Significance Statement
The cause of kidney injury in COVID-19 is unclear. In an autopsy study of a single patient with COVID-19 and acute oliguric renal failure, the authors identified intracellular viral arrays within proximal tubular epithelial cells by electron microscopy, consistent with direct infection of the kidney by SARS-CoV-2. They also found ultrastructural features similar to those described in reports of kidney cell lines infected with the related SARS-CoV-1 virus. Virally infected tubular cells showed isometric vacuolization on light microscopy. These findings provide confirmatory evidence of direct kidney infection by SARS-CoV-2 in a patient. However, this work does not exclude other causes of kidney injury, and additional study is needed to determine the frequency and clinical significance of direct kidney infection on renal failure in COVID-19.
Keywords: COVID-19, SARS-CoV-2, acute kidney failure, autopsy, renal pathology, electron microscopy
Abstract
Background
A significant fraction of patients with coronavirus disease 2019 (COVID-19) display abnormalities in renal function. Retrospective studies of patients hospitalized with COVID-19 in Wuhan, China, report an incidence of 3%–7% progressing to ARF, a marker of poor prognosis. The cause of the renal failure in COVID-19 is unknown, but one hypothesized mechanism is direct renal infection by the causative virus, SARS-CoV-2.
Methods
We performed an autopsy on a single patient who died of COVID-19 after open repair of an aortic dissection, complicated by hypoxic respiratory failure and oliguric renal failure. We used light and electron microscopy to examine renal tissue for evidence of SARS-CoV-2 within renal cells.
Results
Light microscopy of proximal tubules showed geographic isometric vacuolization, corresponding to a focus of tubules with abundant intracellular viral arrays. Individual viruses averaged 76 µm in diameter and had an envelope studded with crown-like, electron-dense spikes. Vacuoles contained double-membrane vesicles suggestive of partially assembled virus.
Conclusions
The presence of viral particles in the renal tubular epithelium that were morphologically identical to SARS-CoV-2, and with viral arrays and other features of virus assembly, provide evidence of a productive direct infection of the kidney by SARS-CoV-2. This finding offers confirmatory evidence that direct renal infection occurs in the setting of AKI in COVID-19. However, the frequency and clinical significance of direct infection in COVID-19 is unclear. Tubular isometric vacuolization observed with light microscopy, which correlates with double-membrane vesicles containing vacuoles observed with electronic microscopy, may be a useful histologic marker for active SARS-CoV-2 infection in kidney biopsy or autopsy specimens.
The association between coronavirus disease 2019 (COVID-19) and ARF is an evolving area of study. Renal dysfunction is common in COVID-19, with a sizeable fraction of patients presenting with proteinuria or elevated BUN.1,2 A minority of patients progress to more severe renal disease; retrospective studies of patients hospitalized with COVID-19 in Wuhan, China, report an incidence of ARF ranging from 3% to 7%.3–6 Early data from Italy suggests a correlation between kidney injury and overall disease severity, with renal failure up to four times more common in patients in intensive care units relative to hospitalized patients not requiring intensive care unit admission.7 Renal failure in COVID-19 appears to be an independent risk factor for death in hospitalized patients, with reported adjusted hazard ratios of 3.5 (95% CI, 1.50 to 8.27) and 4.7 (95% CI, 2.55 to 8.75) for stage 2 and 3 AKI, respectively.1 Preexisting renal disease may also present an increased risk for poor outcome, with an adjusted hazard ratio of 2.0 (95% CI, 1.32 to 3.15) for hospitalized patients. Various mechanisms for kidney injury in COVID-19 have been proposed, including ischemic injury owing to multiorgan failure, inflammatory injury secondary to cytokine storm, and direct infection.2,8 Immunohistochemical and PCR-based approaches are possible approaches to demonstrate direct renal infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but both could be confounded by either filtration of circulating viral antigens or incidental detection of nucleic acids in plasma. Detection of virus within renal cells by electron microscopy has been proposed as a straightforward approach to demonstrate direct renal involvement.
Clinical Presentation
A 53-year-old male with a history of obesity, hyperlipidemia, and a recent sick contact presented with an acute type A aortic dissection, extending from the sinotubular junction to the iliac bifurcation, with compression of the right renal artery. On angiogram, renal arteries had a beaded appearance suspicious for fibromuscular dysplasia. The dissection was repaired via median sternotomy with ascending and hemiarch aortic replacement, and the patient was discharged to intensive care on mechanical ventilation. His postoperative course was notable for leukocytosis (white blood cell count of 14,000–18,000 per µl) and persistently elevated fraction of inspired oxygen requirements. On the fourth postoperative day he was successfully extubated but remained oxygen-dependent on high-flow nasal cannula and Bilevel Positive Airway Pressure.
On the sixth postoperative day, the patient’s hypoxemia progressed, requiring reintubation as well as inhaled nitrous oxide. A chest x-ray showed bilateral patchy opacities, a PCR respiratory viral panel was negative, and a locally performed RT-PCR was positive for SARS-CoV-2. He was initially treated with hydroxychloroquine 600 mg twice daily, then 200 mg three times daily for 3 days. In addition, he enrolled in a randomized, controlled trial of sarilumab (IL-6 inhibitor), although it is not known whether he received therapy or placebo. Despite therapy, the patient developed multiorgan dysfunction, with mildly elevated liver enzymes and new-onset diabetes. On the 11th postoperative day, his respiratory and renal status worsened with progressive hypoxemia and acidosis recalcitrant to intravenous bicarbonate.
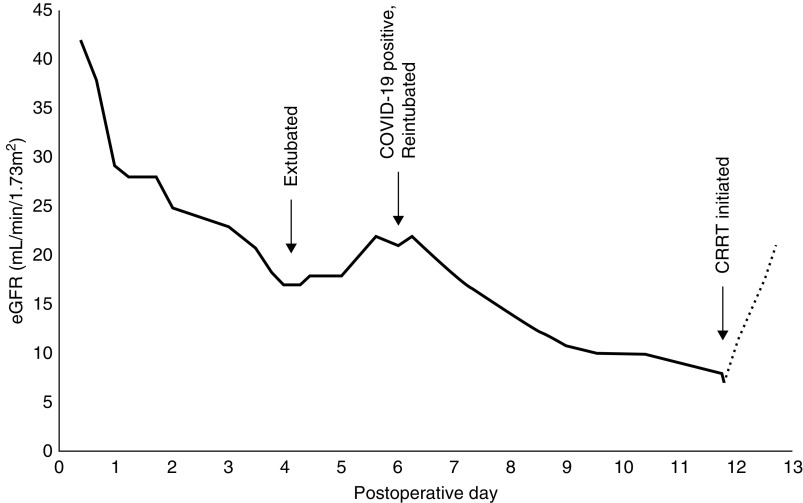
The patient’s renal function closely mirrored his respiratory function (Figure 1). Initially, his eGFR declined acutely after the cardioplegic aortic repair, from 42 ml/min per 1.73 m2 on admission to 29 ml/min per 1.73 m2. In the setting of diuresis with furosemide and metolazone on postoperative day 4, his eGFR further declined to 17 ml/min per 1.73 m2. His renal function briefly improved (eGFR 22 ml/min per 1.73 m2), but subsequently deteriorated on postoperative day 6, simultaneous with the development of acute respiratory distress. Over the course of his illness, his urine output remained between 2 and 4 L per day, but on the 11th postoperative day he became acutely oliguric (100 ml/d), and continuous RRT was initiated in the setting of acidosis and declining respiratory status.
Figure 1.
Kidney function recrudescence with diagnosis of COVID-19. The patient’s eGFR over the course of hospitalization, measured from admission for aortic repair on day 0. eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration equation. CRRT, continuous RRT.
The patient died of cardiac arrest on the 12th postoperative day, and an autopsy was performed in a negative pressure suite, with renal tissue taken for light and electron microscopy. Because of the risk of infectious aerosols, the autopsy was limited to the chest and abdomen, and routine protective equipment (N95 respirator, gown, double gloves, and eye protection) was supplemented with face shields and disposable full-body suits.
Results
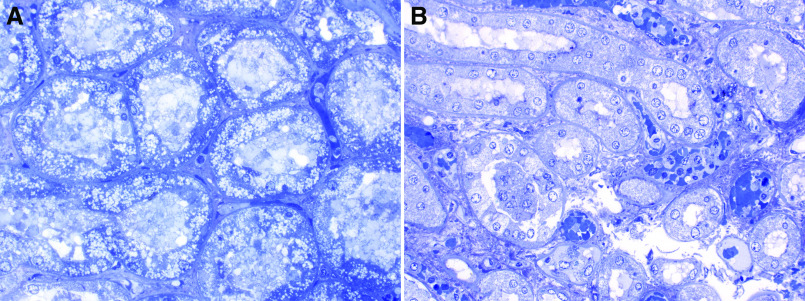
At autopsy, the lungs showed gross and microscopic evidence of diffuse acute alveolar damage with well formed hyaline membranes, edema, and early acute bronchopneumonia, consistent with early reports of patient deaths after SARS-CoV-2 infection. The left and right kidneys weighed 230 g and 240 g, respectively, and were grossly unremarkable. Light microscopic examination of formalin-fixed paraffin sections of the kidneys showed mild autolysis, with no definitive nuclear inclusions (not shown). There was no evidence of glomerulitis, FSGS, or a tubulointerstitial inflammatory infiltrate. Of note, some of the toluidine blue–stained epoxy sections showed focal tubular isometric vacuolization (Figure 2A). Other epoxy sections showed mild-to-moderate necrosis and karyolysis more typical for ischemic injury/autolysis (Figure 2B).
Figure 2.
Proximal tubular isometric vacuolization by light microscopy correlates with SARS-CoV-2 infection. (A) Toluidine blue–stained epoxy section with extensive isometric vacuolization of proximal tubular epithelial cells, corresponding to location of intracellular virus by electron microscopy. (B) Tubular epithelial cells from a section showing mild autolysis and no vacuolization. Electron microscopy showed no virus present in this section. Both images at ×400 magnification.
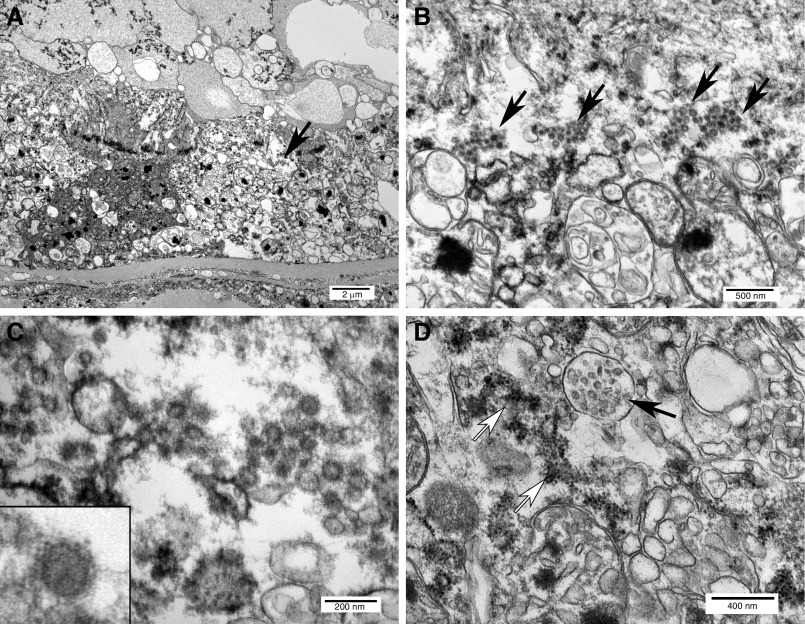
Ultrastructural analysis, limited to the kidney, revealed abundant viral forms within tubular epithelial cells in one of three examined blocks, correlating directly to the areas of isometric vacuolization (Figure 3A). Individual viruses ranged in size from 65 to 91 nm (mean 76 nm). Mature-appearing viruses were predominantly located within the cytoplasm, focally organized into small arrays (Figure 3B). Viral particles were composed of cores with intermediate electron density, surrounded by an envelope studded with abundant crown-like, electron-dense spikes (Figure 3C). Adjacent vacuoles contained abundant ovoid double-membrane vesicles associated with rough endoplasmic reticulum, representing possible viral assembly (Figure 3D). Examined glomeruli and vascular endothelial cells were negative for virus, and coronavirus was not identified in tubular epithelium with more conventional injury and necrosis by light microscopy.
Figure 3.
Ultrastructural features of coronavirus infection and replication in proximal tubular epithelial cells detected after death from COVID-19. (A) Proximal tubule oriented with basement membrane at the bottom and lumen at the top, containing vacuolated and partially degenerated epithelial cells with abundant viral particles (arrow). (B) Intracytoplasmic viral arrays (arrows) within tubular epithelial cells. (C) Detail of viruses showing envelope with crown-like projections. Inset: single virus. (D) Vacuole with double-membrane vesicles (solid arrow) adjacent to ribosome-studded rough endoplasmic reticulum (open arrows), similar to structures reported in SARS-CoV-1–infected cells.
Discussion
There are currently multiple plausible mechanisms for renal injury in COVID-19, including ischemic injury, cytokine storm, and direct infection. Previous research has established a potential mechanism for kidney infection by SARS-CoV-2. The receptor for SARS-CoV-2 cellular entry is angiotensin-converting enzyme 2, which is present at high concentrations in the brush borders of renal tubular epithelial cells, and at lower levels in glomerular and vascular endothelial cells.9 In vitro experiments have demonstrated SARS-CoV-2 viral infection and replication within Vero cells, a primate kidney epithelial cell line.10,11 In humans, SARS-CoV-2 was initially detected by RT-PCR in the urine of a minority of patients with COVID-19; although other studies show no detectible urine shedding.12,13 Recently, Su et al.14 demonstrated coronavirus by electron microscopy in the renal tubular epithelium of seven of 26 patients with COVID-19 at autopsy.
Electron micrographs from this autopsy confirm this finding and provide strong evidence for direct infection. The crown-like morphology is similar to previously published images, and the viral diameter is within the reported 70–90 µm size range of SARS-CoV-2.10 The organization of intracellular viruses into arrays is suggestive of intracellular manufacture and assembly. The presence of double-membrane vesicles with possible viral assembly near the rough endoplasmic reticulum in this case is analogous to the proposed mechanism for viral assembly of SARS-CoV-1.15 Finally, vacuolation of epithelial cells has been reported after infection with SARS-CoV-1, similar to the isometric vacuoles in the proximal tubular epithelium in this patient.16 The isometric vacuoles by light microscopy correlate with double-membrane vesicles containing vacuoles by electron microscopy and were also noted in a recent autopsy series.14 Isometric vacuolization may be a helpful diagnostic clue for the presence of direct renal infection, but this association needs to be confirmed in a larger series using immunohistochemistry or electron microscopy.
There are notable caveats to the findings in this case report. First, although this renal tissue showed only mild postmortem change by light microscopy, there is still significant ultrastructural injury. Disrupted plasma membrane-bound organelles could mimic viral structures. Second, direct kidney infection may be an uncommon occurrence in patients with COVID-19 and renal dysfunction. Indeed, there was no detectible intrarenal virus by electron microscopy in one additional autopsy of a patient with COVID-19, although that patient had nonoliguric stage 2 AKI and interpretation was limited by extensive autolysis. Most importantly, the identification of direct infection by SARS-CoV-2 does not preclude other mechanisms of renal injury in COVID-19. In this case, coronavirus was only identified in a subset of tubules, and direct infection is likely either an incidental finding or a contributing cause of kidney injury. This patient’s renal failure was likely multifactorial, with preexisting ischemic renal injury complicated by progressive hypoxemia and focal viral infection.
This autopsy report is illustrative of the increased morbidity and mortality associated with renal failure and COVID-19. Additional studies are needed to determine the frequency of direct renal infection in patients with COVID-19, and whether there is an association with viral titers in plasma or with preexisting renal comorbidities.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors are indebted to Yoel Bailey and Yinru Sieracki for their invaluable technical assistance with the electron microscopy, as well as to Monique Micallef and Lisa Neal for their expert assistance with the autopsy. Dr. Jeffrey Myers assisted with evaluation of the lung pathology.
Dr. Allecia M. Wilson and Dr. Jeffrey M. Jentzen performed/supervised the autopsy. Dr. Evan A. Farkash supervised the electron microscopy and drafted and revised the manuscript. All authors approved the final version of the manuscript.
Dr. Evan A. Farkash reports personal fees from Novartis, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naicker S, Yang C-W, Hwang S-J, Liu B-C, Chen J-H, Jha V: The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int 97: 824–828, 2020. 10.1016/j.kint.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al.: Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507–513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al.: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 55: 105924, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print Feb 7, 2020]. JAMA doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannuzzella F, Vivekanand J, Canchi A; International Society of Nephrology Academy Online Learning: Webinar: COVID19 for the Nephrologist: Real-Life Experience from Italy. 2020. Available at: https://academy.theisn.org/isn/2020/covid-19/290431/prof.vivekanand.jha.doctor.francesco.iannuzzella.26.doctor.arvind.canchi.html?f=menu%3D13%2Abrowseby%3D8%2Asortby%3D2%2Alabel%3D19791. Accessed March 30, 2020
- 8.Durvasula R, Wellington T, McNamara E, Watnick S: COVID-19 and kidney failure in the acute care setting: Our experience from Seattle [published online ahead of print Apr 7, 2020]. Am J Kidney Dis doi:10.1053/j.ajkd.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuiri S, Ohashi Y: ACE and ACE2 in kidney disease. World J Nephrol 4: 74–82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JM, Chung YS, Jo HJ, Lee NJ, Kim MS, Woo SH, et al.: Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect 11: 3–7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, et al.: Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 117: 7001–7003, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al.: Detection of SARS-CoV-2 in different types of clinical specimens [published online ahead of print Mar 11, 2020]. JAMA doi:10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al.: Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients [published online ahead of print Mar 28, 2020]. Clin Infect Dis doi:10.1093/cid/ciaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoops K, Kikkert M, Worm SH, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, et al.: SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol 6: e226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng ML, Tan SH, See EE, Ooi EE, Ling AE: Early events of SARS coronavirus infection in Vero cells. J Med Virol 71: 323–331, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]