Abstract
Alzheimer’s disease (AD) is a common neurodegenerative disease and a major contributor to progressive cognitive impairment in an aging society. As the pathophysiology of AD involves chronic neuroinflammation, the resolution of inflammation and the group of lipid mediators that actively regulate it—i.e., specialized pro-resolving lipid mediators (SPMs)—attracted attention in recent years as therapeutic targets. This review focuses on the following three specific SPMs and summarizes their relationships to AD, as they were shown to effectively address and reduce the risk of AD-related neuroinflammation: maresin 1 (MaR1), resolvin D1 (RvD1), and neuroprotectin D1 (NPD1). These three SPMs are metabolites of docosahexaenoic acid (DHA), which is contained in fish oils and is thus easily available to the public. They are expected to become incorporated into promising avenues for preventing and treating AD in the future.
Keywords: Alzheimer’s disease, neuroinflammation, resolution of inflammation, SPMs, MaR1, RvD1, NPD1, novel approaches
1. Introduction
Alzheimer’s disease (AD) is currently the most common cause of dementia [1]. While cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists are typically used to attenuate the symptoms and progression of dementia, they do not provide complete treatment nor do any other therapies, till date. Thus, more and more people are developing AD all over the world and obviously it has become a global issue. Hence, the urgent need to stem the increasing prevalence of AD [2] has prompted the improvement of such mainstay prevention and treatment strategies.
Characterized by the deposition of amyloid-β (Aβ) outside neurons [3,4,5,6,7,8] and hyperphosphorylated tau proteins within [9], the pathology of AD is largely ascribed to the activation of immune system, such as microglia [10,11,12], which leads to neuroinflammation induced by the disruption of hypothalamic-pituitary-adrenal hormonal homeostasis by various chronic stress factors [13], including infection, invasive injury, and autoimmune response [14,15,16,17,18,19,20]. In the central nervous system (CNS), glial cells such as oligodendroglia, microglia, and astrocytes play an important role in mediating neuroinflammation by maintaining the balance between extracellular ions and neurotransmitters [21]. Aβ activates microglia and astrocytes, causing chronic inflammation [15].
Attempts to prevent the onset of AD with anti-inflammatory drugs found that the long-term treatment of rheumatoid arthritis with non-steroidal anti-inflammatory drugs (NSAIDs) reduced the incidence of AD [22,23,24,25,26] and that intranasal steroids are effective in mitigating the symptoms of patients with the early stages of AD and olfactory impairment [27]. However, systematic review has not found NSAIDs to be effective in randomized controlled trials, and NSAIDs are not currently recommended for the treatment of AD [28,29].
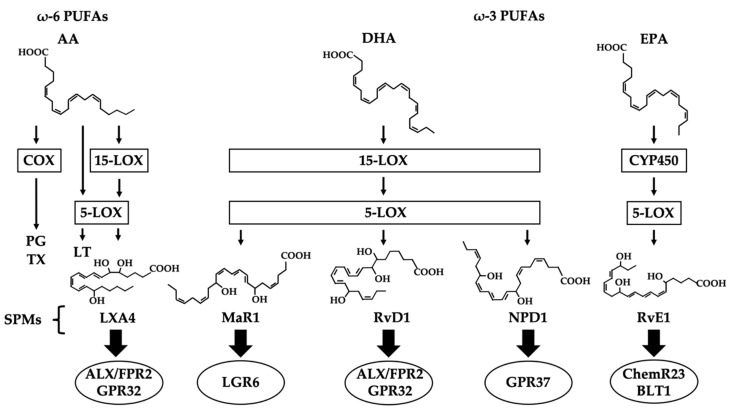
While most of the previous research on inflammation has focused on inhibiting the synthesis of inflammatory substances, the goal of more recent research has shifted to the resolution of inflammation. More specifically, the pathway seems to follow this order: inflammatory substances are synthesized to produce inflammatory lipid mediators, prostaglandins (PGs) and thromboxanes (TXs) via cyclooxygenase enzymes (COXs), and leukotrienes (LTs) via lipoxygenase enzymes (LOXs), by arachidonic acid (AA), one of the ω-6 polyunsaturated fatty acids (ω-6 PUFAs) [30,31,32,33,34]. PGs and LTs are well-known pro-inflammatory lipid mediators that induce fever and pain. As anti-inflammatory drugs, NSAIDs inhibit the activity of COXs. While inflammation is considered to be initiated by an active mechanism, its resolution had been thought of as a passive process. Once lipoxin A4 (LXA4), which is produced from AA by 15-LOX and 5-LOX, was reported to have an anti-inflammatory effect [35,36,37], increasingly more research has focused on the resolution of inflammation. Specialized pro-resolving lipid mediators (SPMs) are a group of lipid mediators that resolve inflammation [38]; they include the ω-3 polyunsaturated fatty acid (ω-3 PUFA) metabolites of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) contained in fish oils [39], which are easily available to the public. DHA is metabolized by 15-LOX and 5-LOX into either maresin 1 (MaR1), resolvin D1 (RvD1), or neuroprotectin D1 (NPD1) [40,41]. EPA is metabolized into resolvin E1 (RvE1) by CYP450 or 15-LOX [42]. These SPMs are produced in human tissue and resolve inflammation [43]. It is called resolution of inflammation [43,44,45,46,47,48].
Recent studies identified receptors for SPMs in the human brain, reduced levels of SPMs in the brains of patients with AD, and a correlation between lower SPM levels and cognitive impairment [49]. Furthermore, SPMs were shown to promote neuronal survival and increase the microglial phagocytosis of Aβ [48,50,51]. The pathways that produce PGs and LTs from AA are aberrantly activated in patients with AD [52,53], and lesser degrees of cognitive impairment among older adults were associated with higher blood concentrations of ω-3 PUFAs [54]; SPMs might be involved in both findings.
The potential use of SPMs to resolve inflammation could be a promising approach to the treatment of AD. The purpose of this review is to compile knowledge concerning the mechanistic role of SPMs in chronic neuroinflammation associated with AD and to summarize future research strategies for novel therapies using SPMs. In addition to an overview of SPMs in general, we focus on three produced in the human brain: MaR1, RvD1, and NPD1 [50,55,56].
2. SPMs (Specialized Pro-resolving Lipid Mediators)
SPMs belong to a superfamily of metabolites derived from ω-3 and ω-6 PUFAs that actively resolve inflammation and promote the return to homeostasis by limiting inflammatory signals and suppressing the natural response during inflammation [57,58,59,60]. Examples of ω-6 PUFAs include AA, which is used by COXs to synthesize PGs and by LOXs to synthesize LTs. These lipid mediators participate in the AA cascade and cause inflammation. However, LXA4, which is synthesized from AA via LOXs, is an SPM that exerts anti-inflammatory effects. The ω-3 PUFAs include DHA and EPA, which are abundant in fish oils [39]. The enzyme induced by fat-1 gene converts ω-6 PUFAs to ω-3 PUFAs, and experiments in mice transfected with this gene showed that lipid mediators, not other ingredients contained in food, not only stimulate inflammation but also help to resolve inflammation [61]: i.e., decrease the levels of pro-inflammatory cytokines and increase the levels of anti-inflammatory cytokines [62]. The discovery of LXA4 revealed that inflammation can be actively resolved through SPMs [35,36,37]. SPMs stimulate physiological signals to resolve and end inflammation and return to physiologically normal conditions [48], whereas it is man-made, not physiological that anti-inflammatory drugs such as NSAIDs block the production of pro-inflammatory chemicals.
Four types of SPMs involved in AD were identified: LXA4, produced from AA by 15-LOX and 5-LOX; MaR1, RvD1 and NPD1, produced from DHA by 15-LOX and 5-LOX; and resolvin E1 (RvE1), produced from EPA by CYP450 and 5-LOX [58,63,64,65]. While the receptors for LXA4, RvD1, and RvE1 were identified, those for MaR1 and NPD1 were not. SPM receptors belong to the 7-transmembrane G protein-coupled receptor (GPCR) [66]. LXA4 and RvD1 bind to LXA4/formyl peptide receptor 2 (ALX/FPR2) and G protein receptor (GPR) 32 [67,68,69,70]. ALX/FPR2 is also a receptor for Aβ, which functions as a ligand that activates microglia and transmitting pro-inflammatory signals [71]. RvE1 binds to chemerin receptor 23 (ChemR23) and the leukotriene B4 receptor 1 (BLT1) [72]. Leucine-rich repeat containing G protein–coupled receptor 6 (LGR6) and G protein–coupled receptor (GPR37) are thought to be possible receptors for MaR1 and NPD1, respectively [73,74,75,76,77] (Figure 1). Since pro-inflammatory ligands aside from SPMs also bind to these receptors [71,78,79], very low concentrations of SPMs can bind to their receptors and signal the resolution of inflammation [77]. A nuclear receptor with anti-inflammatory function, peroxisome proliferator-activated receptor (PPAR)-γ is also a receptor for SPMs such as LXA4, RvD1, and NPD1 that mediates neuroprotective effects in neurons [51,80,81]. PPAR-γ agonists reportedly increase the level of LXA4, and PPAR-γ inhibitors were shown to reduce the level of LXA4 [82]. Retinoic acid-related orphan receptor α (RORα) is also a nuclear receptor with anti-inflammatory function and is thought to be a receptor for MaR1 [74,75].
Figure 1.
Pathways of SPM synthesis.
SPMs inhibit immune cell infiltration, down-regulate pro-inflammatory mediators, up-regulate anti-inflammatory mediators, and promote phagocytosis and tissue repair [50,83,84,85]. The production of pro-inflammatory lipid mediators is class-switched to the production of inflammation-resolving lipid mediators under certain conditions [86,87,88]. Although the detailed mechanism for the class-switching is unclear, this finding indicates that it can be programmed to induce pro-resolving lipid mediators [50]. Moreover, SPMs are detectable in the brain using liquid chromatography mass spectrometry and can be regulated by nutrition such as dietary supplements [89].
Lower levels of LXA4 and NPD1 were reported in patients with severe asthma and decreased concentrations of SPMs were observed in patients with localized aggressive periodontitis relative to healthy controls [90,91,92]. In addition, SPMs inhibit the accumulation of eosinophils and lymphocytes in a mouse model of asthma [93] and inhibit neutrophil infiltration and inflammatory cytokines in a mouse model of sepsis [94]. Furthermore, SPMs reportedly promote the resolution of inflammation in mouse models of peritonitis, enteritis, retinopathy, and inflammatory pain [36,95,96,97].
SPMs repair neurons and promote the microglial phagocytosis of Aβ, changing the microglial phenotype from pro-inflammatory to anti-inflammatory [48]. The levels of LXA4, MaR1, and NPD1 are low in the hippocampi of patients with AD [50], and the concentration of LXA4 is reduced in their cerebrospinal fluid (CSF) [49]. These reductions in SPMs are significant in so far as they present concurrently with increased inflammation. Decreases in LXA4 and RvD1 were correlated with cognitive decline, as measured by scores on the Mini-Mental State Examination (MMSE); however, the detailed mechanism underlying this association remains equivocal [49]. The induction of SPM production in a mouse model of AD promoted the resolution of neuroinflammation, microglial phagocytosis, and memory improvement [98]. Since DHA can cross the blood-brain barrier (BBB) [99], cognitive function could be promoted by administering ω-3 PUFA supplements. Indeed, epidemiological research has shown that the increased intake of ω-3 PUFAs reduces the risk of dementia [100,101,102], while clinical trials in which patients with AD were administered ω-3 PUFAs found that the supplements benefited those with mild cognitive impairment (MCI) but not patients with advanced AD [103,104,105]. This suggests that the factor that metabolizes ω-3 PUFAs into SPMs is deficient in late-stage AD, that the inflammation-resolving effect of SPMs is significant only during the early stages of AD, or that external factors other than ω-3 PUFA pathways are involved such as the ratio of ω-3/ω-6 PUFAs [106]. Although DHA reportedly improves the cognitive impairment of AD patients to a nonsignificant extent [107,108,109], these studies may have been compromised by the nonuniform quality of ω-3 PUFA supplements on the market [110]; larger, more rigorous clinical studies of the potential benefit of DHA in treating AD over longer periods of time are thus warranted. The following sections will focus on MaR1, RvD1, and NPD1, which are produced in the human brain [50,55,56], and discuss the relationship among these SPMs and AD.
3. MaR1 (Maresin 1)
MaR1 is produced from DHA by 15-LOX and 5-LOX. The receptor for MaR1 is thought to be LGR6, and RORα of a nuclear receptor [73,74,75]. (Figure 1) MaR1 exerts a potent anti-inflammatory effect and promotes the macrophage phagocytosis and efferocytosis of apoptotic cells [111,112,113]. In addition to prompting the migration of leukocytes, MaR1 also promotes tissue regeneration at the site of injury [111,113]. MaR1 is produced at the late stage of inflammation [114,115,116] and has a potent analgesic effect that controls not only neuropathic pain but also the resolution of local inflammation and its associated pain [111,113] (Table 1).
Table 1.
Bioaction of MaR1, RvD1, and NPD1.
| SPMs | Receptor | Bioaction in AD | Reference |
|---|---|---|---|
| MaR1 | LGR6 | Promote microglial phagocytosis of Aβ Promote tissue regeneration Analgesic effect |
[50,111,113] |
| RvD1 | ALX/FPR2 & GPR32 | Enhance tissue remodeling in microglia Promote macrophage phagocytosis of Aβ |
[117,118,119] |
| NPD1 | GPR37 | Influence cell survival, neuroinflammatory signaling and transcription Suppress the production of APP |
[51,120,121] |
The level of MaR1 is significantly reduced in the hippocampus and entorhinal cortex of patients with AD [49,50]. MaR1 plays a neuroprotective role in neurons, influencing cell signaling pathways pertinent to inflammatory cell survival, autophagy, axonogenesis, and the inhibition of apoptosis [122,123]. MaR1 up-regulates the level of microglia that are down-regulated by Aβ in the course of AD [122]; in the context of the RvD1- and LXA4-mediated increase in the microglial phagocytosis of Aβ by the action of, this finding suggests that MaR1 plays a distinct role in the microglia-mediated removal of Aβ [50]. These findings suggest that the induction of MaR1 could be a novel, effective approach to treat AD. In model mice, MaR1 treatment decreased the production of pro-inflammatory cytokines, such as TNF-α and IL-6; increased the production of anti-inflammatory cytokines, including IL-2 and IL-10; and mitigated cognitive decline [124].
Furthermore, a prospective human study showed that the intake of DHA, the precursor of MaR1, significantly reduced the incidence of AD [125,126]. On the other hand, there are reports that while DHA treatment improved memory impairment in mice, MaR1 treatment did not [127,128]. Considering that ω-3 PUFAs were effective only in addressing MCI and not advanced AD [103,104,105], it is necessary to elucidate the mechanism by which DHA is metabolized into MaR1 and the biochemical relationship between MaR1 and its receptor.
4. RvD1 (Resolvin D1)
RvD1 is produced from DHA by 15-LOX and 5-LOX [114]. The receptors for RvD1 are the same as those for LXA4: ALX/FPR2 and GPR32 [67,68,69,70] (Figure 1). RvD1 effects anti-inflammation by down-regulating pro-inflammatory cytokines, such as IL-1 and IL-6, and up-regulating anti-inflammatory profiles, such as IL-1 antagonists and NF-κB [117,118,129,130]. RvD1 enhances tissue remodeling in microglia by promoting the activation of the signal transducer and activator of transcription 6 (STAT6), as well as PPAR-γ transcriptional factors, through IL-4 activation [119]. (Table 1.)
Murine research has demonstrated that RvD1 improves inflammation-induced cognitive impairment when administered before or during invasive surgery [131,132]. More research on the relationship between RvD1 and AD-related neuroinflammation is ongoing. In vitro, RvD1 promotes macrophage phagocytosis of Aβ and inhibits apoptosis through GPR32 [117,118]. Recent studies showed that RvD1 promotes the phagocytosis of Aβ by modulating MEK1/2, PKA, and PI3K/Akt/mTOR pathways, which are responsible for supporting cell survival upon exposure to oxidative stress [117,133].
Reduced in the CSF of patients with AD, the levels of RvD1 and LXA4 correlate with lower MMSE scores [49]. The increased expression of ALX/FPR2 in patients with AD suggests enhanced neuroinflammation [134]; however, since Aβ is also a ligand for ALX/FPR2 [71], the increased expression of the receptor may either indicate a compensation for reduced concentrations of SPMs or that ALX/FPR2 transmits different signals depending on the site to which a ligand binds. The administration of ω-3 PUFA supplementation, the precursor of RvD1, to patients with MCI increases the levels of RvD1 and enhances the phagocytosis of Aβ, resulting in improved MMSE scores [135].
5. NPD1 (Neuroprotection D1)
NPD1 is produced from DHA by 15-LOX and 5-LOX. The receptor for NPD1 is thought to be GPR37 and is PPAR-γ of a nuclear receptor [76,77]. (Figure 1) NPD1 is rapidly synthesized in response to inflammation to promote cell survival [51,136], neuroinflammatory signaling and transcription, and homeostatic regulation [120]. (Table 1).
The levels of NPD1 are significantly reduced in the hippocampi of patients with AD [49,137,138]. NPD1 inhibits apoptosis and promotes cell survival by regulating BCL-2 anti-apoptotic protein and microglia levels [139]. NPD1 suppresses the production of amyloid precursor protein (APP) by the mechanism that activating α-secretase and inhibiting β-secretase through PPAR-γ changes APP from an amyloidogenic pathway into a non-amyloidogenic one, and thus NPD1 effects anti-inflammation [51,120,121]. It has also been suggested that NPD1 may also promote cell survive and regulate the NF-κB cyclic response [140] by stimulating the PI3K/Akt/mTOR pathways [141]. Additionally, in vitro studies showed that APP promotes NPD1 production at concentrations exceeding a specific limit—even in the absence of neuronal DHA [137]—suggesting that APP not only shifts the amyloidogenic pathway to the non-amyloidogenic pathway but also activates 15-LOX, which is involved in NPD1 biosynthetic enzymes [121,142,143]. Although the role of NPD1 in cell signal transduction has received much attention, few clinical studies of AD considered the potential use of NPD1 alone or with ω-3 PUFAs.
6. Discussion
As ω-3 PUFAs, the precursor of SPMs, are plentiful in fish oils and readily available to the general public, future developments in the use of ω-3 PUFAs to treat MCI and AD are expected. As SPMs are easily oxidized and converted to other metabolites, it is currently difficult to consider SPMs as a drug target for organs to which they cannot be administered directly. However, there is a great promise for the development of analogs and agonists of SPMs to treat cognitive impairment. Some targeted perspectives for future research are as follows. First, investigating the kinetics of administered DHA after it passes through the BBB will help to determine whether DHA supplements are effective in producing SPMs in the brain and will inform a clearer approach to their application to the prevention and treatment of AD. Second, as few large-scale studies investigated the relationship among AD and SPMs by administering ω-3 PUFAs and DHA, large, long-term clinical studies that use supplements of a uniform quality are warranted. With the accumulation of positive evidence, modulating nutrition could encourage the prevention of AD. Third, since APOEε4 mice reportedly reduced SPM levels [144], research on the relationship among SPMs and APOE, which is highly relevant to AD, could lead to the discovery of a promising prevention strategy. Fourth, although almost all the current methods for measuring neural levels of SPMs are based on the analysis of pathological samples, a noninvasive method of collecting samples with the imaging of magnetic resonance imaging (MRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT) may help to elucidate the dynamics of SPM activity in the brain. Fifth, inhibiting the metabolism of SPMs will provide more anti-inflammatory effects if it is clarified how they are metabolized, even though the metabolic pathways of many lipid mediators are not yet fully understood.
7. Conclusions
This review summarizes the latest developments in research on neural MaR1, RvD1 and NPD1: SPMs that promote the resolution of inflammation by down-regulating pro-inflammatory mediators and up-regulating anti-inflammatory mediators. As their mechanisms have yet to be fully explored, understanding their roles in resolution of inflammation is imperative for their application to the development of preventive and therapeutic strategies to address the incidence and progression of AD.
Abbreviations
| AA | Arachidonic acid |
| AD | Alzheimer’s disease |
| ALX/FPR | Lipoxin A4/formyl peptide receptor |
| APP | Amyloid precursor protein |
| Aβ | Amyloid-beta |
| BBB | Blood-brain barrier |
| BLT | Leukotriene B4 receptor |
| ChemR | Chemerin receptor |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| CSF | Cerebrospinal fluid |
| DHA | docosahexaenoic |
| EPA | eicosapenraenoic |
| GPCR | G protein-coupled receptor |
| GPR | G protein receptor |
| LGR | Leucine-rich repeat containing G protein-coupled receptor |
| LT | Leukotriene |
| LOX | Lipoxygenase |
| LXA4 | Lipoxin A4 |
| MaR1 | Maresin 1 |
| MCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic resonance imaging |
| NMDA | N-methyl-D-aspartate |
| NPD1 | Neuroprotectin D1 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PET | Positron emission tomography |
| PG | Prostaglandin |
| PPAR | Peroxisome proliferator-activated receptor |
| PUFA | Polyunsaturated fatty acid |
| RORα | Retinoic acid-related orphan receptor α |
| RvD1 | Resolvin D1 |
| RvE1 | Resolvin E1 |
| SPECT | Single photon emission computed tomography |
| SPM | Specialized pro-resolving lipid mediator |
| TX | Thromboxane |
Author Contributions
Conceptualization, K.M., H.F. and Y.T. (Yasuko Tatewaki); methodology, H.F. and Y.T. (Yasuko Tatewaki); investigation, K.M.; resources, Y.T. (Yumi Takano) and S.Y.; writing—original draft preparation, K.M., H.F. and Y.T. (Yasuko Tatewaki); writing—review and editing, Y.T. (Yumi Takano), S.Y., T.M., and Y.T. (Yasuyuki Taki); supervision, T.M. and Y.T. (Yasuyuki Taki); project administration, H.F. and Y.T. (Yasuko Tatewaki). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Kaye J., Montine T.J., et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International . World Alzheimer Report 2019: Attitudes to Dementia. Alzheimer’s Disease International; London, UK: 2019. [Google Scholar]
- 3.Rogers J., Cooper N.R., Webster S., Schultz J., McGeer P.L., Styren S.D., Civin W.H., Brachova L., Bradt B., Ward P., et al. Complement activation by β-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H., Burdick D., Glabe C.G., Cotman C.W., Tenner A.J. beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J. Immunol. 1994;152 [PubMed] [Google Scholar]
- 5.Afagh A., Cummings B.J., Cribbs D.H., Cotman C.W., Tenner A.J. Localization and cell association of C1q in Alzheimer’s disease brain. Exp. Neurol. 1996;138:22–32. doi: 10.1006/exnr.1996.0043. [DOI] [PubMed] [Google Scholar]
- 6.Chen S., Frederickson R.C.A., Brunden K.R. Neuroglial-mediated immunoinflammatory responses in Alzheimer’s disease: Complement activation and therapeutic approaches. Neurobiol. Aging. 1996;17:781–787. doi: 10.1016/0197-4580(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 7.Webster S., Bradt B., Rogers J., Cooper N. Aggregation State-Dependent Activation of the Classical Complement Pathway by the Amyloid β Peptide. J. Neurochem. 2002;69:388–398. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- 8.Webster S., Lue L.F., Brachova L., Tenner A.J., McGeer P.L., Terai K., Walker D.G., Bradt B., Cooper N.R., Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol. Aging. 1997;18:415–421. doi: 10.1016/S0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y., Lue L.F., Yang L.B., Roher A., Kuo Y.M., Strohmeyer R., Goux W.J., Lee V., Johnson G.V.W., Webster S.D., et al. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 2001;305:165–168. doi: 10.1016/S0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- 10.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 11.Venegas C., Kumar S., Franklin B.S., Dierkes T., Brinkschulte R., Tejera D., Vieira-Saecker A., Schwartz S., Santarelli F., Kummer M.P., et al. Microglia-derived ASC specks crossseed amyloid-β in Alzheimer’s disease. Nature. 2017;552:355–361. doi: 10.1038/nature25158. [DOI] [PubMed] [Google Scholar]
- 12.Heneka M.T., Carson M.J., Khoury J.E., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet. Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canet G., Hernandez C., Zussy C., Chevallier N., Desrumaux C., Givalois L. Is AD a stress-related disorder? Focus on the HPA axis and its promising therapeutic targets. Front. Aging Neurosci. 2019;11:269. doi: 10.3389/fnagi.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. Reactive microglia are positive for HLA-DR in the: Substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/WNL.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 15.Hu J., Akama K.T., Krafft G.A., Chromy B.A., Van Eldik L.J. Amyloid-β peptide activates cultured astrocytes: Morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/S0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso A., Nicoletti F., Mango D., Saidi A., Orlando R., Scaccianoce S. Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 2018;132:130–134. doi: 10.1016/j.phrs.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Risk Reduction of Cognitive Decline and Dementia WHO Guidelines. WHO Press; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 19.Middleton L.E., Yaffe K. Promising strategies for the prevention of dementia. Arch. Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso A., Nicoletti F., Gaetano A., Scaccianoce S. Risk factors for Alzheimer’s disease: Focus on stress. Front. Pharmacol. 2019;10:976. doi: 10.3389/fphar.2019.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wekerle H., Linington C., Lassmann H., Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. doi: 10.1016/0166-2236(86)90077-9. [DOI] [Google Scholar]
- 22.Mcgeer P.L., Mcgeer E., Rogers J., Sibley J. Anti-inflammatory drugs and Alzheimer disease. Lancet. 1990;335:1037. doi: 10.1016/0140-6736(90)91101-F. [DOI] [PubMed] [Google Scholar]
- 23.Beard C.M., Kokman E., Kurland L.T. Rheumatoid arthritis and susceptibility to Alzheimer’s disease. Lancet. 1991;337:1426. doi: 10.1016/0140-6736(91)93122-P. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M.L., Bliss M.R., Brain A.T., Scott D.L. Rheumatoid Arthritis and Senile Dementia of the Alzheimer’s Type. Br. J. Rheumstol. 1989;28:86–88. doi: 10.1093/rheumatology/28.1.86-b. [DOI] [PubMed] [Google Scholar]
- 25.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozben T., Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer S., Rheinstein P.H. Nasal steroids as a possible treatment for Alzheimer’s disease. Discov. Med. 2017;24:147–152. [PubMed] [Google Scholar]
- 28.Jaturapatporn D., Isaac M.G.E.K.N., McCleery J., Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD006378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J., Tan L., Wang H.F., Tan C.C., Meng X.F., Wang C., Tang S.W., Yu J.T. Anti-inflammatory drugs and risk of Alzheimer’s Disease: An updated systematic review and meta-analysis. J. Alzheimer’s Dis. 2015;44:385–396. doi: 10.3233/JAD-141506. [DOI] [PubMed] [Google Scholar]
- 30.Narumiya S. Physiology and Pathophysiology of Prostanoid Receptors. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2007;83 doi: 10.2183/pjab.83.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas D.W., Mannon R.B., Mannon P.J., Latour A., Oliver J.A., Hoffman M., Smithies O., Koller B.H., Coffman T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moncada S., Gryglewski R., Bunting S., Vane J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 33.Langenbach R., Loftin C.D., Christopher L., Tiano H. Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis. Ann. N. Y. Acad. Sci. 1999:52–61. doi: 10.1111/j.1749-6632.1999.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 34.Sellers R.S., Radi Z.A., Khan N.K. Pathophysiology of cyclooxygenases in cardiovascular homeostasis. Vet. Pathol. 2010;47:601–613. doi: 10.1177/0300985810364389. [DOI] [PubMed] [Google Scholar]
- 35.Serhan C.N., Hamberg M., Samuelsson B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuelsson B., Dahlén S.E., Lindgren J.Å., Rouzer C.A., Serhan C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 38.Serhan C.N., Chiang N., Dalli J., Levy B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yazawa K., Kageyama H. Physiological Activity of Docosahexaenoic Acid. J. Japan Oil Chem. Soc. 1991;40:974–978. doi: 10.5650/jos1956.40.974. [DOI] [Google Scholar]
- 40.Hong S., Gronert K., Devchand P.R., Moussignac R.L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: Autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 41.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serhan C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Aspects Med. 2017;58:1–11. doi: 10.1016/j.mam.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiurchiù V., Leuti A., Dalli J., Jacobsson A., Battistini L., MaCcarrone M., Serhan C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colas R.A., Dalli J., Chiang N., Vlasakov I., Sanger J.M., Riley I.R., Serhan C.N. Identification and Actions of the Maresin 1 Metabolome in Infectious Inflammation. J. Immunol. 2016;197:4444–4452. doi: 10.4049/jimmunol.1600837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q., Zhu B., Li Y. Resolution of cancer-promoting inflammation: A new approach for anticancer therapy. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu M., Wang X., Sun L., Schultzberg M., Hjorth E. Can inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Zhu M., Hjorth E., Cortés-Toro V., Eyjolfsdottir H., Graff C., Nennesmo I., Palmblad J., Eriksdotter M., Sambamurti K., et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimer’s Dement. 2015;11:40–50.e2. doi: 10.1016/j.jalz.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu M., Wang X., Hjorth E., Colas R.A., Schroeder L., Granholm A.C., Serhan C.N., Schultzberg M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol. Neurobiol. 2016;53:2733–2749. doi: 10.1007/s12035-015-9544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y., Calon F., Julien C., Winkler J.W., Petasis N.A., Lukiw W.J., Bazan N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikonomovic M.D., Abrahamson E.E., Uz T., Manev H., DeKosky S.T. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J. Histochem. Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohri I., Kadoyama K., Kanekiyo T., Sato Y., Kagitani-Shimono K., Saito Y., Suzuki K., Kudo T., Takeda M., Urade Y., et al. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2007;66:469–480. doi: 10.1097/01.jnen.0000240472.43038.27. [DOI] [PubMed] [Google Scholar]
- 54.Dullemeijer C., Durga J., Brouwer I.A., van de Rest O., Kok F.J., Brummer R.-J.M., van Boxtel M.P.J. Verhoef, P. n−3 Fatty acid proportions in plasma and cognitive performance in older adults. Am. J. Clin. Nutr. 2007;86:1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen M.M.B., Lambertsen K.L., Clausen B.H., Meyer M., Bhandari D.R., Larsen S.T., Poulsen S.S., Spengler B., Janfelt C., Hansen H.S. Mass spectrometry imaging of biomarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci. Rep. 2016;6 doi: 10.1038/srep39571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serhan C.N., Levy B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gronert K. Lipid autacoids in inflammation and injury responses: A matter of privilege. Mol. Interv. 2008;8:28–35. doi: 10.1124/mi.8.1.7. [DOI] [PubMed] [Google Scholar]
- 58.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romano M., Cianci E., Simiele F., Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 60.Kim C., Livne-Bar I., Gronert K., Sivak J.M. Fair-Weather Friends: Evidence of Lipoxin Dysregulation in Neurodegeneration. Mol. Nutr. Food Res. 2020;64:1801076. doi: 10.1002/mnfr.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang J.X. Fat-1 transgenic mice: A new model for omega-3 research. Prostaglandins Leukot. Essent. Fat. Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am. J. Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serhan C.N. Novel Chemical Mediators in the Resolution of Inflammation: Resolvins and Protectins. Anesthesiol. Clin. North Am. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asatryan A., Bazan N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J. Biol. Chem. 2017;292:12390–12397. doi: 10.1074/jbc.R117.783076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serhan C.N., Krishnamoorthy S., Recchiuti A., Chiang N. Novel Anti-Inflammatory-Pro-Resolving Mediators and Their Receptors. Curr. Top. Med. Chem. 2012;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.H., Yang R., Petasis N.A., Serhan C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiala M., Kooij G., Wagner K., Hammock B., Pellegrini M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017;31:3229–3239. doi: 10.1096/fj.201700065R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruiz A., Sarabia C., Torres M., Juárez E. Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. Int. Immunopharmacol. 2019;74 doi: 10.1016/j.intimp.2019.105694. [DOI] [PubMed] [Google Scholar]
- 70.Maddox J.F., Hachicha M., Takano T., Petasis N.A., Fokin V.V., Serhan C.N. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J. Biol. Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 71.Le Y., Gong W., Tiffany H.L., Tumanov A., Nedospasov S., Shen W., Dunlop N.M., Gao J.L., Murphy P.M., Oppenheim J.J., et al. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emre C., Hjorth E., Bharani K., Carroll S., Granholm A., Schultzberg M. Receptors for pro-resolving mediators are increased in Alzheimer’s disease brain. Brain Pathol. 2020;30:614–640. doi: 10.1111/bpa.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiang N., Libreros S., Norris P.C., De La Rosa X., Serhan C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Invest. 2019;129:5294–5311. doi: 10.1172/JCI129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Im D.S. Maresin-1 resolution with RORα and LGR6. Prog. Lipid Res. 2020;78:101034. doi: 10.1016/j.plipres.2020.101034. [DOI] [PubMed] [Google Scholar]
- 75.Han Y.H., Shin K.O., Kim J.Y., Khadka D.B., Kim H.J., Lee Y.M., Cho W.J., Cha J.Y., Lee B.J., Lee M.O. A maresin 1/RORα/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Invest. 2019;129:1684–1698. doi: 10.1172/JCI124219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bang S., Xie Y.K., Zhang Z.J., Wang Z., Xu Z.Z., Ji R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Invest. 2018;128:3568–3582. doi: 10.1172/JCI99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qu L., Caterina M.J. Accelerating the reversal of inflammatory pain with NPD1 and its receptor GPR37. J. Clin. Invest. 2018;128:3246–3249. doi: 10.1172/JCI122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bondue B., Wittamer V., Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Fülöp P., Seres I., Lorincz H., Harangi M., Somodi S., Paragh G. Association of chemerin with oxidative stress, inflammation and classical adipokines in non-diabetic obese patients. J. Cell. Mol. Med. 2014;18:1313–1320. doi: 10.1111/jcmm.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao Z., Dong J., Wu W., Yang T., Wang T., Guo L., Chen L., Xu D., Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 2012;13 doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinberger B., Quizon C., Vetrano A.M., Archer F., Laskin J.D., Laskin D.L. Mechanisms mediating reduced responsiveness of neonatal neutrophils to lipoxin A4. Pediatr. Res. 2008;64:393–398. doi: 10.1203/PDR.0b013e318180e4af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sobrado M., Pereira M.P., Ballesteros I., Hurtado O., Fernández-López D., Pradillo J.M., Caso J.R., Vivancos J., Nombela F., Serena J., et al. Synthesis of lipoxin A 4 by 5-lipoxygenase mediates pparγ-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J. Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rees D., Miles E.A., Banerjee T., Wells S.J., Roynette C.E., Wahle K.W.J., Calder P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am J. Clin. Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 84.Medeiros R., Kitazawa M., Passos G.F., Baglietto-Vargas D., Cheng D., Cribbs D.H., Laferla F.M. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces alzheimer disease-like pathology in mice. Am. J. Pathol. 2013;182:1780–1789. doi: 10.1016/j.ajpath.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunn H.C., Ager R.R., Baglietto-Vargas D., Cheng D., Kitazawa M., Cribbs D.H., Medeiros R. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. J. Alzheimer’s Dis. 2015;43:893–903. doi: 10.3233/JAD-141335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo M., Jones S.M., Phare S.M., Coffey M.J., Peters-Golden M., Brock T.G. Protein kinase a inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J. Biol. Chem. 2004;279:41512–41520. doi: 10.1074/jbc.M312568200. [DOI] [PubMed] [Google Scholar]
- 87.Ye Y., Lin Y., Perez-Polo J.R., Uretsky B.F., Ye Z., Tieu B.C., Birnbaum Y. Phosphorylation of 5-Lipoxygenase at Ser 523 by Protein Kinase A Determines Whether Pioglitazone and Atorvastatin Induce Proinflammatory Leukotriene B 4 or Anti-Inflammatory 15-Epi-Lipoxin A 4 Production. J. Immunol. 2008;181:3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 88.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joffre C., Rey C., Layé S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 2019;10:1022. doi: 10.3389/fphar.2019.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Planagumà A., Kazani S., Marigowda G., Haworth O., Mariani T.J., Israel E., Bleecker E.R., Curran-Everett D., Erzurum S.C., Calhoun W.J., et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am. J. Respir. Crit. Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyata J., Fukunaga K., Iwamoto R., Isobe Y., Niimi K., Takamiya R., Takihara T., Tomomatsu K., Suzuki Y., Oguma T., et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013;131 doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 92.Fredman G., Oh S.F., Ayilavarapu S., Hasturk H., Serhan C.N., van Dyke T.E. Impaired phagocytosis in localized aggressive periodontitis: Rescue by resolvin E1. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J.N., Petasis N.A., Blumberg R.S., Serhan C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Connor K.M., Sangiovanni J.P., Lofqvist C., Aderman C.M., Chen J., Higuchi A., Hong S., Pravda E.A., Majchrzak S., Carper D., et al. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J.Y., Han S.H., Park M.H., Song I.S., Choi M.K., Yu E., Park C.M., Kim H.J., Kim S.H., Schuchman E.H., et al. N-AS-triggered SPMs are direct regulators of microglia in a model of Alzheimer’s disease. Nat. Commun. 2020;11:1–19. doi: 10.1038/s41467-020-16080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petursdottir A.L., Farr S.A., Morley J.E., Banks W.A., Skuladottir G.V. Effect of Dietary n-3 Polyunsaturated Fatty Acids on Brain Lipid Fatty Acid Composition, Learning Ability, and Memory of Senescence-Accelerated Mouse. J. Gerontol. 2008;63:1153–1160. doi: 10.1093/gerona/63.11.1153. [DOI] [PubMed] [Google Scholar]
- 100.Kalmijn S., Launer L.J., Ott A., Witteman J.C.M., Hofman A., Breteler M.M.B. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Ann. Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 101.Engelhart M.J., Geerlings M.I., Ruitenberg A., Van Swieten J.C., Hofman A., Witteman J.C.M., Breteler M.M.B. Diet and risk of dementia: Does fat matter? The Rotterdam study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.WNL.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 102.Hooper C., de Souto Barreto P., Coley N., Cantet C., Cesari M., Andrieu S., Vellas B. Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J. Nutr. Heal. Aging. 2017;21:988–993. doi: 10.1007/s12603-017-0957-5. [DOI] [PubMed] [Google Scholar]
- 103.Freund-Levi Y., Eriksdotter-Jönhagen M., Cederholm T., Basun H., Faxén-Irving G., Garlind A., Vedin I., Vessby B., Wahlund L.O., Palmblad J. ω-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study—A randomized double-blind trial. Arch. Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 104.Chiu C.C., Su K.P., Cheng T.C., Liu H.C., Chang C.J., Dewey M.E., Stewart R., Huang S.Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 105.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., Salem N., Stedman M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement. 2010;6:456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 106.Loef M., Walach H. The Omega-6/Omega-3 Ratio and Dementia or Cognitive Decline: A Systematic Review of Human Studies and Biological Evidence. J. Nutr. Gerontol. Geriatr. 2013;32:1–23. doi: 10.1080/21551197.2012.752335. [DOI] [PubMed] [Google Scholar]
- 107.Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C., Galvin J.E., Emond J., Jack C.R., Weiner M., et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA - J. Am. Med. Assoc. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Phillips M.A., Childs C.E., Calder P.C., Rogers P.J. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s disease: A randomised controlled trial. Int. J. Mol. Sci. 2015;16:24600–24613. doi: 10.3390/ijms161024600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tabue-Teguo M., de Souza P.B., Cantet C., Andrieu S., Simo N., Fougère B., Dartigues J.F., Vellas B. Effect of Multidomain Intervention, Omega-3 Polyunsaturated Fatty Acids Supplementation or their Combinaison on Cognitive Function in Non-Demented Older Adults According to Frail Status: Results from the MAPT Study. J. Nutr. Heal. Aging. 2018;22:923–927. doi: 10.1007/s12603-018-1024-6. [DOI] [PubMed] [Google Scholar]
- 110.Fiala M., Restrepo L., Pellegrini M. Immunotherapy of Mild Cognitive Impairment by ω-3 Supplementation: Why Are Amyloid-β Antibodies and ω-3 Not Working in Clinical Trials? J. Alzheimer’s Dis. 2018;62:1013–1022. doi: 10.3233/JAD-170579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serhan C.N., Dalli J., Colas R.A., Winkler J.W., Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serhan C.N., Yang R., Martinod K., Kasuga K., Pillai P.S., Porter T.F., Oh S.F., Spite M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Serhan C.N., Dalli J., Karamnov S., Choi A., Park C., Xu Z., Ji R., Zhu M., Petasis N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rathod K.S., Kapil V., Velmurugan S., Khambata R., Siddique U., Khan S., Van Eijl S., Gee L., Bansal J., Pitrola K., et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Invest. 2017;127:169–182. doi: 10.1172/JCI89429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chatterjee A., Sharma A., Chen M., Toy R., Mottola G., Conte M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang T., Xu G., Newton P.T., Chagin A.S., Mkrtchian S., Carlström M., Zhang X.M., Harris R.A., Cooter M., Berger M., et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br. J. Anaesth. 2019;122:350–360. doi: 10.1016/j.bja.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mizwicki M.T., Liu G., Fiala M., Magpantay L., Sayre J., Siani A., Mahanian M., Weitzman R., Hayden E.Y., Rosenthal M.J., et al. 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heras-Sandoval D., Pedraza-Chaverri J., Pérez-Rojas J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflammation. 2016;13 doi: 10.1186/s12974-016-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L., Wu Y., Wang Y., Wu J., Song L., Xian W., Yuan S., Pei L., Shang Y. Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J. Neuroinflammation. 2014;11 doi: 10.1186/1742-2094-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bazan N.G. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation and cell survival. Prostaglandins Leukot. Essent. Fat. Acids. 2013;88:127–129. doi: 10.1016/j.plefa.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stark D.T., Bazan N.G. Neuroprotectin D1 induces neuronal survival and downregulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol. Neurobiol. 2011;43:131–138. doi: 10.1007/s12035-011-8174-4. [DOI] [PubMed] [Google Scholar]
- 122.Yin P., Wang S., Wei Y., Wang X., Zhang J., Yin X., Feng J., Zhu M. Maresin1 Decreased Microglial Chemotaxis and Ameliorated Inflammation Induced by Amyloid-β42 in Neuron-Microglia Co-Culture Models. J. Alzheimer’s Dis. 2020;73:503–515. doi: 10.3233/JAD-190682. [DOI] [PubMed] [Google Scholar]
- 123.Serhan C.N., Yacoubian S., Yang R. Anti-Inflammatory and Proresolving Lipid Mediators. Annu. Rev. Pathol. Mech. Dis. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin P., Wang X., Wang S., Wei Y., Feng J., Zhu M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2019;13 doi: 10.3389/fncel.2019.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Wilson R.S., Aggarwal N., Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 126.Famenini S., Rigali E.A., Olivera-Perez H.M., Dang J., Chang M.T., Halder R., Rao R.V., Pellegrini M., Porter V., Bredesen D., et al. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on v-3 supplementation. FASEB J. 2017;31:148–160. doi: 10.1096/fj.201600677rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu F.J., Xue Y., Liu X.F., Xue C.H., Wang J.F., Du L., Takahashi K., Wang Y.M. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014;64:9–17. doi: 10.1016/j.neuint.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 128.Vela S., Sainz N., Moreno-Aliaga M.J., Solas M., Ramirez M.J. DHA Selectively Protects SAMP-8-Associated Cognitive Deficits Through Inhibition of JNK. Mol. Neurobiol. 2019;56:1618–1627. doi: 10.1007/s12035-018-1185-7. [DOI] [PubMed] [Google Scholar]
- 129.Ren Y.Z., Zhang B.Z., Zhao X.J., Zhang Z.Y. Resolvin D1 ameliorates cognitive impairment following traumatic brain injury via protecting astrocytic mitochondria. J. Neurochem. 2020 doi: 10.1111/jnc.14962. [DOI] [PubMed] [Google Scholar]
- 130.Abdelmoaty S., Wigerblad G., Bas D.B., Codeluppi S., Fernandez-Zafra T., El-Awady E.S., Moustafa Y., Abdelhamid A.E.-d.S., Brodin E., Svensson C.I. Spinal Actions of Lipoxin A4 and 17(R)-Resolvin D1 Attenuate Inflammation-Induced Mechanical Hypersensitivity and Spinal TNF Release. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0075543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Terrando N., Gómez-Galán M., Yang T., Carlström M., Gustavsson D., Harding R.E., Lindskog M., Eriksson L.I. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- 132.Tang J.X., Mardini F., Janik L.S., Garrity S.T., Li R.Q., Bachlani G., Eckenhoff R.G., Eckenhoff M.F. Modulation of murine alzheimer pathogenesis and behavior by surgery. Ann. Surg. 2013;257:439–448. doi: 10.1097/SLA.0b013e318269d623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bathina S., Gundala N.K.V., Rhenghachar P., Polavarapu S., Hari A.D., Sadananda M., Das U.N. Resolvin D1 Ameliorates Nicotinamide-streptozotocin-induced Type 2 Diabetes Mellitus by its Anti-inflammatory Action and Modulating PI3K/Akt/mTOR Pathway in the Brain. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Waechter V., Schmid M., Herova M., Weber A., Günther V., Marti-Jaun J., Wüst S., Rösinger M., Gemperle C., Hersberger M. Characterization of the Promoter and the Transcriptional Regulation of the Lipoxin A4 Receptor (FPR2/ALX) Gene in Human Monocytes and Macrophages. J. Immunol. 2012;188:1856–1867. doi: 10.4049/jimmunol.1101788. [DOI] [PubMed] [Google Scholar]
- 135.Fiala M., Halder R.C., Sagong B., Ross O., Sayre J., Porter V., Bredesen D.E. ω-3 supplementation increases amyloid-β phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J. 2015;29:2681–2689. doi: 10.1096/fj.14-264218. [DOI] [PubMed] [Google Scholar]
- 136.Mukherjee P.K., Marcheselli V.L., Serhan C.N., Bazan N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lukiw W.J., Cui J.G., Marcheselli V.L., Bodker M., Botkjaer A., Gotlinger K., Serhan C.N., Bazan N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zoghbi H.Y., Orr H.T. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, Spinocerebellar ataxia type 1. J. Biol. Chem. 2009;284:7425–7429. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bazan N.G. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Aspects Med. 2018;64:18–33. doi: 10.1016/j.mam.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Calandria J.M., Asatryan A., Balaszczuk V., Knott E.J., Jun B.K., Mukherjee P.K., Belayev L., Bazan N.G. NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Differ. 2015;22:1363–1377. doi: 10.1038/cdd.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Halapin N.A., Bazan N.G. NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem. Res. 2010;35:1944–1947. doi: 10.1007/s11064-010-0351-8. [DOI] [PubMed] [Google Scholar]
- 142.Marcheselli V.L., Hong S., Lukiw W.J., Tian X.H., Gronert K., Musto A., Hardy M., Gimenez J.M., Chiang N., Serhan C.N., et al. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 143.Czapski G.A., Czubowicz K., Strosznajder J.B., Strosznajder R.P. The lipoxygenases: Their regulation and implication in alzheimer’s disease. Neurochem. Res. 2016;41:243–257. doi: 10.1007/s11064-015-1776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinsen A., Tejera N., Vauzour D., Harden G., Dick J., Shinde S., Barden A., Mori T.A., Minihane A.M. Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J. 2019;33:10315–10326. doi: 10.1096/fj.201900423R. [DOI] [PubMed] [Google Scholar]