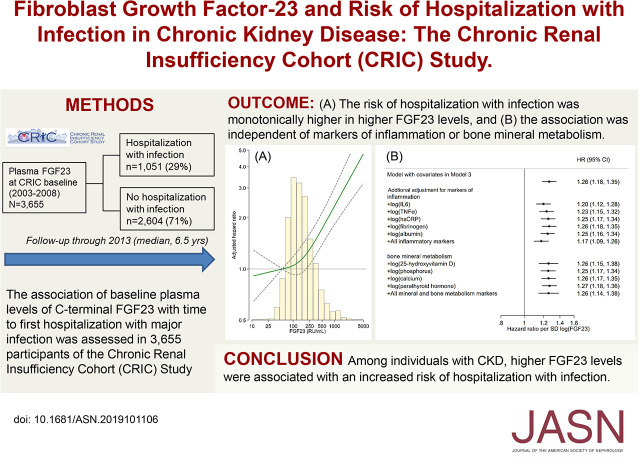
Significance Statement
Association of fibroblast growth factor 23 (FGF23) with risk of infection has not been evaluated in a CKD population. Among 3655 participants of the Chronic Renal Insufficiency Cohort (CRIC) study who had nondialysis-dependent CKD, higher baseline plasma levels of C-terminal FGF23 were significantly and monotonically associated with time to first hospitalization with major infection, independent of biomarkers of inflammation or bone mineral metabolism. These findings suggest a possible role of FGF23 in the increased susceptibility to infection among individuals with CKD. The most frequent infections were of the urinary tract, followed by cellulitis/osteomyelitis, pneumonia, and then bacteremia/septicemia.
Keywords: chronic kidney disease, Chronic inflammation, fibroblast
Visual Abstract
Abstract
Background
Risk of infectious disease is increased among individuals with CKD. Fibroblast growth factor 23 (FGF23) is often elevated in CKD, and may impair immune function directly or indirectly through proinflammatory and vitamin D–suppressing pathways. Whether FGF23 is associated with risk of infection has not been evaluated in a CKD population.
Methods
In 3655 participants of the Chronic Renal Insufficiency Cohort study, we evaluated the association of baseline plasma levels of C-terminal FGF23 with time to first hospitalization with major infection, defined by hospital discharge with a diagnosis code for urinary tract infection, pneumonia, cellulitis/osteomyelitis, or bacteremia/septicemia. Multivariable Cox models were used to estimate hazard ratios (HRs) and adjust for confounding.
Results
During a median follow-up of 6.5 years, 1051 individuals (29%) were hospitalized with major infection. Multivariable Cox analysis indicated a graded increase in the risk of infection with higher levels of FGF23 (HR, 1.51; 95% CI, 1.23 to 1.85 with the highest quartile [≥235.9 RU/ml] versus lowest quartile [<95.3 RU/ml]; HR, 1.26; 95% CI, 1.18 to 1.35 per SD increment in log FGF23). The association was consistent across infection subtypes and demographic and clinical subgroups, and remained significant after additional adjustment for biomarkers of inflammation (IL-6, TNF-α, high-sensitivity C-reactive protein, fibrinogen, and albumin), and bone mineral metabolism (25-hydroxyvitamin D, phosphorus, calcium, and parathyroid hormone). The association was consistent across infection subtypes of urinary tract infection (482 cases), cellulitis/osteomyelitis (422 cases), pneumonia (399 cases), and bacteremia/septicemia (280 cases).
Conclusions
Among individuals with CKD, higher FGF23 levels were independently and monotonically associated with an increased risk of hospitalization with infection.
Infectious disease is a major complication of CKD. Although the risk is highest in ESKD, even mild-to-moderate stages of CKD pose a 30%–50% greater risk of hospitalization with infection.1–4 Infectious disease accounts for one out of five hospitalizations of adults in the United States with nondialysis-dependent CKD, ranking as the second leading cause after cardiovascular disease.5 However, underlying mechanisms behind the increased susceptibility to infection in CKD are not completely understood.
Fibroblast growth factor 23 (FGF23) is an endocrine hormone that regulates phosphate and vitamin D metabolism, but may also have effects on the immune response both directly6 and indirectly through inflammation7 and vitamin D metabolism.8,9 The blood level of FGF23 is often increased from an early stage of CKD to maintain the serum phosphate in the normal range.10 Thus, the role of FGF23 is particularly important in nondialysis-dependent CKD.11 Previous research on the association of FGF23 with the risk of infection has primarily been in general populations that included relatively few individuals with CKD12,13 or ESKD requiring chronic dialysis.14 Whether FGF23 is associated with the risk of infection has not been systematically evaluated in a diverse group of patients with non-dialysis dependent CKD.
We hypothesized that elevated levels of FGF23 would be associated with increased risk of major acute infections, such as pneumonia, urinary tract infection, cellulitis/osteomyelitis, and bacteremia/septicemia, that were severe enough to require hospitalization (hereafter referred to as hospitalization with infection) independently of eGFR and urinary albumin-to-creatinine ratio (ACR) in individuals with CKD. To test this hypothesis, we assessed the association of FGF23 with the risk of first hospitalization with infection among participants in the Chronic Renal Insufficiency Cohort (CRIC) study.
Methods
Study Population
The CRIC study is an ongoing, observational, prospective study that enrolled 3939 participants with mild-to-severe CKD from seven clinical centers across the United States between 2003 and 2008. Details on the design and methods of the CRIC study have previously been described.15 For this study, we excluded 284 individuals with missing covariates, leaving 3655 participants included in the analyzed sample. The CRIC study was approved by institutional review boards at each clinical center and its scientific and data coordinating center; all participants provided written informed consent.
Exposure
The primary exposure was plasma FGF23, which was obtained at baseline visit and measured using a second generation C-terminal assay (Immutopics, San Clemente, CA). All measurements were performed in a central laboratory at the University of Pennsylvania after a single thaw in samples collected at the baseline visit. The mean intraassay coefficient of variation was <5%. The mean interassay coefficient of variation was 7.6%.
Outcome
The primary outcome was first hospitalization with major infection after enrollment in the CRIC study, defined by hospital discharge with a diagnosis code for the four most common infection types16: pneumonia, urinary tract infection, cellulitis/osteomyelitis, and bacteremia/septicemia. Hospitalizations were identified through annual study site visits and bi-annual telephone calls to participants or their proxies. Active surveillance collected hospital discharge records and associated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, regardless of whether a patient was discharged alive or dead. Starting with the date of enrollment in the CRIC study, we identified cases of pneumonia (ICD-9-CM codes 480–486), urinary tract infection (ICD-9-CM codes 590.1, 299.0, and 601.0), cellulitis/osteomyelitis (ICD-9-CM codes 040.0, 681, 682, 730.0, and 730.2), and bacteremia/septicemia (ICD-9-CM codes 038, 003.1, 020.2, 022.3, 036.2, 054.5, 785.52, 790.7, 995.91, and 995.92) at any position. For this study, follow-up was continued through December 31, 2013. Participants who did not have a hospitalization with infection were censored when they died, were lost to follow-up, or at the end of follow-up. For primary analysis, individuals were also censored at incident ESKD because patients with ESKD had unique infection causes compared with those with CKD who were not dependent on RRT.17
Covariates
Demographics (age, sex, and race/ethnicity), smoking history (never or ever smokers), alcohol consumption, past history of cancer, chronic obstructive pulmonary disease, and cardiovascular disease (heart failure, coronary heart disease, stroke, and peripheral artery disease) were ascertained by questionnaire at the baseline visit. Body mass index was calculated as weight in kilograms divided by height in meters squared. Hypertension was defined by participant self-report, use of antihypertensive medication, or a seated BP ≥140/90 mm Hg. Diabetes was defined by participant self-report, use of antidiabetic medications, or a fasting blood glucose ≥126 mg/dl. GFR was estimated using the CKD Epidemiology Collaboration equation incorporating serum creatinine and cystatin C.18 Albuminuria (ACR) was calculated as the ratio of urinary albumin to urinary creatinine.
Statistical Analyses
Baseline characteristics were compared according to quartiles of FGF23, using chi-squared tests for categorical variables and Student t tests for continuous variables. Crude incidence rates (IRs) and their 95% confidence intervals (95% CIs) were estimated using Poisson regression analysis. Multivariable Cox regression analysis was used to characterize the adjusted association between FGF23 levels and infection outcomes. Model 1 adjusted for age, sex, and race/ethnicity. Model 2 also accounted for eGFR and ACR. Model 3 further adjusted for body mass index, smoking status, alcohol consumption, hypertension, diabetes, cancer, chronic obstructive pulmonary disease, and cardiovascular disease. All models were stratified by clinical center to account for regional variability. The level of FGF23 was treated as a categorical variable parameterized into quartiles,12,13 and also as a continuous variable on a log scale to account for its right-skewed distribution. For categorical analysis, the lowest quartile served as the reference category. For continuous analysis, we considered two models. In the first model, we modeled the level of FGF23 as a restricted cubic spline with knots at values corresponding to the fifth percentile, 50th percentile, and 95th percentile, and a value corresponding to the fifth percentile served as the reference. Because we confirmed the overall dose-response association for FGF23, in the second model, we showed the hazard ratios (HRs) per 1 SD increment of log-transformed FGF23.
We performed several sensitivity analyses to explore the robustness of our primary findings. First, to account for the possibility that FGF23 is associated with other causes of hospitalization leading to an increased chance of infection diagnosis, we analyzed the association restricting the outcomes where infection was a primary cause of hospitalization. Second, because previous studies reported the association of FGF23 with an increased risk of cardiovascular disease,19,20 we censored follow-up at the time of incident cardiovascular disease. Third, to account for the possibility of reverse causation (higher levels of FGF23 as an indicator for latent or undiagnosed infection, which could be clinically manifested shortly after the follow-up), we censored events that occurred <1 or 3 years after the baseline visit, respectively. Fourth, we repeated the analysis continuing the follow-up after incident ESKD instead of censoring at the time of incident ESKD. Fifth, we ran a Fine and Gray model to estimate the subdistribution hazard, accounting for death as a competing risk.
We also examined the level of FGF23 as a time-varying exposure in a subset of participants who had repeated measurements of FGF23. In brief, the level of FGF23 was repeatedly measured annually up to 5 years from the baseline visit in a random subset of 1135 participants who were enrolled in the ancillary study.21
Subgroup analyses included those by age (≥65 versus <65 years), sex (men versus women), race/ethnicity (non-Hispanic black versus others), body mass index (<30 versus ≥30 kg/m2), diabetes (yes versus no), history of cardiovascular disease (yes versus no), eGFR (≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2), and ACR (<10, 10–29, 30–299, and ≥300 mg/g). Interaction by these subgroups was statistically assessed using log-likelihood tests.
Next, the association between FGF23 and infection may be mediated by inflammation or vitamin D metabolism,7–9 which have been linked to the risk of infection.22,23 Thus, we explored these putative pathways by assessing whether the association of FGF23 with risk of infection was attenuated by adjustments for biomarkers of inflammation (IL-6, TNF-α, high-sensitivity C-reactive protein, fibrinogen, and albumin) or bone mineral metabolism (25-hydroxyvitamin D, phosphorus, calcium, and parathyroid hormone). Sample size for 25-hydroxyvitamin D was restricted to the 1915 individuals for whom vitamin D levels had been obtained at four of the seven CRIC study clinical centers that implemented this testing.24
Finally, we estimated event rate ratios for hospitalization with infection, incorporating multiple events per person during the follow-up. For this analysis, we ran a zero-inflated, multivariable, negative binominal regression model to account for overdispersion and excess zero counts. A two-sided P value <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 14 (StataCorp, College Station, TX).
Results
Study Population
In the overall sample of 3655 participants, the mean age was 58 years, 55% were men, 43% were non-Hispanic white, and 41% were non-Hispanic black. Table 1 shows baseline characteristics by the quartiles of FGF23. The median FGF23 was 144.2 RU/ml (interquartile range, 95.3–235.9 RU/ml). Participants with higher levels of FGF23 were more likely to be older, women, non-Hispanic black, and current smokers, as well as to have a higher body mass index, hypertension, diabetes, cardiovascular disease, lower eGFR, and higher ACR.
Table 1.
Baseline characteristics by quartiles of FGF23
| Characteristics | Overall, n=3655 | FGF23 Quartile, RU/ml | P Value | |||
|---|---|---|---|---|---|---|
| <95.3, n=913 | 95.3–144.2, n=914 | 144.2–235.9, n=914 | ≥235.9, n=914 | |||
| Age, yr (SD) | 58 (11) | 56 (11) | 58 (11) | 59 (11) | 58 (11) | <0.001 |
| Men, n (%) | 2008 (55) | 580 (64) | 551 (60) | 483 (53) | 394 (43) | <0.001 |
| Race/ethnicity, n (%) | ||||||
| Non-Hispanic white | 1562 (43) | 429 (47) | 401 (44) | 396 (43) | 336 (37) | <0.001 |
| Non-Hispanic black | 1506 (41) | 380 (42) | 344 (38) | 352 (39) | 430 (47) | |
| Other | 587 (16) | 104 (11) | 169 (18) | 166 (18) | 148 (16) | |
| Body mass index, kg/m2 (SD) | 32 (7.8) | 31 (6.7) | 32 (6.9) | 32 (7.8) | 34 (9.1) | <0.001 |
| Current smoking, n (%) | 471 (13) | 68 (7.4) | 92 (10) | 127 (14) | 184 (20) | <0.001 |
| Alcohol use, n (%) | 2313 (63) | 656 (72) | 622 (68) | 558 (61) | 477 (52) | <0.001 |
| Comorbidity, n (%) | ||||||
| Hypertension | 3144 (86) | 689 (76) | 792 (87) | 818 (90) | 845 (93) | <0.001 |
| Diabetes | 1753 (48) | 273 (30) | 415 (45) | 508 (56) | 557 (61) | <0.001 |
| Cardiovascular disease | 1211 (33) | 195 (21) | 279 (31) | 330 (36) | 407 (45) | <0.001 |
| Cancer | 255 (7.0) | 67 (7.3) | 55 (6.0) | 73 (8.0) | 60 (6.6) | 0.37 |
| COPD | 112 (3.1) | 21 (2.3) | 22 (2.4) | 31 (3.4) | 38 (4.2) | 0.07 |
| eGFR, ml/min per 1.73 m2, n (%) | ||||||
| ≥60 | 552 (15) | 300 (33) | 151 (17) | 56 (6) | 45 (5) | <0.001 |
| 45–59 | 1118 (31) | 379 (42) | 349 (38) | 253 (28) | 137 (15) | |
| 30–44 | 1321 (36) | 204 (22) | 343 (38) | 407 (45) | 367 (40) | |
| <30 | 664 (18) | 30 (3) | 71 (8) | 198 (22) | 365 (40) | |
| ACR, mg/g, n (%) | ||||||
| <10 | 1000 (27) | 388 (42) | 308 (34) | 182 (20) | 122 (13) | <0.001 |
| 10–29 | 582 (16) | 170 (19) | 141 (15) | 150 (16) | 121 (13) | |
| 30–299 | 965 (26) | 214 (23) | 249 (27) | 237 (26) | 265 (29) | |
| ≥300 | 1108 (30) | 141 (15) | 216 (24) | 345 (38) | 406 (44) | |
Categorical variables are presented with number (percentage).Continuous variables are presented with mean (SD). COPD, chronic obstructive pulmonary disease.
FGF23 and Hospitalization with Infection
During a median follow-up of 6.5 years, 1051 participants had hospitalizations with infection (crude IR, 48.9; 95% CI, 46.0 to 51.9 per 1000 person-years), of which 49 participants died within 30 days of hospitalization. Table 2 shows the crude IRs and adjusted HRs for hospitalization with infection stratified by quartiles of FGF23. Overall, the IRs were greater in higher quartiles of FGF23 (crude IR, 30.3; 95% CI, 26.4 to 34.8 in the lowest quartile versus crude IR, 85.5; 95% CI, 76.8 to 95.3 per 1000 person-years in the highest quartile). In Cox analysis adjusted for age, sex, and race/ethnicity, participants in the highest quartile had a 2.46-fold (HR, 2.46; 95% CI, 2.05 to 2.96) increased risk of infection compared with those in the lowest quartile. The HRs were also significant for those in the second (HR, 1.24; 95% CI, 1.03 to 1.50) and third (HR, 1.68; 95% CI, 1.39 to 2.02) quartiles. The results were partially attenuated with further adjustment for other confounders, including eGFR and ACR (HR, 1.97; 95% CI, 1.61 to 2.40), but participants in the highest quartile of FGF23 remained at a significantly higher risk in the final model (HR, 1.51; 95% CI, 1.23 to 1.85) of infection compared with those in the lowest quartile (Table 2).
Table 2.
IRs and HRs of risk of hospitalization with infection according to quartiles of FGF23, CRIC study 2003–2013
| Outcomes | FGF23 Quartile, RU/ml | Continuous, Per SD Increase | |||
|---|---|---|---|---|---|
| <95.3 | 95.3–144.2 | 144.2–235.9 | ≥235.9 | ||
| n | 913 | 914 | 914 | 914 | 3655 |
| Events | 200 | 241 | 280 | 330 | 1051 |
| IR per 1000 person-years (95% CI) | 30.3 (26.4–34.8) | 40.0 (35.3–45.4) | 55.8 (49.6–62.7) | 85.5 (76.8–95.3) | 48.9 (46.0–51.9) |
| HR (95% CI) | |||||
| Model 1 | 1 (Reference) | 1.24 (1.03 to 1.50) | 1.68 (1.39 to 2.02) | 2.46 (2.05 to 2.96) | 1.45 (1.37 to 1.54) |
| Model 2 | 1 (Reference) | 1.15 (0.95 to 1.39) | 1.40 (1.15 to 1.70) | 1.97 (1.61 to 2.40) | 1.36 (1.27 to 1.45) |
| Model 3 | 1 (Reference) | 1.06 (0.88 to 1.29) | 1.16 (0.95 to 1.41) | 1.51 (1.23 to 1.85) | 1.26 (1.18 to 1.35) |
Model 1 was adjusted for age, sex, and race. Model 2 was additionally adjusted for eGFR andACR. Model 3 was additionally adjusted for body mass index, smoking status, alcohol consumption, hypertension, diabetes, and past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease). All models were stratified by clinical center.
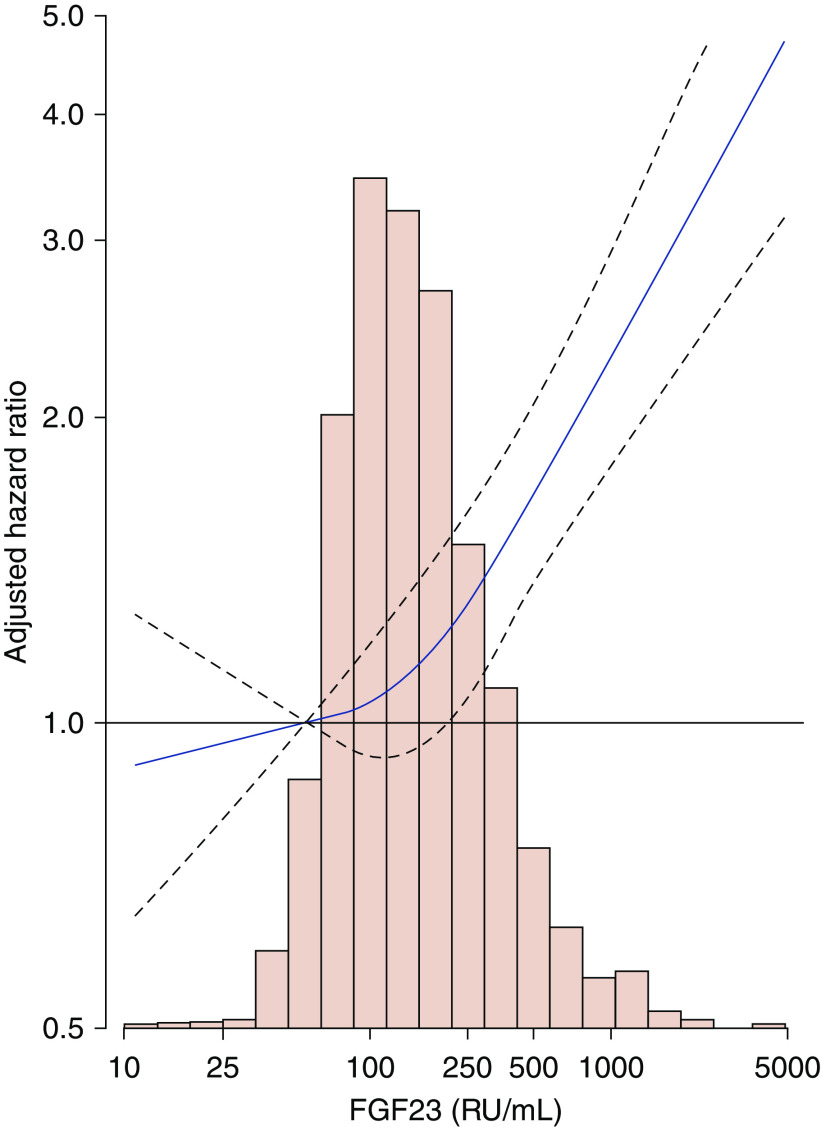
The association was consistent when FGF23 was modeled as a restricted cubic spline (Figure 1). The slope was relatively shallow in the lower levels of FGF23, with the threshold at around the median (144 RU/ml), but became steeper with higher levels of FGF23. Compared with the risk at a value corresponding to the fifth percentile, the HR at the 50th, 75th, 90th, and 99th percentiles were 1.12 (95% CI, 0.94 to 1.34), 1.29 (95% CI, 1.05 to 1.59), 1.57 (95% CI, 1.27 to 1.95), and 2.66 (95% CI, 2.03 to 3.49), respectively (Figure 1).
Figure 1.
Histograms and adjusted HRs of risk of hospitalization with infection for FGF23, CRIC study 2003–2013. The slope was relatively shallow in the lower levels of FGF23, with the threshold at around the median (144 RU/ml), but became steeper with higher levels of FGF23. The level of FGF23 was modeled as a restricted cubic spline with the knots at values corresponding to the fifth, 50th, and 95th percentiles. The value at the fifth percentile served as the referent point. Solid line represents point estimates. Dotted lines represent the corresponding 95% CIs. Model was stratified by center and adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, hypertension, diabetes, past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease), eGFR, and ACR. Histograms show the distribution of FGF23 in the study population.
The association was consistent and remained significant when analyzing infections as a primary diagnosis for hospitalization, after censoring follow-up at the time of incident cardiovascular disease or continuing follow-up after the incident ESKD, and after censoring infectious hospitalizations that occurred in the first 1 or 3 years after measurement of FGF23 (HRs per SD increment in log FGF23 from 1.15 (95% CI, 1.04 to 1.28) to 1.26 (95% CI, 1.18 to 1.34) in model 3) (Table 3). The association was slightly attenuated but consistent after considering death as a competing risk (sub-HR per SD increment in log FGF23, 1.17; 95% CI, 1.09 to 1.26 in model 3) (Supplemental Table 1). A dose response was consistently observed when timely updating the level of FGF23 in a subset of 1135 participants who had repeated FGF23 measurements (Supplemental Figure 1).
Table 3.
HRs of hospitalization with infection according to SD increment in log FGF23 in sensitivity analysis, CRIC study 2003–2013
| Outcomes | Types of Sensitivity Analysis | ||||
|---|---|---|---|---|---|
| Restricting Outcome to Primary Infection Diagnoses | Censoring Incident CVD Cases during Follow-Up | Continuing Follow-Up after Incident ESKD | Censoring Infection Cases in the First <1 yr | Censoring Infection Cases in the First <3 yr | |
| Events, n | 569 | 743 | 1304 | 865 | 551 |
| HR (95% CI), per log SD increase in FGF23 | |||||
| Model 1 | 1.36 (1.25 to 1.47) | 1.38 (1.28 to 1.49) | 1.48 (1.40 to 1.55) | 1.43 (1.34 to 1.53) | 1.33 (1.22 to 1.46) |
| Model 2 | 1.29 (1.18 to 1.42) | 1.31 (1.20 to 1.42) | 1.34 (1.27 to 1.42) | 1.33 (1.24 to 1.44) | 1.25 (1.13 to 1.38) |
| Model 3 | 1.19 (1.08 to 1.31) | 1.22 (1.12 to 1.33) | 1.26 (1.18 to 1.34) | 1.23 (1.14 to 1.33) | 1.15 (1.04 to 1.28) |
The HR was per log SD increase in FGF23. Model 1 was adjusted for age, sex, and race. Model 2 was additionally adjusted for eGFR and ACR. Model 3 was additionally adjusted for body mass index, smoking status, alcohol consumption, hypertension, diabetes, and past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease). All models were stratified by clinical center.
FGF23 and Infection Subtypes
The most frequent infection subtype was urinary tract infection (482 cases), followed by cellulitis/osteomyelitis (422 cases), pneumonia (399 cases), and bacteremia/septicemia (280 cases). The association was significant in all infection subtypes with the largely similar strengths of the association (HR per SD increment in log FGF23 from 1.19 (95% CI, 1.06 to 1.32) to 1.39 (95% CI, 1.22 to 1.58) (Table 4). These associations were generally consistent when we restricted our analysis to infections as a primary diagnosis for hospitalization, although the association of urinary tract infection was not significant (HR per SD increment in log FGF23, 1.05; 95% CI, 0.85 to 1.29 for urinary tract infection versus 1.24 (95% CI, 1.08 to 1.43)–1.27 (95% CI, 1.09 to 1.49) for other infection subtypes) (Supplemental Table 2).
Table 4.
HRs of hospitalization with type-specific infection according to SD increment in log FGF23, CRIC study 2003–2013
| Outcomes | Type-Specific Infection | |||
|---|---|---|---|---|
| Urinary Tract Infection | Cellulitis/Osteomyelitis | Pneumonia | Bacteremia/Septicemia | |
| Events, n | 482 | 422 | 399 | 280 |
| HR (95% CI), per log SD increase in FGF23 | ||||
| Model 1 | 1.44 (1.32 to 1.57) | 1.40 (1.27 to 1.53) | 1.54 (1.41 to 1.69) | 1.59 (1.42 to 1.77) |
| Model 2 | 1.34 (1.22 to 1.47) | 1.35 (1.22 to 1.49) | 1.43 (1.29 to 1.58) | 1.47 (1.30 to 1.66) |
| Model 3 | 1.28 (1.16 to 1.41) | 1.19 (1.06 to 1.32) | 1.33 (1.19 to 1.48) | 1.39 (1.22 to 1.58) |
The HR was per SD increment in loincrease in FGF23. Model 1 was adjusted for age, sex, and race. Model 2 was additionally adjusted for eGFR and urinary ACR. Model 3 was additionally adjusted for body mass index, smoking status, alcohol consumption, hypertension, diabetes, and past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease). All models were stratified by clinical center.
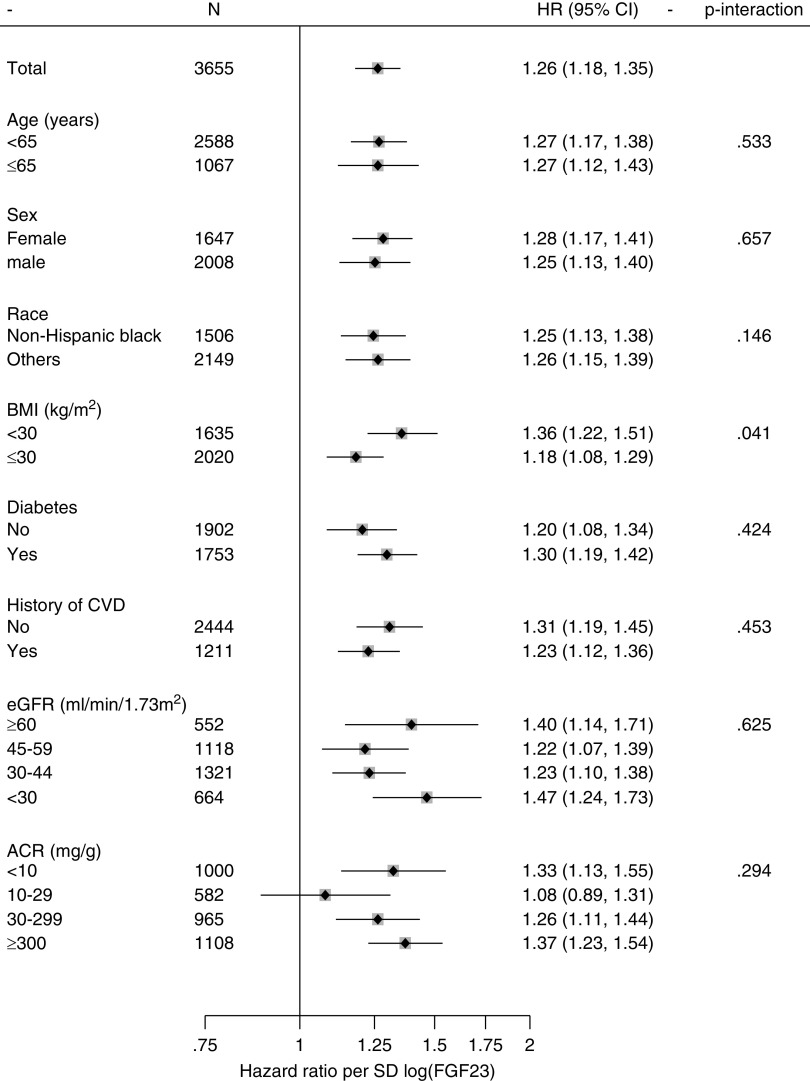
Subgroup Analyses
The association was generally consistent across subgroups by age, sex, race/ethnicity, body mass index, diabetes, history of cardiovascular disease, eGFR, and ACR (Figure 2). The P values for interaction were all >0.05 except for body mass index (P for interaction, 0.04).
Figure 2.
Subgroup analysis for adjusted HRs of hospitalization with infection for FGF23, CRIC study 2003–2013. The association was generally consistent across subgroups by age, sex, race/ethnicity, body mass index, diabetes, history of cardiovascular disease, eGFR, and ACR. Diamonds in square represent point estimates for HRs per SD increment in log FGF23. Models were stratified by center and adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, hypertension, diabetes, chronic obstructive pulmonary disease, cancer, cardiovascular disease, eGFR, and ACR. Horizontal lines represent the range for 95% CIs. BMI, body mass index; CVD, cardiovascular disease.
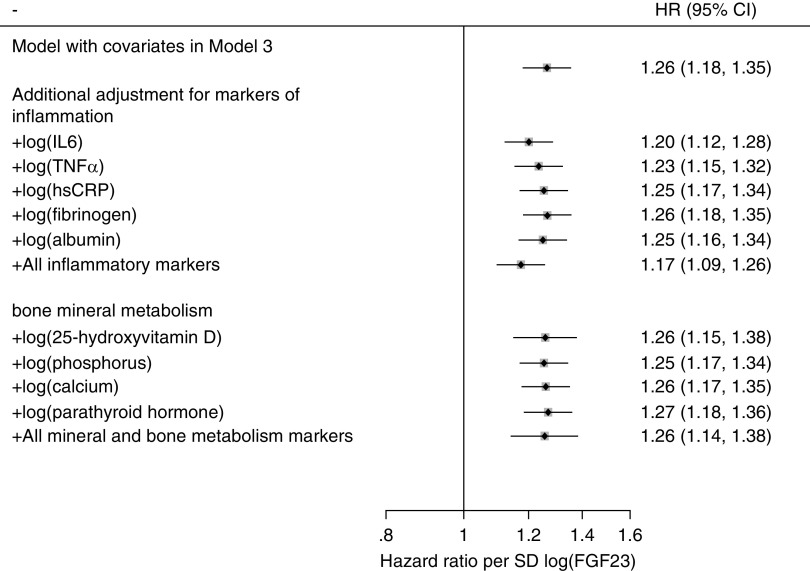
Inflammation, Bone Mineral Metabolism, FGF23, and Risk of Infection
When biomarkers of inflammation (IL-6, TNF-α, high-sensitivity C-reactive protein, fibrinogen, and albumin) and mineral and bone metabolism (25-hydroxyvitamin D, phosphorus, calcium, and parathyroid hormone) were individually or simultaneously added as covariates in model 3, the associations of FGF23 with hospitalization with infection remained significant and largely unchanged. In all models, the risk of infection was 18%–27% higher with every SD increment in log FGF23 (Figure 3), or 36%–51% higher with the highest versus lowest quartile (Supplemental Table 3). The interaction with FGF23 was not significant for any of the biomarkers of inflammation or bone mineral metabolism (P for interaction, all >0.05).
Figure 3.
When biomarkers of inflammation and mineral and bone metabolism were individually or simultaneously added as covariates in model 3, the associations of FGF23 with hospitalization with infection remained significant and largely unchanged. Diamonds in square represent point estimates for HRs per SD increment in log FGF23. Models were adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, hypertension, diabetes, past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease), eGFR, and ACR. Horizontal lines represent the range for 95% CIs. The sample size for analysis for 25-hydroxyvitamin D was restricted to 1915 individuals who participated in an ancillary vitamin D study. hsCRP, high-sensitivity C-reactive protein.
Event Rates for Hospitalization with Infection including Multiple Events per Person
Of 1051 participants, 442 participants had two or more hospitalizations with infection during the follow-up (range, 2–19). The proportion of two or more hospitalizations with infection was higher among participants with higher levels of FGF23 (i.e., 8.4% for <95.3, 11.2% for 95.3–144.2, 13.2% for 144.2–235.9, and 15.5% for ≥235.9 RU/ml) (Table 5). In the multivariable, zero-inflated, negative binomial regression model with the covariates in model 3, participants in the highest quartile of FGF23 had a 71% higher event rate of hospitalization with infection compared with those in the lowest quartile (event rate ratio, 1.71; 95%CI, 1.36 to 2.14).
Table 5.
Adjusted event rate ratios of risk of hospitalization with infection according to quartiles of FGF23, CRIC study 2003–2013
| Outcomes | FGF23 Quartile, RU/ml | |||
|---|---|---|---|---|
| <95.3 | 95.3–144.2 | 144.2–235.9 | ≥235.9 | |
| No. of participants (%) | 913 (100) | 914 (100) | 914 (100) | 914 (100) |
| No. of hospitalizations with infection during follow-up (%) | ||||
| 0 | 713 (78.1) | 673 (73.6) | 634 (69.4) | 584 (63.9) |
| 1 | 123 (13.5) | 139 (15.2) | 159 (17.4) | 188 (20.6) |
| 2 | 37 (4.1) | 47 (5.1) | 66 (7.2) | 67 (7.3) |
| 3 | 23 (2.5) | 23 (2.5) | 30 (3.3) | 26 (2.8) |
| 4 | 4 (0.4) | 15 (1.6) | 16 (1.8) | 22 (2.4) |
| ≥5 | 13 (1.4) | 17 (1.9) | 9 (1.0) | 27 (3.0) |
| Adjusted event rate ratio (95% CI) | 1 (Reference) | 1.12 (0.90 to 1.38) | 1.16 (0.94 to 1.45) | 1.71 (1.36 to 2.14) |
Model was adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, hypertension, diabetes, past medical history (chronic obstructive pulmonary disease, cancer, and cardiovascular disease), eGFR, and ACR.
Discussion
In this cohort study of 3655 individuals with mild-to-severe CKD, higher levels of FGF23 were significantly associated with an increased risk of hospitalization with major infection, although the adjustment for eGFR and ACR and other comorbidity conditions attenuated the association. The risk of infection was greater with higher levels of FGF23, albeit with a shallower risk gradient in the lower range and steeper risk gradient in the higher range. The association was significant across infection subtypes and across demographic and clinical subgroups. Finally, the association of FGF23 was robust and independent of biomarkers of inflammation (i.e., IL-6, TNF-α, high-sensitivity C-reactive protein, fibrinogen, and albumin) and bone mineral metabolism (25-hydroxyvitamin D, phosphorus, calcium, and parathyroid hormone).
Our findings among individuals with nondialysis-dependent CKD are consistent with previous studies in middle- to older-aged adults12,13 and patients on dialysis,14 but strengthen inferences about the relationship of FGF23 and infection for several reasons. First, we studied a diverse set of individuals across a broad spectrum of CKD, a population in which the actions of FGF23 are particularly important. Second, we showed the significant association across infection subtypes, suggesting that FGF23 is broadly implicated in the risk of infection in CKD. Third, a shallow risk gradient in the lower range relative to higher range of FGF23 (e.g., above the median [>144 RU/ml]) may corroborate the previous studies of middle to older adults in the general population reporting the modest association of FGF23 (i.e., 10%–25% increased risk of infection for the highest versus lowest quartile of FGF23),12,13 where the majority (>75%) of individuals had FGF23 levels <100 RU/ml.
The primary role of FGF23 is to lower serum phosphate in CKD by enhancing phosphaturia and suppressing vitamin D synthesis. However, there are several possible biologic mechanisms through which FGF23 increases risk of infection in CKD. In a recent study, FGF23 directly impaired leukocyte recruitment into infected tissue and dampened the host defense in mice with chronic kidney failure.6 Additionally, FGF23 may disrupt the normal immune response by inducing a proinflammatory milieu25 and inhibiting vitamin D–mediated immune pathway.26
Our use of C-terminal FGF23, as opposed to intact FGF23, merits some discussion. C-terminal FGF23 assays measure both intact (i.e., biologically active) and C-terminal fragments of FGF23.27 Previous studies generally showed a strong correlation between C-terminal FGF23 and intact FGF23 levels in patients with CKD.28,29 However, recent studies have suggested that the level of C-terminal FGF23 was disproportionally increased relative to intact FGF23 during the acute inflammatory state.30,31 Therefore, the results of our study may not be generalizable to intact FGF23, and future studies are warranted to confirm the association of intact FGF23 with infection risk.
Our study has limitations. First, observational studies are subject to residual confounding, although CRIC is one of the best available data sets for a CKD population, and we rigorously accounted for major confounders. Second, our outcome ascertainment relied on hospital-generated ICD-9-CM codes, which might have resulted in some misclassification. However, we focused on acute infections which often present with acute symptoms that signal a clear onset of disease and for which the validity of ICD-9 codes is high.32,33 Third, we did not have information on the prevalence of infection at baseline, which may raise the possibility of reverse causality. Nonetheless, we confirmed the consistent association excluding events that occurred in the first year of follow-up. Fourth, although we documented a consistent association of FGF23 with hospitalization for infection, we cannot state that the association is causal. Elevated FGF23 levels may be a nonspecific marker for poor health, although the association of FGF23 was robust to the additional adjustment for serum albumin, another marker of general health. Fifth, whether our results are generalizable to other populations should be evaluated in future studies. For example, previous studies have suggested geographical variations in FGF23 levels.34,35 Finally, because we lacked the granular data regarding the timing of infection (i.e., before or during hospitalization), whether the associations may differ by the cause of infection (e.g., infection severity, hospital-acquired infections) remains unclear and should be explored in future investigations.
Our study provides several important clinical implications. Although FGF23 is not routinely measured in clinical settings, accumulating evidence suggests the usefulness of FGF23 in predicting cardiovascular disease and mortality.11 Our study suggests that FGF23 can also identify individuals at high risk of infection. In addition, because FGF23 synthesis is initially triggered by the decreased capacity of renal phosphate excretion in CKD, interventions to lowering phosphate through medications or diet may be a possible therapeutic approach to reduce the burden of infection in CKD. Importantly, FGF23 itself is an intervention target for a rare hereditary disease36,37; however, its application to CKD requires caution because treatment with reduction of FGF23 in mice with CKD improved hyperparathyroidism but increased mortality.38 Nonetheless, future studies to understand pathways by which FGF23 increases the risk of infection may lead to novel treatments.
In conclusion, in a large cohort of individuals with CKD, there was a direct, progressive relationship between FGF23 and risk of hospitalization with infection, particularly in the higher range of FGF23. These findings suggest a possible role of FGF23 in the increased susceptibility to infection among individuals with CKD.
Disclosures
All authors have nothing to disclose.
Funding
J. Ishigami was supported by National Heart, Lung, and Blood Institute grant T32HL007024. This research was also supported by National Institutes of Health (NIH)/ National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK1100870 (to T. Isakova). Funding for the CRIC study was obtained under a cooperative agreement from NIDDKgrants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902. In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania NIH/National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, National Center for Advancing Translational Sciences and NIH Roadmap for Medical Research grant UL1TR000439, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Center of Biomedical Research Excellence (COBRE) for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente Washington Health Research Institute NIH/National Center for Research Resources (NCRR) University of California, San Francisco - Clinical & Translational Science Institute (UCSF-CTSI) grant UL1 RR-024131. H. Feldman reports grants from National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), during the conduct of the study. R. Townsend reports grants from NIH, during the conduct of the study. L. Appel reports grants from NIDDK, during the conduct of the study.
Supplementary Material
Acknowledgments
The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Junichi Ishigami, Dr. Lawrence J. Appel, and Dr. Kunihiro Matsushita were responsible for research idea and study design. Dr. Junichi Ishigami and Dr. Lawrence J. Appel were responsible for data acquisition. All authors were responsible for data analysis/interpretation. Dr. Junichi Ishigami and Dr. Kunihiro Matsushita were responsible for statistical analysis. Dr. Lawrence J. Appel and Dr. Kunihiro Matsushita were responsible for supervision or mentorship. All authors approved the final version of the manuscript.
Dr. Harold Feldman reports other from Kyowa Hakko Kirin Co, Ltd. and American Journal of Kidney Disease, outside the submitted work. Dr. Anand Srivastava reports personal fees from Horizon Pharma PLC, AstraZeneca, and CVS Caremark, outside the submitted work. Dr. Teresa K. Chen reports grants from NIH/NIDDK and Yale University, outside the submitted work. Dr. Myles Wolf reports personal fees from Amgen, DiaSorin, Akebia Therapeutics, AMAG Pharmaceuticals, Ardelyx, LUTIPOLD Pharmaceuticals, and Keryx Biopharmaceuticals, outside the submitted work. Dr. Tamara Isakova reports personal fees from Kyowa Kirin and LifeSci Capital, outside the submitted work. Dr. Kunihiro Matsushita reports grants from NIH: research funding, personal fees from Akebia, and grants and personal fees from Kyowa Kirin, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Alan S. Go, Jiang He, James P. Lash, and Mahboob Rahman
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101106/-/DCSupplemental.
Supplemental Table 1. IRs and HRs of risk of hospitalization with infection treating death as a competing risk, CRIC study 2003–2013.
Supplemental Table 2. HRs for the association of FGF23 with risk of hospitalization with type specific infections that were recorded as the primary diagnosis, CRIC study 2003–2013.
Supplemental Table 3. HRs of risk of hospitalization with infection for FGF23 after additional adjustment for markers of inflammation and bone mineral metabolism, CRIC study 2003–2013.
Supplemental Figure 1. Histograms and adjusted HRs of risk of hospitalization with infection in a subset of participants who had repeated FGF23 measurements, CRIC study 2003–2013.
References
- 1.James MT, Laupland KB, Tonelli M, Manns BJ, Culleton BF, Hemmelgarn BR; Alberta Kidney Disease Network : Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med 168: 2333–2339, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Dalrymple LS, Katz R, Kestenbaum B, de Boer IH, Fried L, Sarnak MJ, et al.: The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis 59: 356–363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, Gasparini A, Ishigami J, Mzayen K, Su G, Barany P, et al.: eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol 12: 1399–1408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K: CKD and risk for hospitalization with infection: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 69: 752–761, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saran R, Robinson B, Abbott KC, et al.: US renal data system 2016 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 69: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstädt HJ, Meersch M, et al.: FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126: 962–974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al.: Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985–996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe E, Miyaura C, Tanaka H, Shiina Y, Kuribayashi T, Suda S, et al.: 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci U S A 80: 5583–5587, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al.: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al.: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowak KL, Bartz TM, Dalrymple L, et al.: Fibroblast growth factor 23 and the risk of infection-related hospitalization in older adults. J Am Soc Nephrol 28: 1239–1246, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishigami J, Jaar BG, Rebholz CM, Grams ME, Michos ED, Wolf M, et al.: Biomarkers of mineral and bone metabolism and 20-year risk of hospitalization with infection: The atherosclerosis risk in communities study. J Clin Endocrinol Metab 102: 4648–4657, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK: Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 27: 227–237, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB: Infectious disease hospitalizations in the United States. Clin Infect Dis 49: 1025–1035, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, et al.: Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis 56: 522–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al.; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakova T, Cai X, Lee J, et al.: Associations of FGF23 with change in bone mineral density and fracture risk in older individuals. J Bone Miner Res 31: 742–748, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishigami J, Taliercio J, Feldman H, Srivastava A, Townsend R, Cohen D, et al.; CRIC Study Investigators: Inflammatory markers and incidence of hospitalization with infection in chronic kidney disease: The Chronic Renal Insufficiency Cohort (CRIC) study. Am J Epidemiol kwz246, 2019 [Google Scholar]
- 23.Plantinga LC, Fink NE, Melamed ML, Briggs WA, Powe NR, Jaar BG: Serum phosphate levels and risk of infection in incident dialysis patients. Clin J Am Soc Nephrol 3: 1398–1406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ky B, Shults J, Keane MG, Sutton MS, Wolf M, Feldman HI, et al.; CRIC Study Investigators: FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail 6: 817–824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossaint J, Unruh M, Zarbock A: Fibroblast growth factor 23 actions in inflammation: A key factor in CKD outcomes. Nephrol Dial Transplant 32: 1448–1453, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Bacchetta J, Sea JL, Chun RF, et al.: Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28: 46–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith ER, Ford ML, Tomlinson LA, et al.: Instability of fibroblast growth factor-23 (FGF-23): Implications for clinical studies. Clin Chim Acta 412: 1008–1011, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al.: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith ER, Cai MM, McMahon LP, Holt SG: Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97: 3357–3365, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, et al.: Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol 399: 208–218, 2015 [DOI] [PubMed] [Google Scholar]
- 31.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al.: Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber C, Lacaille D, Fortin PR: Systematic review of validation studies of the use of administrative data to identify serious infections. Arthritis Care Res (Hoboken) 65: 1343–1357, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH: Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 60: 397–409, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S, et al.: Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol 7: 810–819, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Liabeuf S, Ryckelynck JP, El Esper N, Ureña P, Combe C, Dussol B, et al.; FRENCH Study Collaborators: Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol 12: 1930–1940, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, et al.: Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest 124: 1587–1597, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppe MD, Zhang X, Imel EA, Weber TJ, Klausner MA, Ito T, et al.: Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep 5: 158–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, et al.: FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122: 2543–2553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.