Abstract
Novel coronavirus disease 2019 (COVID‐19) is a highly infectious, rapidly spreading viral disease that typically presents with greater severity in patients with underlying medical conditions or those who are immunosuppressed. We present a novel case series of three kidney transplant recipients with COVID‐19 who recovered after receiving COVID‐19 convalescent plasma (CCP) therapy. Physicians should be aware of this potentially useful treatment option. Larger clinical registries and randomized clinical trials should be conducted to further explore the clinical and allograft outcomes associated with CCP use in this population.
Keywords: convalescent plasma, COVID‐19, immunosuppression, kidney transplant recipient, viral pneumonia
1. BACKGROUND
The novel corona virus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first identified in Wuhan, China, in December 2019 and was declared a pandemic by the World Health Organization on March 11, 2020. 1 This disease has been identified as affecting diverse populations from healthy individuals to those with underlying comorbidities and compromised immune systems; clinical presentations vary from an asymptomatic course to acute respiratory hypoxic failure requiring invasive mechanical ventilation and extracorporeal membrane oxygenation. 2 This pandemic has by now infected millions; with countless hospitalizations and mortality rate of 1%‐3%, it represents an extreme burden on the healthcare system. 3 We present a novel case series of three kidney transplant (KT) recipients with COVID‐19 who recovered after receiving COVID‐19 convalescent plasma (CCP) therapy. At our center, CCP was given under the auspices of the nationwide expanded access program led by the Mayo Clinic, was supported by the generosity of donors at local and regional blood centers (RI Blood Center, NY Blood Center), and was coordinated by our hospital's transfusion medicine team.
2. CASE SERIES
2.1. Patient 1
A 65‐year‐old woman with a history of cardiovascular disease, end‐stage renal disease (ESRD) secondary to familial focal segmental glomerulosclerosis, and cadaver KT (with thymoglobulin induction) 31 months prior to admission on three‐drug immunosuppression (tacrolimus, mycophenolate, prednisone) presented with intermittent diarrhea for 2 days, sharp left lower quadrant of abdominal pain for 3 days, fever of 102.3°F, and fatigue. Blood pressure was 154/85, pulse 81, and respiratory rate was 18. Her SpO2 was 97% on room air. In the ED she was found to have a positive SARS‐CoV‐2 nasopharyngeal RT‐PCR, CT‐chest showed bibasilar sub‐segmental airspace, and CT‐abdomen/pelvis demonstrated evidence of acute diverticulitis of the proximal sigmoid and distal descending colon (Figure 1).
Figure 1.
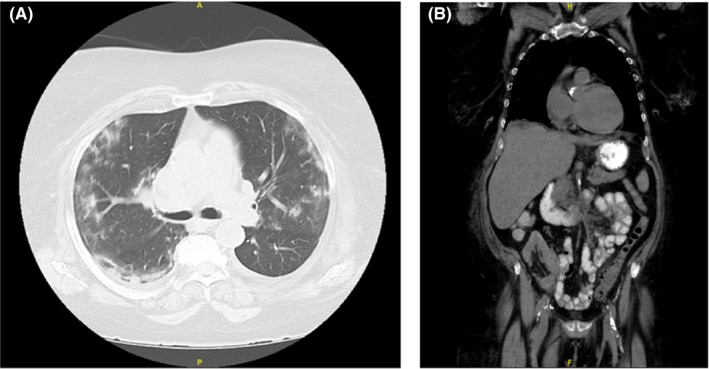
A, Case 1: High‐resolution computed tomography axial view lung window; bilateral, peripheral, multi‐lobar ground glass opacities of rounded morphology, typical imaging features of COVID 19 pneumonia. B, Case 1: High resolution computed tomography coronal view abdomen window; distal descending colon and peripheral sigmoid diverticulitis as evident by wall thickening and surrounding fat stranding; transplanted kidney in the right lower quadrant
She was admitted to the general medicine service with surgery consulting. Her extended GI multiplex PCR panel, stool Clostridium difficile toxin PCR, and serum CMV qPCR were all negative. She received supportive care as well as piperacillin‐tazobactam (HD 1‐7) and amoxicillin‐clavulanate (HD 7‐9) and her abdominal pain and diarrhea slowly improved. She developed mild dyspnea (HD 3) and recurrent fever of 102.0°F (HD 5), repeat CT‐chest showed diffuse bilateral peripheral ground glass opacities in the lungs (HD 5). At this time, the patient still had no respiratory symptoms and her SpO2 was 95% on room air. Laboratory testing was notable for lymphopenia and elevated inflammatory markers (Table 1). Mycophenolate was held; tacrolimus and prednisone were continued. She received one unit of CCP which she tolerated well (HD 7). She remained clinically stable and was discharged home soon thereafter (HD 9).
Table 1.
Clinical characteristics of COVID‐19 patients who received convalescent plasma
| Reference | Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|---|
| Age (y)/Gender | 65/F | 35/F | 36/M | |
| Date of transplant | September 2017 | August 2018 | April 2020 | |
| Comorbidity | Obesity, ESRD, non‐smoker | HTN, ESRD, non‐smoker. | HTN. ESRD, anemia of chronic disease, non‐smoker | |
| Baseline medication | MPA, tacrolimus, prednisone | Azathioprine, tacrolimus, prednisone | MPA, tacrolimus, prednisone | |
| Treatment during hospital stay (immunosuppressant/antiviral/antibiotic) | Tacrolimus, prednisone, piperacillin‐tazobactam, amoxicillin‐clavulanate | Tacrolimus, prednisone, remdesivir, tocilizumab, ceftriaxone, azithromycin, vancomycin, piperacillin/tazobactam | Tacrolimus, prednisone, azithromycin, vancomycin, piperacillin/tazobactam, sulfamethoxazole‐trimethoprim, valganciclovir | |
| Interval between symptom onset and CP therapy (days) | 9 | 4 | 7 | |
| Transfused CP unit(s) | 1 (hd 7) | 2 (hd 4) | 1 (hd 2) | |
| a Antibody levels in plasma (index) | 8.68 | 5.70, 8.15 | 5.67 | |
| Ventilation | Room air | NIPPV, Mechanical ventilation | NIPPV, HF O2 | |
| Complications | None | ARFH | None | |
| Clinical outcome | Improved | Improved | Improved | |
| Length of hospital stay (days) | 9 | 25 | 16 | |
| Blood parameters | ||||
| Alanine aminotransferases (ALT) | 6‐45 IU/L | 24 (hd 1), 17 (pdc) | 15 (hd 1), 25 (hd 5), 93 (ad) | 152 (hd 2), 131 (hd 3), 69 (hd 11) |
| Aspartate aminotransferases (AST) | 10‐42 IU/L | 23 (hd 1), 11 (pdc) | 34 (hd 1), 36 (hd 5), 55 (ad) | 35 (hd 2), 27 (hd 3), 33 (hd 11) |
| Total bilirubin | 0.2‐1.3 MG/DL | 0.4 (hd 1), 0.3 (pdc) | 0.6 (hd 1), 0.8 (hd 5), 0.5 (ad) | 0.6 (hd 2), 0.5 (hd 3), 0.3 (hd 11) |
| Albumin | 3.5‐5.0 G/DL | 3.9 (hd 1), 3.3 (hd 8), 4 (pdc) | 4.3 (hd 1), 2.9 (hd 5), 3.8 (ad) | 2.8 (hd 2), 3.2 (hd 3), 3.2 (ad) |
| Hemoglobin (Hb) | 11.0‐15.0 G/DL | 10.6 (hd 1), 9.2 (hd 8), 9.5 (pdc) | 14.2 (hd 1), (hd 5), 10.7 (ad) | 9.3 (hd 1), 8.9 (hd 3), 10.9 (ad) |
| Hematocrit | 32.0%‐45.0% | 33.9 (hd 1), 30 (hd 8), 31.4 (pdc) | 42 (hd 1), (hd 5), 32.1 (ad) | 28 (hd 1), 27 (hd 3), 32.3 (ad) |
| White blood cells (WBC) | 3.5‐11.0 x10exp9/L | 9.5 (hd1), 9.5 (hd 8), 6.9 (pdc) | 13.7 (hd 1), (hd 5), 13.8 (ad) | 9 (hd 1), 10.2 (hd 3), 7.5 (ad) |
| Absolute neutrophil count | 1.5‐7.5 x10exp9/L | 8.6 (hd 1), 8.4 (hd 8), 5 (pdc) | 12.5 (hd 1), (hd 5), 8.6 (ad) | 8.5 (hd 1), 9.7 (hd 3), 5.4 (ad) |
| Lymphocyte absolute | 1.0‐4.0 x10exp9/L | 0.4 (hd 1), 0.4 (hd 8), 1 (pdc) | 0.8 (hd 1), (hd 5), 3.3 (ad) | 0.2 (hd 1), 0.3 (hd 3), 1.8 (ad) |
| Platelets | 150‐400 x10exp9/L | 226 (hd 1), 322 (hd 8), 354 (pdc) | 219 (hd 1), (hd 5), 333 (ad) | 211 (hd 1), 227 (hd 3), 324 (ad) |
| Serum creatinine (SCr) | 0.44‐1.03 MG/DL | 3.32 (hd 1), 2.49(hd 8), 1.98 (pdc) | 1.27 (hd 1), 0.94 (hd 5), 0.70 (ad) | 1.44 (hd 1), 1.84 (hd 3), 2.4 (ad) |
| Blood urea nitrogen | 6‐24 MG/DL | 52 (hd 1), 22 (hd 8), 38 (pdc) | 14 (hd 1), 20 (hd 5), 15 (ad) | 37 (hd 1), 41(hd 3), 51 (ad) |
| eGFR | Abnormal < 60 ML/MIN/1.73M | 14 (hd 1), 19 (hd 8), 25 (pdc) | 48 (hd 1),> 60 (hd 5), >60 (ad) | 55 (hd 1), 42 (hd 3), 31 (ad) |
| Serum inflammatory markers | ||||
| Ferritin | 10‐120 NG/ML | 2649 (hd 1), 2460 (ad) | 1447 (hd 1), 785 (ad) | 556 (hd 1), 731 (hd 3), 294 (ad) |
| C‐reactive protein (CRP) | 0.00‐10.00 MG/L | 199 (hd 1), 204 (hd 8), 20 (pdc) | 267 (hd 1), 14.32 (hd 11), 4.5 (ad) | 127.8 (hd 1), 138 (hd 3), 9 (ad) |
| D‐dimer | 0‐300 ng/mL | 478 (hd 1), 790 (ad), 432 (pdc) | 396 (hd 2), 439(hd 3), 242 (ad) | 419 (hd 1), 523 (hd 3), 201 (ad) |
| Interleukin‐6 (IL‐6) | <5.00 pg/mL | 12 (hd 1), 20 (ad), 8 (pdc) | 177.5 (hd 2), 303.6 (hd 10) | 157.5 (hd 2), 8.65 (hd 12) |
| Lactate dehydrogenase (LDH) | 100‐220 IU/L | 229 (hd 1), 321 (ad), 181 (pdc) | 383 (hd 3), 215 (ad) | 175 (hd 1), 151 (hd 5), 102 (ad) |
| Erythrocyte sedimentation rate (ESR) | 0‐30 mm/h | 82 (hd 1), 97 (ad), 66 (pdc) | 46 (hd 1), 63 (hd 3), 58 (ad) | 83 (hd 5), 54 (ad) |
Abbreviations: ad, at discharge; ARFH, acute respiratory failure due to hypoxia; hd, hospital day; HF, high flow; HTN, hypertension; MPA, Mycophenolate; NIPPV, non‐invasive positive pressure ventilation; pdc, post discharge.
Abbott SARS‐CoV‐2 IgG Assay, cut‐off for positive is ≥1.40.
She had acute kidney injury (AKI) on admission (Cr 3.32 from baseline 1.88 mg/dL), however, her allograft function improved slowly throughout her hospitalization (Cr 2.41 mg/dL at discharge) and has continued to recover since then (most recent Cr 1.98 mg/dL). She otherwise had no evident infectious (or non‐infectious) complications throughout her hospitalization.
2.2. Patient 2
A 35‐year‐old woman with a history of hypertension, ESRD, and cadaver KT (with basiliximab induction) 21 months prior to admission on three‐drug immunosuppression (tacrolimus, azathioprine, prednisone) presented with several days of fevers, chills, dyspnea, cough, nausea, and diarrhea. She initially presented to an outside hospital ED where chest x‐ray (CXR) was reportedly clear and SARS‐CoV‐2 testing was obtained. Two days later, she presented to our ED with worsening symptoms and was found to have a positive SARS‐CoV‐2 nasopharyngeal RT‐PCR. She was noted to be ill‐appearing and had a fever to 102.4°F. Her SpO2 was 91% on room air. She was in mild respiratory distress with a respiratory rate of 31, her initial ABG demonstrated pH 7.33, pCO2 24 mmHg, pO2 65 mmHg. Initial laboratory testing was notable for lymphopenia and elevated inflammatory markers (Table 1). Repeat CXR showed patchy ill‐defined multifocal airspace disease (Figure 2).
Figure 2.
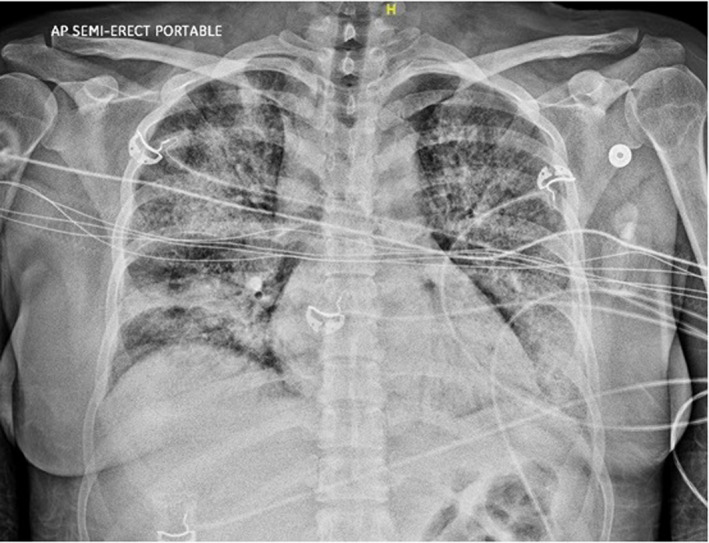
Case 2: AP semi‐erect portable view of the chest demonstrating bilateral diffuse patchy airspace opacities consistent with multifocal pneumonia
She was briefly admitted to the general medicine service before escalating O2 requirements prompted a transfer to the ICU (HD 1). Progressive hypoxic respiratory failure prompted transition to non‐invasive positive pressure ventilation (HD 3) and eventual intubation (HD 6). Diagnostic testing for concomitant infections was unrevealing, CTA‐chest showed progressive multifocal pneumonia but no pulmonary embolus. Azathioprine was held, tacrolimus trough goal was lowered to 4‐6 ng/mL, and prednisone was continued. She was started on therapeutic anticoagulation given rising d‐Dimer (HD 6). She received ceftriaxone (HD 1‐5) and azithromycin (HD 1‐3), vancomycin (HD 1‐3, 6‐7) and piperacillin/tazobactam (HD 6‐12). She received two units of CCP (HD 3), 400mg of tocilizumab (HD 5), and 10 days of remdesivir (HD 5‐14). Clinical recovery from her critical illness progressed slowly, but she was eventually extubated (HD 15), transitioned back to room air (HD 24), and discharged home soon thereafter (HD 26).
She had AKI on admission (Cr 1.27 from baseline 0.91 mg/dL), but her allograft function soon improved and remained stable throughout her hospitalization (Cr 0.70 mg/dL on discharge). She was found to have polymicrobial bacteriuria (HD 16:10‐50k E coli and 50‐100k E faecalis) and was empirically treated for a concomitant bacterial pneumonia as described above (though serial sputum cultures showed only mixed respiratory flora), but she otherwise had no evident infectious (or non‐infectious) complications throughout her initial hospitalization. She was readmitted to the hospital two weeks later with allograft pyelonephritis and E coli bacteremia but was discharged again after an otherwise uneventful three‐day re‐hospitalization.
2.3. Patient 3
A 36‐year‐old man with a history of hypertension, ESRD, and cadaver KT (with basiliximab induction) 17 days prior to admission on three‐drug immunosuppression (tacrolimus, mycophenolate, and prednisone) presented with several days of dyspnea. In the ED he was found to have a positive SARS‐CoV‐2 nasopharyngeal RT‐PCR. He was noted to be ill‐appearing but was afebrile. His ambulatory SpO2 was 80% on room air, and he was noted to have respiratory distress associated with minimal exertion prompting initiation of high‐flow O2 supplementation. Initial laboratory testing was notable for lymphopenia and elevated inflammatory markers (Table 1). CXR showed patchy lower lobe predominant multifocal airspace disease (Figure 3).
Figure 3.
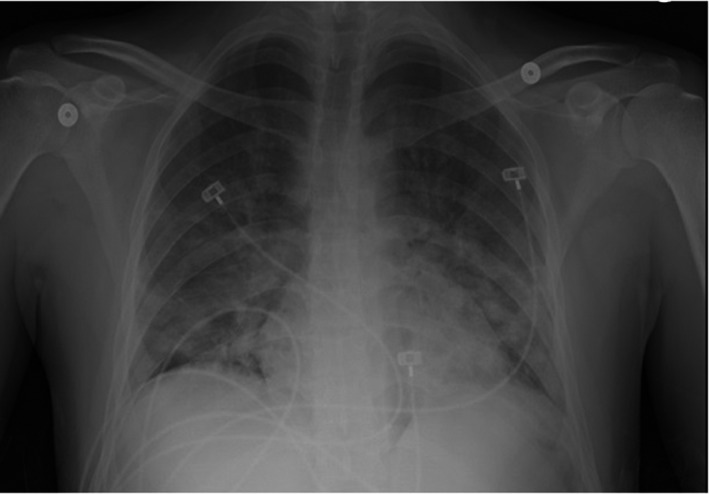
Case 3: AP view of the chest demonstrating lower lobe predominant patchy airspace opacities consistent with multifocal pneumonia
He was briefly admitted to the general medicine service before escalating O2 requirements prompted transfer to ICU for a higher level of care. Diagnostic testing for concomitant infections was unrevealing; repeat CXR showed progressive multifocal pneumonia. Mycophenolate was held, tacrolimus and prednisone were continued. He received azithromycin (HD 2‐4), piperacillin/tazobactam (HD 1‐10) and vancomycin (HD 1‐4). He continued sulfamethoxazole‐trimethoprim and valganciclovir for prophylaxis against Pneumocystis and cytomegalovirus infections. He received one unit of CCP (HD 2) which was complicated by acute chest pain and dyspnea with accompanying tachypnea and worsening hypoxemia; these symptoms improved over the following 12‐24 hours. He also received 10 days of remdesivir (HD 2‐11). He continued to require high‐flow O2 supplementation but eventually had clinical improvement prompting transition back to nasal cannula (HD 10)) and subsequently room air (HD 14); he was discharged home soon thereafter (HD 15).
His creatinine on admission was at a post‐transplant nadir (Cr 1.44 from 10.3 mg/dL pre‐transplant). He had AKI during his hospitalization (Cr peak 2.49 mg/dL) which was thought to be multifactorial in the setting of his critical illness, but this has continued to recover since then (most recent Cr 1.96 mg/dL). Otherwise he had no evident infectious (or non‐infectious) complications throughout his hospitalization.
3. DISCUSSION
Worldwide, multiple treatment options for COVID‐19 are under investigation including large clinical trials for antiviral and anti‐inflammatory drugs, 4 , 5 however, there are at present no specific therapeutic agents or vaccines available for COVID‐19. The use of CP therapy is a standard passive immunization method that has been used to treat infectious diseases for more than a century. Use of CP for treating Middle Eastern respiratory syndrome (MERS), 6 syndrome of acute respiratory distress (SARS), 7 and Influenza A (H1N1) 8 may have contributed to improved survival rates in infected patients. Since MERS, SARS‐CoV‐1, and SARS‐CoV‐2 share many similar virulence and clinical characteristics, 9 CP therapy may be a potentially efficacious treatment for COVID‐19 patients. 10
Outcomes of CP therapy are dependent on multiple factors. Firstly, in an effective CP product there should be an optimum level of neutralizing antibody titers. Investigation on SARS‐CoV‐1 and MERS‐Co‐V infected patients showed that the level of specific neutralizing antibodies decreased rapidly within months after infection, suggesting that SARS‐CoV‐2 neutralizing antibodies may also wane; therefore, plasma from recently recovered patients may be optimal for use in infected individuals undergoing active treatment. 11 , 12 , 13 Duan et al report that recently recovered COVID‐19 patients with neutralizing antibody titers above 1:640 are considered ideal CP donors. 10 The CCP used in these three patients contained high levels of SARS‐CoV‐2 antibodies, as measured by a chemiluminescent immunoassay for the nucleocapsid protein (Table 1). Secondly, CP is expected to be more effective when given earlier in the disease course; our case series (with administration of CCP nine, four, and seven days after symptom onset) is consistent with a previous study where CCP transfusion was given 14‐day post onset of illness with good effect. 10 It is unlikely these three patients had significantly high antibody titers prior to their CCP transfusion given the timing of administration relative to symptom onset. Lastly, CP contains, in addition to neutralizing antibodies, other proteins such as anti‐inflammatory cytokines, anticoagulant factors, natural defensins, and other proteins that are transferred from the donor to the recipient 14 ; all of these proteins may have independent effects in strengthening the immune system of the recipient by neutralizing an overactive immune system that contributes to the pathogenesis of COVID‐19 in many patients. 15
Risks associated with the transfusion of CP include allergic reactions, which commonly are minor such as urticaria or erythema and very rarely severe (eg anaphylaxis) circulatory overload (TACO) in at risk patients, and transfusion related acute lung injury (TRALI). The risk of the latter is abrogated by choosing only males or females who are HLA antibody negatives as donors of CP. The risk of viral disease transfusion is currently almost negligible. 16 Of note, in one of our cases (patient 3), the patient developed acute dyspnea after transfusion of their first unit of CCP, which improved slowly over the following 12‐24 hours. Clinically, this was considered as a TRALI reaction but in view of the TRALI mitigation approaches, likely a TRALI type II. 17 Distinguishing the cause of acute dyspnea in transfusion recipients is known to be problematic. 18 Additionally, there is a theoretical risk that the use of CCP may treat or prevent COVID‐19 disease in a way that mitigates the native humoral immune response, leaving these individuals vulnerable to subsequent reinfection. 19 Furthermore, for solid organ transplant recipients in particular, there is some evidence of a risk of developing donor‐specific antibodies (and subsequent antibody mediated rejection) after receiving allogeneic blood products, 20 but this risk is likely very low with CCP given the expected minimal HLA antigen load in a single‐donor plasma product.
Treating KT recipients who have COVID‐19 can be challenging given the need for ongoing immunosuppressive medications in these patients. 4 When treating immunosuppressed individuals with severe infections, it is a common management approach to reduce or discontinue immunosuppressive medications (if able) so that their immune system may be better able to fight the disease. Anti‐metabolite treatment was discontinued in all of our patients at admission. Prednisone has anti‐inflammatory effects that may act to reduce inflammatory cell infiltration in the alveoli and reduce the risk of acute respiratory distress syndrome (ARDS). 21 It is important to note that prednisone may also delay recovery due to inhibition of antiviral immune response from the host and clinical evidence does not currently support corticosteroids as adjunctive treatment in COVID‐19 patients. 22 However, in our patients, we continued low dose maintenance prednisone daily throughout their hospital stay to avoid adrenal insufficiency. It is unclear if immunosuppressed patients are in any way protected from the hyper‐inflammatory syndrome affecting some patients with COVID‐19.
There are several limitations to our case series of the use of CCP in KT recipients. First, given its descriptive nature, this small case series cannot define efficacy of CCP therapy in KT recipients or others with compromised immune systems. Second, our patients were on multiple other medications that may have altered their immune response and affected their recovery from COVID‐19. Two patients received remdesivir, which may have some direct antiviral efficacy, thus confounding any possible contribution from CCP to their clinical outcome. Despite these limitations, we report here the successful recovery from COVID‐19 in three KT recipients who received CCP therapy without a detectable untoward effect on their allograft function. Physicians should be aware of this potentially useful treatment option for COVID‐19 in KT recipients. Larger clinical registries and randomized clinical trials should be conducted to further explore the clinical and allograft outcomes associated with CCP use in this population as compared to the general population.
AUTHORS’ CONTRIBUTION
SN, RG, and RR wrote the manuscript, contributed to the conception and design of the manuscript; GB, CC, DF, BM, PM, AO, JAB, JS, contributed to the revisions and final approval of the manuscript.
Naeem S, Gohh R, Bayliss G, et al. Successful recovery from COVID‐19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2021;23:e13451. 10.1111/tid.13451
REFERENCES
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta bio‐medica: Atenei Parmensis. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID‐19. JAMA. 2020;323(15):1499. [DOI] [PubMed] [Google Scholar]
- 3. Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case‐fatality risk estimates for COVID‐19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020;26(6):1339‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 5. Lu H. Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Bioscience Trends. 2020;14(1):69‐71. [DOI] [PubMed] [Google Scholar]
- 6. Ko J‐H, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617‐622. [DOI] [PubMed] [Google Scholar]
- 7. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hung IFN, To KKW, Lee C‐K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee P‐I, Hsueh P‐R. Emerging threats from zoonotic coronaviruses‐from SARS and MERS to 2019‐nCoV. J Microbiol Immunol Infect. 2020;53(3):365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao W‐C, Liu W, Zhang P‐H, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162‐1163. [DOI] [PubMed] [Google Scholar]
- 13. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garraud O, Heshmati F, Pozzetto B, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lünemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology—mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80. [DOI] [PubMed] [Google Scholar]
- 16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52:65S‐79S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vlaar APJ, Toy P, Fung M, et al. A consensus redefinition of transfusion‐related acute lung injury. Transfusion. 2019;59(7):2465‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nixon CP, Sweeney JD. Discriminating different causes of transfusion‐associated pulmonary edema. Transfusion. 2015;55(8):1825‐1828. [DOI] [PubMed] [Google Scholar]
- 19. Crowe JE, Firestone C‐Y, Murphy BR. Passively acquired antibodies suppress humoral but not cell‐mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910‐3918. [DOI] [PubMed] [Google Scholar]
- 20. Hassan S, Regan F, Brown C, et al. Shared alloimmune responses against blood and transplant donors result in adverse clinical outcomes following blood transfusion post‐renal transplantation. Am J Transplant. 2019;19(6):1720‐1729. [DOI] [PubMed] [Google Scholar]
- 21. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. [DOI] [PubMed] [Google Scholar]
- 22. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. The Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]


