Significance Statement
Kidney involvement may occur in coronavirus disease 2019 (COVID-19), and can be severe among Black individuals. In this study of collapsing glomerulopathy in six Black patients with COVID-19, the authors found that all six had variants in the gene encoding apo L1 (APOL1) that are more common among those of African descent and linked by past research to susceptibility to collapsing glomerulopathy in non–COVID-19 patients. They found no evidence of direct kidney viral infection but observed changes in gene expression in kidney biopsy samples suggesting that the mechanism is likely driven by a host response. These findings suggest that Black individuals with an APOL1 high-risk genotype and severe acute respiratory syndrome coronavirus 2 infection are at increased risk for experiencing an aggressive form of kidney disease associated with high rates of kidney failure.
Keywords: collapsing glomerulopathy, APOL1, kidney biopsy, SARS-CoV-2, nephrotic, COVID-19
Visual Abstract
Abstract
Background
Kidney involvement is a feature of COVID-19 and it can be severe in Black patients. Previous research linked increased susceptibility to collapsing glomerulopathy, including in patients with HIV-associated nephropathy, to apo L1 (APOL1) variants that are more common in those of African descent.
Methods
To investigate genetic, histopathologic, and molecular features in six Black patients with COVID-19 presenting with AKI and de novo nephrotic-range proteinuria, we obtained biopsied kidney tissue, which was examined by in situ hybridization for viral detection and by NanoString for COVID-19 and acute tubular injury–associated genes. We also collected peripheral blood for APOL1 genotyping.
Results
This case series included six Black patients with COVID-19 (four men, two women), mean age 55 years. At biopsy day, mean serum creatinine was 6.5 mg/dl and mean urine protein-creatinine ratio was 11.5 g. Kidney biopsy specimens showed collapsing glomerulopathy, extensive foot process effacement, and focal/diffuse acute tubular injury. Three patients had endothelial reticular aggregates. We found no evidence of viral particles or SARS-CoV-2 RNA. NanoString showed elevated chemokine gene expression and changes in expression of genes associated with acute tubular injury compared with controls. All six patients had an APOL1 high-risk genotype. Five patients needed dialysis (two of whom died); one partially recovered without dialysis.
Conclusions
Collapsing glomerulopathy in Black patients with COVID-19 was associated with high-risk APOL1 variants. We found no direct viral infection in the kidneys, suggesting a possible alternative mechanism: a “two-hit” combination of genetic predisposition and cytokine-mediated host response to SARS-CoV-2 infection. Given this entity’s resemblance with HIV-associated nephropathy, we propose the term COVID-19–associated nephropathy to describe it.
AKI occurs in 0.1%–29% of patients hospitalized with coronavirus disease 2019 (COVID-19) and is associated with an increased risk of death.1–6 The clinical characteristics include increased serum creatinine with variable degrees of proteinuria and hematuria.4,5 Reports describe patients with COVID-19 with nephrotic-range proteinuria and collapsing glomerulopathy.7–9 We have also encountered such patients, all of whom were of African descent. Therefore, we hypothesized that collapsing glomerulopathy constitutes a new renal manifestation of COVID-19 that may arise from genetic predisposition to injurious second hits caused directly or indirectly by COVID-19. Accordingly, we assessed apo 1 (APOL1) risk allele variants in affected patients and performed in situ hybridization (ISH) and NanoString analysis to assess for virus in the kidney biopsies. Our case series describes six patients with COVID-19 and kidney biopsy specimens in the setting of AKI and proteinuria.
Methods
Six patients with COVID-19 were included in this case series. The diagnosis of COVID-19 was confirmed with a positive PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal swabs. This study was conducted in compliance with the Declaration of Helsinki and approved by the institutional review board. Informed consent was obtained from all patients.
Renal biopsies were processed by standard light microscopy, immunofluorescence (IF), and electron microscopy (EM). Hematoxylin and eosin, Periodic acid–Schiff, and Jones methenamine silver stains were performed on sections from paraffin blocks. IF was performed on frozen sections for IgG, IgA, IgM, C3, C1q, κ light chain, and λ light chain, and EM analysis was done.
DNA was extracted from peripheral blood or renal biopsy tissue and genotyped for APOL1 risk alleles on a ViiA 7 Real-Time PCR System (Thermo Fischer, Waltham, MA) using TaqMan assays.10
ISH was performed with RNAScope (ACD, Newark, CA) using probes directed against SARS-CoV-2 on formalin-fixed, paraffin-embedded, 3-µm tissue sections (ACDBio probes, catalog number 848561, targeted S-gene with target region 21631–23303).11 A negative control probe (bacterial gene dapB) assessed background signals and positive control probes to the housekeeping gene peptidylprolyl isomerase B confirmed RNA integrity. The ISH sections were counterstained using Periodic acid–Schiff.
Three 20-µm sections from the formalin-fixed, paraffin-embedded biopsy block were deparaffinized and RNA extracted for NanoString analysis.12 RNA was analyzed on an nCounter Max System (NanoString, Seattle. WA) using the 770 gene Human Organ Transplant Panel of probes13 supplemented with the 10-gene probe COVID-19 Panel Plus Beta (NanoString). Eight probes are included for SARS-CoV-2 (envelope protein, membrane glycoprotein, nucleocapsid phosphoprotein, surface glycoprotein, and four open reading frames [orf1ab, 3a, 7a, and 8]). The complete gene list for these panels is available at https://www.NanoString.com/products/gene-expression-panels/gene-expression-panels-overview/human-organ-transplant-panel, https://www.NanoString.com/COVID19. Normalized counts of transcripts were compared with those from eight control renal biopsies processed with the Human Organ Transplant Panel in the same way (four with thin basement membrane disease, two with idiopathic hematuria, one minimal change disease in remission, and one potential donor). As a positive control for the SARS-CoV-2 probes, autopsy lung and kidney samples from four COVID-19+ decedents were analyzed.
Results
Clinical Presentation
The clinical characteristics are summarized in Table 1. All patients presented with a febrile illness, with overt respiratory symptoms in five patients. COVID-19 was confirmed with a positive PCR for SARS-CoV-2 from nasopharyngeal swabs. Three patients had CKD stage 3A at baseline, whereas no preexisting CKD was documented for the remaining three patients. Four patients had essential hypertension and three had type 2 diabetes mellitus. Patient 1 had an acquired solitary kidney due to donation to his sister 12 years before the encounter. Otherwise, no remarkable medical or family history was identified. Three patients presented with an elevated serum creatinine and AKI on admission, whereas the remaining three developed a rise in serum creatinine during hospitalization. New-onset, nephrotic-range proteinuria based on urine protein-creatinine ratio and dipstick developed in five patients, and with dipstick only in one patient. All patients had adequate urine output, and none showed hemodynamic instability or shock in parallel with or before AKI. In contrast, two patients were hypertensive. Only two progressed to acute hypoxic respiratory failure requiring mechanical ventilation. Antinuclear antibody was positive in one patient and ANCA was negative in all six patients. Serum complements, assessed in five patients, were within normal limits. AKI did not resolve, and kidney biopsies were performed to establish a diagnosis for the renal manifestations.
Table 1.
Clinical information of six patients with COVID-19, AKI, and nephrotic-range proteinuria
| Patient Identifier | Age (y) | Sex | Race | APOL1 Risk Variants | Serum Creatinine(mg/dl) | Urine | Hgb (mg/dl) | WBC (/ul) | Platelet (/ul) | Albumin (g/dl) | Ferritin (ng/ml) | Final Disposition | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | At Biopsy | Dipstick Protein | Blood (cells/hpf) | WBC (cells/hpf) | UPCR (g/g) | Urine Sediment Microscopy | |||||||||||
| 1 | 63 | M | B | G1/G1 | 1.3 | 4.9 | 3+ | 1–3 | 0–5 | 12.7 | Abundant waxy and muddy brown granular casts | 15.6 | 8.8 | 244 | 2.1 | 2147 | Discharged dependent on dialysis |
| 2 | 64 | F | B | G2/G2 | 1.5 | 4.2 | 3+ | Negative | Negative | 4.6 | Some waxy and coarse granular casts | 8.8 | 7.4 | 421 | 2.4 | 6875 | Discharged home with improving serum creatinine, did not need dialysis |
| 3 | 65 | F | B | G1/G1 | 1.3 | 2.9 | 3+ | Negative | Negative | 13.6 | Many coarse granular and some muddy brown granular casts | 8.3 | 16.6 | 299 | 2.6 | 4934 | Needed dialysis, died with suspected pulmonary embolus |
| 4 | 44 | M | B | G1/G1 | 1.4 | 11.4 | 3+ | >100 | 0–5 | 25 | NP | 8.1 | 4.1 | 241 | 2.5 | 443 | Discharged dependent on dialysis |
| 5 | 37 | M | B | G1/G2 | 1 | 9 | 3+ | 0–2 | 11–20 | NP | NP | 11.7 | 8 | 64 | 3 | 1450 | Needed dialysis; then died of ventricular arrhythmia |
| 6 | 56 | M | B | G1/G1 | 1.2 | 6.7 | 3+ | 50–100 | 5–10 | 3.6 | NP | 13 | 7 | 113 | 2.9 | 1620 | Needed dialysis; discharged off dialysis |
Kidney Biopsy Findings
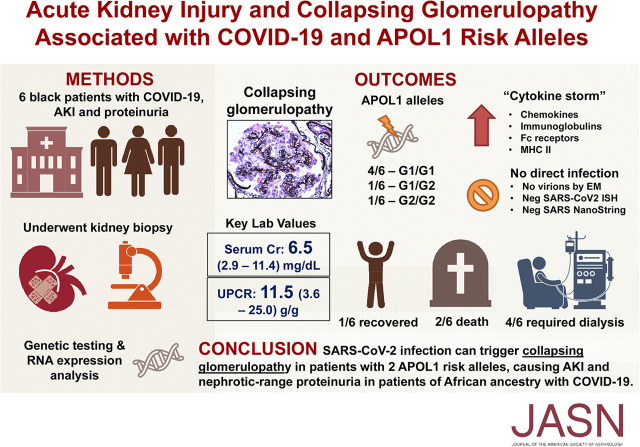
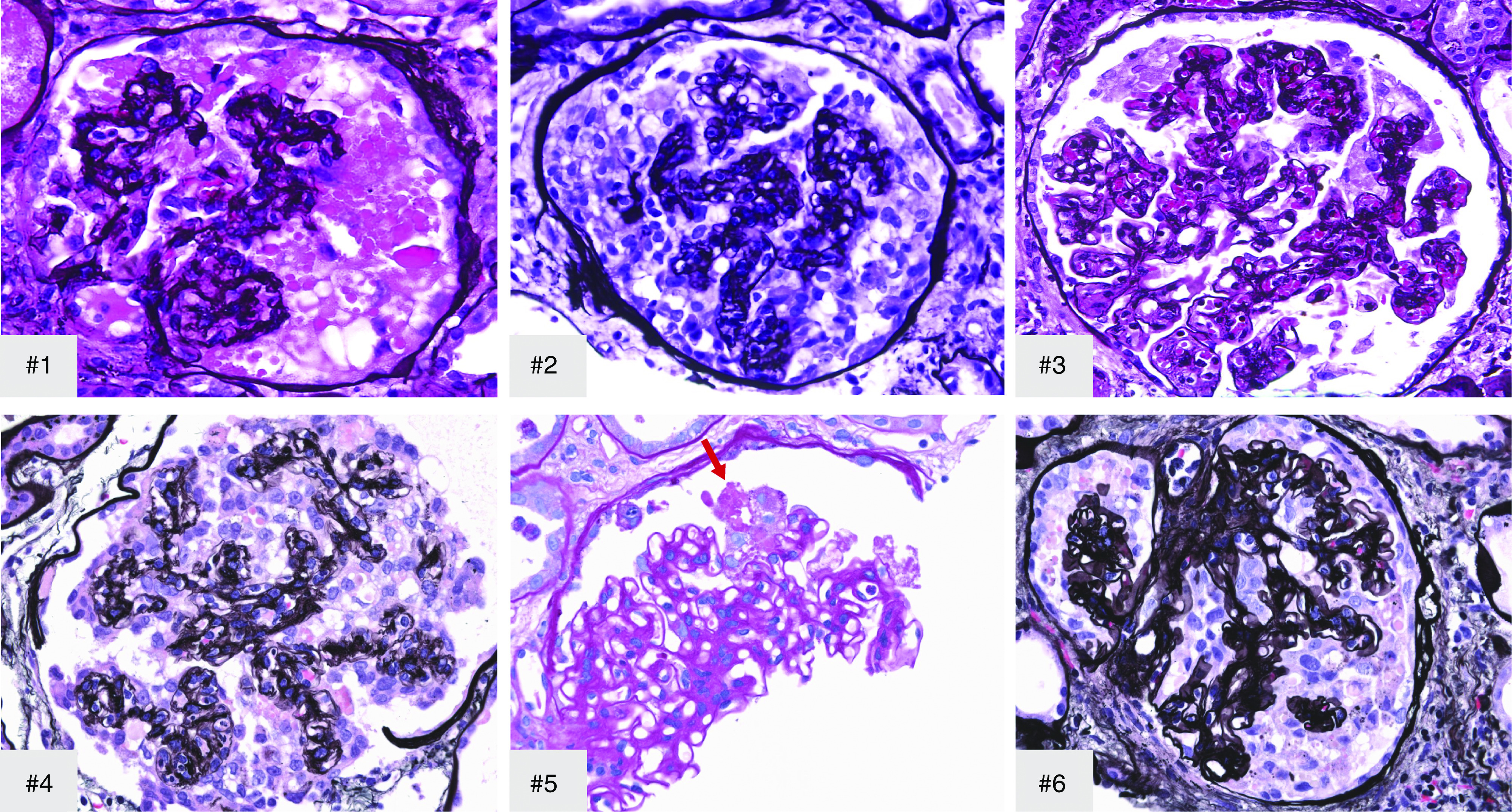
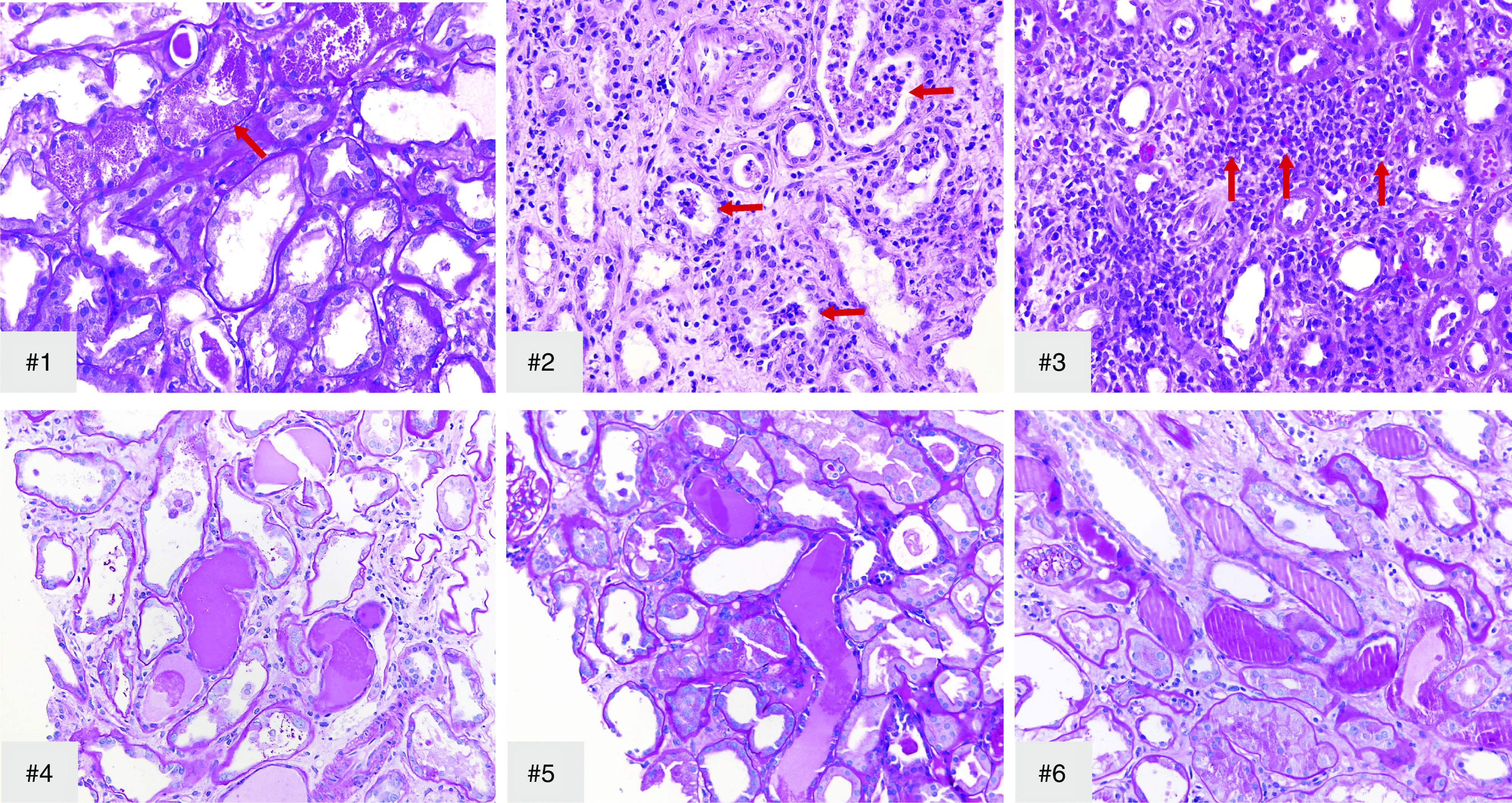
Kidney biopsy findings are summarized in Table 2. All patients showed collapsing lesions with overlying hypertrophy and hyperplasia of visceral epithelial cells with marked eosinophilic protein droplets (Figure 1). There was focal to diffuse acute tubular injury with frequent protein droplets in tubules and very focal microcystic dilation (Figure 2). In patient 2, a diffuse, patchy, pleomorphic interstitial infiltrate composed mostly of lymphocytes with plasma cells and occasional neutrophils along with tubulitis and rare foci of neutrophilic tubular cuffing was observed (Figure 2, case 2), suggesting an infectious etiology. In patient 3, the biopsy showed characteristic features of arterionephrosclerosis and diabetic nephropathy, and also focal acute interstitial nephritis with a lymphocytic infiltrate with occasional foci of eosinophils (ten in a high-power field) and tubulitis with acute tubular injury and interstitial edema (Figure 2, case 3), suggesting drug-induced hypersensitivity. In one patient, focal peritubular capillaries showed red blood cell aggregates but without any fibrin. IF microscopy showed no deposits in any of the cases. EM was performed in five patients and showed extensive foot process effacement and microvillous transformation of podocytes (Figure 3). Endothelial cells were swollen, with occasional small-to-medium reticular aggregates in the cytoplasm in three patients (Figure 3). No definitive viral particles were identified. No electron-dense deposits were present.
Table 2.
Pathologic findings in kidney biopsies
| Patient Identifier | Light Microscopy | EM | ISH | NanoString | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Glomeruli (no.) | Global Sclerosis (no.) | Segmental Sclerosis (no.) | Collapsing Lesions (no.) | ATI | Interstitial Fibrosis | Virus Particles | Foot Process Effacement (%) | Reticular Aggregates | SARS-CoV-2 RNA | SARS-CoV-2 RNA | |
| 1 | 18 | 4 | 6 | 6 | Focal | Mild | None | 100 | Present | Negative | Negative |
| 2 | 25 | 2 | 4 | 3 | Diffuse | Mild | None | 80 | Absent | Negative | Negative |
| 3 | 18 | 2 | 1 | 1 | Diffuse | Mild to moderate | None | 80 | Present | Negative | Negative |
| 4 | 24 | 14 | 2 | 2 | Diffuse | Moderate | None | 100 | Present | Negative | N/A |
| 5 | 14 | 2 | 4 | 3 | Diffuse | Moderate | None | 100 | Absent | Negative | N/A |
| 6 | 7 | 1 | 4 | 3 | Diffuse | Mild | N/A | N/A | N/A | Negative | N/A |
Additional significant findings: Patient 2 had diffuse patchy pleomorphic interstitial infiltrate with neutrophils and tubulitis; patient 3 had mild-to moderate arterionephrosclerosis, mild diabetic nephropathy, and focal acute interstitial nephritis. ATI, acute tubular injury; N/A, not available.
Figure 1.
Collapsing glomerulopathy, showing collapse of glomerular tuft with overlying hypertrophy and hyperplasia of visceral epithelial cells and marked protein droplets in cases 1–6. Arrow, early segmental collapsing lesion. #1–#4 and #6, Jones methenamine silver; #5, Periodic acid–Schiff; original magnification, ×500 in #1 and ×400 in #2–#6.
Figure 2.
All biopsies show acute tubular injury with frequent tubular protein droplets. Arrow in #1, tubular protein droplets. In addition, case 2 (#2) showed diffuse patchy pleomorphic interstitial infiltrate with neutrophils and tubulitis, and rare tubules with intratubular neutrophils (arrows); case 3 (#3) showed focal acute interstitial nephritis with occasional foci of eosinophils (arrows), and microcystic tubules were present in all cases except #3. #1, #4–#6, Periodic acid–Schiff; #2 and #3, hematoxylin and eosin; original magnification, ×400 in #1–#3 and ×200 in #4–#6.
Figure 3.
Transmission EM shows extensive foot process effacement and moderate microvillous transformation of podocytes in all cases. EM shows occasional reticular aggregates (arrow) in cytoplasm of endothelial cells (representative picture from #1). Original magnification, ×4400 in #1, top left; ×2200 in #2–#3; ×6000 in #4; ×8000 in #5; ×14,000 in #1, bottom right.
ISH was performed in all six patients for the presence of SARS-CoV-2 RNA and it failed to show evidence of viral RNA in the kidney, with appropriate staining of controls. No significant signal above the control biopsies was detected for any of the eight SARS-CoV-2 probes by NanoString analysis. For example, the envelop protein RNA-normalized counts were 13.1±3.9 (mean±SD) for the three FSGS cases versus 21.9±16.7 for the 11 negative controls. The control COVID19+ autopsy samples were strongly positive, with envelop protein RNA-normalized counts of 111.3±76.8 for kidney samples and 6924.0±13,528.7 for lung samples. Prominent differential gene expression (more than fourfold versus the controls) was detected for genes related to tubular injury (HAVCR1, HIF1A, LCN2), whereas expression of several normal tubular genes were reduced (AQP2, ASB15, FABP1, MME, MUC1, SLC12A3, SLC4A1), indicative of acute tubular injury. Among the other notable expression increases (>4×) were IL6, numerous chemokines (CCL2, CCL5, CCL19, CCL20, CXCL1/2, CXCL2, CXCL10, CXCL16), Fc receptors (FCER1G, FCGR1A, FCGR2A, FCGR2B, FCGR3A/B), Ig (IGHG1, IGHG2, IGHG3, IGHG4, IGHM, IGKC, IGLC1), and MHC class II antigens (HLA-DQA1, HLA-DRA, HLA-DRB1). No increase in IFNG or IFNA1 transcripts was detected.
Follow-up Clinical Course
Peripheral blood specimens or kidney tissue were used to perform genotyping for APOL1 G1 and G2 risk alleles. All patients showed two risk alleles for APOL1 (G1/G1 in four patients, G1/G2 in one, and G2/G2 in one). Five (83%) patients progressed to require hemodialysis during the hospitalization. Patients 3 and 5 died in the hospital due to suspected pulmonary embolism and ventricular arrhythmia, respectively. Of the four patients who were discharged home, two were still dependent on hemodialysis at discharge. Patient 1 eventually came off dialysis 4 weeks later. Patient 2 did not require hemodialysis, had a gradual recovery, and was discharged with serum creatinine of 4.2 mg/dl. At a follow-up visit 2 weeks later, serum creatinine improved to 3.1 mg/dl, but she remained nephrotic with a urine protein-creatinine ratio of 3.7 g/g. Patient 4 remains dependent on dialysis 6 weeks postdischarge, but hemodialysis has been reduced from three times to twice weekly because of increase in urine output. Patient 6 has shown further improvement in serum creatinine, down to 2.8 mg/dl, but his proteinuria has not been reassessed.
Discussion
AKI is relatively common in patients with COVID-19, especially in those with critical illness.5,14,15 This report expands on the recognition and etiology of AKI and proteinuria in patients with COVID-19 and strongly suggests that SARS-CoV-2 infection may play a role in the development of collapsing glomerulopathy in susceptible individuals. Of note, three recent publications from the United States and Europe each reported a single case of collapsing glomerulopathy in Black patients with COVID-19, and APOL1 G1 risk allele homozygosity was assessed and detected in two of the patients.7–9 Although acute tubular injury resulting from hemodynamic instability is likely the main driver for AKI in critically ill patients with COVID-19, the possibility of direct infection of kidney parenchyma by this virus has been entertained. Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound peptidase that acts as protein ligand for COVID-19 binding in humans, thus allowing viral cell entry and damage of target organs.16 ACE2 is highly expressed in the kidney, especially in the proximal renal tubules, and relatively weakly in the glomeruli.17–19 The tubular ACE2 expression in normal kidneys is nearly 100 times higher than that in the respiratory tract (Z. Li, M. Wu, J. Yao, J. Guo, X. Liao, S. Song, et al.: Caution on kidney dysfunctions of 2019-nCoV patients, https://doi.org/10.1101/2020.02.08.20021212).20 Su et al.21 recently reported an autopsy study of patients with COVID-19 who died of severe respiratory failure with multiorgan complications. In this study, only a small number of peritubular capillaries showed red blood cell aggregates in one patient, a lesion described in the autopsy series from China.21 A subset of patients in the Chinese autopsy series had new-onset kidney disease, but without nephrotic syndrome or collapsing glomerulopathy. Tissue from a few of these patients showed occasional viral-like particles in podocytes and in tubules. However, those structures were not confirmed to be of coronavirus origin by either ISH or immunogold EM.21 Interestingly, aberrantly increased ACE2 expression was observed in some of these patients’ proximal tubule cells, with mild increase in podocytes and de novo expression in parietal epithelial cells.21 These autopsy findings suggested the possibility that, in some patients, SARS-CoV-2 could be contributing directly to podocyte and tubular cell injury. Recently, further evidence of direct infection of the virus into the kidneys has been reported in autopsy specimens without collapsing lesions.22 However, in our case series, we did not find any evidence of kidney infection of SARS-CoV-2 RNA by EM, ISH, or NanoString. Thus, although the possibility that the virus was present below the level of detection cannot be entirely excluded, our findings indicate that direct damage by SARS-CoV-2 is not the mechanism triggering the collapsing glomerulopathy in this setting.
A host response—i.e., a “cytokine storm”–induced injury—may be an alternative mechanism for COVID-19–associated collapsing glomerulopathy. Patients with COVID-19 in the intensive-care unit typically show elevated plasma levels of proinflammatory cytokines including IL-2, IL-7, IL-10, granulocyte cell-stimulating factor, IP-10, MCP1, MIP1A, and TNF-α versus non-ICU patients with COVID-19.23 Moreover, COVID-19 induced several proinflammatory cytokines also induced by SARS-CoV-2, including IL1B, IFN, IP10, and MCP1. We also observed a marked increase in gene expression of IL6 and numerous chemokines including CCL2 and CXCL10 (also known as MCP1 and IP10, respectively) in the kidney tissue by NanoString. These cytokines can cause enhanced apoptosis of target cells, suboptimal T cell reactions, impaired virus clearance, and increased vascular leakage—the classic consequences of this immune storm.23,24 In addition, occasional reticular aggregates were observed by EM in some of the patients, although no increase in IFNG or IFNA1 transcripts was detected by NanoString. Reticular aggregates are a marker associated with high levels of the cytokine α-IFN, and they are particularly numerous in patients with SLE, HIV infection, and IFN treatment. Therefore, we speculate that the SARS-CoV-2 infection could initiate a systemic cytokine activation and immune cascade. Our observation confirms the report by Larsen et al.7 who also observed reticular aggregates in a case of collapsing glomerulopathy linked to SARS-CoV-2 infection.
Collapsing glomerulopathy has numerous etiologies including viral infections, such as HIV, cytomegalovirus, and parvovirus B19; severe ischemia; medications, such as pamidronate, anabolic steroids; and IFN.25–26 Whereas HIV can directly infect renal parenchymal cells (tubular epithelial cells and glomerular visceral epithelial cells) in cases of HIV-associated nephropathy,27,28 such definitive evidence for coronavirus glomerular infection is lacking. Increased susceptibility to collapsing glomerulopathy, including HIV-associated nephropathy, has been linked to risk variants of APOL1 (G1, G2), which are increased in those of African descent and are protective against trypanosomal disease.26,29–31 Our patients were Black with two risk alleles for APOL1, suggesting the possibility that COVID-19 may increase the risk of collapsing glomerulopathy in those patients with risk variants of APOL1, i.e., a “second-hit” phenomenon. In one patient, a single kidney status due to living donation more than a decade earlier likely further increased the risk of adverse response to additional hits.
In summary, we present six Black patients with COVID-19 and two APOL1 risk alleles who presented with rapid worsening in renal function and proteinuria, with renal biopsies showing collapsing glomerulopathy without evidence of kidney parenchymal viral infection. Because collapsing glomerulopathy can often result in irreversible kidney damage and ESKD, this observation may have important public health implications for individuals with genetic risk factors. Further study is needed both from biopsy and autopsy to fully investigate the renal morphologic changes in patients with COVID-19 worldwide and uncover potential mechanisms with implications for treatment.
Disclosures
J.M. Gimenez reports he belongs to the Boston Scientific Interventional Oncology Advisory Board, is a Yttrium-90 proctor for Boston Scientific Therasphere, and is a speaker for Boston Scientific in Interventional Oncology. He does not believe any of these are related to the submitted work. His institution receives funds for these activities, not him. I. Rosales reports consultancy for eGenesis. J.C. Velez has participated in advisory board engagements with Mallinckrodt Pharmaceuticals and Retrophin and is a member of a speaker bureau for Otsuka Pharmaceuticals; none of the products related those engagements are discussed in this manuscript. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
We thank Lyndsey Buckner from the Ochsner BioBank for facilitating blood collection and shipping.
Dr. Agnes B. Fogo, Dr. Juan Carlos Q. Velez, and Dr. Huijuan Wu wrote and edited the manuscript; Dr. Tiffany Nicole Caza, Dr. Agnes B. Fogo, Dr. Christopher Patrick Larsen, Dr. Mark A. Lusco, Dr. Huijan Wu, and Dr. Haichun Yang interpreted kidney biopsies and prepared tissue for molecular studies; Dr. Tiffany Nicole Caza and Dr. Christopher Patrick Larsen performed APOL1 and ISH; Dr. Ellen Acheampong, Dr. Robert Colvin, and Dr. Ivy A. Rosales performed NanoString analysis; Dr. Asim Chughtai, Dr. Juan M. Gimenez, Dr. Cesar F. Hernandez-Arroyo, Dr. Muner M.B. Mohamed, Dr. Tyler A. Sandow, Dr. Moh’d Sharshir, Dr. Juan Carlos Q. Velez, and Dr. Liping Xie provided clinical information; Dr. Juan M. Gimenez and Dr. Tyler A. Sandow performed three of the kidney biopsies.
Dr. Juan Carlos Q. Velez reports personal fees from Mallinckrodt Pharmaceuticals, personal fees from Otsuka Pharmaceuticals, and personal fees from Retrophin, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al.: Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al.: Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al. : Acute kidney injury associated with Coronavirus disease 2019 in Urban New Orleans [published online ahead of print May 13, 2020]. Kidney360 doi:10.34067/KID.0002652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al.: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med 8: e26, 2020]. Lancet Respir Med 8: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with (COVID-19). Kidney Int Rep 5: 935–939, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al.: Collapsing glomerulopathy in a COVID-19 patient [published online ahead of print April 15, 2020]. Kidney Int doi:10.1016/j.kint.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, et al.: Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection [published online ahead of print April 28, 2020]. Kidney Int Rep doi:10.1016/j.ekir.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al.: RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14: 22–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RN, Matsunami M, Adam BA, Rosales IA, Oura T, Cosimi AB, et al.: RNA expression profiling of nonhuman primate renal allograft rejection identifies tolerance. Am J Transplant 18: 1328–1339, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, et al.: Banff 2019 Meeting Report: Molecular diagnostics in solid organ transplantation – Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation [published online ahead of print May 19, 2020]. Am J Transplant doi:10.1111/ajt.16059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabb H: Kidney diseases in the time of COVID-19: major challenges to patient care. J Clin Invest 130: 2749–2751, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V: The novel coronavirus 2019 epidemic and kidneys. Kidney Int 97: 824–828, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al.: Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565–574, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H: Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG: Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Zou X, Chen K, Zou J, Han P, Hao J, Han Z: Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China [published online ahead of print April 9, 2020]. Kidney Int doi:10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. : Multiorgan and renal tropism of SARS-CoV-2 [published online ahead of print May 13, 2020]. N Eng J Med doi:10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al.: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Channappanavar R, Perlman S: Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529–539, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra P, Kopp JB: Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J 6: 1–5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopp JB: Expanding the spectrum of APOL1-related renal disease: de novo collapsing glomerulopathy following kidney transplant. Kidney Int 94: 1048–1050, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen AH, Sun NC, Shapshak P, Imagawa DT: Demonstration of human immunodeficiency virus in renal epithelium in HIV-associated nephropathy. Mod Pathol 2: 125–128, 1989. [PubMed] [Google Scholar]
- 28.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, et al.: Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med 344: 1979–1984, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Abid Q, Best Rocha A, Larsen CP, Schulert G, Marsh R, Yasin S, et al.: APOL1-Associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-Associated vasculopathy with onset in infancy (SAVI). Am J Kidney Dis 75: 287–290, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al.: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]