Kidney injury is a known and relatively common sequalae of end stage heart failure,1 and ESKD can occur or be potentiated in the setting of heart transplant.2 Dialysis after heart transplant adds to complexity of care, cost, and morbidity.3,4 Simultaneous heart kidney transplant (SHK) can mitigate the effects of suboptimal kidney function during the perioperative period, and several retrospective studies have demonstrated improved survival after SHK compared with heart transplant alone among patients on dialysis or with reduced GFR.5–8 Perhaps in response to the emerging recognition of these benefits, SHK has experienced the largest increase in frequency of all multiorgan transplants (MOTs) in the past 5 years.9 From 2004 to 2018, the number of SHKs has increased nearly fivefold (from 44 to 202), and the proportion of all heart transplants that are SHK has increased 2.5 times (from 2.6% to 6.9%) (Figure 1A). In the same time period, the proportion of SHK recipients on dialysis during this time has stayed constant at approximately 50% (Figure 1B). Additionally, the proportion of deceased donor kidneys allocated to SHK recipients has tripled from 0.46% to 1.37%, whereas the proportion of deceased donor kidney recipients receiving MOT has stayed constant (from 12.2% to 11.6%) (Figure 1C).
Figure 1.
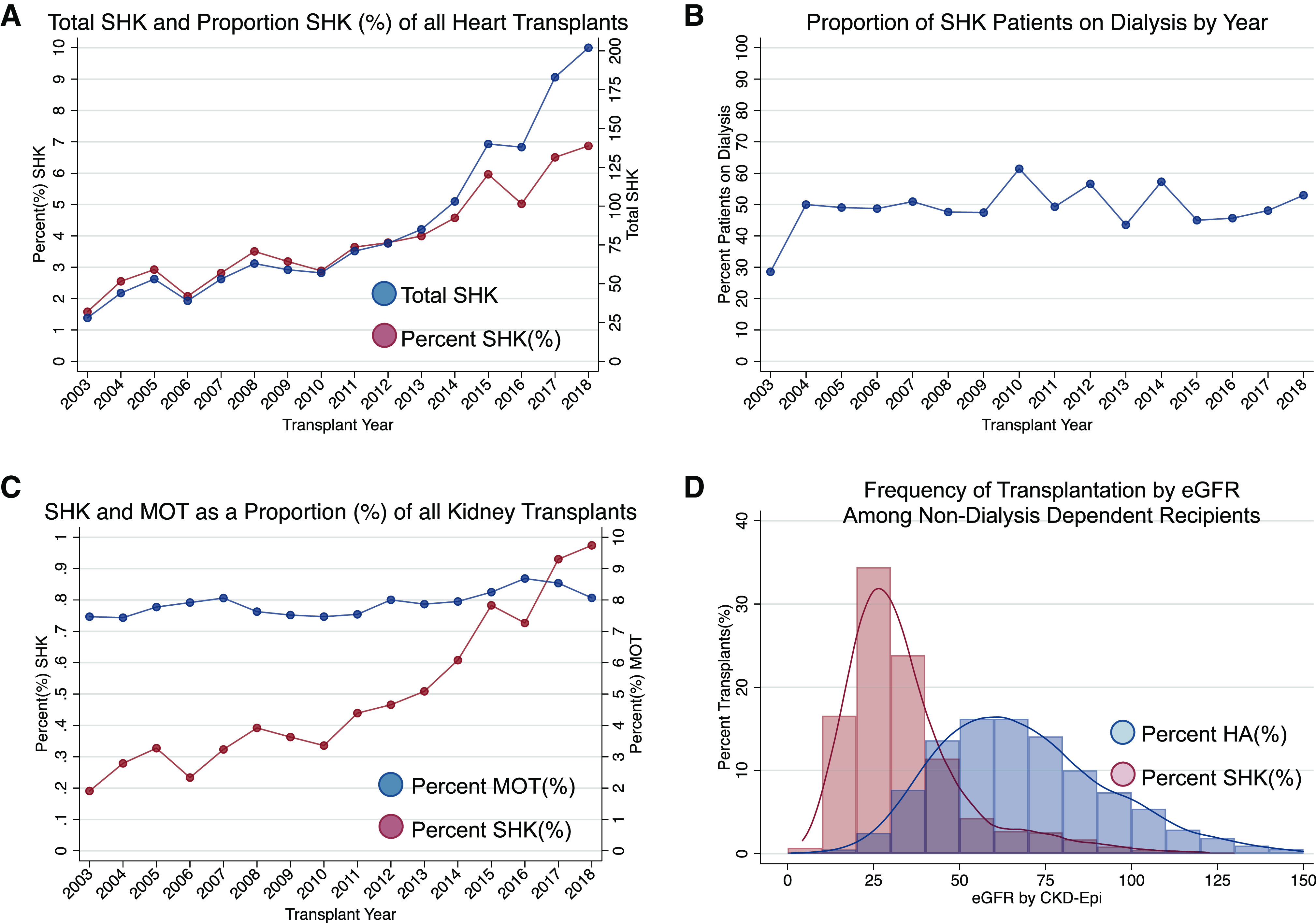
SHK is increasing at a faster rate than MOT overall. (A) Total number and proportion of SHK among all heart transplants. (B) Proportion of patients undergoing SHK on dialysis at the time of transplant. (C) Proportion of all kidney transplants that are SHKs or MOTs (includes simultaneous kidney pancreas transplant). (D) Distribution of eGFR among patients undergoing SHK or heart alone (HA) transplantation among non-dialysis depdent patients (on the basis of Organ Procurement and Transplantation Network data as of June 2019). CKD-EPIpi, Chronic Kidney Disease Epidemiology Collaboration.
There are no universally agreed upon guidelines for eligibility for SHK, and the ability to predict renal recovery in patients with heart failure is limited. Therefore, kidney transplant at the time of heart transplant may be unnecessary in some patients who undergo SHK. Many patients with renal insufficiency in the setting of heart failure have cardiorenal syndrome, and heart transplantation alone may lead to renal recovery.10,11 In these patients, a staged approach, with the potential for kidney transplant after heart transplant, may be the most judicious use of scarce organs. Additionally, implantation of a kidney at the time of heart transplant may actually lead to worse kidney graft survival given the hemodynamic shifts, vasopressor requirement, and use of cardiopulmonary bypass in the perioperative period.6 Finally, there are likely patients who meet reasonable criteria for SHK but lack the physiologic reserve to benefit regardless of if the operation is pursued because worsening renal function in the peritransplant period portends inferior outcomes.12 All SHK candidates must be evaluated by a selection committee prior to being added to the wait list, but individual centers may define their SHK evaluation processes. For example, SHK candidacy can be determined by the heart selection committee, kidney selection committee, or both; some centers have developed special multiorgan selection committees. Inconsistent evaluation processes increase the challenge of determining whether current practices are optimal both for individual patients and for the transplant system as a whole.
It is imperative to closely examine current approaches to patient selection for SHK. Any criteria used should maximize both beneficence—what is best for the individual patient—and utility—what creates the most good for the greatest number of patients. The benefit of SHK varies by degree of renal insufficiency,5–8 and there is wide variation in the range of eGFR at which SHK is performed in non-dialysis dependent patients (Figure 1D). Meanwhile, patients without heart failure but with ESKD on dialysis have a 5-year survival of 60% that improves to nearly 90% with kidney transplantation.13 Therefore, listing patients with moderate renal insufficiency for SHK may allocate kidneys to patients who receive less benefit from the graft in terms of life-years gained. This occurs because kidneys intended for SHK are allocated ahead of those intended for patients undergoing kidney-only transplantation. Additionally, recipients who receive kidneys as part of MOT operations generally receive higher-quality kidneys as assessed by the Kidney Donor Profile Index, regardless of their age or functional status.14 This undermines matching schemes used in the current kidney allocation system, which pair kidneys with recipients with appropriate predicted lifespans.15 In attempting to balance beneficence and utility, the SHK population may benefit from looking to the work done in simultaneous liver kidney (SLK). One conceptualization in SLK, a willingness to transplant threshold, specifies the acceptable number of quality-adjusted life-years (QALYs) gained per organ transplanted and then determines the policy that yields the highest “net transplant benefit” at a given QALY threshold.16 When optimizing utility, MOT is restricted because these recipients are more likely to experience early morbidity and mortality. When optimizing beneficence, MOT is favored because these recipients more urgently need an organ at a given time. Also, a “safety net” has been created, which allows for the allocation of a kidney up to 12 months after transplant if the liver recipient experiences sustained renal failure.17 Experience with both kidney after liver transplant and kidney after heart transplant is growing and generally positive, but both require continued evaluation.18,19
Certainly, SHK is the appropriate operation in many patients, and we should continue to prioritize those patients who urgently require it while refining our understanding of the short- and long-term benefits. An allocation system similar to SLK may be the most appropriate intermediate step, where demonstrated renal failure that is unlikely to recover, as determined by a nephrologist, is required for SHK eligibility. In SLK, this is defined as CKD (GFR<60 for 90 consecutive days as well as either a GFR ≤30 at or after registration for kidney transplant or dialysis in the setting of ESKD), AKI (either dialysis or a GFR≤25 for six consecutive weeks or a combination of dialysis and low GFR for six consecutive weeks), or the presence of certain metabolic diseases (including hyperoxaluria).17 However, we acknowledge that the criteria may require adjustment for the heart failure population. A safety net system, similar to the one outlined in the SLK allocation policy, would expedite kidney allocation for patients with persistent renal failure postoperatively. We do not advocate for regulations so stringent as to arrest the practice of SHK. Indeed, recent changes to allocation in SLK have not affected the overall volume of SLK in the United States but rather, are a starting point for continued evaluation and refinement of selection criteria.9
Further research should be performed to assist in defining criteria under which SHK should be performed. Examples include (1) observational cohort studies that better characterize the relationship between kidney function and heart function, identify risk factors for worsening renal function, and elucidate mediators of renal recovery in patients with heart failure; (2) prospective studies of objective measures of kidney function—such as biopsy or nuclear medicine testing—to assess native renal function before and after SHK; and (3) retrospective studies modeling the utility of allocating a given kidney graft to a kidney alone candidate versus an SHK candidate to inform costs, health care resource utilization, and QALY cutoffs at which a given transplant (SHK versus kidney alone) should proceed. A substantial research effort exists to identify biomarkers associated with renal recovery in patients with heart failure, but the extent to which these results can be applied to the heart transplant population is less well defined and deserves investigation.10 Finally, studies to define which patients with significant—but not end stage—heart disease who do not qualify for primary renal transplant but could benefit from SHK should be constructed.
With demand for organs continuing to dwarf supply, considerations of the optimal use of each organ are paramount. More evidence is needed to understand when and how SHK ought to be performed. All transplants not only involve a scarce resource but also begin in an act of altruism. We owe it to our donors to maximize the utility of their gifts.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C.
Acknowledgments
The authors thank the Duke Transplant Center for support of this project.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, et al.: Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: Insights from the get with the guidelines-heart failure registry. Circ Heart Fail 11: e004646, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J; ADHERE Scientific Advisory Committee and Investigators : High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J Card Fail 13: 422–430, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cantarovich M, Hirsh A, Alam A, Giannetti N, Cecere R, Carroll P, et al.: The clinical impact of an early decline in kidney function in patients following heart transplantation. Am J Transplant 9: 348–354, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Hornberger J, Best J, Geppert J, McClellan M: Risks and costs of end-stage renal disease after heart transplantation. Transplantation 66: 1763–1770, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Gill J, Shah T, Hristea I, Chavalitdhamrong D, Anastasi B, Takemoto SK, et al.: Outcomes of simultaneous heart-kidney transplant in the US: A retrospective analysis using OPTN/UNOS data. Am J Transplant 9: 844–852, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR: Heart and combined heart-kidney transplantation in patients with concomitant renal insufficiency and end-stage heart failure. Am J Transplant 14: 384–396, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Karamlou T, Welke KF, McMullan DM, Cohen GA, Gelow J, Tibayan FA, et al.: Combined heart-kidney transplant improves post-transplant survival compared with isolated heart transplant in recipients with reduced glomerular filtration rate: Analysis of 593 combined heart-kidney transplants from the United Network Organ Sharing Database. J Thorac Cardiovasc Surg 147: 456–461.e1, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Kilic A, Grimm JC, Whitman GJ, Shah AS, Mandal K, Conte JV, et al.: The survival benefit of simultaneous heart-kidney transplantation extends beyond dialysis-dependent patients. Ann Thorac Surg 99: 1321–1327, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Fox A: Ethical implications of multi organ transplant. OPTN/UNOS Public Comment Proposal.1-29, 2019. Available at: https://optn.transplant.hrsa.gov/media/2801/ethics_publiccomment_20190122.pdf. Accessed February 20, 2020
- 10.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al.; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology: Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American heart association. Circulation 139: e840–e878, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Taiwo AA, Khush KK, Stedman MR, Zheng Y, Tan JC: Longitudinal changes in kidney function following heart transplantation: Stanford experience. Clin Transplant 32: e13414, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolsrud O, Karason K, Holmberg E, Ricksten S-E, Felldin M, Samuelsson O, et al.: Renal function and outcome after heart transplantation. J Thorac Cardiovasc Surg 155: 1593–1604.e1, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang S-H, et al.: An economic assessment of contemporary kidney transplant practice. Am J Transplant 18: 1168–1176, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Reese PP, Veatch RM, Abt PL, Amaral S: Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant 14: 21–26, 2014. [DOI] [PubMed] [Google Scholar]
- 15.UNOS/OPTN: The New Kidney Allocation System (KAS) frequently asked questions. 1–17, 2016. Available at: https://optn.transplant.hrsa.gov/media/1235/kas_faqs.pdf. Accessed February 27, 2020
- 16.Cheng XS, Goldhaber-Fiebert J, Tan JC, Chertow GM, Kim WR, Wall AE: Defining a willingness-to-transplant threshold in an era of organ scarcity: Simultaneous liver-kidney transplant as a case example. Transplantation 104: 387–394, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNOS/OPT: Simultaneous Liver Kidney (SLK) Policy. 1-19, 2017. Available at: https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf. Accessed April 23, 2020
- 18.Gallo M, Trivedi JR, Schumer EM, Slaughter MS: Combined heart-kidney transplant versus sequential kidney transplant in heart transplant recipients [published online ahead of print March 9, 2020]. J Card Fail doi:10.1016/j.cardfail.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Jay CL, Washburn WK, Rogers J, Harriman D, Heimbach J, Stratta RJ: Difference in survival in early kidney after liver transplantation compared with simultaneous liver-kidney transplantation: Evaluating the potential of the “safety net”. J Am Coll Surg 230: 463–473, 2020. [DOI] [PubMed] [Google Scholar]


