Abstract
Human endothelial nitric oxide synthase (NOS3) gene polymorphism at Exon 7 (Glu298Asp) has been linked to vascular endothelial dysfunction, but the mechanisms are not defined. Shear is a key modulator of NOS3 function in vivo and association with caveolae is important for the control of NOS3 protein activity. Here we tested the hypothesis that altered enrichment of NOS3 in the caveolar membrane defines Glu298Asp genotype-specific responses and NOS3 activity. Basal caveolar membrane enrichment was carried out to quantitate the NOS3 enrichment in caveolae. Cells were subjected to shear and NOS3 protein levels, phosphorylation, enzyme function were investigated. Variant genotypes had lower NOx production pre- and post-shear, but no genotype-dependent alterations in pNOS3 were observed. Asp variants had significantly lower NOS3 enrichment in the caveolar membrane fraction. Further, immunoprecipitation studies demonstrated that Asp variants had substantially less NOS3/Cav-1 association (~40%) during static conditions. Furthermore, acute shear causes impaired NOS3/Cav-1 dissociation in Asp variants. The results from immuno-precipitation studies were in complete agreement with caveolar membrane preparation findings. Collectively, these data demonstrate functional consequences of the Glu298Asp NOS3 variation and further define disruption of NOS3 caveolar localization and shear-induced mobilization as the primary mechanism responsible for these differences.—Joshi, M. S., Mineo, C., Shaul, P. W., Bauer, J. A. Biochemical consequences of the NOS3 Glu298Asp variation in human endothelium: altered caveolar localization and impaired response to shear.
Keywords: caveolin-1, endothelial nitric oxide synthase, functional genomics, gene polymorphism, protein-protein interactions
The vascular endothelium plays a vital role in regulating local blood flow and hemostasis, and many studies have shown that endothelial dysfunction predisposes patients of all ages to a variety of cardiac and vascular disease states (1, 2). Of particular importance for normal endothelial function is the formation of nitric oxide (NO) via a constitutively expressed isoform of nitric oxide synthase (NOS3 or “endothelial NOS”) (3, 4). The expression and/or activity of the NOS3 enzyme in endothelial cells is influenced by a variety of local stimuli (hypoxia, shear, growth factors, and others) (3, 5, 6) and diminished NO availability has been associated with most settings of endothelial impairment. Although NO dysregulation in endothelial cells is a known contributor to many forms of cardiovascular disease (7–9), the mechanisms of this phenomenon and the initiating or predisposing factors are not well defined.
In recent years the complicated regulation of NOS3 in endothelial cells has begun to be ascertained. This regulatory cycle involves a series of post-translational protein modifications and intracellular compartmentalization to allow for coordinated substrate availability, calcium-dependent activation, enzyme catalysis, and NO release (10, 11). Of these steps, specific localization of NOS3 with caveolae, cholesterol, and glycosphingo-lipid-rich membrane microdomains in the plasma membrane is critical for enzyme regulation and activity (10, 11). In endothelial cells, caveolin-1 (Cav-1) is the major protein constituent of caveolae; this protein is thought to hold or “store” NOS3 in an inactive state wherein phosphorylation causes dissociation and coordinated NOS3 activation (11). Basal NOS3 bound to caveolin-1 is therefore essential for its activation, leading to controlled endothelial cell NO production (12).
Since the sequencing of the human NOS3 gene in the mid-1990s, several specific allelic variations have been identified and investigated for possible links to cardiovascular disease. In general, three classes of variations have been identified: those in intron regions, those in 5’-flanking DNA (promoter), and those within the open reading frame (13). A total of eight variations in the human NOS3 gene have been identified, although only one code for a variant form of the enzyme: a single nucleotide polymorphism (SNP) at position 894 (G>T), leading to the sequence change Glu298Asp (13). Using a “candidate gene” approach in which patient genotypes are related to the incidence of a disease and/or clinical outcome to test for statistically significant associations, many reports have indicated that NOS3 polymorphisms are related to increased occurrence of CVD, including coronary artery disease (14), myocardial infarction (15), hypertension (16), stroke (17), or coronary artery spasm (18). In particular, the Glu298Asp polymorphism has been most consistently implicated in these studies, and the T allele has also been linked to more severe outcomes or to a lesser level of recovery after an acute cardiovascular event (13).
The NOS3 Glu298Asp variation is fairly widespread in the human population, with 35–40% prevalence of the T allele in Caucasians and less prevalence in African Americans and Asians (13–18). The disease states most consistently implicating this SNP have typically been shown to involve endothelial dysfunction. However, specific evidence that the Glu298Asp variation causes a meaningful change in endothelial cell function or vulnerability has been lacking. Furthermore, the biochemical consequences of this single amino acid variation have not been defined. In recent studies from transfected bacteria, purified NOS3 enzymes containing the Glu298 vs. Asp298 sequence were found to be identical with respect to catalytic activity, suggesting that other aspects of NOS3 regulation may be affected (19). Other studies suggested that the Asp298 version was less stable in cellular homogenates (20), but these findings were later suggested to be a technical artifact (19). Since NOS3 is exquisitely regulated in its native endothelial cell environment, we postulated that the Glu298Asp variation might influence this regulation rather than affect the enzyme’s catalytic activity per se. To address this postulate, we developed a library of genotyped primary human endothelial cell isolates and investigated genotype-specific cellular characteristics including cell size, shape, and growth, basal NOS3 activities, changes in NOS3 expression and enzymatic activity in response to shear stimuli, and NOS3 localization in caveolae.
MATERIALS AND METHODS
General study design
Single donor human umbilical vein endothelial cells (ECs) were initially genotyped and maintained as individual cell lines throughout all phases of this study. In all cases, cell culture and stimulation conditions were identical for each genotyped cell preparation. All data shown are 9–15 replicate measurements from 3–5 individual cell preparations in each genotype.
NOS3 genotyping
Individual donor ECs were screened for NOS3 genotype and categorized as homozygous wild-type (Glu/Glu), heterozygous variant (Glu/Asp), and homozygous variant (Asp/Asp) using polymerase chain reaction-based DNA amplification, followed by restriction enzyme digestion (15). DNA was extracted from human umbilical vein endothelial cells. To detect Glu298Asp polymorphism of the NOS3 gene, we used primer pairs to amplify a part of the NOS3 gene containing exon 7 by polymerase chain reaction (PCR). Primer pairs for PCR were as follows: sense, 5’TCC CTG AGG AGG GCA TGA GGC T-3’; antisense, 5’TGA GGG TCA CAC AGG TTC CT-3’. Samples were amplified for 30 cycles consisting of denaturation at 94°C for 1 min, annealing at 61°C for 1 min, and extension at 72°C for 1 min. The resulting 457 bp amplification product was incubated at 37°C for 3 h with 10U of the restriction enzyme BanII (Roche, Nutley, NJ, USA). The amplified fragments were digested by BanII into smaller fragments (137 and 320 bp). In the case of a G to T substitution, a BanII restriction site is lost. The restricted fragments were separated on 6% TBE gels with ethidium bromide staining. Genotyping was also confirmed via direct sequencing.
Initial characterizations of genotyped cells
Unstimulated growth curve studies were carried out in ECM-2 medium. ECs were initially seeded in 96-well plates (1000 cells/well) and incubated at 37°C for up to 7 days. After each 24 h period, cells were fixed with formalin and the number of viable cells was determined via crystal violet staining assay, as described (21). Time-dependent cell data were fitted to the modified Gompertz equation for exponential cell growth to provide statistical comparisons of cell doubling time per Glu298Asp genotype (22).
Measurements of cell size were performed by digital image analysis and automated edge detection using research-based image analysis software (ImagePro Plus, Media Cybernetics, Silver Spring, MD, USA). Total protein and DNA content from cell homogenates were determined using the following.
RT-PCR and NOS3 mRNA
ECs cultured in a monolayer were lysed with Trizol reagent, then RNA was extracted and recovered by ethanol precipitation. RNA was dissolved in water and its concentration was determined by absorbance of an aliquot at 260 nm. RNA (5 µg) was reverse transcribed with AMV reverse transcriptase and deoxynucleotides at 42°C for 1 h. These reaction conditions were optimized for linear quantitation of the RT-PCR product. Resulting cDNA was amplified using primers specific for the NOS3 gene and β-actin; β -actin: sense primer, 5’-ATG GAT GAC GAT ATC GCT-3’ and antisense primer, 5’-ATG AGG TAG TCT GTC AGG T-3’ to produce a 538 bp product. The amplified PCR product was subjected to agarose electrophoresis and ethidium bromide staining. Gels were digitally photographed and signal intensities were quantified by image analysis (Image Pro). The ratio of NOS3 signal to β-actin for each sample was calculated.
Exposure of ECs to shear
ECs were grown to confluence in 6-well cell culture plates and subjected to physiologically relevant shear stress (20 dynes/cm2) for 4 h using a cone plate viscosimeter. Briefly, this assembly consists of a cone on a propeller rotating on top of a cell culture dish. Laminar shear stress was applied by a constant angular velocity in a humidified environment with 5% CO2 at 37°C. Each experiment was accompanied by parallel EC preparations under identical conditions and time intervals, but without propeller rotation, to serve as controls.
NOx production
Cellular NO production was assessed by indirect measurement of nitrate/nitrite in cell culture supernatant according to the manufacturer’s protocol (enzymatic nitric oxide assay kit; Oxford Biomedical Research, Oxford, MI, USA).
Caveolar fraction isolation
Caveolae membrane fractions were isolated by a previously described detergent-free method (23). The cells were washed in ice-cold buffer A (20 mM Tricine pH 7.8, 1 mM EDTA, and 250 mM sucrose) and collected by scraping in the same buffer. Cells were lysed by Dounce homogenization in buffer A containing protease inhibitor cocktails (Protease Inhibitor Cocktail Set III; Calbiochem, San Diego, CA, USA). A postnuclear supernatant fraction was prepared by spinning the lysate at 90 g for 10 min. The postnuclear supernatant was layered over 23 ml of ice-cold 30% Percoll (Amersham Biosciences, Arlington Heights, IL, USA) in buffer A. After centrifugation at 85,000 g for 30 min, the cytosol and plasma membrane fractions were collected. The plasma membrane fraction was briefly sonicated (six 50 Joule bursts at 10 W each), mixed with 50% w/v OptiPrep (Axis-Shield PoC As, Oslo, Norway) in buffer A plus 40 mM sucrose to a final OptiPrep concentration of 23%, and overlaid with 6 ml of linear (10–20%) gradient of OptiPrep in buffer A. Samples were centrifuged at 53,000 g for 90 min. The bottom 4 ml of the gradient was pooled and designated noncaveolae membrane. The top 5 ml of the gradient were mixed with 4 ml of buffer B, overlaid with 1 ml of 15% w/v OptiPrep in buffer A, followed by 0.5 ml of 5% w/v OptiPrep in buffer A. The gradients were centrifuged at 53,000 g for 90 min and caveolae were collected from the 5%/15% interface (0.5 ml).
Cell lysis and immunoprecipitation
After washing three times with ice-cold PBS, cells were lysed in Nonidet P-40 lysis buffer. The cell extracts were used for an immunoprecipitation reaction. A Protein G Immunoprecipitation Kit from Sigma (St. Louis, MO, USA) was used. The cell extracts were incubated overnight at 4°C, with NOS3 pAb on protein G agarose beads. The beads were washed thoroughly after incubation and a sample was prepared for SDS-PAGE. The blots were probed for Cav-1 protein. The amount of cav-1 associated with NOS3 was calculated as a ratio of caveolin-1 band optical density to NOS3 band optical density. The optical density analysis was carried out using Labworks image analysis software.
Western blot
Samples prepared as described above were separated on 4–20% Tris-glycine gel. After electrophoresis, the proteins were transferred onto nitrocellulose or PVDF membranes. The membranes were blocked with 5% nonfat milk in Tween 20 (0.1%)-Tris-buffered saline (10 mM Tris, pH 7.5; 150 mM NaCl) and incubated with primary antibody for 1 h at room temperature: anti-NOS3 rabbit polyclonal antibody for NOS3 basal and post-shear levels (Transduction Labs, Lexington, KY, USA), antiphosphoserine polyclonal antibody for phosphorylation status (Upstate Biotechnology, Lake Placid, NY, USA), anticaveolin-1 polyclonal antibody for caveolar membrane studies, and anti-NOS3 monoclonal antibody for caveolar association (Transduction Labs). HRP-conjugated secondary antibody (Vector Laboratories, Burlingame, CA, USA), enhanced chemiluminescence reaction (PerkinElmer Life Sciences, Norwalk, CT, USA), image capture using UVP EpiChemi darkroom system, and image analysis (ImagePro) were used to assess the relative amount of target at the appropriate molecular weight.
Statistical analysis
All data are expressed as mean ± se of 9–15 total replicates from 3–5 individuals for each genotype. Each cell line was treated as an individual sample. GraphPad Prism software was used to fit the data and carry out the statistical analysis. Comparisons between groups were performed by 1-way analyses of variance, with Student-Newman-Keuls post hoc testing. Analyses of relationships between variables were performed using Spearman’s nonparametric correlation analysis. In all cases, significance was defined as P < 0.05.
RESULTS
NOS3 Glu298Asp genotyping and initial characterizations of ECs
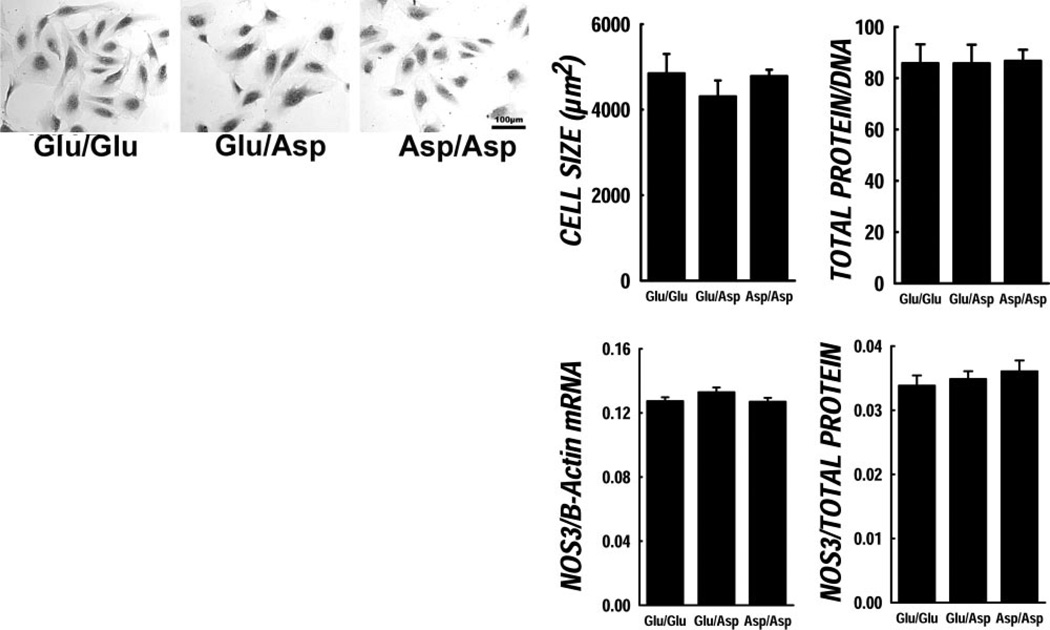
Initial studies were performed to compare genotype-specific cell characteristics regarding cellular morphology and growth properties under static basal conditions (data shown in Fig. 1). There were no obvious morphological differences among genotypes under light microscopy (see representative images in Fig. 1). Digital imaging methods also revealed no significant differences in cell size or shape. Protein/DNA ratio (a marker of cell hypertrophy) was not different among genotypes either, nor was unstimulated cell replication different among genotypes (doubling times; Glu/Glu 12±3.87 h; Glu/Asp 14.98±5.05 h; Asp/Asp 12.40±1.69 h, NS).
Figure 1.
Characterization of Glu298Asp genotyped cells. General characteristics of the genotyped ECs are shown here. Top: Representative images of ECs from the three NOS3 Glu298Asp genotypes. No apparent morphological differences were observed across the three genotypes. There were no significant differences in cell size or shape. Protein/DNA ratio was not different among genotypes either. Basal NOS3 mRNA and protein levels in the genotyped ECs were not significantly different.
Basal NOS3 protein and mRNA levels are not different
We investigated the total NOS3 mRNA and protein level genotyped ECs under basal unstimulated conditions. The basal NOS3 mRNA levels were not significantly different in the ECs with NOS3 Glu298Asp polymorphism, nor were NOS3 basal protein levels different in the genotyped ECs (data shown in Fig. 1).
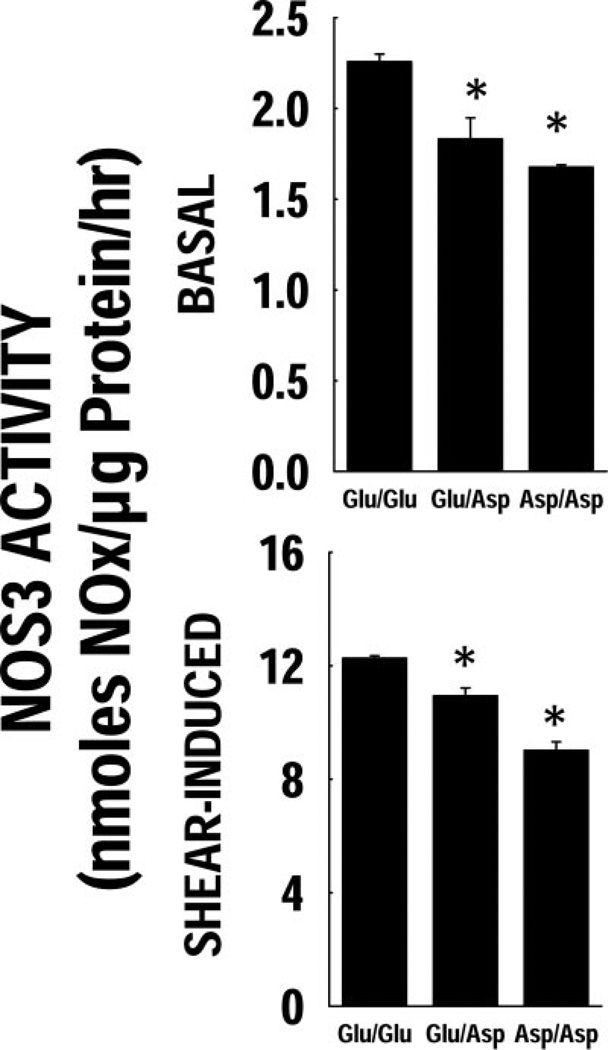
Basal and shear-induced NOS3 activity
Shown in Fig. 2 are effects of shear (20 dynes/cm2 for 4 h) on EC NOS3 activity. Basal NOx was diminished in the variant genotypes (Glu/Asp or Asp/Asp) compared with Glu/Glu (Fig. 2A). Treatment of Glu/Glu genotype cells with 4 h of shear caused a nearly 6-fold increase in apparent NOS3 activity, whereas this shear-induced activity was lower in the variant genotypes (Fig. 2B). In additional analyses, a statistically significant relationship (e.g., a positive correlation) was found between basal NOS3 activity and the extent of shear-induced NOS3 activation (Spearman’s correlation analysis, P <0.01)
Figure 2.
Cellular NOx production post-shear. The NOx production rate pre- and post-shear is shown. The basal NOx levels were significantly lower in the Asp variant ECs. There was a significant increase in the NOx levels post-high shear in all three genotypes. The extent of NOS3 activity induction post-shear was lower in the Asp variant cells.
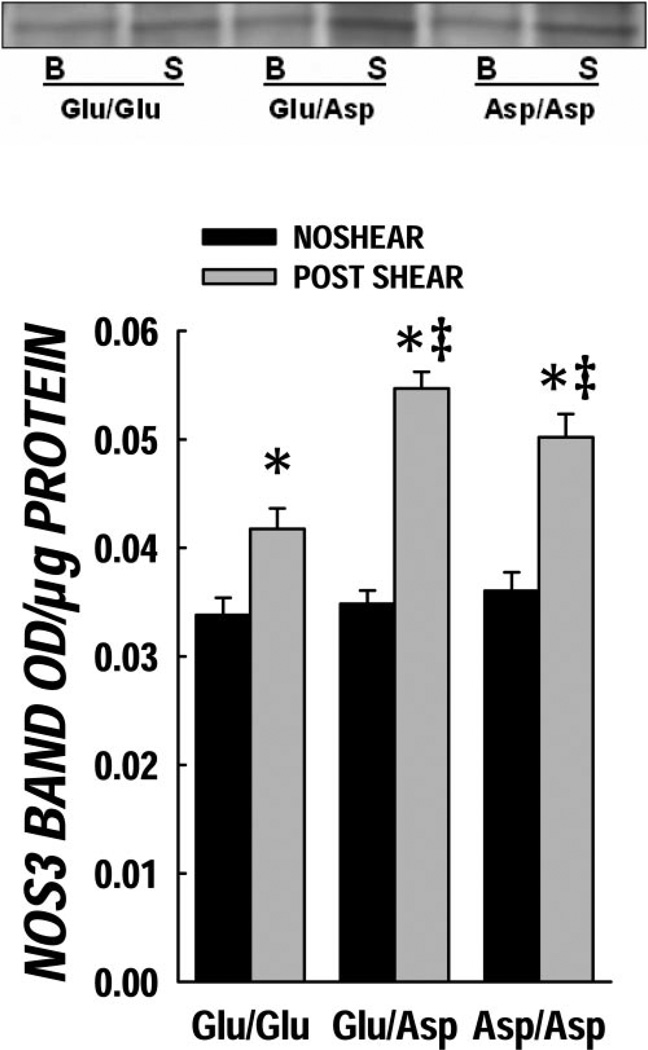
NOS3 protein levels
Total cellular NOS3 protein was determined in genotyped endothelial cells before and after 4 h of shear (Fig. 3). The Glu/Glu genotype had a slight but statistically significant increase in total NOS3 after shear stimulation; in contrast, the shear-induced effects were more pronounced in the variant genotypes (P < 0.05 compared with Glu/Glu). Relationships of total cellular NOS3 protein content (by Western blot) vs. NOx production post-shear response were investigated among the genotypes. No significant relationship was observed between these variables, demonstrating the importance of NOS3 regulation rather than total cellular content as the key determinant of enzyme activity.
Figure 3.
Cellular NOS3 protein content post-shear. The NOS3 protein levels pre- and post-shear are shown here. The basal NOS3 levels (black bars) were not different in the three genotypes. There was a significant increase in the NOS3 protein level post-high shear (gray bars). *P < 0.05 vs. basal level and ‡P < 0.05 vs. Glu/Glu (wild-type) post-shear.
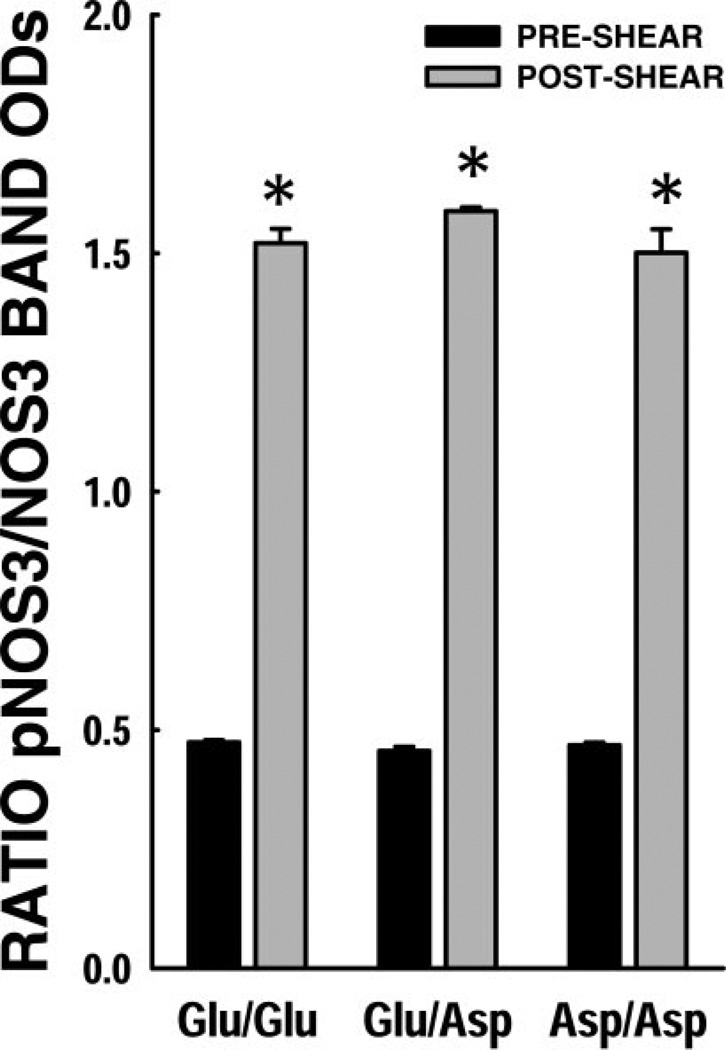
NOS3 phosphorylation status post-shear
Shown in Fig. 4 are shear effects on NOS3 phosphorylation status among the NOS3 genotypes. The basal phosphorylated NOS3 levels were not significantly different in the genotypes, whereas significant increases were observed in all three genotypes after 4 h of shear. No genotype-dependent differences in the extent of NOS3 phosphorylation were observed.
Figure 4.
NOS3 phosphorylation status post-shear. Ratio of pNOS3 band OD to NOS3 protein band OD was calculated as a measure of NOS3 phosphorylation. Basal NOS3 phosphorylation levels (black bars) were not significantly different among the NOS3 genotypes. There was significantly higher pNOS3 post-shear in these ECs (gray bars). No significant differences were observed across the NOS3 genotypes in terms of shear-induced NOS3 phosphorylation. *P < 0.05 compared to basal phosphorylation levels.
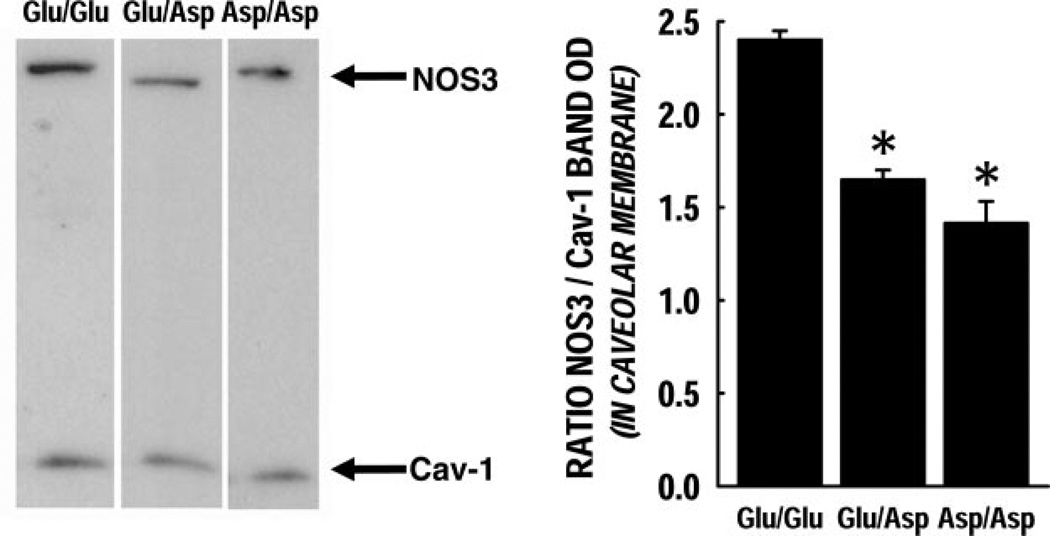
Caveolar membrane enrichment of NOS3
Caveolae membrane fractions were isolated and probed for NOS3 and Cav-1 protein content (Fig. 5). The NOS3 protein enrichment in the caveolar membrane preparation was significantly lower in the Glu/Asp and Asp/Asp genotypes compared with Glu/Glu (P < 0.05).
Figure 5.
Caveolar membrane enrichment in ECs under basal conditions. Caveolar membranes were isolated and the ratio of NO3 to Cav-1 in the membranes was calculated as their respective band optical densities. Each group had n = 3; these data are represented as mean ± se. The NOS3 protein enrichment in the caveolar membrane fraction was significantly lower in both Glu/Asp and Asp/Asp variants (P < 0.05).
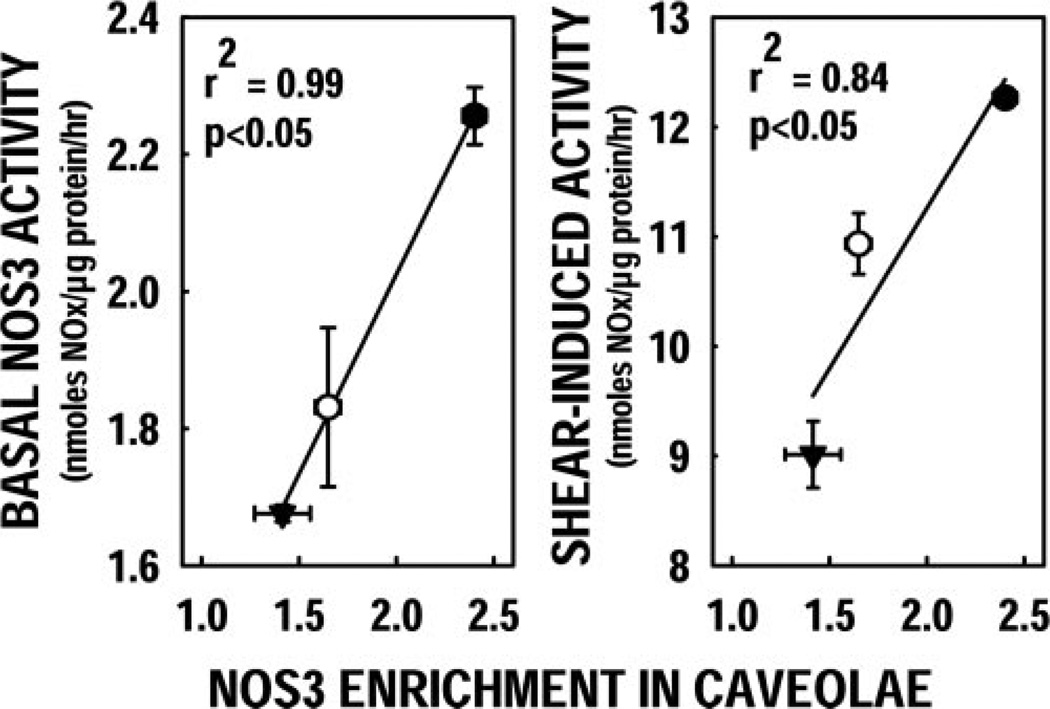
Using Spearman’s nonparametric correlation analysis, we investigated the relationships between NOS3 activity and key attributes of NOS3 protein status. The extent of NOS3 enrichment in caveolar fractions among NOS3 genotypes was also found to be correlated with the observed genotype-dependent variations in basal NOS activity (Fig. 6A) and with shear-induced NOS3 stimulation (Fig. 6B). In contrast, no other relationships were found to be statistically significant, including NOS3 activity vs. total NOS3 protein (pre- or post-shear) or NOS3 activity vs. NOS3 phosphorylation status (pre- or post-shear).
Figure 6.
Shear-dependent NOx production correlates with caveolar NOS3 enrichment. The extent of NOS3 enrichment in caveolar fractions correlated significantly with basal NOS3 activity and with shear-induced NOS3 stimulation.
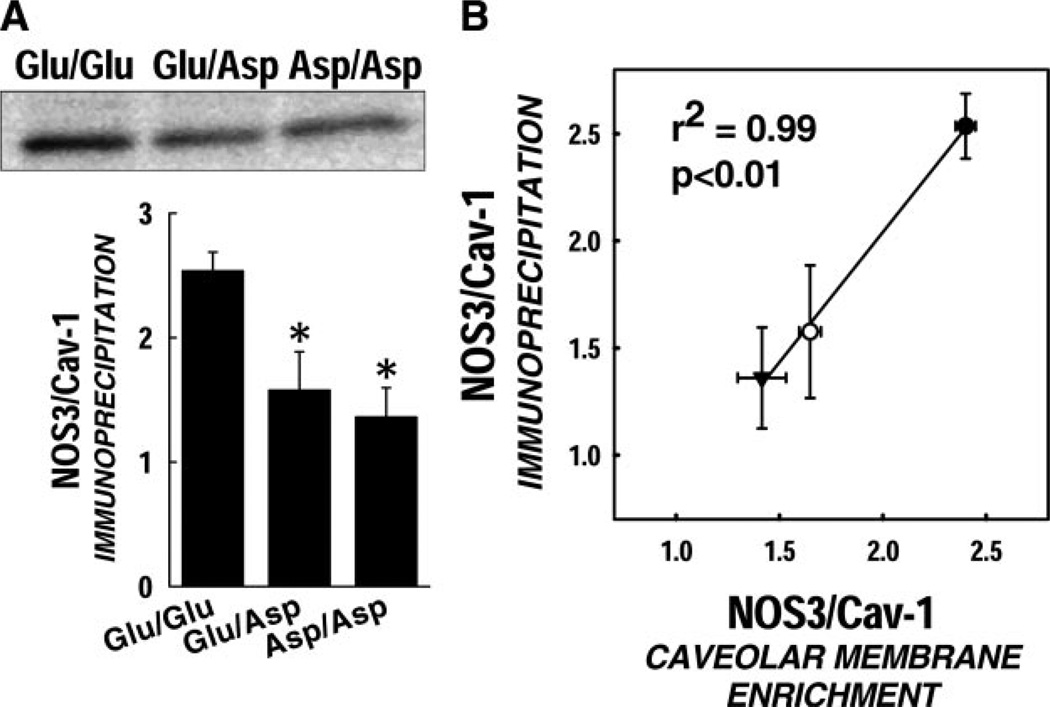
Caveolin-1 association of NOS3
NOS3 immunoprecipitation was carried out in cells exposed to high shear, and samples were then probed for the amount of Cav-1 associated with NOS3 protein. Under static conditions, the basal Cav-1/NOS3 association was significantly lower in the variant genotypes (P < 0.05) (Fig. 7A). Upon exposure to shear, the Cav-1/NOS3 complex is dissociated to a significantly greater extent in the Glu/Glu wild-type ECs compared with the Asp variants. We estimate that the actual amount of NOS3 mobilized during our shear conditions is ~30% less for Asp variants then for the Glu/Glu genotype. A significant correlation was observed between the amount of NOS3 in the caveolar membrane fraction and the Cav-1 association with NOS3 as detected by immunoprecipitation (P < 0.05) (Fig. 7B).
Figure 7.
Diminished Cav-1 and NOS3 association. A) Immunoprecipitation studies demonstrate that the amount of Cav-1 associated with NOS3 under static conditions is ~40% lower in the Asp variants. Data are represented as mean ± se (P < 0.05). B) Spearman’s correlation analysis detected a significant correlation between NOS3 enrichment in caveolar fraction and the Cav-1 association with NOS3 detected by immunoprecipitation.
DISCUSSION
Endothelial dysfunction is an important contributor to many forms of cardiovascular disease (2, 24). The mechanisms of endothelial dysfunction in these varied disease states are not defined, but the dysregulation of NOS3 has been consistently implicated as a contributing factor (24). Several clinical reports have shown a potential contribution of the NOS3 Glu298Asp mutation with respect to disease risk or poorer outcomes (15, 16). Here we investigated possible mechanistic aspects that could explain these clinical observations. One previous report by Tesauro et al. 20) demonstrated that the presence of the SNP at nucleotide 894 generates protein products with differing susceptibility to cleavage. Later, Fairchild et al. 19) carried out extensive functional studies documenting that Glu298Asp polymorphism in NOS3 does not influence the stability, half-life, or biological activity of the protein when isolated, and provided adequate cofactor availability (19). Perhaps this is not surprising since the amino acid position 298 is distant from the catalytic domain of the homodimer. In our studies, we emphasized an approach to study the impact of the Glu298Asp mutation in its native endothelial cell environment.
Although it is well known that exposure of endothelial cells to shear stress stimulates production of NO from NOS3 in both cultured cells and intact vessels (25), the molecular mechanisms by which shear stress regulates NO production have not been clearly elucidated and the key rate-limiting step (or steps) have not been defined. It has been shown that shear stress stimulates NO production from endothelial cells in two distinct phases: first, the Ca2+/CaM-dependent NO burst phase (lasting from seconds to ~30 min on shear initiation), followed by the Ca2+-independent phase (lasting as long as shear is imposed) in which NO production rate is maintained at a much reduced level (26, 27). Although conflicting reports exist, shear stress appears to induce a transient increase in intracellular free Ca2+ level. Upon exposure to increased shear stress, NOS3 has been shown to dissociate from caveolin-1 and to associate with CaM, activating the enzyme activity (12). During this first phase of NOS3 activation, phosphorylation of NOS3-S1179 also occurs and plays a critical role in early NO production. Initial evidence suggested that phosphorylation of NOS3 at Ser-1179 by a sequential activation of the PI3K and Akt pathway is the underlying mechanism by which shear stress stimulates NO production in a Ca2+/CaM-insensitive manner (28). Given the importance of shear as a controller of short-term NOS3 activity and longer term protein expression and localization, we used changes in endothelial cell shear environments to test for genotype-specific functional and mechanistic responses.
In our initial studies, we found that ECs possessing the variant NOS3 protein (Glu/Asp and Asp/Asp) were not morphologically different from the Glu/Glu genotype. Furthermore, cell growth under basal conditions was not altered, suggesting that this mutation does not cause overt perturbations of normal cellular function. This is consistent with the general clinical observations that genetic variation is fairly common among human population and may serve as a modifier of disease risk or progression rather than a specific disease mediator. Biochemical properties of the individual genotypes with respect to total NOS3 protein were not different. RT-PCR and Western blot showed that the steady-state levels of NOS3 message and NOS3 protein (in total homogenates) were identical among groups under basal static conditions. These observations suggest that the presence of this SNP does not affect NOS3 steady-state transcription in endothelial cells, and is consistent with previous reports in other cell types.
Using a well-defined setting of increased shear stress, we found that total NOS3 protein content is increased within 4 h at 20 dynes/cm2; others have shown that this change in protein content is primarily a transcriptional response rather than a change in protein stability (29). The degree of shear-induced NOS3 protein induction was statistically greater in the Glu/Asp and Asp/Asp genotypes, suggesting that the variant genotype may affect some components of the shear-mediated signaling process. In contrast to the observed increases in protein content, the variant genotype was associated with significant decreases in measured NOS3 activity. This key observation suggested there might be discordance between the total cellular content of NOS3 vs. activity of the enzyme with respect to NO production. Using homogenates from placental tissues, Wang et al. similarly found that NOS3 enzyme activities were decreased with the presence of the T allele (30). This decrease was more prominent for the NOS3 activities adjusted by NOS3 protein levels. The NOS3 activities in TT homozygotes and GT heterozygotes were only ~20% and 63% of the activities in GG homozygotes (30). This issue is readily apparent in our finding that the extent of shear-induced NO production was not statistically related to total cellular NOS3 among the genotypes investigated. This discordance likely is related to genotype-driven differences in NOS3 regulation, and supports the findings of others demonstrating that this protein is spatially and temporally regulated in endothelial cells.
After increased [Ca2+]i, NOS3 complexes with Ca2+/calmodulin and dissociates from caveolin-1, thereby increasing NOS3 activity and NO production (12). NOS3 is also regulated by phosphorylation mediated by the Ser/Thr kinase Akt. Selective phosphorylation of sites on the enzyme can either increase or decrease NOS3 activity; that is, phosphorylation of Ser-1179 increases NOS3 activity and sensitivity to Ca2+/calmodulin whereas phosphorylation at Thr497 negatively regulates NOS3 activity (28, 31, 32). In parallel experiments, we therefore investigated whether genotype-dependent shear effects were mediated by NOS3 phosphorylation status. Previous reports have shown that serine 1177 phosphorylation is a key step for full activity of the NOS3 dimer and that impaired phosphorylation limits shear responses in vitro. Recent studies by Boo et al. have also shown that endothelial NOS3 activity is coordinated via both phosphorylation and subcellular localization (33). We therefore investigated these potential components of the NOS3 regulatory cycle in relation to the Glu298Asp genotype. Using an antiphosphoserine antibody that detects both NOS3 phosphorylation sites, we found no evidence of genotype-dependent differences in protein phosphorylation under static conditions or after 4 h of shear. The only biochemical end point we measured that was found to statistically correlate with shear-induced NOS3 activity in our studies was the basal quantity of NOS3 associated with caveolar structures (e.g., not total cellular NOS3 protein or mRNA, phosphorylation status, or relative change in these end points under shear conditions). This finding is consistent with the likelihood that the sites of phosphorylation are distant from the variant position 298, and therefore are not influenced with respect to kinase or phosphatase binding affinities, etc. Although additional studies to address site-specific phosphorylation status may be warranted in order to define their potential contributions, the data available so far are internally consistent and strongly support our original hypothesis. Collectively, this series of observations supports a critical role for NOS3 caveolar localization (rather than total protein levels or phosphorylation status) as the primary controller of NO production in response to shear, and suggests that this aspect is altered in the Glu298Asp variant proteins.
NOS3 is enriched in caveolae, where it associates with caveolin-1 by interacting with the scaffolding domain located between amino acids 82 and 101. Binding of NOS3 to caveolin-1 in vitro holds the enzyme in an inactive state (34). NOS3 caveolin complex can be rapidly disrupted and subsequently restored after agonist activation. There is a dynamic cycle of NOS3 regulation in the intact endothelial cells. Previous reports in the literature have demonstrated that expression of caveolin-1 in endothelial cells is associated with inhibition of NO release (34). Based on these reports, we investigated the extent of caveolar NOS3 enrichment in our library of genotyped ECs. Significant reductions in NOS3 in caveolar membrane fractions were observed for the Glu/Asp and Asp/Asp variations compared with Glu/Glu. Our finding supports the potential importance of this difference that the extent of caveolar membrane enrichment was highly correlated with both basal and shear-induced NOS3 activity, whereas other biochemical attributes (total NOS3, phosphorylation status) were not related. By the immunoprecipitation studies carried out in cells before subjecting them to shear, we observed that under basal conditions Cav-1/NOS3 association was ~40% lower in the variant genotypes. Statistically significant correlation was also observed between the two independent methods employed, conducted in separate experiments by two different laboratories. Cav-1 association by immunoprecipitation and NOS3 enrichment in the caveolar membrane fraction were found to produce identical conclusions with respect to genotype-specific caveolar associations. These observations are consistent with other studies showing that Cav-1 is specifically confined to caveolar structures in endothelial cells and further support our assertion that the NOS3 association with Cav-1 is significantly altered in the variant genotypes. Moreover, upon exposure to high shear, the amount of NOS3 mobilized from Cav-1 was significantly higher in wild-type cells. The shear-dependent dissociation of NOS3 from Cav-1 was ~35% lower in the variant genotypes, which was similar in magnitude to the differences observed in shear-induced NOx production. Based on our observations, apparent differences in the endothelial cell NOS3 protein activity can therefore be mechanistically explained by deficient coordination at the caveolae, wherein a lesser basal association of NOS3 causes a less coordinated response to shear (despite the same total cellular NOS3 protein content). NOS3 localization at caveolae is therefore central for protein activity and endothelial cell shear-dependent response; furthermore, the NOS3 Glu/Asp variation apparently perturbs this key component of NOS3 regulation. NOS3 regulation is affected by many external stimuli, including estrogen, HDL, and caveolae endocytosis. Recent investigations have demonstrated that caveolae internalization is a major regulator of NOS3 activation in a Ca2+-independent manner (35). The altered interactions of variant NOS3 with caveolin-1 may also contribute to the NOS3 functional alterations due to caveolar internalization. The Glu298Asp variation does not occur in the enzyme active site, but is in the side chain region. This likely affects the NOS3 binding to Cav-1 and results in diminished basal enrichment in the caveolar fraction. We believe that the NOS3 variation primarily affects the first (Ca2+-dependent) phase and, to a lesser extent, the second (phosphorylation-dependent) phase of NOS3 activation. Ca2+/CaM complex is responsible for dissociation of NOS3 from its binding to Cav-1. Diminished binding of NOS3 to the caveolar fraction presents reduced pools of NOS3 available for Ca2+-dependent activation. Our studies show that the first step of NOS3 activation is substantially diminished in the variant genotypes, whereas once in the cytosolic pool, the phosphorylation status was not changed substantially among the NOS3 variants. Although the roles of phosphorylation/dephosphorylation may also contribute to some degree to our observed alterations in cellular responses, these may play a lesser role.
In summary, endothelial dysfunction is an important contributor to many disease settings; the NOS3 Glu298Asp mutation has been suggested to contribute in these settings, but the functional consequences have not been defined. Using a comprehensive approach to investigate the impact of this variation in intact primary endothelial cells, we have provided first-time evidence of NOS3 dysregulation in the variant genotypes (Glu/Asp and Asp/Asp) relative to the Glu/Glu genotype. The primary mechanistic difference was found to be an altered localization of the protein at caveolae leading to diminished shear-dependent responses and impaired coordination of the NOS3 regulatory cycle. These studies define the mechanistic basis for several clinical reports of increased cardiovascular disease risk with the presence of this fairly common NOS3 polymorphism. They also demonstrate the potential importance of protein interactions and subcellular localizations as potential mediators of functional genomics outcomes.
Acknowledgments
Supported in part by grants from the National Institutes of Health HL58888 (P.W.S.), HL63067 (J.A.B.), DK55053 (J.A.B.), and Columbus Children’s Research Institute.
REFERENCES
- 1.Aird WC. Endothelium as an organ system. Crit. Care Med. 2004;32:S271–S279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 2.Angus JA. Role of the endothelium in the genesis of cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 1996;23:S16–S22. doi: 10.1111/j.1440-1681.1996.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Andrews KL, Triggle CR, Ellis A. NO and the vasculature: where does it come from and what does it do? Heart Fail. Rev. 2002;7:423–445. doi: 10.1023/a:1020702215520. [DOI] [PubMed] [Google Scholar]
- 5.Busse R, Fleming I. Regulation and functional consequences of endothelial nitric oxide formation. Ann. Med. 1995;27:331–340. doi: 10.3109/07853899509002586. [DOI] [PubMed] [Google Scholar]
- 6.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J. Biol. Chem. 2002;277:44085–44092. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- 7.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am. J. Physiol. 2004;286:L1244–L1254. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 8.Andrews KL, Pannirselvam M, Anderson TJ, Jenkins AJ, Triggle CR, Hill MA. The vascular endothelium in diabetes: a practical target for drug treatment? Expert Opin. Ther. Targets. 2005;9:101–117. doi: 10.1517/14728222.9.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Raij L. Nitric oxide in hypertension: relationship with renal injury and left ventricular hypertrophy. Hypertension. 1998;31:189–193. doi: 10.1161/01.hyp.31.1.189. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 11.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 12.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. 2001;280:F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 13.Wattanapitayakul SK, Mihm MJ, Young AP, Bauer JA. Therapeutic implications of human endothelial nitric oxide synthase gene polymorphism. Trends Pharmacol. Sci. 2001;22:361–368. doi: 10.1016/s0165-6147(00)01692-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DE. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat. Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 15.Hibi K, Ishigami T, Tamura K, Mizushima S, Nyui N, Fujita T, Ochiai H, Kosuge M, Watanabe Y, Yoshii Y, et al. Endothelial nitric oxide synthase gene polymorphism and acute myocardial infarction. Hypertension. 1998;32:521–526. doi: 10.1161/01.hyp.32.3.521. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani AD. Endothelial nitric oxide synthase polymorphisms and hypertension. Curr. Hypertens. Rep. 2003;5:19–25. doi: 10.1007/s11906-003-0006-0. [DOI] [PubMed] [Google Scholar]
- 17.Markus HS, Ruigrok Y, Ali N, Powell JF. Endothelial nitric oxide synthase exon 7 polymorphism, ischemic cerebrovascular disease, and carotid atheroma. Stroke. 1998;29:1908–1911. doi: 10.1161/01.str.29.9.1908. [DOI] [PubMed] [Google Scholar]
- 18.Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, Parsons A, Haydock S, Hopper RV, Stephens NG, O’Shaughnessy KM, Brown MJ. A common variant of the endothelial nitric oxide synthase (Glu298->Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999;100:1515–1520. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- 19.Fairchild TA, Fulton D, Fontana JT, Gratton JP, McCabe TJ, Sessa WC. Acidic hydrolysis as a mechanism for the cleavage of the Glu(298)-> Asp variant of human endothelial nitric-oxide synthase. J. Biol. Chem. 2001;276:26674–26679. doi: 10.1074/jbc.M103647200. [DOI] [PubMed] [Google Scholar]
- 20.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2832–2835. doi: 10.1073/pnas.97.6.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihm MJ, Wattanapitayakul SK, Piao SF, Hoyt DG, Bauer JA. Effects of angiotensin II on vascular endothelial cells: formation of receptor-mediated reactive nitrogen species. Biochem. Pharmacol. 2003;65:1189–1197. doi: 10.1016/s0006-2952(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Zwietering MH, Jongenburger I, Rombouts FM, van’t Riet K. Modeling of the bacterial growth curve. Appl. Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 24.Arnal JF, Dinh-Xuan AT, Pueyo M, Darblade B, Rami J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell. Mol. Life Sci. 1999;55:1078–1087. doi: 10.1007/s000180050358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awolesi MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439–444. discussion 444-435. [PubMed] [Google Scholar]
- 26.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int. Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 28.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser 1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 29.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am. J. Physiol. 2006;291:C803–C816. doi: 10.1152/ajpcell.00457.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wang XL, Sim AS, Wang MX, Murrell GA, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471:45–50. doi: 10.1016/s0014-5793(00)01356-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 32.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J. Biol. Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 33.Boo YC, Kim HJ, Song H, Fulton D, Sessa W, Jo H. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic. Biol. Med. 2006;41:144–153. doi: 10.1016/j.freeradbiomed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am. J. Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 35.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, Vogel SM, Skidgel RA, Malik AB, Minshall RD. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ. Res. 2006;99:870–877. doi: 10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]