The SARS‐CoV‐2 pandemic poses unique and unprecedented challenges to patients, providers, and healthcare systems. Coronavirus disease 2019 (COVID‐19) appears to be high‐risk in patients with cardiovascular disease and in those on immunosuppressive agents in the setting of organ transplantation. 1 Patients with COVID‐19 have been shown to develop acute myocarditis, characterized by either viral or immune‐mediated cardiac injury. 2
In heart and kidney transplant recipients, a noninvasive strategy to rule out acute rejection has been employed using gene expression profiling (GEP; AlloMap™) and measurements of donor‐derived cell‐free DNA (dd‐cfDNA; AlloSure™). Plasma dd‐cfDNA has shown a high negative predictive value for acute rejection 3 and is postulated to be similarly effective to identify other forms of cardiac injury such as vasculopathy. In the setting of COVID‐19, noninvasive monitoring of rejection is advantageous to minimize patient contact with the healthcare system. We present a case of a heart transplant recipient who developed COVID‐19 and subsequent results of GEP and dd‐cfDNA. This case report is exempt from IRB and ethics board approval.
The patient is a 37‐year‐old male who initially underwent cardiac transplantation in 2006 followed by retransplantation in July 2018 for cardiac allograft vasculopathy. At the time of the most recent transplant, his calculated panel reactive antibodies were 70%, no antibodies were crossed, and direct crossmatch was negative. He underwent induction with basiliximab followed by tacrolimus, mycophenolate mofetil, and a protocol‐driven prednisone taper to 5 mg per day. After 4 months, the mycophenolate mofetil was switched to sirolimus. The post‐transplant course has been uncomplicated without evidence of rejection, allograft dysfunction, donor‐specific antibodies, or infections.
At the end of April 2020, the patient developed dyspnea on exertion, sore throat, headache, and body aches and tested positive for SARS‐CoV‐2. He self‐quarantined and was managed symptomatically. Troponin I level was <0.01 ng/mL, and NTproBNP level was 69 pg/mL. During SARS‐CoV‐2 infection, dd‐cfDNA doubled from previous baseline and then returned to baseline on subsequent testing (Figure 1). GEP had been elevated and variable, likely due to the history of retransplantation. We considered echocardiography and endomyocardial biopsy, but deferred given the lack of heart failure symptoms, low biomarker levels, and concern for viral transmission. He was followed with virtual visits, and follow‐up SARS‐CoV‐2 PCR testing remained positive until 5 weeks after developing symptoms. He has improved symptomatically, but continues to have dyspnea on exertion and fatigue 7 weeks after developing symptoms.
Figure 1.
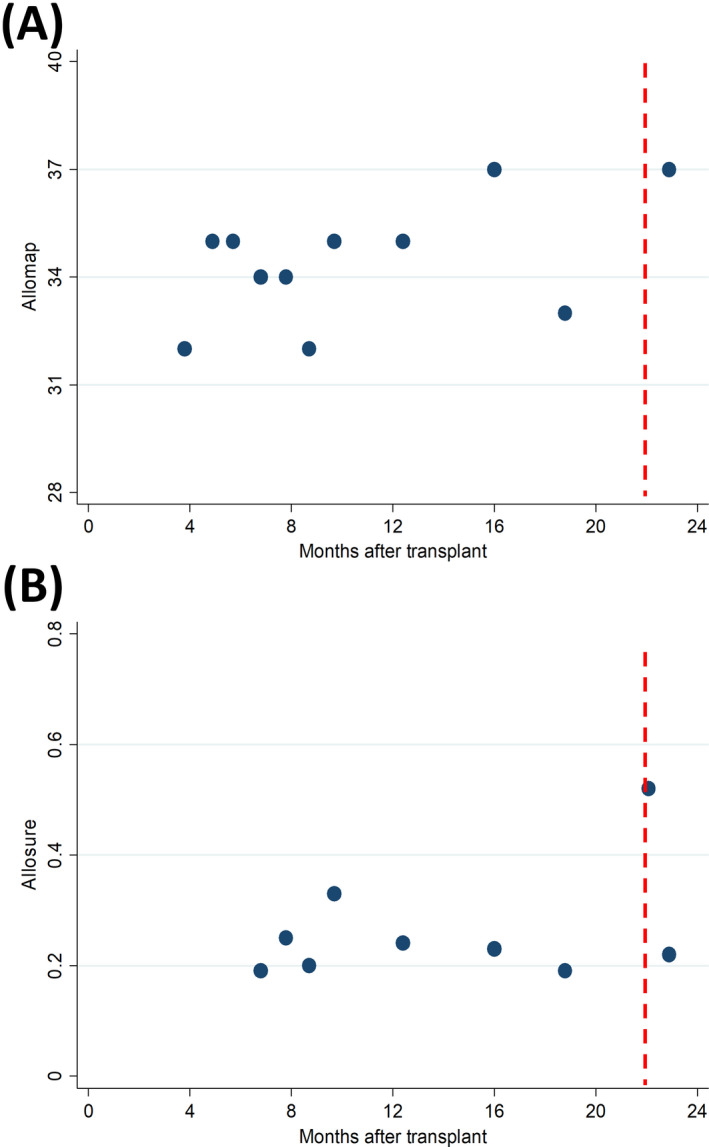
Results from GEP (AlloMap™) and dd‐cfDNA (AlloSure™) testing in a patient with COVID‐19. The dotted line represents symptom onset. The dd‐cfDNA level doubled during the acute phase of the infection but returned to baseline levels 24 d later
This case highlights several interesting findings with respect to COVID‐19. First, GEP and dd‐cfDNA analyses can be helpful in the noninvasive diagnosis of allograft dysfunction and rejection in an environment with limited in‐person interaction with providers. Second, there are reports of SARS‐CoV‐2 viral infection leading to myocarditis, but this is the first report showing evidence of an increase in dd‐cfDNA in a heart transplant patient, suggesting the subclinical allograft damage related to viral infection. Third, patients on immunosuppression may have persistently positive viral testing, making it difficult to provide recommendations as far as how long to self‐quarantine and when to safely resume in‐person cardiovascular appointments and testing. While this case is hypothesis‐generating, we did not assess for overt cardiac dysfunction with echo or for rejection with biopsy due to concerns over infectivity early in the pandemic. Our current approach involves the judicious use of dd‐cfDNA and GEP testing to monitor for rejection; in the setting of active COVID‐19 disease, we would avoid additional cardiac diagnostic testing unless there are additional signs or symptoms of cardiac dysfunction or heart failure.
CONFLICTS OF INTEREST
None.
AUTHORS’ CONTRIBUTIONS
Brett W. Sperry: Drafted the first version of the manuscript and performed data collection; and Taiyeb M. Khumri and Andrew C. Kao: Assisted in writing and editing of the paper.
ACKNOWLEDGEMENTS
None.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
REFERENCES
- 1. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19. Circulation. 2020;142(1):68‐78. [DOI] [PubMed] [Google Scholar]
- 3. Khush KK, Patel J, Pinney S, et al. Noninvasive detection of graft injury after heart transplant using donor‐derived cell‐free DNA: a prospective multicenter study. Am J Transplant. 2019;19(10):2889‐2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


