Visual Abstract
Keywords: dialysis, peritoneal dialysis, acute renal failure, COVID-19
Hospitalized patients with AKI rarely initiate peritoneal dialysis (PD) because of the limited availability of surgeons or interventionists with experience in placing PD catheters as well as of nephrologists and trained nursing staff who can manage and perform PD. Moreover, to minimize the incidence of mechanical complications, pericatheter leaks, and infection, the European Best Practice Guidelines for PD recommend not using PD catheters until 2 weeks after insertion,1 which is not possible in the inpatient setting where there may be an urgent need for dialysis. The slow clearance of PD, a gentle form of RRT, also makes it impractical in these circumstances. As a result, patients with AKI usually undergo placement of a central venous catheter to start hemodialysis or continuous RRT (CRRT) instead.
This process runs smoothly under normal circumstances, when demand for RRT is not at the unprecedented levels experienced in New York City hospitals during the novel coronavirus disease 2019 (COVID-19) pandemic. However, because of such challenges as staffing issues, supply shortages, and dialysis circuit clotting during this health care crisis, many hospitals turned to acute PD to augment their RRT capacity.
At the Mount Sinai Hospital in New York City, as demand for RRT more than doubled during the COVID-19 pandemic, an acute PD program was swiftly planned and implemented. This article describes the implementation process and this program’s outcomes.
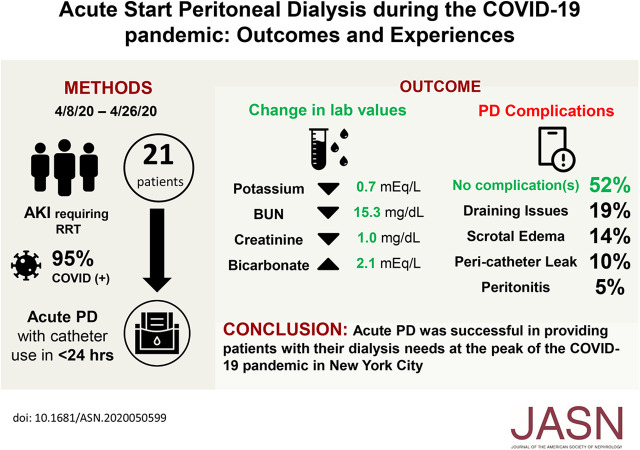
We inserted 21 PD catheters in 21 patients. Table 1 shows patient demographics and characteristics: mean age (62 years), sex (57% men), race (14% white, 52% black, and 33% Hispanic), mean weight and body mass index (BMI; 95 kg and 33 kg/m2), and comorbidities (diabetes mellitus, 12 patients [57%]; hypertension, 11 patients [52%]). Four had a history of abdominal surgery. At the time of catheter placement, 16 (76%) were receiving mechanical ventilation, with mean ventilator settings of tidal volume of 402 ml, respiratory rate of 29 breaths per minute, fraction of inspired oxygen of 53%, and positive end expiratory pressure of 7 cm H2O.
Table 1.
Demographics, baseline characteristics, and PD catheter outcomes
| Characteristic | Outcome |
|---|---|
| Age, yr | 62 (IQR, 53–67.5) |
| Men | 12 (57%) |
| Race | |
| White | 3 (14%) |
| Black | 11 (52%) |
| Other (Latino) | 7 (33%) |
| Weight, kg | 95 (IQR, 79–114.5) |
| BMI, kg/m2 | 33 (IQR, 26.5–40.9) |
| Past medical history | |
| Diabetes mellitus | 12 (57%) |
| Hypertension | 11 (52%) |
| Abdominal surgery | 4 (19%) |
| COVID-19 positive | 20 (95%) |
| Ventilated | 16 (76%) |
| Catheter insertion | |
| Surgery | 16 (76%) |
| Interventional radiology | 5 (24%) |
| Time to catheter use, h | |
| <6 | 2 (10%) |
| 6–12 | 8 (38%) |
| 12–24 | 11 (52%) |
| Complications | |
| Mechanical flow disruption | 4 (19%) |
| Scrotal edema | 3 (14%) |
| Pericatheter leak | 2 (10%) |
| Peritonitis | 1 (5%) |
| No complications | 11 (52%) |
IQR, interquartile range.
Patients with obesity are a high-risk group to treat for COVID-19.2 Moreover, hypertension and diabetes mellitus are both known risk factors for poor outcomes in patients with COVID-19.3,4 In addition, COVID-19 acute respiratory distress syndrome is associated with prolonged mechanical ventilation and a high short-term mortality.5
To the date of submission, nine (43%) of the patients have died. Of the 12 who remained alive, 3 (25%) had renal recovery, 2 (17%) were discharged on RRT, and 7 (58%) remained hospitalized on RRT. The high proportion of patients with risk factors helps explain why so many died during their hospitalization. There are some similarities in our numbers to those reported in a case series of 30 patients from Brazil, in which 57% of 30 patients with AKI died and 23% had renal recovery, although only 7% remained on dialysis after 30 days.6 It is important to note that the Brazilian patients were on high-volume PD, and the study took place before the COVID-19 pandemic, which may affect renal recovery rates.
Table 2 shows baseline laboratory data, both mean values from day 3 of PD onward and the difference between values on the day before initiating PD and the day of PD termination. The mean change in potassium was consistent with a decrease of 0.7 meq/L; serum bicarbonate increased by 2.1 meq/L, and serum albumin levels were unchanged. However, there was a greater decrease in BUN in the last day of treatment compared with the mean of day 3 onward (−15.3 versus −9.9 meq/L, respectively). Factors that may have blunted an otherwise even larger drop in BUN included incremental increases in average protein intake in both tube and oral feeds (as patients’ BUN values decreased while on dialysis) and the initiation of intravenous methylprednisolone in three patients. Medical management largely maintained normoglycemia, although one patient who developed severe hyperglycemia on day 3 of PD was converted to a prescription of exclusively icodextrin and had no further glycemic issues.
Table 2.
Laboratory data prior to and changes achieved while on PD
| Serum Laboratory | Mean | Pre-PD − Post-PDa |
|---|---|---|
| Potassium, meq/L | 4.7 | −0.7 |
| BUN, mg/dl | 129.2 | −15.3 |
| Creatinine, mg/dl | 8.2 | −1.0 |
| Bicarbonate, meq/L | 19.4 | 2.1 |
| Glucose, mg/dl | 188 | 4.0 |
| Albumin, g/dl | 1.8 | 0.0 |
The difference between values from the day prior to PD initiation to the day of PD termination.
Prior to PD initiation, 12 patients required hemodialysis, continuous venovenous hemofiltration (CVVH), or both. Nineteen central venous catheters were placed for 21 hemodialysis and 6 CVVH treatments. Mean times on hemodialysis and CVVH were 1.9 and 57 hours, respectively. Mean blood flow rate was 251.2 ml/min. Nine hemodialysis treatments (43%) were terminated early because of hypotension or access clotting issues. Severe hypercoagulability is a well documented complication of COVID-19, with patients demonstrating markedly elevated fibrinogen and d-dimer plasma levels.7 Only one patient required another form of RRT during time on PD. Acute PD provided an alternative form of RRT that bypassed the issue of hypercoagulability.
On average, six patients were on PD per day; mean PD duration was 8 days. For catheter placement, 76% were placed surgically at the bedside, and 24% were placed percutaneously in the interventional radiology suite. Eleven patients had no PD-related complications, and three developed scrotal edema. Four had mechanical flow disruptions; tidal PD may have helped remediate this issue in two patients, whereas the other two, both of whom had a past abdominal surgery, were unable to start PD. A limitation of bedside catheter placement is the inability to lyse intra-abdominal adhesions or perform an omentopexy, if needed. Despite the initiation of PD <24 hours after catheter placement in a cohort with a high BMI, only two patients had a pericatheter leak. One leak resolved after 48 hours with lowering of the fill volume to 0.75 L before gradually increasing it; the other developed on day 19 of PD treatment and resulted in treatment termination. One patient developed bacterial peritonitis, which responded to intraperitoneal antibiotics.
The average PD prescription was five exchanges: 1.5-L fills of 2.5% dextrose solution over 10 hours, with a last fill of 1.5 L of 7.5% icodextrin. The mean total cycle fill volume was 7049 ml; dwell time was 86 minutes; drain time was 30 minutes; and the ultrafiltration rate was 405 ml/d, with a mean urine output of 622 ml/d. The average protein intake was 75.4 g/d.
We calculated weekly Kt/V values for eight patients from 16 urine and 17 dialysate samples, finding a mean total weekly Kt/V of 2.18, meeting the International Society for Peritoneal Dialysis recommendation of 2.10 Kt/V or greater.8 The authors recognize, however, that urea clearance may be a suboptimal marker for dialysis adequacy.9 A comparison of hemodialysis needs before and during acute PD is in Supplemental Material, section 5.
The acute PD program successfully helped mitigate the demand for RRT during the COVID-19 pandemic’s peak at Mount Sinai Hospital. It was a challenging process that required implementation of a treatment protocol, collaboration with interventional radiology and surgery for catheter placement, and hiring of additional nursing staff. We were able to provide our patients with this life-sustaining treatment and aid in their care. We hope that our experience demonstrates the feasibility of initiating an acute PD program during a health care crisis that results in increased demand for RRT.
Methods
From April 8, 2020 to April 26, 2020, we considered PD catheter placement for patients who required RRT and met one of the following criteria, regardless of their COVID-19 status, weight, BMI, or past medical history: AKI necessitating initiation of RRT; AKI already on RRT, with an expected prolonged hospital stay; or AKI on RRT with multiple dialysis circuit clotting issues. Prior abdominal surgery was not an absolute contraindication. Prone patients were excluded.
Patients on ventilators underwent bedside surgical catheter placement, whereas those who were on room air or oxygen supplementation via nasal cannula underwent percutaneous catheter placement by interventional radiology (Supplemental Material, section 1). Of note, these procedures did not involve either a purse-string suture or a paramedian incision.
Initiation of PD occurred within 24 hours of catheter insertion. We used the HomeChoice cycler and lactate-based PD solutions. We used low fill volumes (1 L) for the first treatment, with five to six exchanges over a 10-hour period, and we added heparin at a dose of 500 U/L to all peritoneal fluid bags. If there was no pericatheter leak, we gradually increased fill volumes to 1.5–2 L. All patients were prescribed an extended dwell of icodextrin to limit overnight troubleshooting.
PD nurses documented the exit site’s condition, the PD effluent’s appearance, the daily treatment total fill and drain volumes, initial drain volumes, average dwell and drain times, and total ultrafiltration achieved. We also obtained and recorded routine laboratory data and urine output, and we noted ventilator settings for patients requiring mechanical ventilation, protein intake from either tube feeds or regular oral intake, and steroid dosage delivered. Urine and PD effluent samples were collected, and urea clearance was calculated from April 17, 2020 onward (Supplemental Material, section 2).
We also documented the number of treatments, average blood flow rates, treatment durations, and the number of central venous catheters placements for patients who were on hemodialysis or CVVH prior to PD initiation as well as the number of dialysis treatments performed in the hospital in the 19 days prior to and during the acute PD program’s duration.
A PD staff member was always present to troubleshoot any issues arising during the patients’ treatments (Supplemental Material, section 3). Reasons for treatment termination included persistent peritonitis, mechanical flow disruptions, development of hernias or leaks, inadequate clearance and ultrafiltration, and prone positioning. Discharge planning is described in Supplemental Material, section 4.
We used Microsoft Excel 16.30 for data collection and basic analysis. This project was approved by the institutional review board of the Mount Sinai School of Medicine (IRB-20–03479).
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors acknowledge the hard work, dedication, and extraordinary efforts of the Mount Sinai Home Dialysis Unit PD nursing staff, without whom this project would not have been possible.
Dr. Osama El Shamy, Dr. Shuchita Sharma, and Dr. Jaime Uribarri designed the study; Dr. Mohamed Abdelbaset, Dr. Osama El Shamy, and Dr. Niralee Patel analyzed the data; Dr. Osama El Shamy made the tables; Dr. Linda Chenet, Dr. Noah Cohen, Dr. Osama El Shamy, Dr. David Lee, Dr. Robert Lookstein, Dr. Niralee Patel, Dr. Shuchita Sharma, Dr. Joji Tokita, and Dr. Jaime Uribarri drafted and revised the paper; and Dr. Mohamed Abdelbaset, Dr. Linda Chenet, Dr. Noah Cohen, Dr. Osama El Shamy, Dr. David Lee, Dr. Robert Lookstein, Dr. Niralee Patel, Dr. Shuchita Sharma, Dr. Joji Tokita, and Dr. Jaime Uribarri approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050599/-/DCSupplemental.
Supplemental Material. Catheter placement and use, total Kt/V calculation method, ancillary staff roles and cycler troubleshooting, discharge planning, and hemodialysis demands before and during the acute peritoneal dialysis program.
References
- 1.Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al.: EBPG Expert Group on Peritoneal Dialysis : European best practice guidelines for peritoneal dialysis. 1 General guidelines. Nephrol Dial Transplant 20[Suppl 9]: ix2, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A: Comentary: Obesity: The “Achilles heel” for COVID-19? Metabolism 108: 154251, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al.: COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options [published online ahead of print April 30, 2020]. Cardiovasc Res doi:10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Yang Y, Dong K, Yan Y, Zhang S, Ren H, et al.: Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China [published online ahead of print May 1, 2020]. Endocr Pract doi:10.4158/EP-2020-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, et al.: Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy [published online ahead of print April 23, 2020]. Crit Care Resusc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponce D, Caramori JT, Barretti P, Balbi AL: Peritoneal dialysis in acute kidney injury: Brazilian experience. Perit Dial Int 32: 242–246, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al.: COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 120: 998–1000, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, et al.: Peritoneal dialysis for acute kidney injury. Perit Dial Int 34: 494–517, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perl J, Dember LM, Bargman JM, Browne T, Charytan DM, Flythe JE, et al.: American Society of Nephrology Dialysis Advisory Group : The use of a multidimensional measure of dialysis adequacy-moving beyond small solute kinetics. Clin J Am Soc Nephrol 12: 839–847, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.