Summary
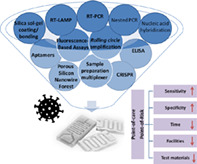
There is a long way to go before the coronavirus disease 2019 (Covid‐19) outbreak comes under control. qRT‐PCR is currently used for the detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of Covid‐19, but it is expensive, time‐consuming, and not as sensitive as it should be. Finding a rapid, easy‐to‐use, and cheap diagnostic method is necessary to help control the current outbreak. Microfluidic systems provide a platform for many diagnostic tests, including RT‐PCR, RT‐LAMP, nested‐PCR, nucleic acid hybridization, ELISA, fluorescence‐Based Assays, rolling circle amplification, aptamers, sample preparation multiplexer (SPM), Porous Silicon Nanowire Forest, silica sol‐gel coating/bonding, and CRISPR. They promise faster, cheaper, and easy‐to‐use methods with higher sensitivity, so microfluidic devices have a high potential to be an alternative method for the detection of viral RNA. These devices have previously been used to detect RNA viruses such as H1N1, Zika, HAV, HIV, and norovirus, with acceptable results. This paper provides an overview of microfluidic systems as diagnostic methods for RNA viruses with a focus on SARS‐CoV‐2.
Keywords: coronaviruses, covid‐19, diagnosis, microfluidic devices, RNA viruses, RT‐PCR
1. INTRODUCTION
Viruses that have RNA as their genetic core material can cause diseases like Ebola, hepatitis C, influenza, severe acute respiratory syndrome (SARS) and poliomyelitis. 1 Coronaviruses are enveloped, single‐stranded RNA viruses that can cause diseases in both humans and animals, mostly affecting the respiratory system. 2 Coronaviruses originate from multiple species. Four strains of human coronaviruses (hCoVs), including 229E, ‐NL63, ‐OC43, and ‐HKU1, cause common cold‐like symptoms in humans. The other three hCoVs can result in potentially fatal lower respiratory tract diseases. They have caused three outbreaks: severe acute respiratory syndrome coronavirus (SARS‐COV) in 2002–2003, the Middle East respiratory syndrome coronavirus (MERS‐COV) in 2012, and the novel coronavirus disease in 2019 (Covid‐19). 3 , 4 In 2007, it was stated that another disastrous SARS epidemic would probably break out in the coming years because of the vast reservoir of SARS‐like coronaviruses in horseshoe bats and the tradition of eating exotic mammals in southern China. 4 Subsequently, Covid‐19 became pandemic. 5 , 6
To date, Covid‐19 has infected more than 14 million people involving healthcare and non‐healthcare settings. 7 It causes a multisystem infectious disorder 8 , 9 , 10 that is more likely to arise in genetically susceptible individuals 11 , 12 and people with pre‐existing conditions associated with immune dysregulation. 13 , 14 , 15 , 16 , 17 , 18 After 7 months of the outbreak and pandemic of Covid‐19, 19 , 20 no specific treatment and prevention exist, 21 and supportive care is the only option along with anti‐inflammatory and antiviral agents. 14 , 17 , 22 , 23 , 24 The condition at being the cause of more than 600 000 deaths has promoted international investigative efforts 25 , 26 , 27 to find tools for earlier diagnosis of Covid‐19, allowing us to consider isolation practices as well as apply for supportive care earlier.
1.1. Conventional diagnostic methods
Early diagnosis of viral diseases can lead to better and more accurate treatment. Cell culture‐based techniques are the gold standard for viral detection. 28 Rapid molecular techniques with high sensitivity involve the amplification of viral genomic material and may detect several viruses simultaneously. 28 The two most important types of nucleic acid‐based amplification tests (NATs) are nucleic acid sequence‐based amplification (NASBA) and real‐time polymerase chain reaction (real‐time PCR). NASBA is an isothermal and continuous amplification reaction in which three different enzymes are applied: RNase‐H, AMV‐RT, and T7‐RNA polymerase. 29 Real‐time PCR involves the amplification of complementary DNA (cDNA) prepared from viral RNA in a real‐time manner and is appropriate for the detection of minute amounts of nucleic acids. 30 , 31 Another method is a biosensor that has high sensitivity and specificity, and most of the biosensors are based on electrochemical transduction. 32
Covid‐19 can be diagnosed in different ways, including CT‐Scan and RT‐PCR. 33 CT‐Scan results indicate bilateral ground‐glass and consolidative pulmonary opacities. 34 , 35 qRT‐PCR is currently used for the detection of SARS‐CoV‐2, but it is expensive, time‐consuming, and not as sensitive as it should be. The shortage of equipment in healthcare centers and the need for better disease management require the development of more convenient and more reliable methods of diagnosis. This review aims to provide an overview of the microfluidic systems as a diagnostic method for RNA viruses with a focus on SARS‐CoV‐2.
1.2. New and rapid diagnostic methods
The gold standard for the detection of the novel coronavirus is qRT‐PCR. 36 However, there may be other methods that allow fast and inexpensive diagnosis. One study used reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) that was conducted in under 30 min. 36 Microfluidic devices might be a rapid diagnostic approach in the future as they are cheap and easy to use. In recent years, such devices have been applied to the diagnosis of several viruses such as influenza, SARS‐coronavirus, and smallpox. 37 , 38 , 39 As a result, these devices may also help detect SARS‐CoV‐2 and accelerate the process of diagnosis and rehabilitation, ultimately lowering the death rate. These devices can use several techniques such as RT‐PCR, RT‐LAMP, nested PCR, nucleic acid hybridization, ELISA, or fluorescence‐based Assays. 40 , 41 , 42 , 43 , 44
2. MICROFLUIDIC DEVICES
2.1. An introduction and history
The computer industry has been transformed by microfabricated integrated circuits that significantly reduce the space, effort, and time for computations. Biology and chemistry have the potential to develop through microfluidic systems, which use small amounts of reagent, rapidly 45 , 46 . An excellent example of such devices is the home pregnancy test, which detects hormones in urine and is the most commonly used example of the lateral flow strip assays (LFSAs), which are made to detect specific biomolecules. Such devices can also be used for the detection of bacterial cells and cancer cells. 46 Microfluidic devices coated with virus‐capturing antibodies can be used to detect viruses present in a solution. 47 Researchers have also developed a microfluidic chip that detects RNA‐based viruses from throat swab samples; the H1N1 virus was used as a model for this experiment. 39 Another research developed a microfluidic chip system that can detect SARS‐CoV. 38
2.2. Fabrication methods
Generally, there are two different kinds of microfluidic devices; channel‐based and paper‐based. The paper‐based tool is made of a series of hydrophilic cellulose or nitrocellulose fibers that guide liquid in a paper by absorption. The channel‐based one could be fabricated using four main methods, including laminate, molding, 3D‐printing, and nanofabrication. 48 Channel‐based microfluidic devices need channels to create a bed for the integration of reagents.
2.2.1. Laminate
Layers cut separately by using a knife or laser are fused to form the channel. Although it is an easy method, it is impossible to achieve sub‐micrometer levels. 49 , 50 , 51
2.2.2. Moulding
Moulding is a technique that consists of four main steps, including shaping the mould, choosing the appropriate polymer, curing the polymer, and releasing the material from the mould. It includes three different methods, including replica moulding, injection moulding, and hot embossing. 52
3D‐p3rinting
This is a method of fabricating layer by layer. It has two main parts. One is computer aided design. The second part is a 3D‐printer that uses the computer format of stereolithography (STL), building up in 2D layers based on its resolution. 53 , 54 There are different methods of 3D‐printing, such as fused deposition modeling (FDM), STL and digital micromirror device‐based projection printing (DMD‐PP), multi‐jet modeling, and two‐photon polymerization.
Although 3D‐printing has some limitations, such as the material which can be used 55 and the resolution and biocompatibility of the models, 56 it is a single‐step method that does not require the manual working of some other methods such as soft lithography. 57 It will probably become the most common way in laboratories in the future.
2.2.3. Nanofabrication
In the top‐down approach, the model size is reduced to the nanoscale until the desired shape and dimensions are achieved. In contrast, the bottom‐up approach starts from atomic and molecular levels to finally shape the model. 58 , 59 , 60 Extreme ultraviolet lithography (EUL), electron beam lithography (EBL), and nanoimprint lithography (NIL) are three different methods used in nanofabrication. EUL and EBL are not common in microfluidic fabrication, and the main reason is high costs. 61 , 62 However, NIL, which is a special kind of replica moulding with the resolution of sub 15 nm, is affordable and has many applications in microfluidic fabrication. 60 , 63
2.3. Useful strategies for RNA virus detection
In recent years, portable microfluidic devices have reduced global cost per analysis and reagent consumption and also led to faster analyses due to shorter reactions. 64 , 65 , 66 Among conventional methods for detecting RNA viruses, traditional cultural methods, serological methods, and molecular biology techniques can be mentioned. According to different studies, so much time and money can be spared when these methods are integrated into a microfluidic‐based device. Table 1 provides a summary of microfluidic devices useful for the detection of RNA viruses.
TABLE 1.
Methods used in Microfluidic devices for RNA virus detection
| Method integrated microfluidic device | Types of the method | Detected virus | Advantages | References |
|---|---|---|---|---|
| PCR and RT‐PCR‐based | PCR | Rotavirus | Fast (30 min overall), low‐cost, easy to use, detection limit: 1 × 103 copies/mL, highly sensitive and specific (100%) | Ye, Xu 92 |
| Nested PCR | RNA viruses | Detection limit range: 100 to 103 copies/μL, simultaneous detection and genotyping of RNA virus, sampling from human feces, sewage, and artificially contaminated oysters | Oshiki, Miura 73 | |
| Single‐Cell‐in‐Droplet PCR | HIV‐1 | High sensitivity | Yucha, Hobbs 93 | |
| in situ PCR and RT‐PCR | Zika virus | Recovery of the virus at very low concentrations of 50 transducing units (TU)/mL from human saliva, the captured ZIKV RNA is directly used for downstream PCR without any loss | Zhu, Zhao 68 | |
| RT‐qPCR and qPCR | HCV, HIV, Zika, HPV 16, and HPV 18 viruses | Rapid and sensitive, reaction times: 25 min | Powell, Wiederkehr 94 | |
| RT‐PCR | Ebola virus |
Disposable and low‐cost. Same sensitivity (10 RNA copies per microliter) and efficiency (90–110%) Amplification with high sensitivity was achieved in 30–50 min. Faster amplifications were possible (20 min), but sensitivity was reduced |
Fernández‐Carballo, McBeth 95 | |
| RT‐PCR | Hepatitis A virus and norovirus | An end‐point, sensitive, accurate absolute quantification approach, determination of target copy numbers without external quantitative standards | Fraisse, Coudray‐Meunier 69 | |
| LAMP and RT‐LAMP‐based | Smartphone Detection of Loop‐mediated Isothermal Amplification | Zika virus | Limit of detection: 1 copy/μL, simple, rapid(15 min), easily quantified using a smartphone | Kaarj, Akarapipad 96 |
| RT‐LAMP | MS2 virus | Easy to use, Low cost (less than 0.10 $ per piece), fluorescence intensities 100 times more than other methods in differentiation between positive and negative pores | Lin, Huang 97 | |
| RT‐LAMP | Zika, Chikungunya, and Dengue viruses | Clinically relevant sensitivity. Detection of Zika virus as low as 1.56e5 PFU/mL from whole blood, Low reagent consumption | Ganguli, Ornob 98 | |
| RT‐LAMP | HIV | Disposable, flexible, inexpensive, light, high sensitivity and specificity, faster amplification, higher stability, and lower complexity | Safavieh, Kaul 41 | |
| RT‐LAMP | Zika virus | High sensitivity and inexpensive | Song, Mauk 72 | |
| RPA and RT‐RPA | RPA |
HIV‐1 |
High rapidity, portable and independence on electricity | Kong, Li 99 |
| RPA | Zika virus | Good sensitivity and selectivity, the detection limit of 10 copies/μL, well‐defined accuracy, feasible by human trials | Yang, Kong 100 | |
| RT‐RPA | Ebola virus | Lower reaction time for low viral load detection as compared to paper, high sensitivity (90%) without unduly damaging the specificity (60.8%) | Magro, Jacquelin 101 | |
| Immunoassay‐based | Immunoassay |
Citrus tristeza Virus |
Rapid, low‐cost, high sensitivity and specificity | Freitas, Proença 102 |
| Sandwich immunoassay |
HIV‐1 |
Low‐cost, simple and efficient operation, limits of detection (LODs) of 0.17 and 0.11 ng/mL for p24 antigen | Li, Zheng 103 | |
| Scattering‐based Immunoassay | Influenza virus | High sensitivity | Wang, Ruan 75 | |
| Immunoassay | AIV | Detection of H5N2 AIV at virus concentration as low as 3.6 × 103 EID50/mL, high sensitivity. | Yu, Xia 42 | |
| Bead‐based immunofluorescence‐assay |
Dengue virus |
rapid on‐chip detection (5 min), small required sample (≈15 μL), long life‐time (>50× reusable) | Iswardy, Tsai 104 | |
| RGO‐based electrochemical immunosensor |
H1N1 |
High selectivity and specificity for H1N1 viruses | Singh, Hong 105 | |
| Custom inkjet printing and roll‐coating process‐immunoassay | Rubella virus | Materials cost for the new devices of only US $0.63 per device, 100% clinical sensitivity and specificity for RV IgG and IgM in a panel of serum samples | Dixon, Ng 106 | |
| Electrochemical immunoassay | Rubella virus | High sensitivity | Rackus, Dryden 107 | |
| Aptasensor | Impedance Aptasensor | H5N1 Avian Influenza | High specificity and rapid | Lum, Wang 108 |
| Graphene‐gold nano‐composite aptasensor | norovirus | The detection limit of 100 pmol for recombinant norovirus‐like particles, total detection time less than 35 min. | Chand and Neethirajan 81 | |
| Nano‐based | Nanoparticle‐enhanced electrical detection | Zika virus | Highly specificity, the detection limit of 101 virus particles/μl, simple, rapid, and cost‐effective | Draz, Venkataramani 109 |
| Porous silicon nanowire (pSiNW) | H5N2 avian influenza viruses | A virus with specific size could be isolated from 100 μL in 30 min | Xia, Tang 84 | |
| Fluorescence‐Based | Internal reflection fluorescence microscopy | HIV‐1 | Highly sensitive, high speed | Lau, Walsh 110 |
| Custom integrated fluorometer | Ebola virus | Rapid, amplification‐free, simple, and sensitive, the detection limit of 20 pfu/mL (5.45 × 107 copies/mL) of purified Ebola RNA in 5 min | Qin, Park 111 | |
| Barcode Fluorescence Reporter and a Photocleavable Capture Probe | Ebola virus | High specificity., detection time less than 90 min | Du, Park 112 | |
| Fluorescence‐Based Assays | Influenza A | Detection time less than 2 h. | Shah and Yager 76 | |
| Combination of several techniques | Immunomagnetic separation and RT‐PCR | H1N1 | High sensitivity, rapid, and straightforward | Kim, Abafogi 40 |
| Glycan‐coated magnetic beads and RT‐PCR | Influenza A | Simultaneous detection of 12 viruses, Fast detection (under 100 min), Limit of detection ranging from 40 to 3000 | Shen, Sabbavarapu 113 | |
| RT‐LAMP‐lateral flow immunoassay (LFIA) | HIV‐1 | Low‐cost and portable platform, rapid and autonomous analysis of HIV‐1 virus | Phillips, Moehling 114 | |
| Reverse‐transcription LAMP coupled with reverse dot blot analysis | Zika virus | Rapid, sensitive, the limit of detection of the RT‐LAMP assay using spiked saliva samples was found to be ≈2 × 103 RNA copies/mL (6.6 RNA copies/reaction, RNA detection time between 3 and 10 min | Sabalza, Yasmin 115 | |
| Fluorescent‐labeled universal aptamer | H1N1, H3N2, and influenza B | Rapid, simple, and inexpensive | Wang, Chang 77 | |
| ELISA and fluorescence‐based | Hendra virus | Simple and rapid | Gao, Pallister 74 | |
| Novel time‐resolved fluorescence (TRF) europium nanoparticle immunoassay | HIV‐1 | High sensitivity, rapid and straightforward | Haleyur Giri Setty, Liu 116 | |
| Isothermal amplification and a real‐time colorimetric method | Influenza A and influenza B virus, and human adenoviruses | Faster (the entire process takes an hour), high specificity and sensitivity | Wang, Zhao 117 | |
| PLP and RCA | Tropical viruses like Ebola, Zika, and Dengue | High specificity, sensitivity, and multiplexing capability | Ciftci, Neumann 118 | |
| RNA viruses (NDV, IBV and AIV) | High specificity and sensitivity, multiple detections, the detection limit of less than 10 | Ciftci, Neumann 78 | ||
| Other techniques | Capillary Flow Dynamics‐Based method | Zika virus | Clinically relevant sensitivity and specificity, detecting down to 1 log CFU/mL E. coli in water samples and 20 pg/mL ZIKV in serum samples at an operating time of 30s, easy‐to‐use and affordable | Klug, Reynolds 119 |
| Nucleic acid hybridization | Influenza A | Detection time 80 min, very low reagent consumption (only 3 μL), high sensitivity | Zhang, Hong 43 | |
| SPM | Ebola virus | High sensitivity and selectivity, rapid, using a small volume of samples at the microliter scale (~60 μL for 3× and ≈800 μL for 80×, with 0.021 pfu/mL sensitivity, the ability for early clinical decisions | Du, Cai 82 | |
| CRISPR/Cas9 | Zika virus | Simple and inexpensive | Meagher, Negrete 86 | |
| High‐throughput drop‐based microfluidics | murine noroviruses (MNV) | High specificity and sensitivity and simple | Tao, Rotem 120 | |
| Simple epoxy silica sol‐gel coating/bonding method | Influenza virus | High sensitivity and inexpensive | Liu, Zhao 85 | |
| Isothermal nucleic acid amplification | HIV | High sensitivity, specificity, reproducibility, high amplification efficiency, and easy detection | Mauk, Song 121 | |
| RCA | Influenza and Ebola viruses | Little need for pre‐amplified sample, Portable, affordable, the possibility of detection of several pathogens, Elongation time from 10 to 120 min | Soares, Neumann 44 |
2.3.1. RT‐PCR integrated microfluidic device
RT‐PCR can be carried out in two ways: a one‐step and two‐step. Using the former assay, reverse transcription and PCR occur in a single reaction chamber. The two processes take place in different reaction chambers on the two‐step procedure. Colorimetric methods, such as immunochromatographic strips, can be used for RT‐PCR product detection in microfluidic chips. 67
Kim et al recently designed a microfluidic‐based method for detecting H1N1 influenza, and the results suggested that the limit of detection (LOD) of molecular diagnostics for the virus can be lowered by systematically combining immunomagnetic separation and RT‐PCR in one microfluidic device. 40 Moreover, RT‐PCR in situ has been successfully used for the diagnosis of Zika virus. 68 Digital microfluidic RT‐PCR has been performed in a study to detect Hepatitis A and noroviruses in the gut, and the results showed that absolute quantification by digital RT‐PCR may be an appropriate alternative method to standardize quantification of enteric viruses in foodstuffs. 69
2.3.2. RT‐LAMP integrated microfluidic
RT‐LAMP versus commonly‐used PCR does not require thermal cycles and is performed at a constant temperature between 60 and 65°C. 70
Safavieh et al. designed cellulose‐based paper microchips and amplified the target RNA using the RT‐LAMP technique and detected the HIV‐1 virus through the electrical sensing of LAMP amplicons. They developed an RT‐LAMP paper microchip assay, which could be used as a simple and affordable method for the detection of HIV‐1. 41 Two other studies have shown that microfluidic‐based RT‐LAMP assay can affordably detect the Zika virus and Bacteriophage MS2 virus. 71 , 72
2.3.3. Nested PCR integrated microfluidic
Nested PCR is a modification of PCR, which involves the use of two primer sets and two successive PCR reactions. Therefore, it profits from higher sensitivity and specificity compared to conventional PCR.
Oshiki et al. used a microfluidic nested‐PCR device and next‐generation sequencer to develop high‐throughput detection and genotyping tool for 11 human RNA viruses including Aichi virus, astrovirus, enterovirus, norovirus (genogroups I, II, and IV), hepatitis A virus, hepatitis E virus, rotavirus, sapovirus, and human parechovirus. The results of this study showed that microfluidic nested PCR followed by MiSeq sequencing enabled efficient tracking of the fate of multiple RNA viruses in various environments like feces, sewage, and oysters. 73
2.3.4. Nucleic acid hybridization
Nucleic acid hybridization on a microfluidic chip integrated with the controllable micro‐magnetic field has been reported as a rapid method for simultaneously detecting and subtyping multiple influenza viruses. The subtypes H1N1, H3N2, and H9N2 could be simultaneously detected in 80 min with detection limits about 0.21, 0.16, 0.12 nM, respectively. Therefore, this method can be a reliable technology platform with the ability of rapid diagnosis and subtyping of influenza viruses. 43
2.3.5. ELISA
The enzyme‐linked immunosorbent assay (ELISA) is a plate‐based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies, and hormones. Recently, it has been widely used with microfluidic devices resulting in a fast and affordable method of diagnosing RNA viruses.
The commonly used ELISA and fluorescence‐based Luminex assay typically consists of three steps and takes several hours to complete, but combining this method with the microfluidic system has led to efficient and rapid diagnosis. Gao et al. used ELISA microfluidic system for detecting Hendra virus IgG antibody within 60 min. 74
In another study performed by Yu et al., detection of avian influenza virus (AIV) took only 1.5 h with the help of an ELISA‐based microfluidic platform. 42 Sandwich immunoassay based‐microfluidic device has been used for detecting influenza as well. 75
2.3.6. Fluorescence‐based assays
A fluorescence‐based microfluidic device decreased the limitation of detection of influenza (A) nucleoprotein immunoassay by over 50%. 76 Wang and colleagues showed that the fluorescent‐labeled universal aptamer integrated with a microfluidic device could distinguish and detect three different influenza viruses (influenza A H1N1, H3N2, and influenza B) simultaneously in 20 min. 77
2.3.7. Rolling circle amplification
Rolling circle replication is a process of rapid unidirectional replication of circular molecules of DNA and RNA, such as plasmids and the RNA genome of viroids. When mixed with microfluidic systems, some benefits like rapidity and cheapness are present. Rolling circle amplification combined with on‐chip size‐selective trapping of amplicons on silica beads showed that this system could be applied to diagnosing Ebola and influenza viruses. 44 In another study, Ciftci et al. showed that traditional approaches like virus isolation, serology, immunoassays, and RT‐PCR are difficult and limited in terms of specificity and sensitivity for detecting RNA viruses. However, rolling circle amplification, in combination with padlock probes, had a higher specificity for detecting RNA viruses like Newcastle disease virus, avian coronavirus, and avian influenza virus. 78
2.3.8. Aptamers
Aptamers are single‐stranded artificial oligonucleotides (DNA or RNA) with a high affinity for binding to specific targets. They are of short length from 20 to 100 nucleotides and can bind to a variety of small (amino acids, antibiotics, and nucleotides) and large molecules (proteins, 79 viruses, and bacteria 80 ).
According to a study performed by Chand et al., aptasensor integrated with a microfluidic‐based device could achieve a detection limit of 100 pmol with a detection range from 100 pmol to 3.5 nM for noroviruses. 81
2.3.9. Sample preparation multiplexer
According to a study performed by Du et al. an automated microfluidic sample preparation multiplexer (SPM) can be used for Ebola virus detection. This multiplexed, miniaturized sample preparation microdevice is considered as a critical technology that is believed to have a significant role in the next generation point‐of‐care (POC) detection system. 82
2.3.10. The microfluidic device integrated with porous silicon nanowire forest
The nanoscale features in silicon nanowires (SiNWs) can suppress phonon propagation, which is referred to when phonons propagate through a lattice, and sharply reduce their thermal conductivities compared to the bulk value. 83 Xia et al. developed a microfluidic device embedded with porous silicon nanowire (pSiNW) forest for label‐free size‐based point‐of‐care virus capture in a continuous curved flow design. They worked on Influenza virus (H5N1) and demonstrated that this method could have high potentials for virus discovery, isolation, and culture. 84
2.3.11. Silica sol‐gel coating/bonding method
Liu et al. fabricated a polycarbonate (PC)‐polydimethylsiloxane (PDMS) hybrid microchip using a simple epoxy silica sol‐gel coating/bonding method. They showed that infectious reference viruses and nasopharyngeal swab patient specimens could be successfully tested using microchip Europium nanoparticle immunoassay (μENIA) on hybrid microchip platforms. The potential of this unique microchip nanoparticle assay was demonstrated in the clinical diagnosis of influenza viruses. 85
2.3.12. Clustered regularly interspaced short palindromic repeats (CRISPR)
Repetitive DNA sequences found in prokaryotic genomes contain DNA fragments of bacteriophages. Meagher et al. highlighted the potential of paper‐based sensors coupled with CRISPR/Cas9 for the detection of Zika virus. 86
3. THE APPLICATION OF MICROFLUIDIC DEVICES FOR SARS‐COV‐2 DETECTION
Lamb et al. developed a faster and cheaper method based on RT‐LAMP as an alternative process to qRT‐PCR that could be performed in less than 30 min, and its specificity was investigated using various types of coronaviruses. Also, the simplicity of the assay allows individuals at home to use it without special equipment. 87
El‐Tholoth et al. presented another fast‐molecular test with high sensitivity and point‐of‐care (POC) suitable for home‐use. The method is based on LAMP two‐stage isothermal amplification (SARS‐CoV‐2 Penn‐RAMP) in closed tubes to create more sensitivity. Finally, detection by fluorescence or colorimetry leads to an easy diagnosis without specific instruments. The sensitivity of RAMP is 10–100 times more than that of LAMP and RT‐PCR for SARS‐CoV. 88
Nguyen et al. described a POC device, which is rapid, robust, and affordable, with minimal training for emergencies such as the outbreaks. This device uses a LAMP reaction in combination with a lateral flow strip (LFS) to detect the virus in less than 1 h. 89 Another example of using LFS is the BioMedomics COVID‐19 IgM/IgG Rapid test. 90
Yang and colleagues designed an RNA‐based POC device for the diagnosis of SARS‐CoV‐2 using both a LAMP assay and a paper‐based POC diagnostic device. It was integrated with a smartphone to provide a fast, sensitive, and more accessible tool. This method utilizes a small sample volume, and the fluorescent probe selection can be evaluated by a smartphone to facilitate the recording and sharing of the test results. 91
In conclusion, microfluidic devices offer a wide range of methods, including RT‐PCR, RT‐LAMP, Nested PCR, Nucleic acid hybridization, ELISA, and Fluorescence‐Based Assays, for detection of RNA viruses such as H1N1, H3N2, and H9N2, Hendra, and influenza B viruses. These accurate methods of detecting RNA viruses might also have the potential for detecting the novel coronavirus that has caused a global issue of Covid‐19.
CONFLICT OF INTEREST
The authors have no competing interest.
Abbreviations
- AIV
avian influenza virus
- AMV‐RT
avian myeloblastosis virus reverse transcriptase
- cDNA
complementary DNA
- COVID‐19
coronavirus disease 2019
- CRISPR
clustered regularly interspaced short palindromic
- DMD‐PP
digital micromirror device‐based projection
- DNV
dengue virus
- EBL
electron beam lithography
- ELISA
enzyme‐linked immunosorbent assay
- EUL
extreme ultraviolet lithography
- FDM
fused deposition molding
- HIV‐1
human immunodeficiency virus‐1
- IBV
infectious bronchitis virus
- Ig
immunoglobin
- LFS
lateral flow strip
- LFSA
lateral flow strip assay
- LOD
limit of detection
- MERS
middle east respiratory syndrome
- NASBA
nucleic acid sequence‐based amplification
- NATs
nucleic acid‐based amplification tests
- NDV
Newcastle disease virus
- NIL
nanoimprint lithography
- NS1
nonstructural protein 1
- PC
polycarbonate
- PDMS
polydimethylsiloxane
- PLP
padlock probes
- POC
point‐of‐care
- pSiNW
porous silicon nanowire
- qRT‐PCR
quantitative reverse transcription‐polymerase chain reaction
- RCA
rolling circle amplification
- RT‐LAMP
reverse transcription loop‐mediated isothermal amplification
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SARS‐coronavirus
severe acute respiratory syndrome‐related coronavirus
- SiNWs
silicon nanowires
- SPM
sample preparation multiplexer
- STL
stereolithography
- WHO
The World Health Organization
- μENIA
microchip Europium nanoparticles immunoassay
Basiri A, Heidari A, Nadi MF, et al. Microfluidic devices for detection of RNA viruses. Rev Med Virol. 2021;31:e2154. 10.1002/rmv.2154
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Weiss SR, Navas‐Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ji W, Wang W, Zhao X, Zai J, Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross‐species transmission from snake to human. J Med Virol. 2020;92(4). 10.1002/jmv.25682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Kielian M, Mettenleiter TC, Roossinck M, eds, Advances in Virus Research. Vol 100. Cambridge, MA: Academic Press; 2018;163‐188. 10.1016/bs.aivir.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID‐19) and the probable outbreak size on the diamond princess cruise ship: a data‐driven analysis. Int J Infect Dis. 2020;93:201‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis. 2020;20:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rezaei N. COVID‐19 affects healthy pediatricians more than pediatric patients. Infect Control Hosp Epidemiol. 2020;1‐1. 10.1017/ice.2020.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jahanshahlu L, Rezaei N. Central nervous system involvement in COVID‐19. Arch Med Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lotfi M, Rezaei N. SARS‐CoV‐2: a comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. 2020. 10.1002/jmv.26123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saleki K, Banazadeh M, Saghazadeh A, Rezaei N. The involvement of the central nervous system in patients with COVID‐19. Rev Neurosci. 2020;31(4):453‐456. [DOI] [PubMed] [Google Scholar]
- 11. Darbeheshti F, Rezaei N. Genetic predisposition models to COVID‐19 infection. Med Hypotheses. 2020;142:109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yousefzadegan S, Rezaei N. Case report: death due to COVID‐19 in three brothers. Am J Trop Med Hyg. 2020;102(6):1203‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahrami A, Vafapour M, Moazzami B, Rezaei N. Hyperinflammatory shock related to COVID‐19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. 2020. 10.1111/jpc.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fathi N, Rezaei N. Lymphopenia in COVID‐19: therapeutic opportunities. Cell Biol Int. 2020;44(9):1792‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nasab MG, Saghazadeh A, Rezaei N. SARS‐CoV‐2‐a tough opponent for the immune system. Arch Med Res. 2020. 10.1016/j.arcmed.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saghazadeh A, Rezaei N. Immune‐epidemiological parameters of the novel coronavirus ‐ a perspective. Expert Rev Clin Immunol. 2020;16(5):465‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahu KK, Siddiqui AD, Rezaei N, Cerny J. Challenges for management of immune thrombocytopenia during COVID‐19 pandemic. J Med Virol. 2020;1‐6. 10.1002/jmv.26251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID‐19: friend or foe? Life Sci. 2020;256:117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanaei S, Rezaei N. COVID‐19: developing from an outbreak to a pandemic. Arch Med Res. 2020. 10.1016/j.arcmed.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jabbari P, Jabbari F, Ebrahimi S, Rezaei N. COVID‐19: a chimera of two pandemics. Disaster Med Public Health Prep. 2020;1‐3. 10.1017/dmp.2020.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saghazadeh A, Rezaei N. Towards treatment planning of COVID‐19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti‐antibodies, immunoglobulins, and corticosteroids. Int Immunopharmacol. 2020;84:106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basiri A, Pazhouhnia Z, Beheshtizadeh N, Hoseinpour M, Saghazadeh A, Rezaei N. Regenerative medicine in COVID‐19 treatment: real opportunities and range of promises. Stem Cell Rev Rep. 2020;1‐13. 10.1007/s12015-020-09994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jahanshahlu L, Rezaei N. Monoclonal antibody as a potential anti‐COVID‐19. Biomed Pharmacother. 2020;129:110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lotfi M, Hamblin MR, Rezaei N. COVID‐19: transmission, prevention, and potential therapeutic opportunities. Clinica Chimica Acta. 2020;508:254‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed K, Rodríguez‐Román E, Rahmani F, et al. Borderless collaboration is needed for COVID‐19‐a disease that knows no borders. Infect Control Hosp Epidemiol. 2020;1‐2. 10.1017/ice.2020.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Momtazmanesh S, Ochs HD, Uddin LQ, et al. All together to fight COVID‐19. Am J Trop Med Hyg. 2020;102(6):1181‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moradian N, Ochs HD, Sedikies C, et al. The urgent need for integrated science to fight COVID‐19 pandemic and beyond. J Transl Med. 2020;18(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cella LN, Blackstock D, Yates MA, Mulchandani A, Chen W. Detection of RNA viruses: current technologies and future perspectives. Crit Rev Eukaryot Gene Expr. 2013;23(2):125‐137. [DOI] [PubMed] [Google Scholar]
- 29. Compton J. Nucleic acid sequence‐based amplification. Nature. 1991;350(6313):91‐92. [DOI] [PubMed] [Google Scholar]
- 30. Gurukumar K, Priyadarshini D, Patil J, et al. Development of real time PCR for detection and quantitation of dengue viruses. Virol J. 2009;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watzinger F, Ebner K, Lion T. Detection and monitoring of virus infections by real‐time PCR. Mol Aspects Med. 2006;27(2–3):254‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anusha J, Kim BC, Yu K‐H, Raj CJ. Electrochemical biosensing of mosquito‐borne viral disease, dengue: a review. Biosens Bioelectron. 2019;142:111511. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Gayle AA, Wilder‐Smith A, Rocklov J. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2). 10.1093/jtm/taaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutierrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus Disease‐19 (COVID‐19): relationship to duration of infection. Radiology. 2020;295:200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laura Elaine Lamb PDBH, Royal Oak MUS. Rapid detection of novel coronavirus (COVID‐19) by reverse transcription‐loop‐ mediated isothermal amplification. The Lancet. 2020. 10.1101/2020.02.19.20025155 [DOI] [Google Scholar]
- 37. Sofi Ibrahim M, Kulesh DA, Saleh SS, et al. Real‐time PCR assay to detect smallpox virus. J Clin Microbiol. 2003;41(8):3835‐3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou X, Liu D, Zhong R, et al. Determination of SARS‐coronavirus by a microfluidic chip system. Electrophoresis. 2004;25(17):3032‐3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferguson BS, Buchsbaum SF, Wu TT, et al. Genetic analysis of H1N1 influenza virus from throat swab samples in a microfluidic system for point‐of‐care diagnostics. J Am Chem Soc. 2011;133(23):9129‐9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, Abafogi AT, Tran BM, et al. Integrated microfluidic Preconcentration and nucleic amplification system for detection of influenza a virus H1N1 in saliva. Micromachines. 2020;11(2):203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Safavieh M, Kaul V, Khetani S, et al. Paper microchip with a graphene‐modified silver nano‐composite electrode for electrical sensing of microbial pathogens. Nanoscale. 2017;9(5):1852‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu X, Xia Y, Tang Y, et al. A nanostructured microfluidic immunoassay platform for highly sensitive infectious pathogen detection. Small. 2017;13(24):1700425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang RQ, Hong SL, Wen CY, Pang DW, Zhang ZL. Rapid detection and subtyping of multiple influenza viruses on a microfluidic chip integrated with controllable micro‐magnetic field. Biosens Bioelectron. 2018;100:348‐354. [DOI] [PubMed] [Google Scholar]
- 44. Soares RRG, Neumann F, Caneira CRF, et al. Silica bead‐based microfluidic device with integrated photodiodes for the rapid capture and detection of rolling circle amplification products in the femtomolar range. Biosens Bioelectron. 2019;128:68‐75. [DOI] [PubMed] [Google Scholar]
- 45. Squires TQ. Stephen. Microfluidics: fluid physics at the nanoliter scale. Rev Mod Phys. 2005;77:977‐1026. [Google Scholar]
- 46. Campbell JM, Balhoff JB, Landwehr GM, Rahman SM, Vaithiyanathan M, Melvin AT. Microfluidic and paper‐based devices for disease detection and diagnostic research. Int J Mol Sci. 2018;19(9):2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu WT, Zhu L, Qin QW, Zhang Q, Feng H, Ang S. Microfluidic device as a new platform for immunofluorescent detection of viruses. Lab Chip. 2005;5(11):1327‐1330. [DOI] [PubMed] [Google Scholar]
- 48. Xia Y, Si J, Li Z. Fabrication techniques for microfluidic paper‐based analytical devices and their applications for biological testing: a review. Biosens Bioelectron. 2016;77:774‐789. [DOI] [PubMed] [Google Scholar]
- 49. Mahmud MA, Blondeel EJM, Kaddoura M, MacDonald BD. Features in microfluidic paper‐based devices made by laser cutting: how small can they be? Micromachines. 2018;9(5):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nath P, Fung D, Kunde YA, Zeytun A, Branch B, Goddard G. Rapid prototyping of robust and versatile microfluidic components using adhesive transfer tapes. Lab Chip. 2010;10(17):2286‐2291. [DOI] [PubMed] [Google Scholar]
- 51. Walsh DI 3rd, Kong DS, Murthy SK, Carr PA. Enabling microfluidics: from clean rooms to Makerspaces. Trends Biotechnol. 2017;35(5):383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li D. Encyclopedia of Microfluidics and Nanofluidics: Springer Science & Business Media, NewYork; 2008. [Google Scholar]
- 53. Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86(7):3240‐3253. [DOI] [PubMed] [Google Scholar]
- 54. Ho CM, Ng SH, Li KH, Yoon YJ. 3D printed microfluidics for biological applications. Lab Chip. 2015;15(18):3627‐3637. [DOI] [PubMed] [Google Scholar]
- 55. Capel AJ, Edmondson S, Christie SDR, Goodridge RD, Bibb RJ, Thurstans M. Design and additive manufacture for flow chemistry. Lab Chip. 2013;13(23):4583‐4590. [DOI] [PubMed] [Google Scholar]
- 56. Bhattacharjee N, Urrios A, Kang S, Folch A. The upcoming 3D‐printing revolution in microfluidics. Lab Chip. 2016;16(10):1720‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan HN, Chen Y, Shu Y, Chen Y, Tian Q, Wu H. Direct, one‐step molding of 3D‐printed structures for convenient fabrication of truly 3D PDMS microfluidic chips. Microfluid Nanofluid. 2015;19(1):9‐18. [Google Scholar]
- 58. Ariga K, Hill JP, Lee MV, Vinu A, Charvet R, Acharya S. Challenges and breakthroughs in recent research on self‐assembly. Sci TechnolAdv Mater. 2008;9(1):014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Biswas A, Bayer IS, Biris AS, Wang T, Dervishi E, Faupel F. Advances in top–down and bottom–up surface nanofabrication: techniques, applications & future prospects. Adv Colloid Interface Sci. 2012;170(1):2‐27. [DOI] [PubMed] [Google Scholar]
- 60. Gale BK, Jafek AR, Lambert CJ, et al. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions. 2018;3(3):60. [Google Scholar]
- 61. Isobe G, Kanno I, Kotera H, Yokokawa R. Perfusable multi‐scale channels fabricated by integration of nanoimprint lighography (NIL) and UV lithography (UVL). Microelectron Eng. 2012;98:58‐63. [Google Scholar]
- 62. Mali P, Sarkar A, Lal R. Facile fabrication of microfluidic systems using electron beam lithography. Lab Chip. 2006;6(2):310‐315. [DOI] [PubMed] [Google Scholar]
- 63. Li Z, Gu Y, Wang L, et al. Hybrid nanoimprint‐soft lithography with sub‐15 nm resolution. Nano Lett. 2009;9(6):2306‐2310. [DOI] [PubMed] [Google Scholar]
- 64. Nguyen N‐T, Wereley ST, Shaegh SAM. Fundamentals and Applications of Microfluidics: Artech House. Norwood, MA: Artech House, Inc; 2019. [Google Scholar]
- 65. Haeberle S, Zengerle R. Microfluidic platforms for lab‐on‐a‐chip applications. Lab Chip. 2007;7(9):1094‐1110. [DOI] [PubMed] [Google Scholar]
- 66. Huang FC, Liao CS, Lee GB. An integrated microfluidic chip for DNA/RNA amplification, electrophoresis separation and on‐line optical detection. Electrophoresis. 2006;27(16):3297‐3305. [DOI] [PubMed] [Google Scholar]
- 67. Kim YT, Chen Y, Choi JY, et al. Integrated microdevice of reverse transcription‐polymerase chain reaction with colorimetric immunochromatographic detection for rapid gene expression analysis of influenza a H1N1 virus. Biosens Bioelectron. 2012;33(1):88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu X, Zhao J, Hu A, et al. A novel microfluidic device integrated with chitosan‐modified capillaries for rapid ZIKV detection. Micromachines. 2020;11(2):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fraisse A, Coudray‐Meunier C, Martin‐Latil S, et al. Digital RT‐PCR method for hepatitis a virus and norovirus quantification in soft berries. Int J Food Microbiol. 2017;243:36‐45. [DOI] [PubMed] [Google Scholar]
- 70. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop‐mediated isothermal amplification of DNA. Nucl Acids Res. 2000;28(12):e63‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roy S, Mohd‐Naim NF, Safavieh M, Ahmed MU. Colorimetric nucleic acid detection on paper microchip using loop mediated isothermal amplification and crystal violet dye. ACS Sensors. 2017;2(11):1713‐1720. [DOI] [PubMed] [Google Scholar]
- 72. Song J, Mauk MG, Hackett BA, Cherry S, Bau HH, Liu C. Instrument‐free point‐of‐care molecular detection of Zika virus. Anal Chem. 2016;88(14):7289‐7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oshiki M, Miura T, Kazama S, et al. Microfluidic PCR amplification and MiSeq amplicon sequencing techniques for high‐throughput detection and genotyping of human pathogenic RNA viruses in human feces, sewage, and oysters. Front Microbiol. 2018;9:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gao Y, Pallister J, Lapierre F, Crameri G, Wang L‐F, Zhu Y. A rapid assay for Hendra virus IgG antibody detection and its titre estimation using magnetic nanoparticles and phycoerythrin. J Virol Methods. 2015;222:170‐177. [DOI] [PubMed] [Google Scholar]
- 75. Wang Y, Ruan Q, Lei Z‐C, et al. Highly sensitive and automated surface enhanced raman scattering‐based immunoassay for H5N1 detection with digital microfluidics. Anal Chem. 2018;90(8):5224‐5231. [DOI] [PubMed] [Google Scholar]
- 76. Shah KG, Yager P. Wavelengths and lifetimes of paper autofluorescence: a simple substrate screening process to enhance the sensitivity of fluorescence‐based assays in paper. Anal Chem. 2017;89(22):12023‐12029. [DOI] [PubMed] [Google Scholar]
- 77. Wang C‐H, Chang C‐P, Lee G‐B. Integrated microfluidic device using a single universal aptamer to detect multiple types of influenza viruses. Biosens Bioelectron. 2016;86:247‐254. [DOI] [PubMed] [Google Scholar]
- 78. Ciftci S, Neumann F, Hernández‐Neuta I, et al. A novel mutation tolerant padlock probe design for multiplexed detection of hypervariable RNA viruses. Sci Rep. 2019;9(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schneider D, Tuerk C, Gold L. Selection of high affinity RNA ligands to the bacteriophage R17 coat protein. J Mol Biol. 1992;228(3):862‐869. [DOI] [PubMed] [Google Scholar]
- 80. Torres‐Chavolla E, Alocilja EC. Aptasensors for detection of microbial and viral pathogens. Biosens Bioelectron. 2009;24(11):3175‐3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chand R, Neethirajan S. Microfluidic platform integrated with graphene‐gold nano‐composite aptasensor for one‐step detection of norovirus. Biosens Bioelectron. 2017;98:47‐53. [DOI] [PubMed] [Google Scholar]
- 82. Du K, Cai H, Park M, et al. Multiplexed efficient on‐chip sample preparation and sensitive amplification‐free detection of Ebola virus. Biosens Bioelectron. 2017;91:489‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weisse JM, Marconnet AM, Kim DR, et al. Thermal conductivity in porous silicon nanowire arrays. Nanoscale Res Lett. 2012;7(1):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xia Y, Tang Y, Yu X, et al. Label‐free virus capture and release by a microfluidic device integrated with porous silicon nanowire Forest. Small. 2017;13(6):1603135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu J, Zhao J, Petrochenko P, Zheng J, Hewlett I. Sensitive detection of influenza viruses with europium nanoparticles on an epoxy silica sol‐gel functionalized polycarbonate‐polydimethylsiloxane hybrid microchip. Biosens Bioelectron. 2016;86:150‐155. [DOI] [PubMed] [Google Scholar]
- 86. Meagher RJ, Negrete OA, Van Rompay KK. Engineering paper‐based sensors for Zika virus. Trends Mol Med. 2016;22(7):529‐530. [DOI] [PubMed] [Google Scholar]
- 87. Lamb LE, Bartolone SN, Ward E, Chancellor MB. Rapid Detection of Novel Coronavirus (COVID19) by Reverse Transcription‐Loop‐Mediated Isothermal Amplification. Available at SSRN 3539654. 2020. Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. El‐Tholoth M, Bau HH, Song J. A single and two‐Stage, closed‐tube, molecular test for the 2019 Novel Coronavirus (COVID‐19) at home, clinic, and points of entry. ChemRxiv. 2020. Feb 19. [Google Scholar]
- 89. Nguyen T, Duong Bang D, Wolff A. 2019 novel coronavirus disease (COVID‐19): paving the road for rapid detection and point‐of‐care diagnostics. Micromachines. 2020;11(3):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. https://www.biomedomics.com/products/infectiousdisease/covid-19-rt/
- 91. Yang T, Wang Y‐C, Shen C‐F, Cheng C‐M. Point‐of‐care RNA‐based diagnostic device for COVID‐19. Point‐of‐Care RNA‐Based Diagnostic Device for COVID‐19. Multidisciplinary Digital Publishing Institute; 2020:165. www.mdpi.com/journal/diagnostics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ye X, Xu J, Lu L, Li X, Fang X, Kong J. Equipment‐free nucleic acid extraction and amplification on a simple paper disc for point‐of‐care diagnosis of rotavirus a. Anal Chim Acta. 2018;1018:78‐85. [DOI] [PubMed] [Google Scholar]
- 93. Yucha RW, Hobbs KS, Hanhauser E, et al. High‐throughput characterization of HIV‐1 reservoir reactivation using a single‐cell‐in‐droplet PCR assay. EBioMedicine. 2017; 20:217‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Powell L, Wiederkehr RS, Fauvart M, et al. Rapid and sensitive detection of viral nucleic acids using silicon microchips. Analyst. 2018;143(11):2596‐2603. [DOI] [PubMed] [Google Scholar]
- 95. Fernández‐Carballo BL, McBeth C, McGuiness I, et al. Continuous‐flow, microfluidic, qRT‐PCR system for RNA virus detection. Anal Bioanal Chem. 2018;410(1):33‐43. [DOI] [PubMed] [Google Scholar]
- 96. Kaarj K, Akarapipad P, Yoon J‐Y. Simpler, faster, and sensitive Zika virus assay using smartphone detection of loop‐mediated isothermal amplification on paper microfluidic chips. Sci Rep. 2018;8(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lin X, Huang X, Urmann K, Xie X, Hoffmann MR. Digital loop‐mediated isothermal amplification on a commercial membrane. ACS Sensors. 2019;4(1):242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ganguli A, Ornob A, Yu H, et al. Hands‐free smartphone‐based diagnostics for simultaneous detection of Zika, Chikungunya, and dengue at point‐of‐care. Biomed Microdevices. 2017;19(4):73. [DOI] [PubMed] [Google Scholar]
- 99. Kong M, Li Z, Wu J, et al. A wearable microfluidic device for rapid detection of HIV‐1 DNA using recombinase polymerase amplification. Talanta. 2019;205:120155. [DOI] [PubMed] [Google Scholar]
- 100. Yang B, Kong J, Fang X. Bandage‐like wearable flexible microfluidic recombinase polymerase amplification sensor for the rapid visual detection of nucleic acids. Talanta. 2019;204:685‐692. [DOI] [PubMed] [Google Scholar]
- 101. Magro L, Jacquelin B, Escadafal C, et al. Based RNA detection and multiplexed analysis for Ebola virus diagnostics. Sci Rep. 2017;7(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Freitas TA, Proença CA, Baldo TA, et al. Ultrasensitive immunoassay for detection of citrus tristeza virus in citrus sample using disposable microfluidic electrochemical device. Talanta. 2019;205:120110. [DOI] [PubMed] [Google Scholar]
- 103. Li F, Zheng Y, Wu J, et al. Smartphone assisted immunodetection of HIV p24 antigen using reusable, centrifugal microchannel array chip. Talanta. 2019;203:83‐89. [DOI] [PubMed] [Google Scholar]
- 104. Iswardy E, Tsai T‐C, Cheng I‐F, Ho T‐C, Perng GC, Chang H‐C. A bead‐based immunofluorescence‐assay on a microfluidic dielectrophoresis platform for rapid dengue virus detection. Biosens Bioelectron. 2017;95:174‐180. [DOI] [PubMed] [Google Scholar]
- 105. Singh R, Hong S, Jang J. Label‐free detection of influenza viruses using a reduced graphene oxide‐based electrochemical immunosensor integrated with a microfluidic platform. Sci Rep. 2017;7:42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dixon C, Ng AH, Fobel R, Miltenburg MB, Wheeler AR. An inkjet printed, roll‐coated digital microfluidic device for inexpensive, miniaturized diagnostic assays. Lab Chip. 2016;16(23):4560‐4568. [DOI] [PubMed] [Google Scholar]
- 107. Rackus DG, Dryden MD, Lamanna J, et al. A digital microfluidic device with integrated nanostructured microelectrodes for electrochemical immunoassays. Lab Chip. 2015;15(18):3776‐3784. [DOI] [PubMed] [Google Scholar]
- 108. Lum J, Wang R, Hargis B, et al. An impedance aptasensor with microfluidic chips for specific detection of H5N1 avian influenza virus. Sensors. 2015;15(8):18565‐18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Draz MS, Venkataramani M, Lakshminarayanan H, et al. Nanoparticle‐enhanced electrical detection of Zika virus on paper microchips. Nanoscale. 2018;10(25):11841‐11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lau D, Walsh JC, Peng W, et al. Fluorescence biosensor for real‐time interaction dynamics of host proteins with HIV‐1 capsid tubes. ACS Appl Mater Interfaces. 2019;11(38):34586‐34594. [DOI] [PubMed] [Google Scholar]
- 111. Qin P, Park M, Alfson KJ, et al. Rapid and fully microfluidic Ebola virus detection with CRISPR‐Cas13a. ACS Sensors. 2019;4(4):1048‐1054. [DOI] [PubMed] [Google Scholar]
- 112. Du K, Park M, Griffiths A, et al. Microfluidic system for detection of viral RNA in blood using a barcode fluorescence reporter and a photocleavable capture probe. Anal Chem. 2017;89(22):12433‐12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shen K‐M, Sabbavarapu NM, Fu C‐Y, et al. An integrated microfluidic system for rapid detection and multiple subtyping of influenza a viruses by using glycan‐coated magnetic beads and RT‐PCR. Lab Chip. 2019;19(7):1277‐1286. [DOI] [PubMed] [Google Scholar]
- 114. Phillips EA, Moehling TJ, Ejendal KF, et al. Microfluidic rapid and autonomous analytical device (microRAAD) to detect HIV from whole blood samples. Lab Chip. 2019;19(20):3375‐3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sabalza M, Yasmin R, Barber CA, et al. Detection of Zika virus using reverse‐transcription LAMP coupled with reverse dot blot analysis in saliva. PloS One. 2018;13(2):e0192398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Haleyur Giri Setty MK, Liu J, Mahtani P, et al. Novel time‐resolved fluorescence europium nanoparticle immunoassay for detection of human immunodeficiency virus‐1 group O viruses using microplate and microchip platforms. AIDS Res Hum Retroviruses. 2016;32(6):612‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang R, Zhao R, Li Y, et al. Rapid detection of multiple respiratory viruses based on microfluidic isothermal amplification and a real‐time colorimetric method. Lab Chip. 2018;18(22):3507‐3515. [DOI] [PubMed] [Google Scholar]
- 118. Ciftci S, Neumann F, Abdurahman S, et al. Digital rolling circle amplification–based detection of Ebola and other tropical viruses. J Mol Diagn. 2020;22(2):272‐283. [DOI] [PubMed] [Google Scholar]
- 119. Klug KE, Reynolds KA, Yoon JY. A capillary flow dynamics‐based sensing modality for direct environmental pathogen monitoring. Chemistry. 2018;24(23):6025‐6029. [DOI] [PubMed] [Google Scholar]
- 120. Tao Y, Rotem A, Zhang H, et al. Rapid, targeted and culture‐free viral infectivity assay in drop‐based microfluidics. Lab Chip. 2015;15(19):3934‐3940. [DOI] [PubMed] [Google Scholar]
- 121. Mauk M, Song J, Bau HH, et al. Miniaturized devices for point of care molecular detection of HIV. Lab Chip. 2017;17(3):382‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


