Significance Statement
Social distancing is an important tool in preventing the spread of SARS-CoV-2 during the COVID-19 pandemic. Dialysis centers that treat patients undergoing hemodialysis typically are not conducive to social distancing, and there is limited published experience available for guidance. The authors describe control measures—including universal protective equipment, a regular screening process, and case isolation—implemented during a rapidly developing COVID-19 epidemic in a large dialysis center, as well as service pressures experienced. Risk factors for infection included older age and infection rates within specific satellite units; aspects of unit design might help explain clustering of cases in those units. After the third week, COVID-19 cases fell short of the projected epidemic course, suggesting that control was achieved and that early adoption of control measures can help protect patients.
Keywords: clinical epidemiology, haemodialysis, COVID-19
Abstract
Background
During the coronavirus disease 2019 (COVID-19) epidemic, many countries have instituted population-wide measures for social distancing. The requirement of patients on dialysis for regular treatment in settings typically not conducive to social distancing may increase their vulnerability to COVID-19.
Methods
Over a 6-week period, we recorded new COVID-19 infections and outcomes for all adult patients receiving dialysis in a large dialysis center. Rapidly introduced control measures included a two-stage routine screening process at dialysis entry (temperature and symptom check, with possible cases segregated within the unit and tested for SARS-CoV-2), isolated dialysis in a separate unit for patients with infection, and universal precautions that included masks for dialysis nursing staff.
Results
Of 1530 patients (median age 66 years; 58.2% men) receiving dialysis, 300 (19.6%) developed COVID-19 infection, creating a large demand for isolated outpatient dialysis and inpatient beds. An analysis that included 1219 patients attending satellite dialysis clinics found that older age was a risk factor for infection. COVID-19 infection was substantially more likely to occur among patients on in-center dialysis compared with those dialyzing at home. We observed clustering in specific units and on specific shifts, with possible implications for aspects of service design, and high rates of nursing staff illness. A predictive epidemic model estimated a reproduction number of 2.2; cumulative cases deviated favorably from the model from the fourth week, suggesting that the implemented measures controlled transmission.
Conclusions
The COVID-19 epidemic affected a large proportion of patients at this dialysis center, creating service pressures exacerbated by nursing staff illness. Details of the control strategy and characteristics of this epidemic may be useful for dialysis providers and other institutions providing patient care.
Following the appearance of a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) and its associated disease (coronavirus disease 2019 [COVID-19]) in Wuhan, China on December 27th,1 there has been rapid viral dissemination leading to a global pandemic. COVID-19 is highly contagious, and human-to-human transmission has been recognized from the outset.2,3 As a result, quarantine measures have played a central role in controlling cluster outbreaks, particularly in confined locations.4 With evidence of significant presymptomatic transmission,5 many countries have instituted population-wide policies to increase social distance, often termed “lockdown.”
The treatment of many long-term conditions requires regular contact with health care staff, preventing social distancing. Most patients receiving hemodialysis need to continue to attend their dialysis unit three times per week, where they encounter nursing staff and a number of other patients. Moreover, this predominantly elderly population has a high burden of comorbidity, including cardiovascular disease and diabetes: these are associated with a higher case-fatality rate6 in those with COVID-19. This combination makes the delivery of dialysis uniquely challenging in the face of the pandemic.
Though dialysis-specific guidance on COVID-19 is available,7–9 there is limited published experience of COVID-19 in dialysis populations. A single-center report from Wuhan (Ma Y, Diao B, Lv X, Zhu J, Liang W, Liu L, et al. [2020] 2019 Novel coronavirus disease in hemodialysis [HD] patients: Report from one HD center in Wuhan, China. 10.1101/2020.02.24.20027201) described an outbreak of 37 cases from a dialysis population of over 200, which was brought under control with radical measures. In two other coronavirus diseases (severe acute respiratory syndrome and Middle East respiratory syndrome), transmission during dialysis was reported, but both epidemics were substantially smaller than COVID-19.10,11
The first laboratory-confirmed case of COVID-19 in the United Kingdom occurred on January 30th. The United Kingdom, with a largely urban-centered epidemic, has responded with progressive social distancing measures, including the closure of social venues and schools from March 20th. We report the effect of the epidemic on a large urban dialysis population, identifying risk factors for infection and characteristics of transmission.
Methods
Setting
Imperial College Healthcare NHS Trust provides RRTs for a catchment population of 2.5 million living in northwest London, including over 1500 patients on dialysis.12 Dialysis care is delivered from a central hospital (with inpatient wards, a hospital dialysis unit, and an area for training and supervision of patients on home dialysis) with eight satellite hemodialysis units in other locations. Institutional transport is provided for patients unable to travel to dialysis, with up to six patients sharing a vehicle. Home dialysis includes peritoneal dialysis and home hemodialysis.
Study Design
This was a cohort study in which data were collected from electronic records from hospitals and dialysis units. The service evaluation and analysis were approved by the Renal Quality and Safety (Governance) Committee of Imperial College Healthcare NHS Trust in view of the project being an evaluation of a service change during the COVID-19 pandemic. Only fully anonymized patient data were used.
Study Population
The cohort included all adults with established renal failure treated with dialysis in satellite units, in the hospital, or at home starting before March 1st, 2020.
Outcome Assessment
Patient data were collected over 6 weeks from March 9th to April 19th, 2020, including all dialysis attendance and all admissions to hospital of cohort patients. Infection with SARS-CoV-2 was confirmed through real-time RT-PCR assay of nasopharyngeal swab specimens, and only laboratory-confirmed cases are included in the analysis.
Covariate Definitions
Dialysis unit, shift (day/time), and transplant status (active, suspended, or not listed) were defined at the start of the observation period. There was no loss to follow-up. Data were complete apart from ethnic status in 1.2% (excluded from the Cox model) and institutional transport: status was complete, but vehicle allocation was available only for 77.8%. Community risk of COVID-19 infection was determined by identifying the upper tier local authority for each patient’s domestic address combined with COVID-19 cumulative incidence by the end of the observation period for each local authority13 standardized against population size, divided into low-, medium-, and high-risk categories. Satellite unit area was defined as the total floor area of both dialysis delivery rooms and waiting rooms divided by the number of available dialysis stations. Station distance was defined as the linear (sight-line) distance between a station and the nearest neighboring station (measured from the center of each chair).
Statistical Analyses
Covariate data were compared using unpaired t tests and chi-squared tests. Multivariable Cox proportional hazards models assessed risk factors for acquisition of COVID-19 in patients treated in satellite hemodialysis units, with proportional hazards assumption tested by Schoenfeld residuals. Factors with a P value <0.10 on univariate analysis were entered into the model. The expected distribution of infection among occupants in shared institutional transport was calculated using the binomial distribution using the observed incidence across satellite units. Funnel plot control limits were calculated using a normal approximation of the binomial distribution. The basic reproduction number R0 was estimated from log cumulative case number by least squares regression using a serial interval of 4 days. Epidemic modeling was performed in Excel with susceptible-infectious-removed (SIR) compartments, and all patients were assumed to be susceptible at the start, with transmission according to the equation S(t+1)=S(t)–β×S(t)×I(t)/N.
Results
The first COVID-19 case was identified on March 13th. Over the next 6 weeks, of 1530 patients (median age 66; 58.2% men) receiving dialysis, 300 developed infection with SARS-CoV-2 (19.6%). Most patients (290) were receiving in-center hemodialysis before the start of the epidemic, but 8 were on peritoneal dialysis and 2 were on home hemodialysis, with patient characteristics given in Table 1. Most infection was acquired outside the hospital setting, but 12 patients acquired infection after hospital admission for a non–COVID-19 condition.
Table 1.
Clinical characteristics of patients
| Characteristics | Total Population | COVID-19 Cases |
|---|---|---|
| No. (% of total cohort) | 1530 (100) | 300 (19.6) |
| Age, yr, median (IQR) | 66 (55–75) | 67 (57–77) |
| Sex (%) | ||
| Men | 891 (58.2) | 180 (60.0) |
| Women | 639 (41.8) | 120 (40.0) |
| Ethnicity (%) | ||
| Asian | 642 (42.0) | 137 (45.7) |
| White | 470 (30.7) | 84 (28.0) |
| Black | 402 (26.2) | 75 (25.0) |
| Unknown | 28 (1.2) | 4 (1.3) |
| Diabetes (%) | 696 (45.4) | 155 (51.7) |
| Mode of dialysis (%) | ||
| Satellite unit HD | 1222 (79.9) | 273 (91.0) |
| Hospital HD | 128 (8.3) | 17 (5.67) |
| Home dialysis, HD or PD | 180 (11.7) | 10 (3.33) |
| Access | ||
| Peritoneal catheter | 155 (10.1) | 8 (2.7) |
| Fistula | 384 (25.1) | 82 (27.3) |
| Graft | 19 (1.2) | 7 (2.3) |
| Tunneled vascular catheter | 972 (63.5) | 203 (67.7) |
| Institutional transport (%) | 675 (44.1) | 163 (54.3) |
| Nursing home (%) | 29 (1.8) | 5 (1.7) |
HD, hemodialysis; PD, peritoneal dialysis.
Tests for SARS-CoV-2 were carried out according to clinical indication and local practice in those presenting to hospital services and following screening in dialysis units (Figure 1). A routine two-stage screening process was introduced at every dialysis session for every patient at the start of the observation period from March 9th. Patients were first screened by temperature and symptom inquiry: those with possible COVID-19 were segregated immediately within the dialysis unit and tested for SARS-CoV-2 by nasopharyngeal swab taken during dialysis. Unless hospitalized on the basis of clinical decision, patients with positive tests were then treated in isolation at a separate unit specifically for patients carrying SARS-CoV-2 starting from their next dialysis session. Initially, a hospital ward was used, but from March 17th, a dedicated isolation unit was opened (by displacing patients to an evening shift in another satellite unit); two further hemodialysis isolation units were similarly opened on March 30th and April 8th. There was no screening process for patients on home dialysis who may have had minor symptoms without presenting to hospital services. From a total of 457 tests for SARS-CoV-2 carried out in the cohort, including 363 in dialysis units following positive symptom report on screening, 65.6% were positive. Nineteen patients with a positive test also had a previous negative test carried out at a median (interquartile range [IQR]) of 9 (5–17) days earlier.
Figure 1.
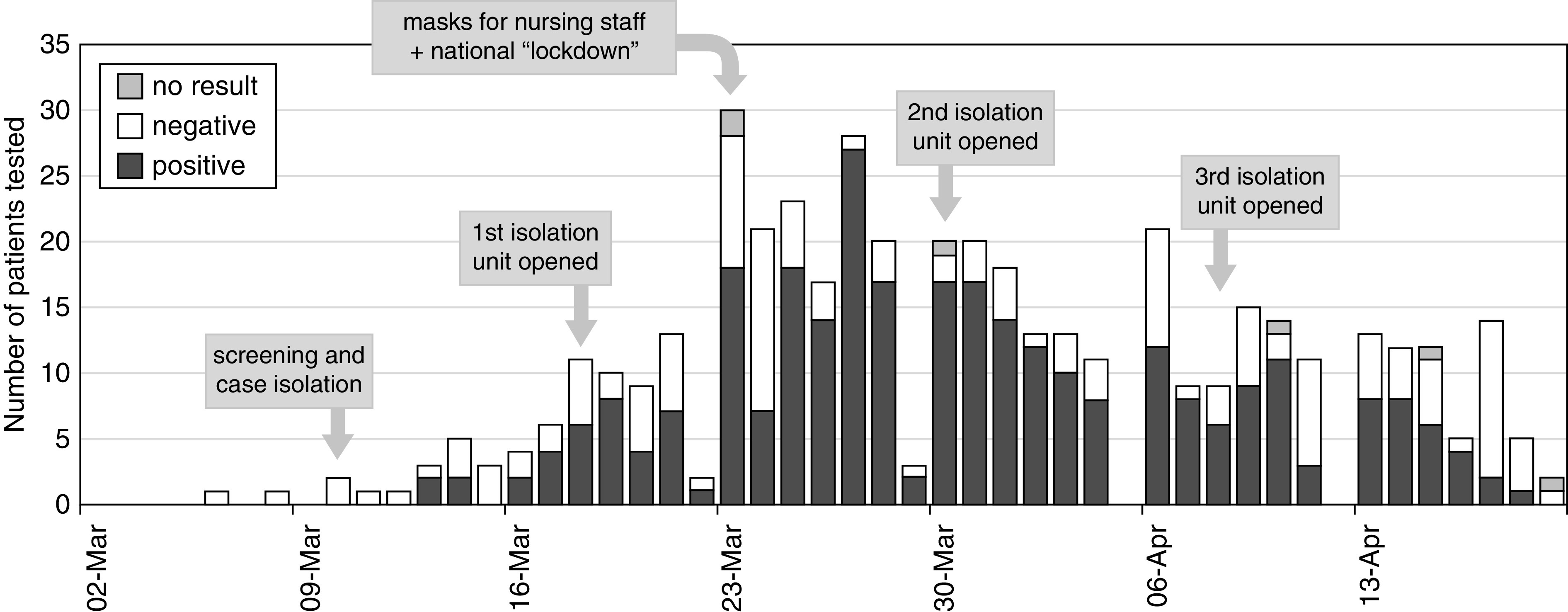
Epidemic timeline by incident patient testing. Epidemic timeline in patients on dialysis showing counts of patients with symptoms suggestive of COVID-19 and testing for SARS-CoV-2 according to date of test. From March 10, all patients on in-center hemodialysis were screened before each dialysis session (symptom questions and temperature) to select those for SARS-CoV-2 testing and dialysis in a segregated area of the unit: patients with positive tests were treated in a dedicated isolation unit starting from their next session. Tests were performed as clinically indicated in patients on home dialysis and those presenting to hospital emergency departments.
Nursing staff members in isolation units wore enhanced protective equipment, including sleeved gowns and FFP3 masks. Nursing staff members in other units wore surgical masks for all patient contact from the third week. Dialysis staff members were encouraged to report any febrile illness and to self-isolate at home for at least 7 days. For the majority of staff, no confirmatory test for SARS-CoV-2 was carried out in accordance with national guidance.
Following diagnosis, patients on in-center hemodialysis who were not hospitalized were isolated for outpatient dialysis for at least 14 days. Deisolation was considered after 14 days in patients without fever or ongoing symptoms, with first nonisolated dialysis occurring at median (IQR) interval of 18 (14–28) days after diagnosis. This led to a need for isolated outpatient dialysis, which peaked at 135 patients during week 4 and started to diminish during the sixth week (Figure 2).
Figure 2.
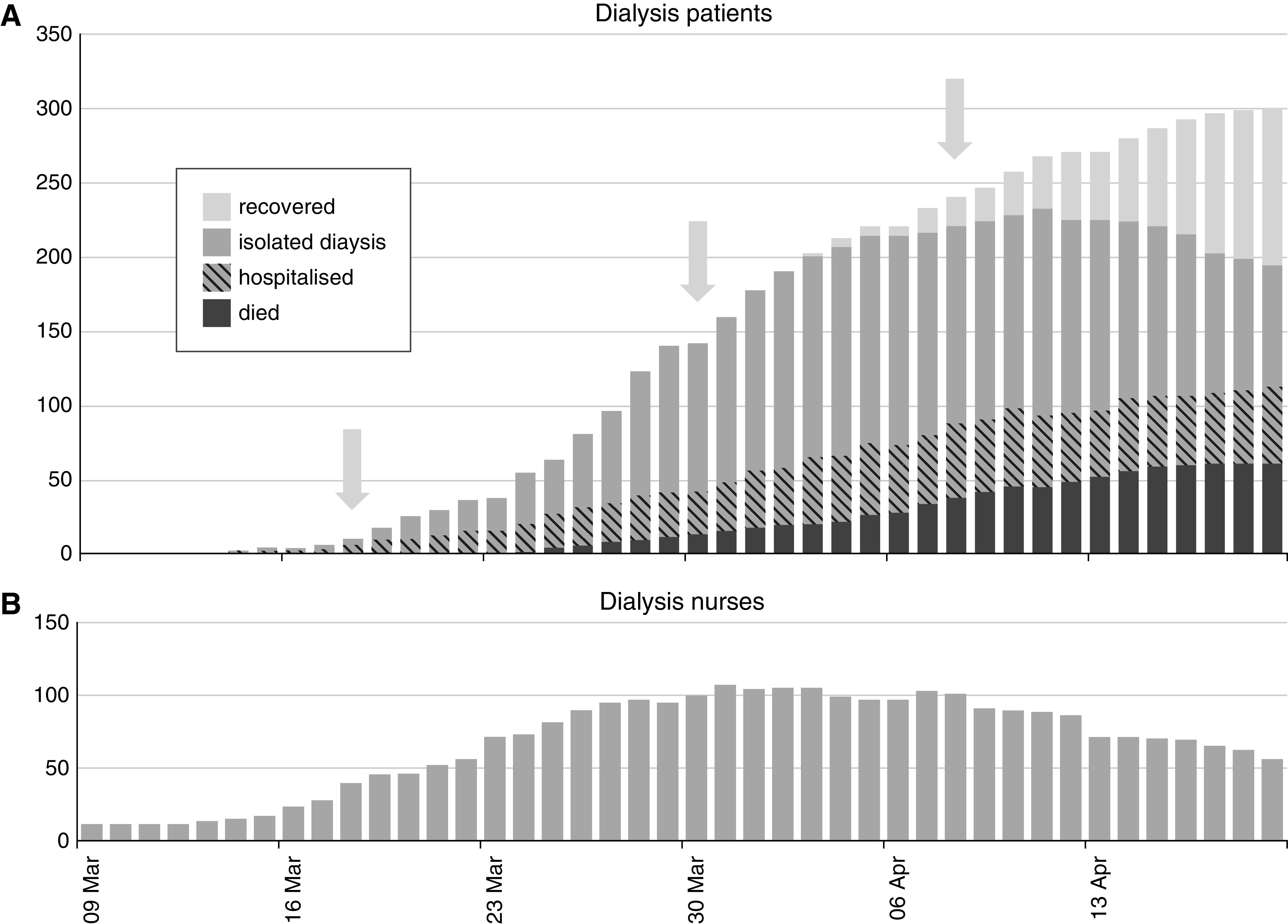
Cumulative counts of patients positive for SARS-CoV-2 according to clinical status and absent nursing staff during the epidemic between March 9 and April 20, 2020. (A) Clinical status of all patients on dialysis developing COVID-19. Patients on in-center hemodialysis who were not hospitalized were isolated for outpatient dialysis for at least 14 days. Prior to March 17, a hospital ward was used, after which three dedicated isolation units were created (arrows) by moving patients between units. (B) Absence from work due to illness (usually without SARS-CoV-2 testing) in dialysis nursing staff.
By the end of the observation period, after a median (IQR) postdiagnosis period of 21 (14–26) days, 239 patients with COVID-19 survived (79.7%), with 51 patients (17.0%) remaining in the hospital (Figure 2). Compared with those surviving, patients who died were older (72.0 versus 63.9 years; P<0.001) and less likely to be recorded as currently active on the deceased donor transplant register (10.7% versus 25.6%; P=0.009). There was no evidence of differences with regards to sex, weight, ethnicity, comorbidity (diabetes), or dialysis duration.
Risk factors for SARS-CoV-2 infection were analyzed for patients attending satellite hemodialysis units (n=1219; median age 66; 59.8% men); infection developed in 270 (22.1%). Compared with those unaffected, patients with detected infection were slightly older (67.2 versus 64.9; P=0.02). There was no evidence of a difference in sex, comorbidity (diabetes), dialysis duration, or activity on the deceased donor transplant register, and there was no evidence of a difference in infection proportion between ethnicities or differing levels of community infection according to residential location (Table 2). The use of institutional transport to attend dialysis was not associated with risk of infection, and there was no evidence of infection clustering within vehicle groups, with the distribution of infection among occupants closely resembling that expected by random dispersion (Table 3).
Table 2.
Risk factors for time to COVID-19 infection in patients attending satellite dialysis units (n=1219)
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Variables included | ||||
| Age, per decade increase | 1.11 (1.02 to 1.21) | 0.02 | 1.09 (0.99 to 1.20) | 0.06 |
| Unit, reference Unit D | ||||
| Unit A | 0.24 (0.11 to 0.50) | <0.001 | 0.24 (0.12 to 0.51) | <0.001 |
| Unit B | 0.67 (0.38 to 1.18) | 0.17 | 0.68 (0.39 to 1.21) | 0.22 |
| Unit C | 1.02 (0.66 to 1.59) | 0.91 | 1.01 (0.65 to 1.57) | 0.97 |
| Unit E | 1.07 (0.76 to 1.49) | 0.71 | 1.04 (0.75 to 1.46) | 0.81 |
| Unit F | 1.14 (0.72 to 1.81) | 0.56 | 1.14 (0.72 to 1.81) | 0.57 |
| Unit G | 2.20 (1.52 to 3.18) | <0.001 | 2.20 (1.52 to 3.18) | <0.001 |
| Variables excluded | ||||
| Sex, women | 0.97 (1.03 to 1.24) | 0.81 | ||
| Ethnicity, reference Asian | ||||
| Black | 0.87 (0.64 to 1.17) | 0.35 | ||
| White | 0.91 (0.68 to 1.20) | 0.50 | ||
| Diabetes | 1.23 (0.97 to 1.57) | 0.08 | ||
| Transport, institutional | 1.12 (0.88 to 1.42) | 0.37 | ||
| Vascular access, other versus catheter | 0.95 (0.74 to 1.23) | 0.71 | ||
| Community risk, reference medium | ||||
| Low | 1.06 (0.76 to 1.49) | 0.72 | ||
| High | 0.90 (0.68 to 1.19) | 0.44 | ||
Community risk is defined by residential address. HR, hazard ratio; 95% CI, 95% confidence interval.
Table 3.
Infection proportion among within-vehicle groups: observed (expected) values
| No. of Patient Occupants Sharing | No. of Vehicle Groups of This Type | No. of Patient Occupants Developing COVID-19 | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 1 | 56 | 43 (43.5) | 13 (12.5) | ||||
| 2 | 49 | 27 (29.6) | 19 (17.0) | 3 (2.4) | |||
| 3 | 66 | 30 (31.0) | 23 (26.6) | 10 (7.7) | 3 (0.7) | ||
| 4 | 32 | 13 (11.7) | 12 (13.4) | 5 (5.8) | 1 (1.1) | 1 (0.1) | |
| 5 | 9 | 3 (2.5) | 2 (3.6) | 3 (2.1) | 1 (0.6) | 0 (0.1) | 0 (0.0) |
Data are the number of vehicle groups in each category given as observed (expected). Expected values are calculated from the binomial distribution, with probability 0.221. Number of patients =525. Number of vehicle groups =212.
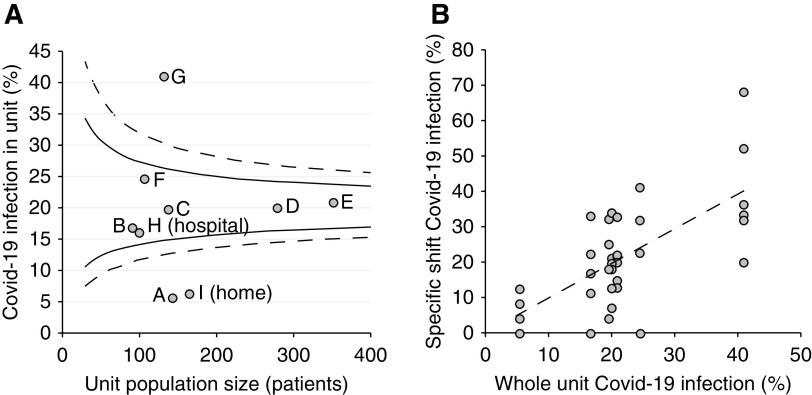
Between satellite units, differences in the proportions developing infection were observed, ranging from 5.6% to 41.5%. Age and dialysis unit were independently predictive of time to infection in a Cox proportional hazards model (Table 2). The role of chance in unit variability is visually demonstrated using a funnel plot to define control limits on the expected variation by unit size (Figure 3A). Possible explanations for unit differences were explored, including the proportion of self-reported staff illness, which followed the same pattern as patient infection (R=0.89; P=0.007). The satellite unit with the smallest number of infections was also the most spacious, but evidence of a linear relationship generally between unit space and infection was not seen (Table 4).
Figure 3.
Variation in infection proportion between satellite unit and shift. (A) Funnel plot showing unit infection by unit size (number of patients) for the seven nonisolation satellite units (indicated as A–G), with hospital dialysis (indicated as H) and home dialysis (indicated as I) included for comparison along with 90% (solid lines) and 99% (dashed lines) control limits. (B) Infection in specific shifts (n=39) of satellite units by proportion with infection in the whole unit, with linear regression line.
Table 4.
Infection proportion and characteristics of satellite dialysis units
| Characteristic | Dialysis Satellite Unit | R | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |||
| COVID-19 diagnosis | |||||||||
| Shift 1, N/at risk | 3/48 | 6/36 | 13/54 | 21/109 | 24/117 | 12/44 | 21/50 | ||
| Shift 2, N/at risk | 3/48 | 7/36 | 14/53 | 33/115 | 35/120 | 14/44 | 22/48 | ||
| Shift 3, N/at risk | 2/46 | 2/28 | 1/24 | 7/47 | 19/102 | 0/18 | 11/32 | ||
| COVID-19 cases, % of unit | 5.6 | 15.0 | 21.4 | 22.5 | 23.0 | 24.5 | 41.5 | ||
| COVID-19 exposure | |||||||||
| Date of first case (March) | 19th | 17th | 14th | 14th | 13th | 21st | 16th | ||
| Staff illness, % | 35.7 | 40.0 | 43.2 | 56.0 | 60.7 | 72.7 | 84.0 | 0.90 | 0.006 |
| Community COVID-19, % | 0.18 | 0.22 | 0.33 | 0.24 | 0.28 | 0.14 | 0.23 | 0.16 | 0.73 |
| Patient population | |||||||||
| Age | 61.4 (14.4) | 64.4 (13.6) | 66.9 (13.4) | 64.7 (14.3) | 66.7 (14.3) | 65.4 (14.4) | 63.3 (13.8) | 0.25 | 0.59 |
| Ethnicity, % Asian/Black | 68.3 | 65.3 | 84.2 | 72.7 | 76.5 | 37.9 | 76.1 | 0.10 | 0.83 |
| Diabetes, % | 49.7 | 36.0 | 45.8 | 43.9 | 47.6 | 46.7 | 50.8 | 0.33 | 0.47 |
| Unit size | |||||||||
| Stations | 24 | 19 | 28 | 66 | 62 | 22 | 25 | 0.08 | 0.86 |
| Dialysis space, m2 per station | 15.2 | 9.2 | 11.0 | 13.6 | 11.3 | 9.9 | 10.5 | −0.48 | 0.27 |
| Waiting room, m2 per station | 2.8 | 0.9 | 1.6 | 1.4 | 1.3 | 1.9 | 0.9 | −0.65 | 0.11 |
| Station distance, m | 3.0 (0.6) | 2.1 (0.4) | 2.1 (0.3) | 2.3 (0.1) | 1.8 (0.3) | 1.9 (0.2) | 1.9 (0.1) | −0.74 | 0.06 |
All correlations are between the variable of interest and proportion of patients with COVID-19. Age and station distance are given as mean (SD). Station distance is defined as the linear distance to the nearest neighboring station measured from the center of each chair. R, Pearson correlation coefficient.
Infection proportion also differed between the 39 specific dialysis-unit shifts, ranging from 0.0% to 68.0%. Shift infection was associated with underlying unit infection (Figure 3B), suggesting that part of the variation between shifts was explained by variation between units. Evidence for “vertical” transmission (e.g., from morning to afternoon patients in the same unit) was not observed, with no association between infection proportions in adjacent shifts within units (R=−0.16; P=0.33).
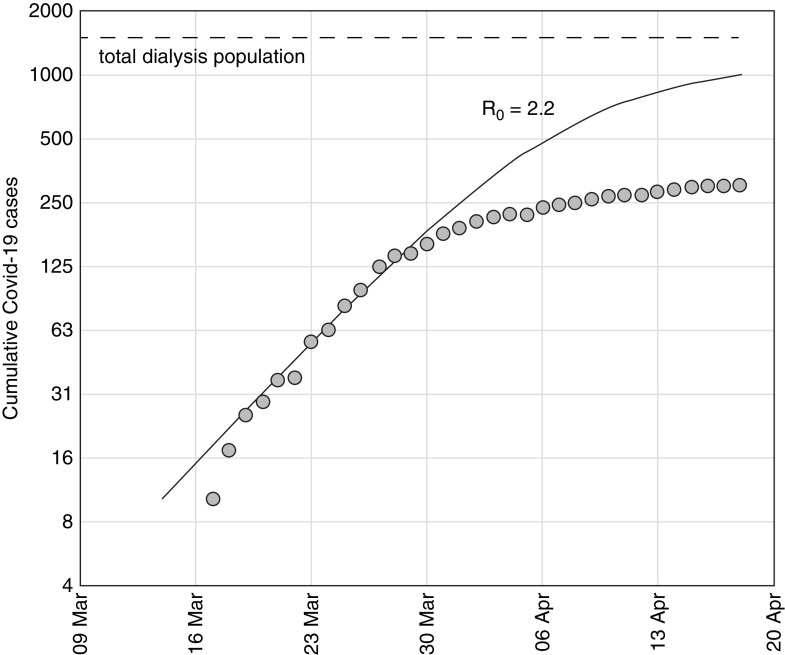
Using cumulative case numbers from the first 2 weeks, an initial reproduction number was estimated (R0=2.2), and an SIR model using this value was fitted to the observed number of cases at the end of the second week. The model predicted case numbers accurately during the third week, but thereafter, substantial deviation started to occur, with fewer cases observed than predicted by the model (Figure 4).
Figure 4.
Deviation from predictive model suggesting control of the epidemic. Cumulative number of COVID-19 cases between March 9 and April 20. Observed cumulative cases (log scale) starting from the tenth case alongside the modeled epidemic curve assuming an R0 of 2.2 (solid line), showing deviation from predicted course after the third week. The total dialysis population is also shown (dashed line).
Discussion
We report the abrupt appearance of COVID-19 within an urban dialysis center, showing its effect in a population unable to remain isolated at home. Although the epidemic was anticipated, it brought multiple challenges for the protection and safe treatment of patients needing dialysis. The characteristics of the epidemic in this setting are therefore worthy of detailed analysis, with implications for dialysis providers as well as other institutions where self-isolation is not possible, such as prisons and long-term care facilities.14
In the dialysis population described, COVID-19 was seen in over 20% of patients within 6 weeks of the first detected case. In contrast, by the end of observation, infection had been detected in <0.5% of the local adult population, and accounting for nondetected illness, it was estimated to have affected <5% of the population.13 Infection may be more severe and therefore, more often detected in populations with long-term medical conditions, particularly when screening for symptoms and fever, or alternatively, infection may be more commonly asymptomatic and missed when not all patients are tested. The estimated R0 of 2.2 is similar to that observed in other local COVID-19 epidemics; therefore, infection does not seem to be accelerated in this group. The relative sparing of patients on home dialysis suggests that transmission may be occurring particularly in those receiving in-center hemodialysis. Variation in risk between units may result from random variation in the introduction of infection from the community, but it also supports the possibility of within-unit transmission, suggesting that to understand this epidemiology, one might examine unit-level factors, such as nursing practices, unit design, and transport.
Though variation in transmission was observed, nursing practices are uniform across the satellite dialysis units studied. The variation observed between shifts suggests that any within-unit transmission is predominantly horizontal (within dialysis shifts) rather than vertical (from one shift to the next). Vertical transmission might be one’s main concern given the evidence of dispersion of SARS-CoV-2 on surfaces within health care settings15 and viral persistence16 on plastics, but this is reduced by well established cleaning practices to prevent blood-borne virus transmission.
Nursing practices cannot prevent airborne droplet transmission as patients arrive, dialyze, and leave, spending 5 or more hours in the unit, during which time SARS-CoV-2 remains viable.16 Given that droplets and aerosols can disperse >2 m,17 unit design is a more likely explanation for some of the variation: for example, the threefold difference between waiting area size of units A and G. Though no clear association between unit area and infection was observed, this study may be too small to detect the effect of unit size, which is likely to play a role in maintaining transmission despite the implementation of control measures.
The high rate of staff illness, mostly without confirmatory testing, is explained by responsible self-isolation in line with United Kingdom government advice, though some of this may not have been due to COVID-19. Dialysis staff risks have been noted in other infections, ranging from hepatitis at the inception of hospital-based dialysis18 to Middle East respiratory syndrome coronavirus more recently.19 Nursing illness was variable, with absences for the whole period ranging from 35% to 84% of staff in different units; it was not possible to infer the direction of the relationship, if any, between infection in patients and staff. However, the shared environment of patients and staff is highlighted by the similarity of their illness distributions.
One could speculate that universal screening of all patients might have mitigated transmission, particularly from presymptomatic patients,20 but this would have overwhelmed testing facilities available at the time. Screening clinically rather than by virus detection was also contemplated—a Wuhan dialysis unit reported screening the entire dialysis population (and all staff) using chest computed tomography21—but this was considered impractical. Because asymptomatic transmission is well recognized,22,23 with heavy viral shedding before symptom onset,5 patients may transmit infection on the dialysis session previous to diagnosis, and universal precautions, including the use of simple surgical masks, may be the most effective measures.24 Surgical masks were routinely used by nurses in all dialysis units from the third week, but masks were generally not in routine use for patients during the observation period, including on institutional transport.
Other suggested options for reducing transmission8 were considered but not adopted: reducing dialysis frequency may have improved distancing within units, but there was concern that patients might be more vulnerable at the onset of infection. Opening nonstandard shifts, such as overnight, might similarly have improved distancing but would have further stretched a nursing group already depleted by illness.
Only a single center was studied, albeit one with several satellite units, which limits the generalizability of conclusions, but it carries the advantage of uniformity of clinical practice across the study. Control lines in the funnel plot are likely to be too conservative due to overdispersion of results, and more centers would be needed to derive accurate confidence limits for outlier detection. The study was not primarily designed to assess mortality, and though age-specific survival seems broadly similar to other comorbid groups,25 no firm conclusions can be drawn: survival data presented should be interpreted with caution.
Dialysis centers experiencing cases of COVID-19 may prevent spread within units by the early adoption of universal protective equipment, a regular patient screening process, and case isolation, though there is no way of assessing the effectiveness or otherwise of interventions adopted in this study. Analysis of cumulative case numbers did suggest a departure from the epidemic course predicted from the early R0 estimation of 2.2, an estimation comparable with COVID-19 outbreaks in cruise ships.26 Three theories could explain this apparent reduction in expected cases. First, the control measures adopted may have been sufficient to substantially modify transmission, with the effect only expected after an interval of 2–3 weeks. Second, reduced numbers may reflect control of the epidemic within the wider community. Third, departure from the prediction may reflect limitations of SIR modeling, which assumes homogenous mixing and uniform susceptibility across a large population. In moderate-sized dialysis units with significant interpatient variability, these assumptions may not be valid,27 and models with variable susceptibility would predict a course closer to that observed.
COVID-19 caused an abrupt epidemic in this group of patients whose essential treatment makes them vulnerable. Details of the control strategy adopted, transmission characteristics, and epidemic course are of relevance to dialysis providers and other institutions as they face the same viral threat.
Disclosures
R. Corbett has the patent “A device for maintaining vascular access connections” (WO2017148836A1) issued to Imperial Innovations Limited. D. Nitsch reports grants from GlaxoSmithKline outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
Dr. Damien Ashby reports personal fees from Fibrogen outside the submitted work. We acknowledge both the patients involved in this work as well as their support by many colleagues during the pandemic. Infrastructure support for this work was provided by the National Institute for Health Research Imperial Biomedical Research Centre Imperial Biomedical Research Centre.
A list of senior clinicians at West London Renal and Transplant Centre caring for these patients and collaborating with this investigation is provided in the Supplemental Material.
Dr. Damien Ashby and Dr. Neill Duncan conceived the study; Dr. Damien Ashby, Dr. Sarah Blakey, Dr. Richard Corbett, Dr. Neill Duncan, Dr. Marina Loucaidou, and Dr. Adam McLean curated the data; Dr. Damien Ashby, Dr. Sarah Blakey, Dr. Richard Corbett, and Dr. Dorothea Nitsch analysed the data; Dr. Damien Ashby, Dr. Richard Corbett, and Dr. Dorothea Nitsch drafted and revised the paper; and Dr. Damien Ashby, Dr. Sarah Blakey, Dr. Richard Corbett, Dr. Neill Duncan, Dr. Marina Loucaidou, Dr. Adam McLean, and Dr. Dorothea Nitsch approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040534/-/DCSupplemental.
Supplemental Material. List of collaborating clinicians.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.: China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, et al.: A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395: 514–523, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.:. China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocklöv J, Sjödin H, Wilder-Smith A: COVID-19 outbreak on the Diamond Princess cruise ship: Estimating the epidemic potential and effectiveness of public health countermeasures. J Travel Med 27: taaa030, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al.: Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26: 672–675, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323: 1239–1242, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, et al.: Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 35: 737–741, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliger AS, Silberzweig J: Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol 15: 707–709, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE : COVID-19 Rapid Guideline: Dialysis Service Delivery, London, National Institute for Health and Care Excellence, 2020 [PubMed] [Google Scholar]
- 10.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DAT, et al.:. KSA MERS-CoV Investigation Team: Hospital outbreak of Middle East respiratory syndrome coronavirus [published correction appears in N Engl J Med 369: 886, 2013]. N Engl J Med 369: 407–416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong PN, Mak SK, Lo KY, Tong GMW, Wong Y, Watt CL, et al.: Clinical presentation and outcome of severe acute respiratory syndrome in dialysis patients. Am J Kidney Dis 42: 1075–1081, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Renal Registry : UK Renal Registry 21st Annual Report, Bristol, United Kingdom, UK Renal Registry, 2019 [Google Scholar]
- 13.Her Majesty’s Government : Coronavirus in the UK. 2020. Available at: https://coronavirus.data.gov.uk/#local-authorities. Accessed April 17, 2020
- 14.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al.:. Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team: Epidemiology of covid-19 in a long-term care facility in king county, Washington. N Engl J Med 382: 2005–2011, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z-D, Wang Z-Y, Zhang S-F, Li X, Li L, Li C, et al.: Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020 [published online ahead of print April 10, 2020]. Emerg Infect Dis 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al.: Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382: 1564–1567, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR: Airborne or droplet precautions for health workers treating COVID-19? [published online ahead of print April 16, 2020]. J Infect Dis 10.1093/infdis/jiaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight AH, Fox RA, Baillod RA, Niazi SP, Sherlock S, Moorhead JF: Hepatitis-associated antigen and antibody in haemodialysis patients and staff. BMJ 3: 603–606, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assiri A, Abedi GR, Saeed AAB, Abdalla MA, Choudhry AJ, Lu X, et al.: Multifacility outbreak of middle east respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis 22: 32–40, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C: COVID-19: The case for health-care worker screening to prevent hospital transmission [published correction appears in Lancet 395: 1422, 2020]. Lancet 395: 1418–1420, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Xu G: Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol 15: 717–719, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al.: Presumed asymptomatic carrier transmission of COVID-19. JAMA 323: 1406–1407, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al.: Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med 382: 970–971, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung NHL, Chu DKW, Shiu EYC, Chan K-H, McDevitt JJ, Hau BJP, et al.: Respiratory virus shedding in exhaled breath and efficacy of face masks [published correction appears in Nat Med 27: 1, 2020]. Nat Med 26: 676–680, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al.:. the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323: 2052–2059, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D: Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the diamond princess cruise ship: A data-driven analysis. Int J Infect Dis 93: 201–204, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss JR, Holley P, Crooke PS: Analyzing pathogen transmission in the dialysis unit: Time for a (schedule) change? Clin J Am Soc Nephrol 2: 1176–1185, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.