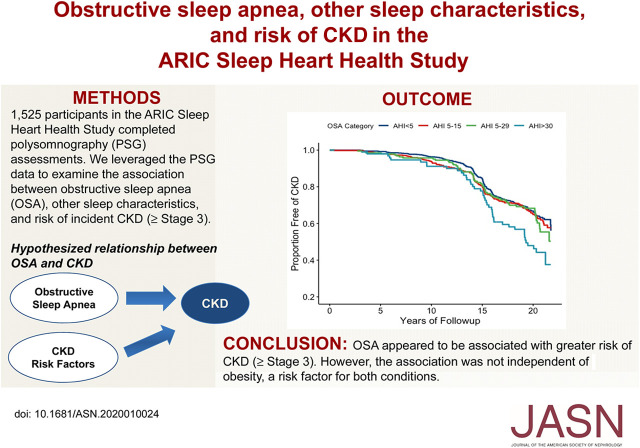
Significance Statement
Obstructive sleep apnea may be associated with development of CKD, but because individuals with this sleep disorder also are at increased risk for established CKD risk factors such as obesity, hypertension, and type 2 diabetes, more evidence is needed to clarify the relationship. To examine the association between this common sleep disorder and risk of incident CKD (stage ≥3) in late midlife, the authors leveraged polysomnography data from a prospective community-based cohort of 1525 adults who were free of CKD and followed for a median of 19 years. Although severe obstructive sleep apnea was associated with an increased risk of incident CKD, this association was not independent of obesity. Given the high prevalence of obstructive sleep apnea, CKD, and obesity, further investigation in this area is needed.
Keywords: sleep, aging, nocturnal hypoxemia, obstructive sleep apnea
Visual Abstract
Abstract
Background
Obstructive sleep apnea may be associated with development of CKD through hypoxia, inflammation, and oxidative stress. Individuals with this sleep disorder are also at increased risk for established CKD risk factors, including obesity, hypertension, and type 2 diabetes.
Methods
We examined the association between obstructive sleep apnea, other sleep characteristics, and risk of incident CKD (stage 3 or higher) in 1525 participants (mean age, 62.5 years; 52.4% women) in the Atherosclerosis Risk in Communities (ARIC) study who completed in-home polysomnography assessments. We used the apnea-hypopnea index (events per hour) to define obstructive sleep apnea severity (normal, <5.0; mild, 5.0–14.9; moderate, 15.0–29.9; and severe, ≥30.0) and defined incident CKD (stage 3 or higher) as eGFR<60 ml/min per 1.73 m2 and ≥25% decline from baseline, CKD-related hospitalization or death, or ESKD. Cox proportional hazards regression was used to estimate obstructive sleep apnea severity with risk of incident CKD, adjusting for demographics, lifestyle behaviors, and cardiometabolic conditions.
Results
During 19 years (median) of follow-up, 461 CKD events occurred. After adjustment for demographics and lifestyle behaviors, severe obstructive sleep apnea associated with increased risk of CKD (hazard ratio [HR], 1.51; 95% confidence interval [95% CI], 1.08 to 2.10), which was attenuated after adjustment for body mass index (HR, 1.07; 95% CI, 0.75 to 1.52). No other sleep characteristics associated with incident CKD.
Conclusions
We found a link between obstructive sleep apnea and an elevated risk of stage 3 CKD or higher, but this association was no longer significant after adjusting for obesity, a risk factor for both conditions. Given the high prevalence of obstructive sleep apnea and CKD among adults, further investigation is warranted.
Sleep plays a critical role in everyday health and well-being. Poor sleep quality and sleep disorders, such as obstructive sleep apnea (OSA), can contribute to the development of chronic cardiometabolic conditions including hypertension, diabetes, cardiovascular disease (CVD), and obesity.1 OSA is a condition characterized by the obstruction of the upper airway during sleep, resulting in repetitive arousals, oxygen desaturation, and intermittent hypoxia. OSA is a prevalent sleep disorder in the United States population, affecting approximately 9%–24% of adults.2 The condition is more common among men, and prevalence increases with both age and obesity.3 On the basis of evidence from community-based samples, OSA and other sleep disorders are believed to be highly underdiagnosed among Americans.3,4
CKD, which affects approximately 14% of the United States population, is a costly public health burden because it is associated with substantial morbidity, mortality, and comorbid conditions.5,6 Recently, reducing the incidence of CKD has become a top public health priority.7 Identification of novel lifestyle-related risk factors linked to CKD risk may lead to improved strategies for prevention. Accumulating evidence suggests that OSA and characteristics of poor sleep may be associated with the development of CKD.8,9 In a 2017 meta-analysis and systematic review of 18 studies investigating OSA and renal outcomes, patients with diagnosed OSA were at a 77% higher odds (pooled) of poorer kidney function.9 However, this meta-analysis consisted primarily of cross-sectional analyses, with one small prospective study in patients with type 2 diabetes.9 In cross-sectional analyses, a high prevalence of OSA and sleep disorders has been documented among patients with prevalent CKD, particularly among patients with ESKD.8,10,11 To date, few longitudinal studies have demonstrated a consistent association between OSA and incident CKD.12,13 In a large prospective cohort study of over 3 million United States veterans, participants with physician-diagnosed OSA had an approximately 2.3-times higher risk of incident CKD (defined as repetitive eGFR measures <60 ml/min per 1.73 m2) compared with veterans with normal breathing.13 In animal models, the intermittent hypoxia reflective of OSA has led to hypoxia-induced systemic and intraglomerular pressure, inflammation, oxidative stress, and endothelial dysfunction, all resulting in long-term damage to kidneys.8,14 Additionally, individuals with OSA are at increased risk for established CKD risk factors including obesity, hypertension, type 2 diabetes, oxidative stress, and inflammation.15–17 Although epidemiologic and pathophysiologic evidence suggests an association between OSA and risk of incident CKD,18 understanding of the association remains incomplete.11
Beyond OSA, an inconsistent association has been reported between other characteristics of poor sleep with incident CKD,19,20 despite evidence suggesting that poor sleep is related to kidney function decline.21 In a 2017 meta-analysis of observational studies, the investigators reported an increased, but nonsignificant, risk for CKD in participants with short sleep durations compared with healthy sleep durations (measured by self-report).20
To date, the research investigating the association of OSA and poor sleep characteristics with incident CKD has focused primarily on individual dimensions of sleep and has relied upon either previous clinical diagnoses of OSA or self-reported measures of sleep. It has been estimated that up to 80% of individuals who meet the diagnostic criteria for OSA remain undiagnosed.4,22 As such, more research is needed including both objective measures of OSA and additional characteristics of poor sleep. Over 1500 participants of the prospective Atherosclerosis Risk in Communities (ARIC) study had objective sleep assessments through the Sleep Heart Health Study (SHHS). Using these data, we tested the hypotheses that OSA severity and other characteristics of poor sleep were associated with a greater risk of incident CKD (stage ≥3) over approximately 20 years of follow-up. Furthermore, we evaluated whether the associations were independent of related CKD risk factors including hypertension, type 2 diabetes, inflammation, and obesity (Figure 1A). Determining if OSA is independently associated with incident CKD may have significant implications for clinical practice and population health.
Figure 1.
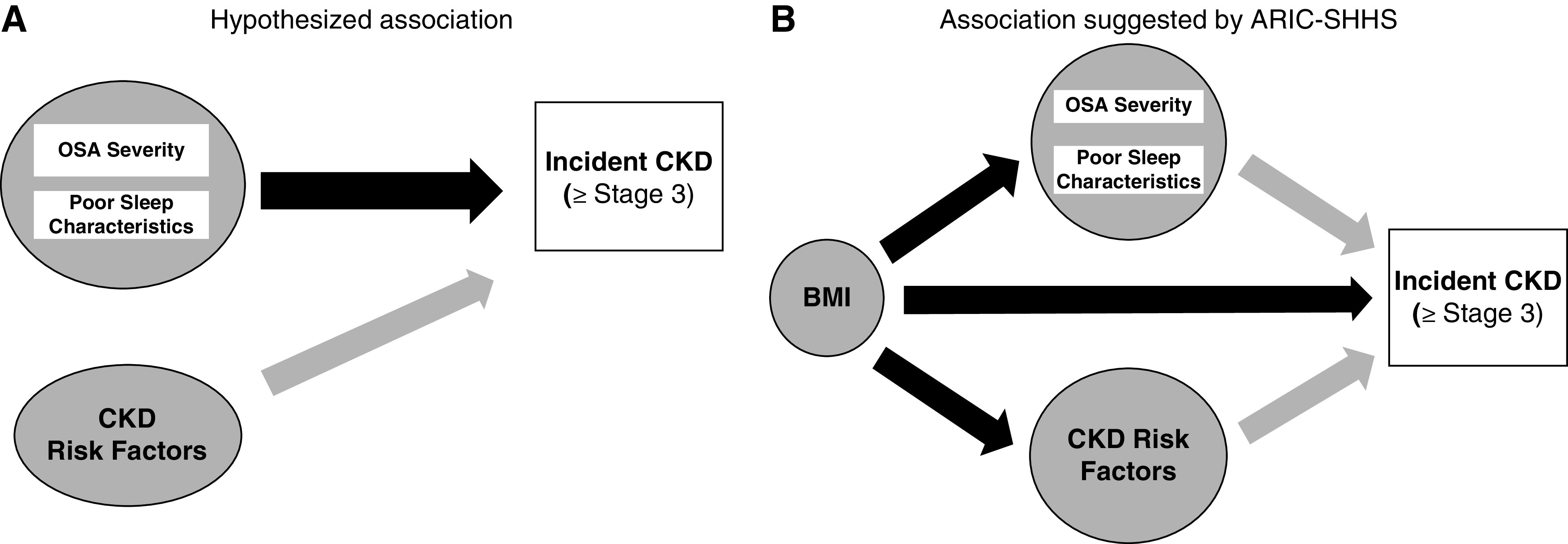
Hypothesized versus data-supported relationship between OSA and CKD. (A) Simplified directed acyclic graph of hypothesized relationship between OSA and CKD. (B) Simplified directed acyclic graph depicting relationship between OSA and CKD suggested by analysis.
Methods
Study Design
Between 1987 and 1989, ARIC recruited and enrolled 15,792 adults aged 45–64 years from four United States communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD) to participate in a prospective, community-based cohort study to investigate the etiology and natural history of atherosclerotic disease.23 At present, seven follow-up clinic visits have taken place, participants are routinely contacted by phone (annually before 2012; semiannually since), and there is continuous surveillance on the entire cohort for all hospitalizations. Approximately 90% of the living cohort continue to participate in phone calls where hospitalization information is provided; hospitalizations can also be identified through surveillance of local hospitals in participant catchment areas and follow-up on linkage to death identified via national and state death indices. More details on the ARIC examination, phone questionnaire, and community-wide surveillance procedures have been previously described elsewhere.23 Participants provided written informed consent at each ARIC visit, and institutional review boards from participating institutions approved study protocols. The ARIC visit 4 (1996–1998), which is baseline for this analysis, was attended by approximately 80% of the original cohort participants who were still living.
At ARIC visit 4, a subset of participants from two ARIC community field centers (suburban Minneapolis, MN and Washington County, MD) participated in an in-home overnight polysomnography (PSG) recording as part of the SHHS.24 Participants at these field centers were almost exclusively white. The SHHS aimed to enroll middle-aged and older adults who had previous risk factor data collected in other cohort studies (including the ARIC) to prospectively assess the relations between sleep-disordered breathing, including OSA, and new or recurrent CVD.24 To be included in the SHHS, participants must be at least 40 years of age and not receiving active treatment for OSA (e.g., continuous positive airway pressure, oral appliance, and oxygen therapy).
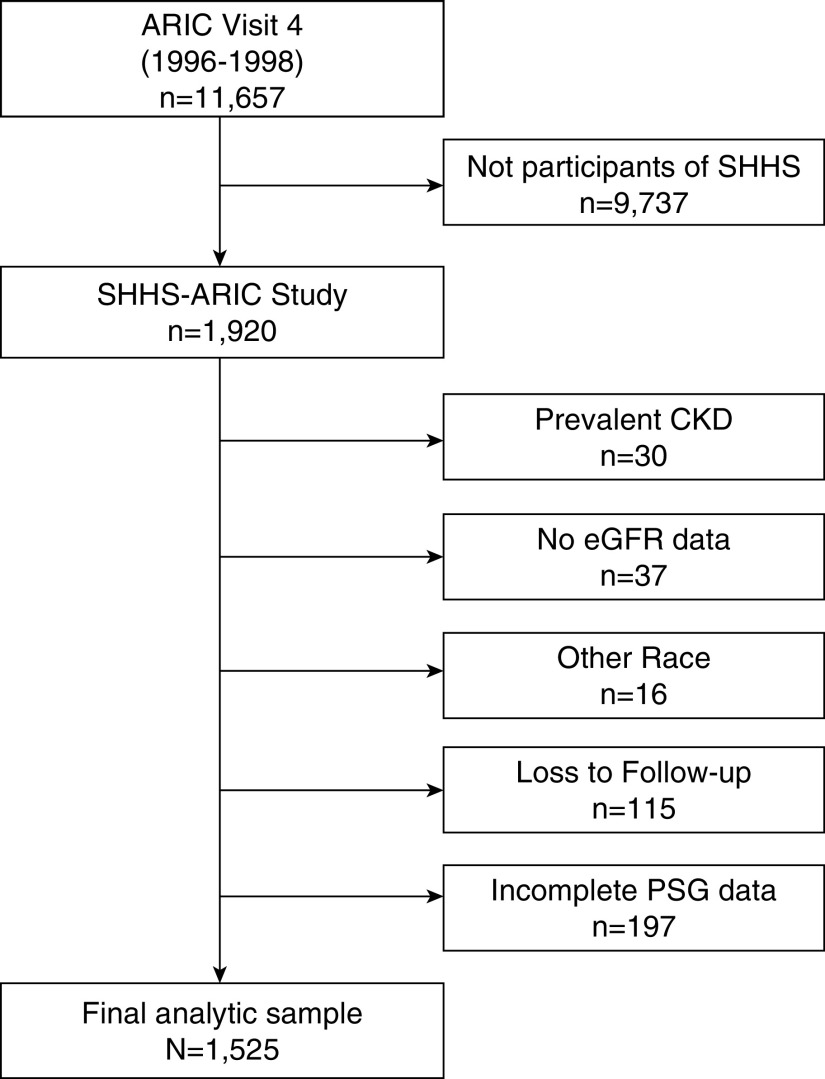
This study only included participants from the ARIC portion of the SHHS sample. From the ARIC-SHHS sample (n=1920), we excluded participants with prevalent CKD, defined by an eGFR<60 ml/min per 1.73 m2 (n=30), or no eGFR data at ARIC visit 4 (n=37); who identified as nonwhite race (n=16); and who had no follow-up data (n=115) or incomplete PSG data (n=197). A total of 1525 participants were included in the analytic sample (Figure 2). Baseline characteristics of the analytic sample were, overall, similar to those for the whole ARIC-SHHS cohort (Supplemental Table 1). Participants in the analytic sample were somewhat more likely to be current alcohol drinkers and less likely to be taking hypertension medication.
Figure 2.
ARIC-SHHS analytic sample. From the ARIC-SHHS sample, participants were excluded with prevalent CKD, missing eGFR data at ARIC visit 4; who identified as nonwhite race; and who had no follow-up data or incomplete PSG data.
Sleep Measurements
At the SHHS examination, an overnight unattended PSG was conducted in the participants’ homes using a portable monitor (PS-2 System; Compumedics Limited, Abbotsford, Victoria, Australia) as has been previously described by the SHHS investigators.24 OSA severity and additional sleep characteristics were categorized to be consistent with previous SHHS analyses.25,26
OSA severity was defined by the PSG-derived apnea-hypopnea index (AHI). Hypopnea was defined as a decrease in airflow <70% for >10 seconds accompanied with a blood oxygen desaturation of ≥4%. AHI was calculated as the sum of all apneas and hypopneas per hour of sleep. Central sleep-disordered breathing events were excluded from the AHI definition. Participants were categorized according to AHI: <5.0 events per hour (normal; reference group); 5.0 to <15 events per hour (mild); 15.0 to <30 events per hour (moderate); and ≥30.0 events per hour (severe).26 Additionally, we defined OSA severity according to PSG-derived hypoxic burden. Hypoxic burden captures the total amount of respiratory event–related hypoxemia over the sleep period. Hypoxic burden was defined as the proportion of the total sleep time with oxygen saturation <90%, and participants were categorized by tertiles: <1% (reference), 1 to <5% (mild/moderate), and ≥5% (severe).
A central apnea index was computed by dividing the number of central apneas by the total sleep time. Participants with central sleep apnea had a central apnea index greater than or equal to one.
Disrupted sleep was defined by the PSG-measured arousal index. The arousal index was defined as the average number of arousals per hour of sleep. PSG-measured sleep efficiency was defined by the percentage of time in bed classified as sleep. For both arousal index and sleep efficiency, participants were categorized according to quartiles.
Habitual sleep duration and self-reported poor sleep quality were derived from the SHHS questionnaire. Habitual sleep duration per night was derived from two items asking how much sleep the participant gets on a weekday night/work night and on a weekend or nonwork night. The average sleep duration per night was calculated as a weighted average: (workdays ×5+ nonwork days ×2)/7. Participants were categorized on the basis of the distribution of sleep duration in the sample: <7 hours, 7–8 hours, and >8 hours. If a participant responded “often” or “almost always” to any of the items assessing frequency of sleep complications, they would be defined as having self-reported poor sleep quality.
Outcome Ascertainment
In ARIC, hospitalizations are identified through the annual/semiannual phone questionnaires, the clinical visits, continuous surveillance of community hospitals in geographic areas where the ARIC participants were originally recruited, and linkage to state and national death indices. In instances of death, proxies are contacted in and provide information about hospitalizations prior to the death. Hospitalization International Classification of Diseases discharge codes are obtained for all participant hospitalizations.
Participants were followed for incident CKD (stage ≥3) from visit 4 (1996–1998) through December 31, 2017. Incident CKD (stage ≥3) was assessed through the thorough ARIC continuous active surveillance procedures (described previously) and through serum creatinine measurements at the ARIC study visit 5 (2011–2013) and visit 6 (2016–2017). Creatinine values were used to calculate eGFR using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation.27 Incident CKD was defined by (1) having an eGFR<60 ml/min per 1.73 m2 and at least 25% decline in eGFR from baseline (visit 4) to visit 5 (2011–2013) or visit 6 (2016–2017); (2) diagnostic code (International Classification of Diseases, Ninth and Tenth Revision [ICD-9/10]) for a hospitalization related to CKD stage ≥3 identified through active surveillance of the ARIC cohort; (3) ICD-9/10 code for a death related to CKD stage ≥3 identified through linkage to the National Death Index; or (4) ESKD identified by linkage to the US Renal Data System registry. This definition of incident CKD has been previously validated in the ARIC study.28
Covariates
Information on covariates was collected in person by trained ARIC staff at the ARIC visit 4, unless otherwise noted. Interview-administered questionnaires were used to assess demographics (visit 1: 1987–1989), smoking and alcohol consumption (categorized as current, former, or never), and physical activity (visit 3: 1993–1995). The physical activity sport index score (range, one [lowest] to five [highest]) was calculated on the basis of activity intensity and time spent in sport-related exercise. Participants brought medication bottles to the clinic, and medication names were coded. At visit 4, antihypertensive medications taken by participants included β-blockers, calcium-channel blockers, diuretics, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, hydralazine, clonidine, and α-blockers. Participants taking any medications included in these classes were mutually coded as one (zero: no antihypertensive medications).
Trained staff collected participant physiologic measurements including height, weight, BP, and phlebotomy. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. High-sensitivity C-reactive protein was measured in serum using a latex particle–enhanced immunoturbidimetric assay kit (46250; Roche Diagnostics, Indianapolis, IN). Prevalent diabetes was defined as fasting blood glucose concentration ≥126 mg/dl, nonfasting glucose concentration ≥200 mg/dl, self-reported physician diagnosis of diabetes, or pharmacologic treatment for diabetes. BP was measured twice after a 5-minute rest, and the average of the measures was used for analysis. Prevalent coronary heart disease (CHD) at visit 4 was on the basis of prior cardiovascular revascularization, self-reported physician-diagnosed myocardial infarction prior to visit 1, presence of a previous myocardial infarction by electrocardiogram at visit 1, or incident CHD following visit 1 and prior to visit 4 adjudicated by the ARIC Morbidity and Mortality Classification Committee.29
Statistical Analyses
Descriptive statistics for the 1525 participants in this analysis were calculated according to OSA severity categories. Chi-squared tests and ANOVA were used to examine differences in means and proportions across categories. For each individual, follow-up time was calculated from baseline date (date of sleep study) and accrued until incident CKD, loss to follow-up, death, or December 31, 2017.
Estimated years free of incident CKD (stage ≥3), stratified by OSA severity category, were visualized using the Kaplan–Meier method and log rank tests. Cumulative risk of CKD was summarized at 5, 10, 15, and 20 years.
Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) relating OSA severity with risk of incident CKD (stage ≥3). To examine the linear trend, additional models were run, including continuous variables for OSA severity. Cox regression analyses included a series of progressively adjusted models. Model 1 adjusted for age, sex, study center, and education level. The adjustment for study center was included to account for regional differences, and the adjustment for education served as a proxy measure for variations in socioeconomic status. Model 2 further adjusted for lifestyle behaviors that may be associated with both OSA and CKD, including smoking status, alcohol consumption, and physical activity. Model 3 included additional adjustment for BMI. In Model 4, we further adjusted for markers of cardiometabolic conditions, including inflammation (high-sensitivity C-reactive protein), prevalent diabetes, systolic BP, diastolic BP, antihypertensive medication, and prevalent CHD. In secondary analyses, we examined the association between additional sleep characteristics and incident CKD (stage ≥3) by running the same series of adjusted models with the exposures being arousal index (quartiles), sleep efficiency (quartiles), habitual sleep duration (tertiles), and self-reported poor sleep quality (no/yes). The proportional hazards assumption was confirmed by testing interactions between OSA severity categories and the natural log of follow-up time (P values were all <0.05) and by visual inspection of graphs of the survival function versus survival time.
To explore possible effect modification of the association between OSA and CKD by sex and age, we included crossproduct interaction terms (OSA × sex and OSA × age) in Model 2. Results stratified by median age (62 years) are also presented. Additional sensitivity analyses excluded individuals with central sleep apnea. At baseline, <1% of the sample (n=10) had a severely increased urine albuminuria-creatine ratio (UACR; >300 mg/g). To account for individuals with an eGFR>60 but an increased UACR, these participants with severely increased UACR were excluded in an additional sensitivity analysis.
All analyses were performed using R version 3.5.1.
Results
The demographic, behavioral, and health characteristics of the 1525 participants in the final analytic sample are presented in Table 1 by OSA severity category. At the time of the sleep assessment, 52.4% of the sample were women and on average 62.5 (SD=5.4) years old, with a mean eGFR of 89.5 (range, 60–176).
Table 1.
The ARIC-SHHS participant characteristics by OSA severity (n=1525)
| Characteristics | OSA Severity | |||
|---|---|---|---|---|
| Normal: AHI<5, n=776 | Mild: AHI=5 to <15, n=452 | Moderate: AHI=15 to <30, n=197 | Severe: AHI≥30, n=100 | |
| Demographics | ||||
| Baseline age, yr, mean ± SD | 61.7±5.4 | 63.2±5.2 | 63.3±5.4 | 63.1±5.1 |
| Women, n (%) | 505 (65.1) | 199 (44.0) | 60 (30.5) | 35 (35.0) |
| Education level, n (%) | ||||
| Less than high school | 69 (8.9) | 54 (12.0) | 27 (13.7) | 9 (9.0) |
| High school graduate | 372 (47.9) | 208 (46.0) | 85 (43.1) | 47 (47.0) |
| More than high school graduate | 335 (43.2) | 190 (42.0) | 85 (43.2) | 44 (44.0) |
| Lifestyle behavioral characteristics | ||||
| Smoking, n (%) | ||||
| Current | 107 (13.8) | 31 (6.9) | 16 (8.1) | 9 (9.0) |
| Former | 340 (43.8) | 239 (52.9) | 106 (53.8) | 54 (54.0) |
| Never | 329 (42.4) | 182 (40.3) | 75 (38.1) | 37 (37.0) |
| Alcohol consumption, n (%) | ||||
| Current | 542 (69.9) | 313 (69.3) | 123 (62.4) | 64 (64.0) |
| Former | 146 (18.8) | 100 (22.1) | 51 (25.9) | 23 (23.0) |
| Never | 88 (11.3) | 39 (8.6) | 23 (11.7) | 13 (13.0) |
| Physical activity sport index, mean ± SD | 2.7±0.8 | 2.7±0.8 | 2.6±0.7 | 2.4±0.7 |
| Health status characteristics | ||||
| BMI, kg/m2, mean ± SD | 27.0±4.3 | 29.1±4.8 | 31.1±5.5 | 33.8±5.5 |
| hsCRP, mg/L, mean ± SD | 3.7±5.1 | 4.1±6.6 | 3.9±4.9 | 4.8±4.4 |
| Prevalent diabetes, n (%) | 61 (7.9) | 69 (15.3) | 28 (14.2) | 20 (20.0) |
| Systolic BP, mm Hg, mean ± SD | 123.3±17.0 | 127.7±18.6 | 126.2±17.6 | 130.1±16.9 |
| Diastolic BP, mm Hg, mean ± SD | 69.7±9.1 | 71.5±9.8 | 71.3±9.1 | 73.4±10.2 |
| Antihypertensive medication use, n (%) | 192 (24.7) | 124 (27.4) | 66 (33.5) | 41 (41.0) |
| Prevalent CHD, n (%) | 56 (7.2) | 34 (7.5) | 25 (12.7) | 9 (9.0) |
| eGFR, ml/min per 1.73 m2, mean ± SD | 90.4±13.6 | 89.5±15.3 | 88.3±13.2 | 85.5±13.3 |
| Sleep characteristics | ||||
| Arousal index, average no. per night, mean ± SD | 15.6±7.2 | 19.4±8.1 | 23.4±8.7 | 35.7±15.1 |
| Sleep efficiency, mean ± SD | 85.0±8.6 | 83.5±9.3 | 82.2±9.4 | 81.8±10.8 |
| Self-reported poor sleep quality, n (%) | 322 (41.5) | 196 (43.4) | 85 (43.1) | 42 (42.0) |
| Habitual sleep duration, average hours per night, mean ± SD | 7.3±1.1 | 7.24±1.0 | 7.24±1.1 | 7.11±1.1 |
hsCRP, high-sensitivity C-reactive protein.
OSA and Incident CKD
In the SHHS sample, 6.6% (n=100) of participants were classified as having severe OSA, 12.9% (n=197) had moderate OSA, 29.6% (n=452) had mild OSA, and 50.9% (n=776) had normal breathing patterns or few AHI events while sleeping. Relative to participants classified as having normal breathing or few AHI events, participants with severe OSA were more likely to have a higher BMI, have prevalent diabetes, use antihypertensive medications, and have lower eGFR. Those participants with more severe OSA were more likely to be former smokers and less likely to be never smokers than those with less severe OSA.
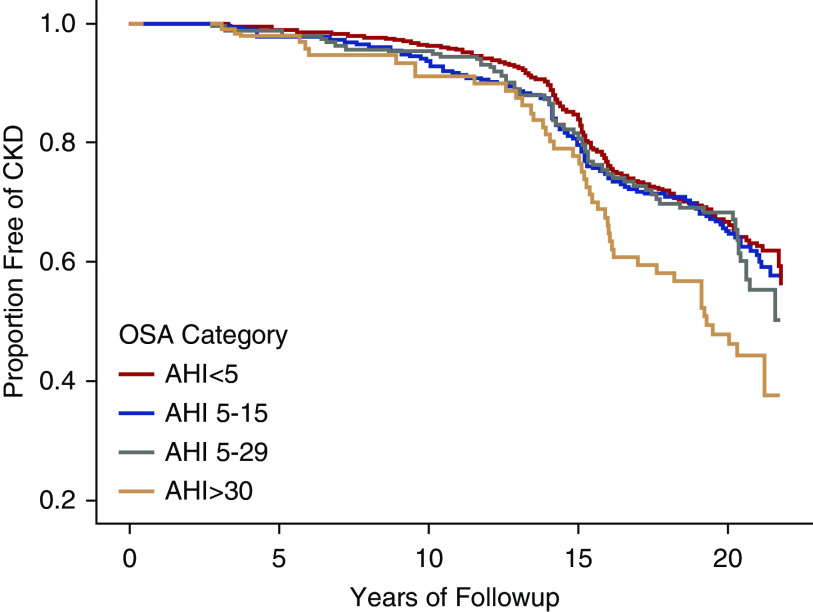
The median follow-up time of participants was 19 years (22 years maximum), and 461 incident CKD cases (stage ≥3) occurred during this period. Figure 3 displays estimated probability of years free of CKD by OSA severity. Results of the log rank test show that risk of CKD significantly differed by OSA severity (P<0.01). Separation in risk became more defined at year 15 of follow-up and beyond. Participant characteristics stratified by CKD (stage ≥3) status are presented in Table 2.
Figure 3.
Estimated probability of years free of CKD (stage ≥3) by OSA severity adjusted for age and sex in the ARIC-SHHS study (n=1525). Kaplan–Meier curves depict the estimated probability of years free of CKD by OSA severity. Log rank test show that risk of CKD significantly differed by OSA severity (P<0.01).
Table 2.
The ARIC-SHHS participant characteristics by CKD status
| Characteristics | Participants with CKD Stage ≥3, n=461 | Participants without CKD, Stage ≥3, n=1064 |
|---|---|---|
| Demographics | ||
| Baseline agea | 63.5±5.4 | 62.0±5.3 |
| Women, n (%)a | 223 (48.4) | 576 (54.1) |
| Education level, n (%) | ||
| Less than high school | 59 (12.8) | 100 (9.4) |
| High school graduate | 216 (46.8) | 496 (46.6) |
| More than high school graduate | 186 (40.3) | 468 (44.0) |
| Lifestyle behavioral characteristics | ||
| Smoking, n (%) | ||
| Current | 56 (12.1) | 107 (10.0) |
| Former | 230 (49.9) | 509 (47.8) |
| Never | 175 (38.0) | 448 (42.1) |
| Alcohol consumption, n (%)a | ||
| Current | 284 (61.6) | 758 (71.2) |
| Former | 126 (27.3) | 194 (18.2) |
| Never | 51 (11.1) | 112 (10.5) |
| Physical activity sport index | 2.6±0.8 | 2.7±0.8 |
| Health status characteristics | ||
| BMI, kg/m2a | 29.5±5.4 | 28.2±4.9 |
| hsCRP, mg/La | 4.3±5.7 | 3.7±5.4 |
| Prevalent diabetes, n (%)a | 80 (17.3) | 98 (9.2) |
| Systolic BP, mm Hga | 130.2±17.9 | 123.3±17.2 |
| Diastolic BP, mm Hga | 71.4±9.6 | 70.4±9.4 |
| Antihypertensive medication use, n (%)a | 170 (36.9) | 253 (23.8) |
| Prevalent CHD, n (%) | 44 (9.5) | 80 (7.5) |
| Baseline eGFR, ml/min per 1.73 m2 | 84.8±13.1 | 91.6±14.1 |
| Sleep characteristics | ||
| Arousal index, average no. per nighta | 19.8±10.6 | 18.7±9.5 |
| Sleep efficiency | 83.8±8.5 | 84.1±9.4 |
| Self-reported poor sleep quality, n (%) | 204 (44.2) | 441 (41.4) |
| Habitual sleep duration, average hours per night | 7.3±1.1 | 7.3±1.1 |
hsCRP, high-sensitivity C-reactive protein.
Signficant differences at P<0.05.
Associations between measures of OSA severity and incident CKD over the 22-year follow-up period are presented in Tables 3 and 4. After adjustment for demographics and lifestyle behaviors (Model 2), participants with severe OSA (defined by AHI≥30) were at a 51% (HR, 1.51; 95% CI, 1.08 to 2.10) greater risk of CKD than participants without OSA (AHI<5) (Table 3). After further adjustment for BMI (Model 3), this association was attenuated and no longer statistically significant (HR, 1.07; 95% CI, 0.75 to 1.52). Excluding individuals with central sleep apnea (n=72) from the analyses did not materially change the results (Model 2 HRsevere OSA versus normal, 1.64; 95% CI, 1.16 to 2.33).
Table 3.
OSA severity (AHI) and risk of incident CKD (stage ≥3): The ARIC-SHHS study (n=1525)
| AHI Category | <5 | 5–14.9 | 15–29 | ≥30 | P Value Trend |
|---|---|---|---|---|---|
| Incident CKD, n | 222 | 135 | 60 | 44 | |
| Model 1 | 1 (reference) | 0.92 (0.74 to 1.15) | 0.93 (0.70 to 1.25) | 1.50 (1.08 to 2.08) | 0.02 |
| Model 2 | 1 (reference) | 0.96 (0.77 to 1.20) | 0.93 (0.69 to 1.25) | 1.51 (1.08 to 2.10) | 0.02 |
| Model 3 | 1 (reference) | 0.89 (0.71 to 1.10) | 0.78 (0.58 to 1.05) | 1.07 (0.75 to 1.52) | 0.98 |
| Model 4 | 1 (reference) | 0.82 (0.65 to 1.03) | 0.79 (0.59 to 1.07) | 1.04 (0.73 to 1.48) | 0.89 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
Table 4.
OSA severity (Hypoxic Burden) and risk of incident CKD (stage ≥3): The ARIC-SHHS study (n=1525)
| Hypoxic Burden Category | <1% | 1 to <5% | ≥5% | P Value Trend |
|---|---|---|---|---|
| Incident CKD, n | 301 | 87 | 73 | |
| Model 1 | 1 (reference) | 1.20 (0.94 to 1.53) | 1.30 (1.01 to 1.69) | 0.19 |
| Model 2 | 1 (reference) | 1.22 (0.95 to 1.55) | 1.29 (0.99 to 1.68) | 0.24 |
| Model 3 | 1 (reference) | 1.06 (0.83 to 1.37) | 1.00 (0.75 to 1.32) | 0.67 |
| Model 4 | 1 (reference) | 1.06 (0.82 to 1.36) | 0.99 (0.74 to 1.31) | 0.74 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
There was evidence for a stronger association of OSA with risk of CKD in younger participants (Model 2 HRsevere OSA versus normal, 2.25; 95% CI, 1.28 to 3.95) compared with participants who were 62 years of age or older (Model 2 HRsevere OSA versus normal, 1.32; 95% CI, 0.87 to 1.99; interaction P=0.03) (Supplemental Table 2). There was no evidence of effect modification for the association of OSA and incident CKD by sex (interaction P=0.93). When individuals with increased UACR were excluded from the analysis, the results remained unchanged (data not shown).
When OSA severity was defined by hypoxic burden, the pattern of associations observed in the models was similar as that for OSA severity defined by AHI category; however, the association between severe hypoxic burden (≥5%) and incident CKD was not statistically significant after adjusting for demographics and health behaviors (Model 2 HRsevere hypoxic burden versus normal, 1.29; 95% CI, 0.99 to 1.68) (Table 4).
Other Sleep Characteristics and CKD
The average number of arousals per hour experienced by participants during their sleep time ranged from 3 to 90 per hour (mean: 19.1±9.9). On average, participants’ sleep efficiency, measured by PSG, was 84.0% ± 9.1%. Of the 1525 participants in the sample, 645 participants (42.3%) self-reported at least one regular sleep complication, and 361 (23.7%) reported a habitual sleep duration <7 hours per night.
We also examined associations between the arousal index, sleep efficiency, sleep quality, and habitual sleep duration and risk of incident CKD. Overall, there was no association between any of these sleep indices and incident CKD, regardless of the level of adjustment for covariates (Tables 5–8). Of these measures, there was an increased risk of CKD for those who self-reported poor sleep quality compared with those who reported good sleep quality (HR, 1.17; 95% CI, 0.97 to 1.41); however, the association was not statistically significant.
Table 5.
PSG-assessed arousal index and risk of incident CKD (stage ≥3): The ARIC-SHHS study
| Arousal Index Quartiles | 1 | 2 | 3 | 4 | P Value Trend |
|---|---|---|---|---|---|
| Range | [3–12.4] | [12.5–16.8] | [16.9–23.7] | [23.8–90.8] | |
| Average no. of arousals per hour | 9.35 | 14.61 | 19.92 | 32.49 | |
| Incident CKD, n | 107 | 108 | 108 | 127 | |
| Model 1 | 1 (reference) | 0.96 (0.73 to 1.25) | 0.92 (0.70 to 1.21) | 1.12 (0.86 to 1.46) | 0.18 |
| Model 2 | 1 (reference) | 0.97 (0.73 to 1.26) | 0.91 (0.70 to 1.20) | 1.12 (0.86 to 1.47) | 0.17 |
| Model 3 | 1 (reference) | 0.97 (0.74 to 1.27) | 0.87 (0.66 to 1.14) | 1.04 (0.80 to 1.36) | 0.54 |
| Model 4 | 1 (reference) | 0.99 (0.75 to 1.30) | 0.85 (0.64 to 1.11) | 1.08 (0.82 to 1.41) | 0.52 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
Table 8.
Poor sleep quality and risk of incident CKD (stage ≥3): The ARIC-SHHS study
| Poor Sleep Quality | No | Yes | P Value |
|---|---|---|---|
| Self-reported poor sleep | |||
| Incident CKD, n | 257 | 204 | |
| Model 1 | 1 (reference) | 1.17 (0.97 to 1.41) | 0.11 |
| Model 2 | 1 (reference) | 1.14 (0.94 to 1.37) | 0.18 |
| Model 3 | 1 (reference) | 1.12 (0.93 to 1.35) | 0.24 |
| Model 4 | 1 (reference) | 1.11 (0.92 to 1.34) | 0.30 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
Table 6.
PSG-assessed sleep efficiency and risk of incident CKD (stage ≥3): The ARIC-SHHS study
| Sleep Efficiency Quartiles | 1 | 2 | 3 | 4 | P Value Trend |
|---|---|---|---|---|---|
| Time in bed asleep, % | 71.36 | 82.80 | 88.31 | 93.48 | |
| Incident CKD, n | 116 | 128 | 119 | 98 | |
| Model 1 | 1 (reference) | 1.04 (0.81 to 1.33) | 0.93 (0.72 to 1.20) | 0.91 (0.69 to 1.20) | 0.88 |
| Model 2 | 1 (reference) | 1.05 (0.82 to 1.36) | 0.91 (0.70 to 1.18) | 0.91 (0.69 to 1.19) | 0.80 |
| Model 3 | 1 (reference) | 1.05 (0.81 to 1.35) | 0.92 (0.71 to 1.20) | 0.95 (0.72 to 1.25) | 0.93 |
| Model 4 | 1 (reference) | 1.09 (0.85 to 1.41) | 0.99 (0.76 to 1.28) | 0.97 (0.73 to 1.28) | 0.57 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
Table 7.
Habitual sleep duration and risk of incident CKD (stage ≥3): The ARIC-SHHS study
| Habitual Sleep Duration | <7 h | 7–8 h | >8 h | P Value Trend |
|---|---|---|---|---|
| Average hours per night | ||||
| Incident CKD, n | 112 | 286 | 63 | |
| Model 1 | 1.06 (0.85 to 1.32) | 1 (reference) | 1.27 (0.96 to 1.66) | 0.48 |
| Model 2 | 1.04 (0.83 to 1.30) | 1 (reference) | 1.26 (0.96 to 1.66) | 0.53 |
| Model 3 | 1.02 (0.82 to 1.27) | 1 (reference) | 1.20 (0.91 to 1.58) | 0.45 |
| Model 4 | 0.98 (0.78 to 1.22) | 1 (reference) | 1.12 (0.85 to 1.48) | 0.47 |
Model 1: Adjusted for age, sex, center, and education. Model 2: Adjusted for Model 1 + smoking, alcohol consumption, and physical activity. Model 3: Adjusted for Model 2 + BMI. Model 4: Adjusted for Model 3 + hsCRP, prevalent diabetes, systolic BP, diastolic BP, antihypertensive medications, and prevalent CHD.
Discussion
The high prevalence of OSA poses a potentially significant health burden to the United States aging population.3 Recent epidemiologic and pathophysiologic evidence has suggested that OSA may be an independent contributor to the development and progression of CKD.18 This prospective study aims to contribute to the evidence of the association between OSA severity and incident CKD using PSG data in a community-based sample of adults in late to midlife. In our analysis, severe OSA was associated with a 51% increased risk of incident CKD (stage ≥3); however, this association was not independent of BMI or prevalent CKD risk factors. The attenuation observed after adjustment for additional covariates suggests that the association between OSA and CKD may be partially explained by obesity and prevalent CKD risk factors. Additional sleep characteristics, measured objectively, were examined in relation to CKD, including measures of disrupted sleep, sleep efficiency, sleep quality, and sleep duration. However, none of these measures of sleep quality and quantity were significantly associated with incident CKD (stage ≥3).
Accumulating evidence indicates OSA may be associated with the development of CKD; however, this relationship is not fully understood. A higher prevalence of OSA and nocturnal hypoxemia, the trademark of OSA, has been well documented in patients with CKD in cross-sectional studies.8,30–32 In a case-control study comparing patients with CKD with controls from a community-based sample, Roumelioti et al.33 reported a two-time higher prevalence of severe OSA (AHI>30) in the patients with CKD versus controls. Likewise, reduced kidney function and a higher prevalence of CKD have been documented among patients with OSA.34,35 To date, the strongest evidence linking OSA to development of CKD has been from prospective studies linking nocturnal hypoxemia to kidney function decline.36,37 In a 2-year prospective study of 858 patients free of CKD, which assessed nocturnal hypoxemia via PSG, hypoxic burden (oxygen saturation <90%) was associated with rapid loss of kidney function (odds ratio, 2.89; 95% CI, 1.25 to 6.67), defined by as eGFR decline ≥4 ml/min per 1.73 m2 per year, after adjustment for BMI, age, diabetes, and CVD.36 The relationship between OSA and kidney decline has also been evaluated in a cohort of over 3 million United States veterans (93% men).13 In this study, physician-diagnosed and treated OSA was associated with both incident CKD and faster kidney function decline after 7 years of follow-up.13 However, as we noted previously, it is estimated that a large proportion of OSA in the community is undiagnosed.4 Therefore, investigations with objective measures of OSA are needed to better understand how OSA, characterized by AHI or sleep apnea–specific hypoxic burden,38 relates to kidney function and decline. Findings from this prospective study, which includes PSG-assessed OSA classified by AHI and hypoxic burden in over 1500 individuals free of CKD (stage ≥3) who were followed for over 20 years, makes a meaningful contribution to the literature. The excess risk for CKD explained by OSA was eliminated after adjustment for CKD risk factors in this analysis. These results, from a relatively large sample, suggest that OSA is not an independent risk factor for CKD. However, it is possible that a small effect may exist.
As depicted in Figure 1A, we hypothesized that the association between OSA and CKD would be independent of existing CKD risk factors, including BMI. However, in our analysis, the association between OSA and incident CKD (stage ≥3) was considerably attenuated after adjustment for BMI. Obesity is an upstream risk factor for both CKD and OSA. This manuscript highlights the difficulty in disentangling the association of OSA with CKD, independent of BMI (Figure 1B). Further, there are multiple pathways through which obesity may increase risk of CKD, including through the development of diabetes, hypertension, and inflammation.39 For example, OSA induces the activation of the sympathetic nervous system while also minimizing the nocturnal dipping of BP, which could lead directly, or indirectly, to the development of hypertension.15
Although it has been primarily hypothesized that the hypoxia associated with OSA is the underlying mechanism that contributes to CKD development, it has also been suggested that other characteristics of poor sleep may contribute to development of the condition.19,21 In particular, short sleep duration and poor sleep quality have been linked to obesity40 and hypertension41,42 as well as ESKD and kidney function decline.21 Among 431 participants of the Chronic Renal Insufficiency Cohort (full cohort n=3459) with documented CKD, shorter sleep duration and greater sleep fragmentation were associated with greater eGFR decline over the 5-year study period (−1.12 and −0.18 ml/min per 1.73 m2 per year, respectively).21 In contrast to our hypothesis and previous evidence, we did not find an association between sleep duration or indices of poor sleep quality and risk of CKD. Differences in these study results may be explained by the limited variation of sleep durations and sleep quality in the study sample or by the fact that the association between various dimensions of poor sleep and CKD is multifactorial and not well captured by the independent assessment of the sleep characteristics.
Strengths of this analysis include the prospective design, relatively large sample size, and objective assessments of OSA as well as multiple dimensions of sleep quality and quantity. A limitation of this study is lack of racial and ethnic diversity among the ARIC-SHHS participants. Some racial/ethnic minority populations are more likely to experience both poor sleep and CKD (especially blacks).43,44 Also, about 7.5% of our sample was lost to follow-up, and selection bias may be present. Limitations related to exposure ascertainment include the absence of information on continuous positive airway pressure treatment of OSA over the follow-up period and the single assessment of sleep at baseline; both have the potential to result in possible misclassification of our exposure, which is likely to bias our results toward the null. Additionally, for CKD identified at clinic visits, we used a definition on the basis of eGFR alone and not also a measure of albuminuria. This could have resulted in missing cases of incident CKD if participants had moderately to severe increases in albuminuria but eGFR>60. However, we did attempt to address this with a sensitivity analysis and found that our results were unchanged. Lastly, the ARIC-SHHS did not assess 24-hour BP; thus, it is possible that nocturnal hypertension is a potential confounder not appropriately addressed in our analyses.
In our community-based sample of over 1500 adults, severe OSA was associated with increased risk of incident CKD stage (≥3) in models adjusted for demographics and lifestyle behaviors; however, this association was not independent of obesity. Disentangling the relations between obesity, OSA, and CKD is challenging given that obesity is upstream from both conditions. OSA remains an underdiagnosed sleep disorder in the aging population; although it does not seem to be an independent risk factor for CKD, it associated with both poor quality of life and cardiometabolic risk, including increased risk of CKD risk factors. As such, improving the diagnosis and treatment of OSA remains a public health priority.
Disclosures
All authors have nothing to disclose.
Funding
The ARIC study has been funded in whole or in part with federal funds from National Heart, Lung, and Blood Institute contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. This work was funded, in part, by National Institute of Environmental Health Sciences Intramural Program grant Z1AES103325-01 (to C. Jackson). K. Full is supported by National Heart, Lung, and Blood Institute training grant T32 HL007779. C. Rebholz is supported by National Institute of Diabetes and Digestive and Kidney Diseases mentored research scientist development award K01 DK107782 and National Heart, Lung, and Blood Institute grant R21 HL143089.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Some of the data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government. A/Prof. Kunihiro Matsushita reports grants and personal fees from Kyowa Kirin and personal fees from Akebia outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020010024/-/DCSupplemental.
Supplemental Table 1. Comparison of participant characteristics in the analytic sample to the Atherosclerosis Risk in Communities Sleep Heart Health Study cohort.
Supplemental Table 2. Associations of obstructive sleep apnea severity and risk of incident CKD (stage 3) stratified by age: the Atherosclerosis Risk in Communities Study (N=1525).
References
- 1.St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al.: Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 134: e367–e386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM: Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108: 246–249, 2009. [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin KA, Lindberg E: Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7: 1311–1322, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram S, Seirawan H, Kumar SKS, Clark GT: Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath 14: 63–70, 2010. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services: Kidney Disease Statistics for the United States | NIDDK, 2016. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease?dkrd=hispt0879. Accessed July 31, 2019
- 6.Collins AJ, Foley RN, Gilbertson DT, Chen S-C: United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 5: 2–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuyassin B, Sharma K, Ayas NT, Laher I: Obstructive sleep apnea and kidney disease: A potential bidirectional relationship? J Clin Sleep Med 11: 915–924, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwu DW, Lin KD, Lin KC, Lee YJ, Chang YH: The association of obstructive sleep apnea and renal outcomes—a systematic review and meta-analysis. BMC Nephrol 18: 313, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adeseun GA, Rosas SE: The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep 12: 378–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams RJ, Appleton SL, Vakulin A, Hanly PJ, McDonald SP, Martin SA, et al.: Chronic kidney disease and sleep apnea association of kidney disease with obstructive sleep apnea in a population study of men. Sleep (Basel) 40: 2017. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y-C, Hung S-Y, Wang H-K, Lin CW, Wang HH, Chen SW, et al.: Sleep apnea and the risk of chronic kidney disease: A nationwide population-based cohort study. Sleep (Basel) 38: 213–221, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molnar MZ, Mucsi I, Novak M, Szabo Z, Freire AX, Huch KM, et al.: Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 70: 888–895, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W, Yin X, Wang Y, Tan Y, Cai L, Wang B, et al.: Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: Hormetic dose response. Dose-Response 11: 385–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Palta M, Skatrud J: Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H, et al.: Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 25: 156–161, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan S, Taylor CT, McNicholas WT: Systemic inflammation: A key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax 64: 631–636, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Voulgaris A, Marrone O, Bonsignore MR, Steiropoulos P: Chronic kidney disease in patients with obstructive sleep apnea. A narrative review. Sleep Med Rev 47: 74–89, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Turek NF, Ricardo AC, Lash JP: Sleep disturbances as nontraditional risk factors for development and progression of CKD: Review of the evidence. Am J Kidney Dis 60: 823–833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheungpasitporn W, Thongprayoon C, Gonzalez-Suarez ML, Srivali N, Ungprasert P, Kittanamongkolchai W, et al.: The effects of short sleep duration on proteinuria and chronic kidney disease: A systematic review and meta-analysis. Nephrol Dial Transplant 32: 991–996, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punjabi NM: The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 136–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ARIC Investigators : The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989. [PubMed] [Google Scholar]
- 24.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al.: The Sleep Heart Health Study: Design, rationale, and methods. Sleep 20: 1077–1085, 1997. [PubMed] [Google Scholar]
- 25.Silva GE, An M-W, Goodwin JL, Shahar E, Redline S, Resnick H, et al.: Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: The Sleep Heart Health Study (SHHS). Sleep 32: 1049–1057, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al.: Sleep characteristics and risk of dementia and alzheimer’s disease: The Atherosclerosis Risk in Communities study. Alzheimers Dement 14: 157–166, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, et al.: Identification of incident CKD stage 3 in research studies. Am J Kidney Dis 64: 214–221, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al.: Community surveillance of coronary heart disease in the Atherosclerosis risk in communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol 49: 223–233, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Nicholl DDM, Ahmed SB, Loewen AHS, Hemmelgarn BR, Sola DY, Beecroft JM, et al.: Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 141: 1422–1430, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Hallett M, Burden S, Stewart D, Mahony J, Farrell P: Sleep apnea in end-stage renal disease patients on hemodialysis and continuous ambulatory peritoneal dialysis. ASAIO J 41: M435–M441, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi Y, Hatta T, Hayashi T, Shoji T, Suzuki A, Tomida K, et al.: Association of nocturnal hypoxemia with progression of CKD. Clin J Am Soc Nephrol 8: 1502–1507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roumelioti M-E, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML: Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol 6: 986–994, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleischmann G, Fillafer G, Matterer H, Skrabal F, Kotanko P: Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant 25: 181–186, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Iseki K, Tohyama K, Matsumoto T, Nakamura H: High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD). Hypertens Res 31: 249–255, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed SB, Ronksley PE, Hemmelgarn BR, Tsai WH, Manns BJ, Tonell M, et al.: Nocturnal hypoxia and loss of kidney function. PLoS One 6: e19029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaussent I, Cristol J-P, Stengel B, Ancelin ML, Dupuy AM, Besset A, et al.: Impact of sleep disturbances on kidney function decline in the elderly. Eur Respir J 47: 860–868, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al.: The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The osteoporotic fractures in men study and the sleep heart health study [published correction appears in Eur Heart J 40: 1157, 2019]. Eur Heart J 40: 1149–1157, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE: Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 7: 75–88, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB: Sleep duration and body mass index and waist circumference among U.S. adults. Obesity (Silver Spring) 22: 598–607, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al.: Short sleep duration as a risk factor for hypertension: Analyses of the first national health and nutrition examination survey. Hypertension 47: 833–839, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, et al.: Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch Intern Med 169: 1055–1061, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al.: Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA study. Am J Epidemiol 164: 5–16, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, et al.: Excess risk of chronic kidney disease among african-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.