Abstract
Introduction
Little is still known about the prognostic impact of incident arrhythmias in hospitalized patients with COVID‐19. The aim of this study was to evaluate the incidence and predictors of sustained tachyarrhythmias in hospitalized patients with COVID‐19, and their potential association with disease severity and in‐hospital mortality.
Materials and methods
This was a retrospective multicenter observation study including consecutive patients with laboratory confirmed COVID‐19 admitted to emergency department of ten Italian Hospitals from 15 February to 15 March 2020. The prevalence and the type of incident sustained arrhythmias have been collected. The correlation between the most prevalent arrhythmias and both baseline characteristics and the development of ARDS and in‐hospital mortality has been evaluated.
Results
414 hospitalized patients with COVID‐19 (66.9 ± 15.0 years, 61.1% male) were included in the present study. During a median follow‐up of 28 days (IQR: 12‐45), the most frequent incident sustained arrhythmia was AF (N: 71; 17.1%), of which 50 (12.1%) were new‐onset and 21 (5.1%) were recurrent, followed by VT (N: 14, 3.4%) and supraventricular arrhythmias (N: 5, 1.2%). Incident AF, both new‐onset and recurrent, did not affect the risk of severe adverse events including ARDS and death during hospitalization; in contrast, incident VT significantly increased the risk of in‐hospital mortality (RR: 2.55; P: .003).
Conclusions
AF is the more frequent incident tachyarrhythmia; however, it not seems associated to ARDS development and death. On the other hand, incident VT is a not frequent but independent predictor of in‐hospital mortality among hospitalized COVID‐19 patients.
Keywords: Atrial Fibrillation, Covid‐19, Covid‐19 and arrhythmia, Covid‐19 and cardiovascular complications, Covid‐19 and cardiovascular System, SARS‐CoV‐2, SARSCoV‐2 and ventricular arrhythmias
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a highly pathogenic human coronavirus recently recognized as the cause of the coronavirus disease 2019 (COVID‐19). The outbreak sparked in China and spread rapidly to other countries, reaching devastating pandemic proportion. 1 Italy is the one of the hardest hit countries by COVID‐19, with more than 236 000 laboratory‐confirmed cases by 14 June 2020. 2 The clinical course of COVID‐19 may be frequently complicated by cardiac arrhythmias, both atrial and ventricular. 3 Actually, little is still known about the clinical characteristics of COVID‐19 patients who developed arrhythmias during hospitalization 4 ; the type and burden of incident sustained tachyarrhythmias; the clinical impact of atrial fibrillation (AF) or ventricular tachycardia (VT) on disease severity and in‐hospital mortality.
The aim of this multicenter study was to evaluate the incidence and the predictors of sustained tachyarrhythmias in hospitalized patients with COVID‐19, and their potential association with disease severity and in‐hospital mortality.
2. MATERIALS AND METHODS
All consecutive patients with laboratory confirmed COVID‐19 admitted to emergency department of ten Italian Hospitals from 15th February to 15th March were included in this retrospective observational study All patients underwent medical history, physical examination, electrocardiographic and laboratory evaluation. Chest X‐Ray and/or computed tomography (CT) scan were also performed to rule out pneumonia in one or multiple sites. The primary aim of our study was to explore the incidence and predictors of most common incident tachyarrhythmias (ie atrial fibrillation and ventricular tachycardias) in hospitalized COVID‐19 patients. The secondary aim was to verify whether atrial fibrillation and/or ventricular tachycardias were independently associated with ARDS and/or in‐hospital mortality. The correlation between the most prevalent arrhythmias and both baseline characteristics and the development of ARDS and in‐hospital mortality has been evaluated. ARDS diagnosis was defined according to the Berlin definition. 5
2.1. Statistical analysis
Distribution of continuous data was tested with the Kolmogorov‐Smirnov and the Shapiro‐Wilk test. Normally distributed variables were expressed as mean ± standard deviation (SD), whereas non‐normal distributed ones as median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. The unadjusted (univariable) and adjusted (multivariable) risk ratios (RR) both for incident tachyarrhythmias and the outcomes of interest were calculated using logistic regression models and presented as RR with their 95% confidence intervals (CI). All independent variables showing a P value <.1 for the association with the response variable at univariable analysis were tested in the multivariable model. For all tests, a P value <.05 was considered statistically significant. Analyses were performed by using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
414 hospitalized patients with COVID‐19 were included in the present study. The mean age was 66.9 ± 15.0 years; 253 (61.1%) were males. The median follow‐up was 28 days (IQR: 12‐45). Incident tachyarrhythmias were reported in 90 cases (21.7%). The most frequent incident sustained arrhythmia was AF (N: 71; 17.1%), of which 50 (12.1%) were new‐onset and 21 (5.1%) were recurrent, followed by VT (N: 14, 3.4%) and supraventricular arrhythmias (N: 5, 1.2%).
COVID‐19 patients who experienced incident AF during the hospitalization were older compared to those without incident AF (P < .001) and showed higher prevalence of smoking habit (P = .035), hypertension (P = .002), history of AF (P = .004), heart failure (P < .001), chronic kidney disease (CKD, P = .034), coronary artery disease (CAD, P < .001), angiotensin‐converting enzyme inhibitors (ACE‐I) and/or angiotensin II receptor blockers (ARBs) use (P = .030) and incident VT (P = .003); however at multivariate regression model only older age (RR: 1.03; P = .002), heart failure (RR: 1.88; P = .023), CAD (RR: 1.75; P = .024) and incident VT (RR: 2.94, P = .008) were associated with increased risk of incident AF (Tables 1, 2, 3).
TABLE 1.
Distribution of patients’ characteristics and their association with incident AF among COVID‐19 study population
| Incident AF free group | Incident AF group | Univariable | Multivariable | |
|---|---|---|---|---|
| RR (CI), P value | RR (CI), P value | |||
| n | 343 | 71 | ||
| Male gender, n (%) | 207 (60.3) | 46 (64.8) | 1.17 (0.75,1.76), P: .485 | ‐ |
| Age (mean (SD)) | 65.54 (15.48) | 73.69 (9.90) | 1.04 (1.02,1.05), P < .001 | 1.03 (1.01,1.05), P: .002 |
| Smoker, n (%) | 63 (18.4) | 21 (29.6) | 1.62 (1.02, 2.36), P: .035 | NS |
| Hypertension, n (%) | 206 (60.2) | 57 (80.3) | 2.08 (1.37, 3.02), P: .002 | NS |
| Diabetes, n (%) | 84 (24.6) | 22 (31.0) | 1.29 (0.81, 1.93), P: .261 | ‐ |
| Dyslipidaemia, n (%) | 94 (27.5) | 24 (33.8) | 1.27 (0.80, 1.88), P: .285 | ‐ |
| Obesity, n (%) | 24 (13.3) | 5 (10.6) | 0.82 (0.30, 1.67), P: .632 | ‐ |
| History of AF, n (%) | 51 (15.0) | 21 (29.6) | 1.93 (1.24, 2.73), P: .004 | NS |
| Heart Failure, n (%) | 29 (8.5) | 17 (23.9) | 2.41 (1.53, 3.35), P < .001 | 1.88 (1.09, 2.86), P: .023 |
| Previous Stroke, n (%) | 25 (7.3) | 10 (14.1) | 1.75 (0.92, 2.78), P: .068 | NS |
| CKD, n (%) | 47 (13.8) | 17 (23.9) | 1.69 (0.61, 5.80), P: .034 | NS |
| CAD, n (%) | 45 (13.2) | 21 (29.6) | 2.12 (1.38, 2.96), P < .001 | 1.75 (1.07, 2.59), P: .024 |
| COPD, n (%) | 65 (19.0) | 23 (32.4) | 1.73 (1.12, 2.48), P: .134 | ‐ |
| ACEI/ARBs, n (%) | 132 (41.4) | 38 (55.9) | 1.57 (1.05, 2.25), P: .030 | NS |
| Ca‐Antagonists, n (%) | 79 (23.0) | 21 (29.6) | 1.32 (0.83, 2.08), P: .236 | ‐ |
| Beta‐Blockers, n (%) | 46 (13.4) | 12 (16.9) | 0.95 (0.83, 1.09), P: .477 | ‐ |
| Incident VT, n (%) | 7 (2.0) | 7 (9.9) | 3.04 (1.55, 4.47), P: .003 | 2.94 (1.37, 4.49), P: .008 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF atrial fibrillation; ARBs, angiotensin II receptor blockers; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DCM dilated cardiomyopathy; VT, ventricular tachycardia.
TABLE 2.
Distribution of patients’ characteristics and their association with VT occurrence among COVID‐19 study population
| Incident VT free group | Incident VT group | Univariable | Multivariable | |
|---|---|---|---|---|
| RR (CI), P value | RR (CI), P value | |||
| n | 400 | 14 | ||
| Male gender, n (%) | 245 (61.3) | 8 (57.1) | 0.85 (0.30,2.47), P: .757 | ‐ |
| Age (mean (SD)) | 66.86 (15.06) | 69.07 (12.95) | 1.01 (0.98,1.05), P: .588 | ‐ |
| Smoker, n (%) | 80 (20.1) | 4 (28.6) | 1.56 (0.44, 4.33), P: .440 | ‐ |
| Hypertension, n (%) | 253 (63.4) | 10 (71.4) | 1.42 (0.48, 4.65), P: .542 | ‐ |
| Diabetes, n (%) | 101 (25.3) | 5 (35.7) | 1.60 (0.50, 4.30), P: .386 | ‐ |
| Dyslipidaemia, n (%) | 111 (27.8) | 7 (50.0) | 2.46 (0.87, 6.30), P: .081 | NS |
| Obesity, n (%) | 27 (12.2) | 2 (33.3) | 3.38 (0.49, 13.09), P: .149 | ‐ |
| History of AF, n (%) | 68 (17.1) | 4 (28.6) | 1.88 (0.53, 5.12), P: .274 | ‐ |
| Heart Failure, n (%) | 44 (11.0) | 2 (14.3) | 1.33 (0.21, 4.51), P: .704 | ‐ |
| Previous Stroke, n (%) | 31 (7.8) | 4 (28.6) | 4.20 (1.23, 10.20), P: .012 | NS |
| CKD, n (%) | 60 (15.1) | 4 (28.6) | 2.16 (0.61, 5.80), P: .181 | ‐ |
| CAD, n (%) | 61 (15.3) | 5 (35.7) | 2.88 (0.92, 7.22), P: .505 | ‐ |
| COPD, n (%) | 85 (21.3) | 3 (21.4) | 1.01 (0.23, 3.07), P: .991 | ‐ |
| ACEI/ARBs, n (%) | 163 (43.7) | 7 (50.0) | 1.28 (0.44, 3.48), P: .642 | ‐ |
| Ca‐Antagonists, n (%) | 96 (24.0) | 4 (28.6) | 1.26 (0.40, 3.92), P: .695 | ‐ |
| Beta‐Blockers, n (%) | 56 (14.0) | 2 (14.3) | 1.02 (0.23, 4.45), P: .976 | ‐ |
| Incident AF, n (%) | 64 (16.0) | 7 (50.0) | 1.70, 10.54), P: .003 | ‐ |
| New‐onset AF, n (%) | 47 (11.8) | 3 (21.4) | 1.98 (0.46, 5.72), P: .284 | ‐ |
| AF recurrence, n (%) | 17 (4.2) | 4 (28.6) | 7.09 (2.20, 15.18), P < .001 | 7.09 (2.20, 15.18), P < .001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARBs angiotensin II receptor blockers; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; VT, ventricular tachycardia.
TABLE 3.
Distribution of patients’ characteristics and their association with death occurrence among COVID‐19 study population
| Survival COVID‐19 Group | Deceased COVID‐19 Group | Univariable | Multivariable | |
|---|---|---|---|---|
| RR (CI), P value | RR (CI), P value | |||
| n | 307 | 107 | ||
| Male gender, n (%) | 177 (57.7) | 76 (71.0) | 1.49 (1.09,1 0.96), P: .015 | 1.63 (1.19, 2.13), P: .004 |
| Age (mean (SD)) | 65.26 (15.67) | 71.75 (11.60) | 1.02 (1.01, 1.04), P < .001 | 1.02 (1.01, 1.04), P: .001 |
| Smoker, n (%) | 62 (20.3) | 22 (20.6) | 1.01 (0.65, 1.46), P: .947 | ‐ |
| Hypertension, n (%) | 184 (60.1) | 79 (73.8) | 1.53 (1.11, 2.00), P: .012 | NS |
| Diabetes, n (%) | 74 (24.2) | 32 (29.9) | 1.23 (0.85, 1.67), P: .244 | ‐ |
| Dyslipidaemia, n (%) | 84 (27.5) | 34 (31.8) | 1.16 (0.81, 1.58), P: .394 | ‐ |
| Obesity, n (%) | 20 (11.1) | 9 (18.8) | 1.57 (0.79, 2.53), P: .163 | ‐ |
| History of AF, n (%) | 51 (16.7) | 21 (19.6) | 1.15 (0.74, 1.64), P: .496 | ‐ |
| Heart Failure, n (%) | 29 (9.5) | 17 (15.9) | 1.49 (0.95, 2.10), P: .073 | NS |
| Previous Stroke, n (%) | 26 (8.5) | 9 (8.4) | 0.99 (0.50, 1.64), P: .970 | ‐ |
| CKD, n (%) | 37 (12.1) | 27 (25.2) | 1.78 (1.27, 2.30), P: .002 | 1.58 (1.06, 2.15), P: .026 |
| CAD, n (%) | 41 (13.4) | 25 (23.4) | 1.57 (1.09, 2.10), P: .017 | NS |
| COPD, n (%) | 63 (20.6) | 25 (23.4) | 1.12 (0.75, 1.58), P: .546 | ‐ |
| ACEI/ARBs, n (%) | 120 (42.6) | 50 (47.6) | 1.16 (0.83, 1.54), P: .372 | ‐ |
| Ca‐Antagonists, n (%) | 76 (24.8) | 24 (22.4) | 0.91 (0.61, 1.35), P: .631 | ‐ |
| Beta‐Blockers, n (%) | 45 (14.7) | 13 (12.1) | 0.85 (0.51, 1.41), P: .528 | ‐ |
| VT, n (%) | 5 (1.6) | 9 (8.4) | 2.55 (1.53, 3.35), P: .003 | 2.55 (1.50, 3.35), P: .003 |
| Incident AF, n (%) | 50 (16.3) | 21 (19.6) | 1.18 (0.76, 1.67), P: .431 | ‐ |
| New‐onset AF, n (%) | 34 (11.1) | 16 (15.0) | 1.28 (0.79, 1.85), P: .291 | ‐ |
| AF recurrence, n (%) | 16 (5.2) | 5 (4.7) | 0.92 (0.35, 1.74), P: .827 | ‐ |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; VT, ventricular tachycardia.
COVID‐19 patients who experienced incident VT during the hospitalization showed more frequently history of previous stroke (P = .024) and AF recurrence during hospitalization (P < .001); however, at multivariate regression model only AF recurrence (RR: 7.09; P < .001) was associated with increased risk of incident VT.
During the hospitalization, 132 patients (31.9%) developed ARDS and 104 patients (25.8%) died. COVID‐19 patients with ARDS were older (70.52 ± 11.89 vs 65.26 ± 15.97 years; P = .001), more frequently male (70.5% vs 56.7%; P = .01) more likely affected by diabetes mellitus (32.6% vs 22.4%; P = .037) and CKD (22.0% vs 12.5%; P = .02) than those without ARDS. No statistically significant difference in both incident AF (22.7% vs 16.3%; P = .15) and VT (3.8% vs 3.2%; P = .98) has been found between the two groups (Figure 1). At multivariate regression model, only older age (RR: 1.02; P = .001) and male gender (RR: 1.47; P = .0006) were significantly associated with increased risk of ARDS.
FIGURE 1.
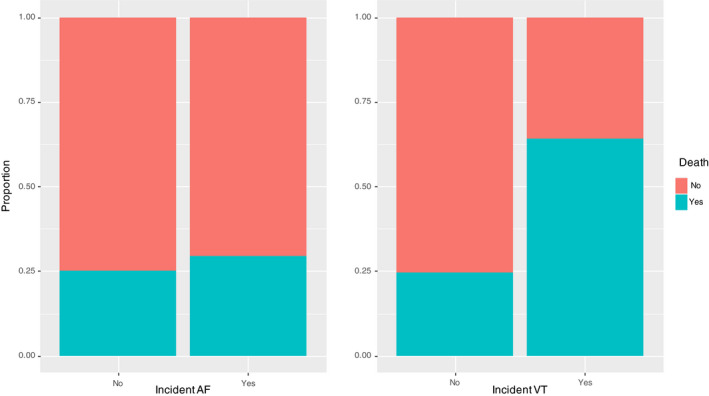
Proportion of incident tachyarrhythmias among survived or not COVID‐19 patients
Not survived COVID‐19 patients were older (P = .001) and more frequently male (P = .02) than those who survived and showed higher prevalence of hypertension (P = .001), CKD (P = .002), CAD (P = .023) and incident VT (P = .002). No statistically significant differences in incident AF (22.7% vs 16.3%; P = .15) have been found between the two groups. Age (RR: 1.63; P = .004), male gender (RR: 1.02; P = .001), CKD (RR: 1.58; P = .026) and incident VT (RR: 2.55; P: .003) have been found to be strong independent predictors of in‐hospital mortality. Figure 2 shows a forest plot visual summary of the results of logistic regression models.
FIGURE 2.
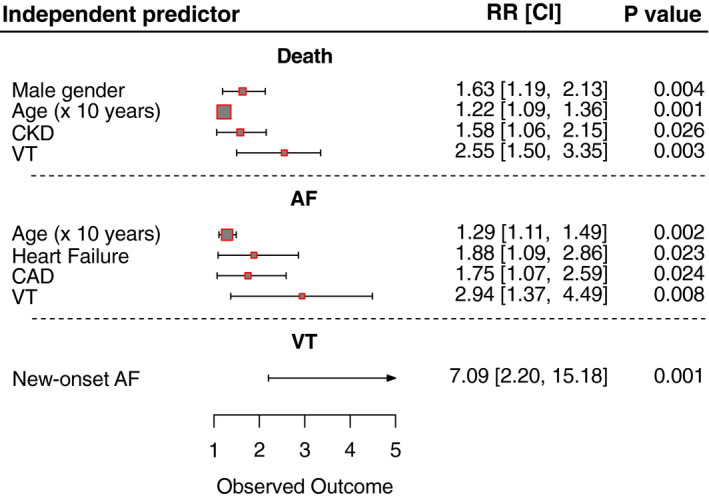
Forest plot visual summary of the results of logistic regression models. AF, atrial fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; VT, ventricular tachycardia
4. DISCUSSION
The main findings of the present study can be summarized as follows: the incident sustained tachyarrhythmias occur in 21% of our study population including COVID‐19 hospitalized patients. AF was the most prevalent arrhythmia accounting for the 18.45% of cases, and its independent predictors were older age, male gender, HF and CAD; moreover, incident AF was a significantly associated with incident VT. VT occurred in a small percentage of COVID‐19 patients (3.4%) and was independently associated with recurrent AF.
Incident AF, both new‐onset and recurrent, did not affect the risk of severe adverse events including ARDS and death during hospitalization; in contrast incident sustained VT significantly increased the risk of in‐hospital mortality.
There are few and conflicting data about the occurrence of arrhythmias in the context of COVID‐19; however, the clinical presentation seems not different from that described in general population. 6 In the cohort study by Wang et al, 7 an arrhythmia has complicated the clinical course of the disease during hospitalization in 16.7% of COVID‐19 patients; moreover, arrhythmias were significantly higher in patients receiving intensive care unit (ICU) care than in those not receiving ICU care (44.4% vs 6.9%; P < .001); however, specifics about the types and duration of arrhythmias have not been provided. Two recent cohort studies by Bhatla et al 8 and Colon et al 9 showed that the incidence of arrhythmias, such as AF and nonsustained VT, among patients with COVID‐19 corresponds to the severity of illness and was not the sole consequence of the viral infection. These results support the hypothesis of the multifactorial pathogenesis of arrhythmias in the clinical context of COVID‐19. If on one hand, the direct effect of SARS‐CoV‐2 on myocardiocytes may lead to myocardial inflammation 10 predisposing per se to cells electrical instability, ischaemia from coronary microvascular disease, gap junction dysfunction and abnormal calcium handling 11 ; on the other, the risk of arrhythmia may increase as the severity of the systemic inflammatory response, 12 which determines an imbalance in autonomic tone, 13 hypoxia, metabolic disarray and significant electrolyte disturbances, leading to the instability of underlying chronic cardiovascular diseases. Moreover, some experimental pharmacological treatments currently administered in COVID‐19 patients may impact on ventricular repolarization, enhancing the susceptibility to QT‐related life‐threatening ventricular arrhythmias. 14
In the present analysis, the incident AF, both new‐onset and recurrent, did not influence the clinical outcome of patients with COVID‐19 in terms of ARDS developing and survival, differently from what happens in critically ill patients with sepsis among general population. 15 In contrast, the incident VT seems to be a strong independent predictor of in‐hospital mortality among COVID‐19 patients; to date, VT has not ever been described as mortality predictor in the clinical contest of sepsis or viral infection. 16 , 17 Based on our results, tachyarrhythmias, in particular AF, could complicate the clinical course of COVID‐19 during hospitalization and, in case of VT, could worsen the prognosis of infected patients. Thereafter, a careful electrocardiographic monitoring would be advisable in COVID‐19 patients to early detect incident tachyarrhythmias that, even though not matching the apparent disease status, might be a red flag of worsening disease. The present study has several limitations: the retrospective nature of the analysis; our limited ability to detect subclinical arrhythmias; the heterogeneity in the characteristics of the different wards where the study patients were hospitalized; and the relatively high number of covariates tested in the regression models in relation of the number of patients. Furthermore, some potential confounders could have been not considered in our analysis that was restricted on available data. Larger multicenter prospective studies are required to confirm our preliminary findings.
5. CONCLUSION
Incident sustained tachyarrhythmias represent a not rare complication of COVID‐19. Among them, AF is the more frequent; however, it not seems associated with ARDS development and death. On the other hand, incident VT is a not frequent but independent predictor of in‐hospital mortality among hospitalized COVID‐19 patients. The careful electrocardiographic monitoring would be advisable in COVID‐19 patients to optimize the clinical management of the disease.
CONFLICT OF INTEREST
No Conflict of Interest.
Russo V, Di Maio M, Mottola F, et al. Clinical characteristics and prognosis of hospitalized COVID‐19 patients with incident sustained tachyarrhythmias: A multicenter observational study. Eur J Clin Invest. 2020;50:e13387. 10.1111/eci.13387
REFERENCES
- 1. Hui DS, I Azhar E, Madani TA, et al. novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2019;2020(91):264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization Coronavirus disease (COVID‐19) . Situation Report—146. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200614‐covid‐19‐sitrep‐146.pdf?sfvrsn=5b89bdad_4. Accessed June 14, 2020.
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo V, Bottino R, Carbone A, et al. COVID‐19 and heart: from clinical features to pharmacological implications. J Clin Med. 2020;9(6):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID‐19 and association between concomitant cardiovascular diseases and severity of COVID‐19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E008. [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatla A, Mayer MM, Adusumalli S, et al. COVID‐19 and Cardiac Arrhythmias [published online ahead of print, 2020 Jun 20]. Heart Rhythm. 2020;17(9):1439‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colon Chad M, Barrios James G, Chiles Joe W, et al. Atrial Arrhythmias in COVID‐19 Patients. JACC: Clinical Electrophysiology. 2020; 10.1016/j.jacep.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peretto G, Sala S, Rizzo S, et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793‐801. [DOI] [PubMed] [Google Scholar]
- 12. Shahreyar M, Fahhoum R, Akinseye O, Bhandari S, Dang G, Khouzam RN. Severe sepsis and cardiac arrhythmias. Ann Transl Med. 2018;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Werdan K, Schmidt H, Ebelt H, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87:266‐274. [DOI] [PubMed] [Google Scholar]
- 14. Lazzerini PE, Boutjdir M, Capecchi PL. COVID‐19, arrhythmic risk and inflammation: mind the gap!. Circulation. 2020;142(1):7‐9. [DOI] [PubMed] [Google Scholar]
- 15. Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23(3):178‐183. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91–95. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;3:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]


