical effects of the NLRP3 inflam-masome in kidney inflammation and fibrosis. Nephrol. Dial. Transplant. 2014;29(1):41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 186.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 187.Sancho D., Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rutherford C., Speirs C., Williams J.J.L., Ewart M-A., Mancini S.J., Hawley S.A., Delles C., Viollet B., Costa-Pereira A.P., Baillie G.S., Salt I.P., Palm-er T.M. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci. Signal. 2016;9(453):ra109. doi: 10.1126/scisignal.aaf8566. [DOI] [PubMed] [Google Scholar]
- 189.Galic S., Fullerton M.D., Schertzer J.D., Sikkema S., Marcinko K., Walkley C.R., Izon D., Honey-man J., Chen Z-P., van Denderen B.J., Kemp B.E., Steinberg G.R. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 2011;121(12):4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stojanovic B., Milovanovic J., Arsenijevic A., Stojanovic B., Strazic Geljic I., Arsenijevic N., Jon-jic S., Lukic M.L., Milovanovic M. Galectin-3 defi-ciency facilitates TNF-α-dependent hepatocyte death and liver inflammation in MCMV infection. Front. Microbiol. 2019;10:185. doi: 10.3389/fmicb.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Means R.T. Anemia of renal failure/chronic kidney disease. Anemia in the Young and Old. Springer; 2019. pp. 147–156. [DOI] [Google Scholar]
- 192.Boeckel J-N., Perret M.F., Glaser S.F., Seeger T., Heumüller A.W., Chen W., John D., Kokot K.E., Katus H.A., Haas J., Lackner M.K., Kayvanpour E., Grabe N., Dieterich C., von Haehling S., Ebner N., Hünecke S., Leuschner F., Fichtlscherer S., Meder B., Zeiher A.M., Dimmeler S., Keller T. Identification and regulation of the long non-coding RNA Heat2 in heart failure. J. Mol. Cell. Cardiol. 2019;126:13–22. doi: 10.1016/j.yjmcc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 193.Jialal I., Chaudhuri A. Targeting inflammation to reduce ASCVD in type 2 diabetes. J. Diabetes Complications. 2019;33(1):1–3. doi: 10.1016/j.jdiacomp.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 194.Kim Y.M., Kim J.H., Park S.W., Kim H.J., Chang K.C. Retinoic acid inhibits tissue factor and HMGB1 via modulation of AMPK activity in TNF-α activated endothelial cells and LPS-injected mice. Atherosclerosis. 2015;241(2):615–623. doi: 10.1016/j.atherosclerosis.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 195.Hsu W-H., Chen T-H., Lee B-H., Hsu Y-W., Pan T-M. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014;64:94–103. doi: 10.1016/j.fct.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 196.Dinarello C.A. Treatment of inflammatory diseases with IL-1 blockade. Curr. Otorhinolaryngol. Rep. 2018;6(1):1–14. doi: 10.1007/s40136-018-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Alaverdi N., Sehy D. Cytokines-master regulators of the immune system. eBioscience Archived from the original (PDF) on 2006, 03-15
- 198.Cudrici C.D., Pelletier M., Siegel R.M. 01.16 Amp-activated protein kinase: An anti-inflammatory target for methotrexate in macrophages. Ann. Rheum. Dis. 2017;76(Suppl. 1):A7–A8. [Google Scholar]
- 199.Kelly B., Tannahill G.M., Murphy M.P., O’Neill L.A. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreduc-tase to limit induction of IL-1β, and boosts IL-10 in LPS-activated macrophages. J. Biol. Chem. 2015:M115–M662114. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhu Y.P., Brown J.R., Sag D., Zhang L., Suttles J. Adenosine 5′-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J. Immunol. 2015;194(2):584–594. doi: 10.4049/jimmunol.1401024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nithipatikom K., Campbell W.B. Roles of eico-sanoids in prostate cancer. Future Lipidol. 2008;3(4):453–467. doi: 10.2217/17460875.3.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jeong G-S., Lee D-S., Li B., Kim J-J., Kim E-C., Kim Y-C. Anti-inflammatory effects of lin-denenyl acetate via heme oxygenase-1 and AMPK in human periodontal ligament cells. Eur. J. Pharmacol. 2011;670(1):295–303. doi: 10.1016/j.ejphar.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 203.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin acti-vates Nrf2 antioxidant pathways and inhibits inflam-matory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015;30(3):747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 204.Zhou Y., Liu S-Q., Yu L., He B., Wu S-H., Zhao Q., Xia S-Q., Mei H-J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015;20(9):1187–1199. doi: 10.1007/s10495-015-1152-y. [DOI] [PubMed] [Google Scholar]
- 205.Place D.E., Kanneganti T-D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu J., Xu X., Li Y., Kou J., Huang F., Liu B., Liu K. Quercetin, luteolin and epigallocatechin gal-late alleviate TXNIP and NLRP3-mediated inflamma-tion and apoptosis with regulation of AMPK in endo-thelial cells. Eur. J. Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 208.Song J., Li J., Hou F., Wang X., Liu B. Mangifer-in inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflam-masome activation with regulation of AMPK in endo-thelial cells. Metabolism. 2015;64(3):428–437. doi: 10.1016/j.metabol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 209.Zimmermann K., Baldinger J., Mayerhofer B., At-anasov A.G., Dirsch V.M., Heiss E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic. Biol. Med. 2015;88(Pt B):417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park S.Y., Jin M.L., Chae S.Y., Ko M.J., Choi Y.H., Park G., Choi Y-W. Novel compound from Polygonum multiflorum inhibits inflammatory re-sponse in LPS-stimulated microglia by upregulating AMPK/Nrf2 pathways. Neurochem. Int. 2016;100:21–29. doi: 10.1016/j.neuint.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 211.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1(6):a001651–a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Inui T., Watanabe M., Nakamoto K., Sada M., Hirata A., Nakamura M., Honda K., Ogawa Y., Takata S., Yokoyama T. Bronchial epithelial cells produce CXCL1 in response to LPS and TNFα: A po-tential role in the pathogenesis of COPD. Exp. Lung Res. 2019:1–9. doi: 10.1080/01902148.2018.1520936. [DOI] [PubMed] [Google Scholar]
- 213.McLachlan D.R.C., Bergeron C., Alexandrov P.N., Walsh W.J., Pogue A.I., Percy M.E., Kruck T.P.A., Fang Z., Sharfman N.M., Jaber V., Zhao Y., Li W., Lukiw W.J. Aluminum in Neurological and Neu-rodegenerative Disease. Mol. Neurobiol. 2019;56(2):1531–1538. doi: 10.1007/s12035-018-1441-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Zhao K., Wen L.B. DMF attenuates cisplatin-induced kidney injury via activating Nrf2 signaling pathway and inhibiting NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(24):8924–8931. doi: 10.26355/eurrev_201812_16662. [DOI] [PubMed] [Google Scholar]
- 215.Park S.Y., Jin M.L., Wang Z., Park G., Choi Y-W. 2,3,4′,5-tetrahydroxystilbene-2-O-β-d-glucoside exerts anti-inflammatory effects on lipopolysaccha-ride-stimulated microglia by inhibiting NF-κB and ac-tivating AMPK/Nrf2 pathways. Food Chem. Toxicol. 2016;97:159–167. doi: 10.1016/j.fct.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 216.Hwang J-T., Park O.J., Lee Y.K., Sung M.J., Hur H.J., Kim M.S., Ha J.H., Kwon D.Y. Anti-tumor ef-fect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int. J. Mol. Med. 2011;28(1):25–31. doi: 10.3892/ijmm.2011.667. [DOI] [PubMed] [Google Scholar]
- 217.Zhu M., Flynt L., Ghosh S., Mellema M., Banerjee A., Williams E., Panettieri R.A., Shore S.A. Anti-inflammatory effects of thiazolidinediones in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2011;45(1):111–119. doi: 10.1165/rcmb.2009-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ong K.W., Hsu A., Tan B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediat-ed by ampk activation. Biochem. Pharmacol. 2013;85(9):1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 219.Benhaddou-Andaloussi A., Martineau L., Vuong T., Meddah B., Madiraju P., Settaf A., Haddad P.S. The in vivo antidiabetic activity of Nigella sativa is medi-ated through activation of the AMPK pathway and in-creased muscle Glut4 content. Evid. Based Complement. Alternat. Med. 2011;2011: 538671. doi: 10.1155/2011/538671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Prattichizzo F., Giuliani A., Mensà E., Sabbatinelli J., De Nigris V., Rippo M.R., La Sala L., Procopio A.D., Olivieri F., Ceriello A. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res. Rev. 2018;48:87–98. doi: 10.1016/j.arr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 221.Gurău F., Baldoni S., Prattichizzo F., Espinosa E., Amenta F., Procopio A.D., Albertini M.C., Bonafè M., Olivieri F. Anti-senescence compounds: A po-tential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018;46:14–31. doi: 10.1016/j.arr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 222.Surmi B.K., Hasty A.H. Macrophage infiltration into adipose tissue: initiation, propagation and remodel-ing. Future Lipidol. 2008;3(5):545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang J., Leng J., Li J-J., Tang J.F., Li Y., Liu B-L., Wen X-D. Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine. 2016;23(2):181–190. doi: 10.1016/j.phymed.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 224.Liu G., Wu K., Zhang L., Dai J., Huang W., Lin L., Ge P., Luo F., Lei H. Metformin attenuated en-dotoxin-induced acute myocarditis via activating AMPK. Int. Immunopharmacol. 2017;47:166–172. doi: 10.1016/j.intimp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 225.Vaez H., Najafi M., Rameshrad M., Toutounchi N.S., Garjani M., Barar J., Garjani A. AMPK acti-vation by metformin inhibits local innate immune re-sponses in the isolated rat heart by suppression of TLR 4-related pathway. Int. Immunopharmacol. 2016;40:501–507. doi: 10.1016/j.intimp.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 226.Zhang M., Fang W-Y., Qu X-K., Yuan F., Wang W-G., Fei J., Wang Z-G. AMPK activity is down-regulated in endothelial cells of GHS-R(-/-) mice. Int. J. Clin. Exp. Pathol. 2013;6(9):1770–1780. [PMC free article] [PubMed] [Google Scholar]
- 227.Aoki C., Hattori Y., Tomizawa A., Jojima T., Ka-sai K. Anti-inflammatory role of cilostazol in vascu-lar smooth muscle cells in vitro and in vivo. J. Atheroscler. Thromb. 2010;17(5):503–509. doi: 10.5551/jat.3392. [DOI] [PubMed] [Google Scholar]
- 228.Lee Y.Y., Park J-S., Lee E-J., Lee S-Y., Kim D-H., Kang J.L., Kim H-S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lip-opolysaccharide-stimulated microglia: critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015;63(13):3472–3480. doi: 10.1021/jf506110y. [DOI] [PubMed] [Google Scholar]
- 229.Park S.Y., Jin M.L., Ko M.J., Park G., Choi Y-W. Anti-neuroinflammatory effect of emodin in LPS-stimulated microglia: involvement of AMPK/Nrf2 ac-tivation. Neurochem. Res. 2016;41(11):2981–2992. doi: 10.1007/s11064-016-2018-6. [DOI] [PubMed] [Google Scholar]
- 230.Chi Y., Li K., Yan Q., Koizumi S., Shi L., Takahashi S., Zhu Y., Matsue H., Takeda M., Kitamura M., Yao J. Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase. J. Pharmacol. Exp. Ther. 2011;339(1):257–266. doi: 10.1124/jpet.111.183020. [DOI] [PubMed] [Google Scholar]
- 231.He C., Li H., Viollet B., Zou M.H., Xie Z. AMPK suppresses vascular inflammation in vivo by inhibit-ing signal transducer and activator of transcription-1. Diabetes. 2015;64(12):4285–4297. doi: 10.2337/db15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Choi I-Y., Ju C., Anthony J.A.M., Lee D.I., Pra-ther P.L., Kim W-K. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway re-duces cerebral ischemic injury. Am. J. Pathol. 2013;182(3):928–939. doi: 10.1016/j.ajpath.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 233.Lee D-H., Lee T.H., Jung C.H., Kim Y-H. Wogonin induces apoptosis by activating the AMPK and p53 signaling pathways in human glioblastoma cells. Cell. Signal. 2012;24(11):2216–2225. doi: 10.1016/j.cellsig.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 234.Li Y., Yang J., Chen M-H., Wang Q., Qin M-J., Zhang T., Chen X-Q., Liu B-L., Wen X-D. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015;99:101–115. doi: 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 235.Vingtdeux V., Chandakkar P., Zhao H., d’Abramo C., Davies P., Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of au-tophagy and amyloid-β peptide degradation. FASEB J. 2011;25(1):219–231. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bess E., Fisslthaler B., Frömel T., Fleming I. Nitric oxide-induced activation of the AMP-activated pro-tein kinase α2 subunit attenuates IκB kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6(6): e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Goodyear L.J. The exercise pill--too good to be true? N. Engl. J. Med. 2008;359(17):1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 238.Fabianowska-Majewska K., Duley J.A., Simmonds H.A. Effects of novel anti-viral adenosine analogues on the activity of S-adenosylhomocysteine hydrolase from human liver. Biochem. Pharmacol. 1994;48(5):897–903. doi: 10.1016/0006-2952(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 239.Charton J., Girault-Mizzi S., Debreu-Fontaine M-A., Foufelle F., Hainault I., Bizot-Espiard J-G., Caignard D-H., Sergheraert C. Synthesis and biolog-ical evaluation of benzimidazole derivatives as potent AMP-activated protein kinase activators. Bioorg. Med. Chem. 2006;14(13):4490–4518. doi: 10.1016/j.bmc.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 240.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y-D., Ma T., Thompson R.C., Zhao Y., Smith L., Gaspa-rini L., Luo Z., Xu H., Liao F.F. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc. Natl. Acad. Sci. USA. 2009;106(10):3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jin H-E., Hong S-S., Choi M-K., Maeng H-J., Kim D-D., Chung S-J., Shim C-K. Reduced anti-diabetic effect of metformin and down-regulation of hepatic Oct1 in rats with ethynylestradiol-induced cholestasis. Pharm. Res. 2009;26(3):549–559. doi: 10.1007/s11095-008-9770-5. [DOI] [PubMed] [Google Scholar]
- 242.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H.S., Frevert E. Identification and characteri-zation of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3(6):403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 243.Scott J.W., van Denderen B.J.W., Jorgensen S.B., Honeyman J.E., Steinberg G.R., Oakhill J.S., Iseli T.J., Koay A., Gooley P.R., Stapleton D., Kemp B.E. Thienopyridone drugs are selective activators of AMP-activated protein kinase β1-containing com-plexes. Chem. Biol. 2008;15(11):1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]