Abstract
Objectives
The presence of high SARS‐Cov‐2 viral loads in the upper airway, including the potential for aerosolized transmission of viral particles, has generated significant concern amongst otolaryngologists worldwide, particularly those performing endoscopic sinus surgery (ESS). We evaluated a simple negative‐pressure mask technique to reduce viral exposure.
Methods
Two models simulating respiratory droplets >5–10 μm and fine respiratory nuclei <5 μm using fluorescein dye and wood smoke, respectively, were utilized in a fixed cadaveric study in a controlled environment. Using ultraviolet light, fluorescein droplet spread was assessed during simulated ESS with powered microdebrider and powered drilling. Wood smoke ejection was used to evaluate fine particulate escape from a negative‐pressure mask using digital subtraction image processing.
Results
The use of a negative‐pressure mask technique resulted in 98% reduction in the fine particulate aerosol simulation and eliminated larger respiratory droplet spread during simulated ESS, including during external drill activation.
Conclusions
As global ear, nose & throat (ENT) services resume routine elective operating, we demonstrate the potential use of a simple negative‐pressure mask technique to reduce the risk of viral exposure for the operator and theatre staff during ESS.
Level of Evidence
5 Laryngoscope, 131:956–960, 2021
Keywords: COVID‐19 virus, endoscopic sinus surgery, rhinology, mask, negative pressure, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) responsible for the coronavirus disease 2019 (COVID‐19) was first reported in Wuhan, China, in December 2019 and has since spread globally in a few short months. 1 At the time of writing, it has claimed nearly 500,000 lives. 2 Thought to primarily spread via respiratory droplets from the upper respiratory tract, 3 , 4 the nose and throat have consistently reported high viral loads. 5 , 6 In addition, van Doremalen et al demonstrated that aerosolized SARS‐Cov‐2 remained viable for at least 3 hours. 7 The World Health Organization (WHO) defines respiratory droplets as particles >5–10 μm and respiratory nuclei <5 μm. 4 Although the primary route of spread is thought to be larger droplets, airborne transmission may be possible during procedures that generate large quantities of aerosolized viral particles. 8 Although evidence of transmission to healthcare workers via aerosolized virus is difficult to prove, and evidence of aerosol transmission is still debatable, 9 it would seem prudent for otolaryngologists to take steps to reduce exposure for theatre staff. 10 , 11 , 12
Concern has thus been raised regarding the safety of otolaryngologists and theatre staff performing surgery within the upper airway, particularly when using powered instrumentation. Both ENT‐UK and the American Academy of Otolaryngology‐Head and Neck Surgery recommended the restriction of aerosol‐generating procedures (AGPs) for this reason. 13 , 14 A few studies have sought to investigate this concern in a simulated setting and have demonstrated significant droplet spread during endoscopic sinus surgery, particularly when using powered drills. 15 , 16 There is therefore an urgent need to develop interventions to mitigate these risks. Using simulations of both aerosols and larger droplets in a cadaveric model, this study investigates the potential for a simple negative‐pressure mask technique to reduce the risk of intraoperative aerosol and droplet exposure for theatre staff.
METHODS
The fixed specimen used in this study had given prior consent for research photography. Formal ethical approval for this study was not required as no living specimens were used. Two models were utilized: smoke to simulate fine particle aerosolization and fluorescein staining to simulate respiratory droplet spread. The study was conducted in the University Hospital Southampton's Anatomy Laboratory on the same cadaveric specimen. We utilized a modified endoscopy mask (VBM Medizintechnik GmbH Ref 30–40‐777, unit cost £31) originally designed for bronchoscopy, connected to a standard operating theatre suction unit operating at 200 mmHg (Fig. 1). The membrane over the instrumentation port was widened to a diameter of 3 cm to allow the passage of a 4‐mm rigid endoscope and powered instruments.
Fig. 1.
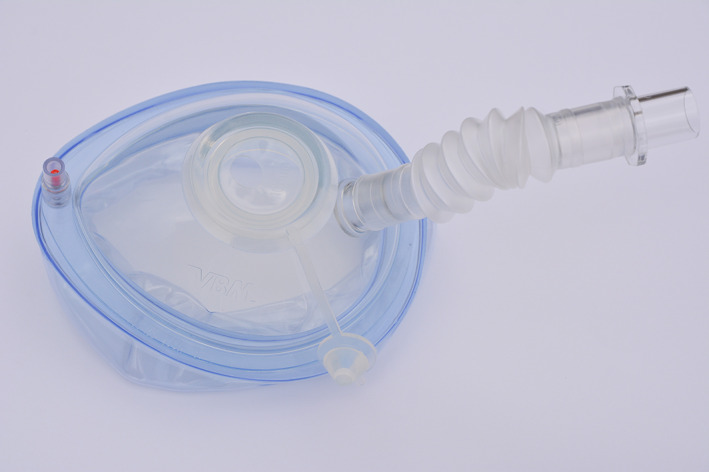
Endoscopy mask.
A wood smoker was used to generate high volumes of smoke expediently (Sage BM600) utilizing hickory wood chips. Particle sizes produced during wood burning peak at 0.1–0.2 μm and thus simulated respiratory nuclei. 17 The smoke was collected and siphoned into a Bag‐Valve‐Mask Respirator (BVM) (Intersurgical UK). A size 7.5 endotracheal tube was inserted into the cadaver head's trachea in reverse, and the cuff was inflated. The mouth was occluded with gauze so that the only point of egress was the nose. The BVM bag was squeezed for over 3 seconds until empty. Five scenarios were tested: 1) a control with no mask; 2) mask fitted without suction; 3) mask fitted with suction; 4) mask fitted with suction and instrument valve removed; and 5) mask fitted with suction, instrument valve removed, and rigid endoscope in position. A digital camera with a CMOS sensor of 24.1 megapixels yielded six images of 2.4 × 107 pixels during each scenario against a black background, with a noise reference image captured before each scenario. The images were processed in Adobe Photoshop 2020. A threshold filter was applied to each reference image to eliminate background noise and create an image of only black or white pixels. The image sequence in each scenario was stacked, a threshold filter was applied to the same level, and the reference image was subtracted to remove any confounding noise. White pixels were counted using ImageJ (imagej.nih.gov).
To simulate intraoperative droplet spread, a 1‐liter solution of 1 mg/ml fluorescein (Alcon Eye Care UK Ltd) was prepared. Of this, 100 ml was instilled both into the nasal cavity and via mini frontal trephines into the frontal sinuses. Saturation was confirmed endoscopically using a blue filter. 18 The remaining 900 ml was used for microdebrider and drill irrigation. The cadaver was placed in a standard supine operating position, and the anatomy lab was set up as a simulated operating theatre. The cadaver was covered in a blue, washable drape and illuminated with an ultraviolet (UV) light emitting diode strip (wavelength 395–405 nm). The drape was marked 10 cm from the edge of the mask with tape for reference and was washed down between each scenario. A total of three scenarios were tested: 1) endoscopic sinus surgery (ESS) (uncinectomy, middle meatal antrostomy, anterior and posterior ethmoidectomy, sphenoidotomy) using a powered suction 4‐mm microdebrider (4 minute duration) with suction mask and repeated without; 2) powered drilling of the frontal recess and beak using a 12,000‐rpm, 55‐degree integrated suction cutting burr with suction mask and repeated without; and 3) drill activation outside cadaver, within the mask aperture for 20 seconds with and without suction applied. All surgical simulations were performed by the same author (PGH). After each scenario, the blue drape was inspected under UV light for droplet spread and photographed.
RESULTS
Respiratory Nuclei Model
In line with results from previous work by Khoury, 19 we demonstrated a significant emission of aerosolized particles without a mask. Table I summarizes the white pixel count for the final subtraction image in each scenario. Figure 2 demonstrates the image processing involved, with Figure 3 depicting each subtraction image. Scenario 2 demonstrated a good mask seal with no visible leak around the edge of the mask. Applying a suction circuit to the mask resulted in a 98% reduction in the white pixel count. Removal of the instrument valve entirely did not result in elevated aerosolized particles. The image for Scenario 4 does demonstrate more smoke at the instrument valve aperture, but these particles were captured by the suction circuit and did not escape into the room. Addition of an endoscope to the setup did not alter this. The residual white pixels in Scenarios 3–5 were produced by motion artefact between image captures as evidenced by a fine white outline around the cadaver/mask. Visual inspection of the images confirms no escaped particles.
TABLE I.
White Pixel Count for the Final Subtraction Image in Each Respiratory Nuclei Scenario.
| Scenario | White Pixel Count |
|---|---|
| 1 No mask | 12,020,889 |
| 2 Mask fitted, no suction | 9,736,099 |
| 3 Mask fitted, suction applied | 216,316 |
| 4 Mask fitted, suction, instrument valve removed | 153,636 |
| 5 Mask fitted, suction, instrument valve removed, with endoscope | 214,731 |
Fig. 2.
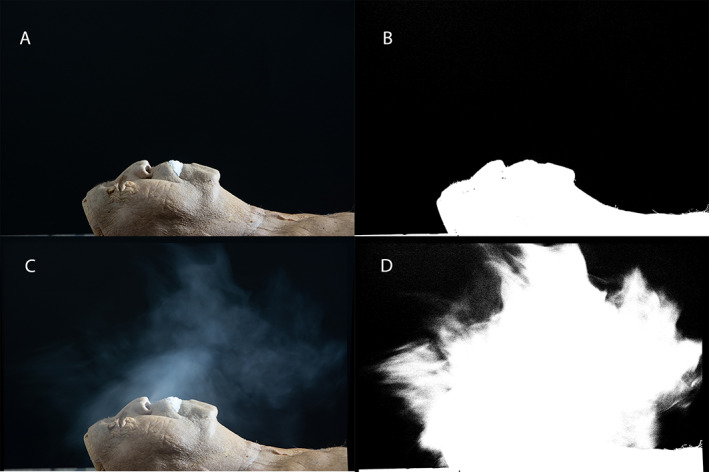
(A) Reference image for Scenario 1, (B) (A) with threshold filter applied, (C) stacked images throughout scenario 1, (D) (C) with threshold filter applied. Subtracting (B) from (D) results in image (A) in Figure 3.
Fig. 3.

Subtraction images for (A) Scenario 1, (B) Scenario 2, (C) Scenario 3, (D) Scenario 4, and (E) Scenario 5.
Respiratory Droplet Model
No fluorescein droplets were observed with or without the negative‐pressure mask during the simulation of powered microdebrider‐assisted ESS. This comprised continuous microdebrider activation for 4 minutes when performing uncinectomy, middle meatal antrostomy, anterior and posterior ethmoidectomy, and sphenoidotomy.
External droplet spread was observed up to the 10‐cm mark during the powered drilling simulation, despite the use of a cutting burr with integrated suction (Fig. 4). However, when the procedure was repeated with the negative‐pressure mask, no contamination was observed.
Fig. 4.
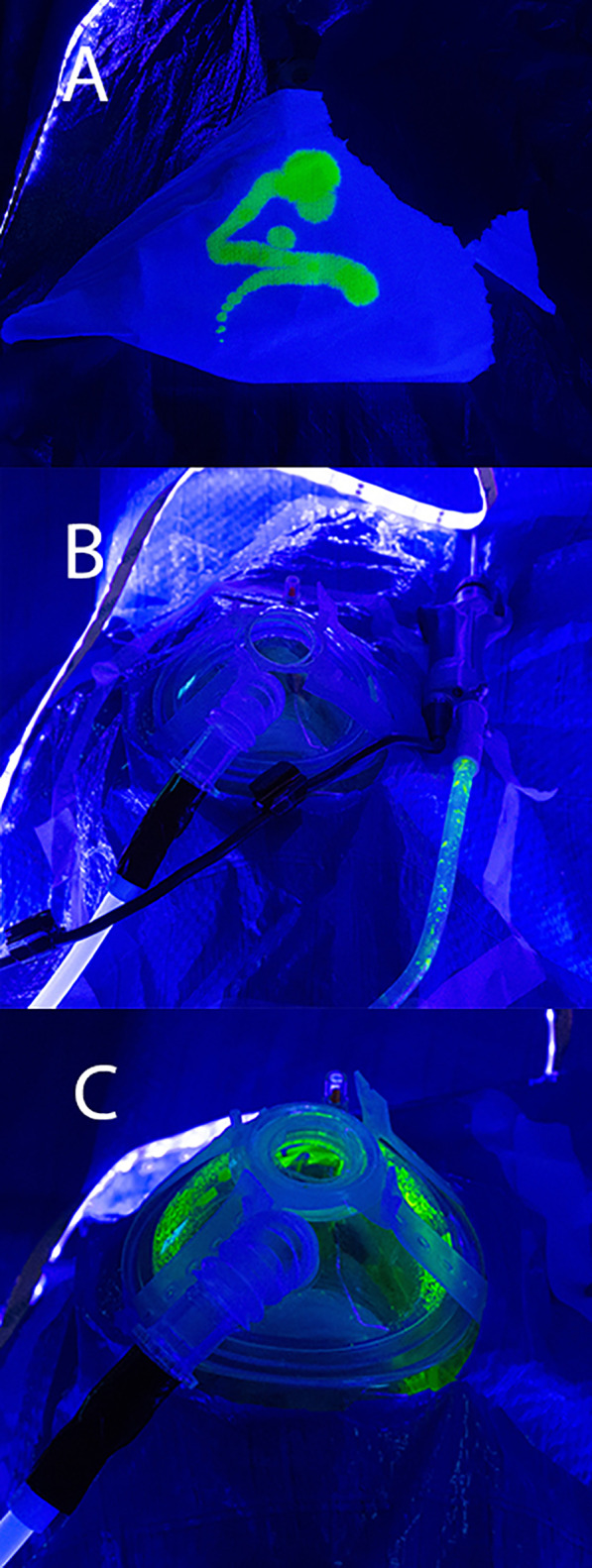
(A) Fluorescein test image under UV light, (B) fluorescein visible in microdebrider suction tubing only, (C) gross contamination within mask after external drill activation.
Accidental external drill activation has been shown to cause gross contamination. 20 In the authors' experience, this most commonly occurs in close proximity to the nares during instrument insertion/removal. To simulate this, we activated the drill external to the cadaver but within the mask instrument aperture, both with and without negative pressure. Significant contamination was observed within the mask, but none was evident externally. Table II summarizes the results.
TABLE II.
Results of the Respiratory Droplet Model.
| Scenario | Procedure | Mask | Duration | Droplet Spread | Maximum Distance |
|---|---|---|---|---|---|
| 1 | Powered microdebrider ESS | No | 4 min | No | — |
| Powered microdebrider ESS | Yes | 4 min | No | — | |
| 2 | Powered drilling of frontal recess & beak | No | 4 min | Yes | 10 cm |
| Powered drilling of frontal recess & beak | Yes | 4 min | No | — | |
| 3 | External drill activation with negative pressure | Yes | 20 sec | Yes | Confined within mask, nil external |
| External drill activation without negative pressure | Yes | 20 sec | Yes | Confined within mask, nil external |
DISCUSSION
As our understanding of SARS‐CoV‐2 transmission evolves, adequate protection of hospital staff from both contracting the virus and unwittingly acting as asymptomatic vectors has become paramount. This is particularly so as the United Kingdom resumes elective ENT services 21 including aerosol‐generating endoscopic procedures.
This proof‐of‐concept study demonstrates that a negative‐pressure mask can effectively reduce both fine droplet nuclei aerosol and larger droplet spread during ESS using powered instruments. In addition, we suggest that sealing the mask around the instruments may not be necessary, which vastly improves the surgeon's range of movement. The use of a mask with a soft face seal also allows for the accommodation of multiple face shapes without needing to manufacture individualized systems. In recent months, several studies have sought to evaluate droplet spread during endoscopic sinus surgery, 16 , 19 , 20 , 22 , 23 , 24 , 25 but this model is the first to examine the performance of a negative‐pressure mask with both respiratory droplet and droplet nuclei simulations.
In the only other study to use smoke simulation of droplet nuclei, Khoury et al. 19 used incense Joss sticks to generate aerosol particles of a diameter of 0.28 μm. 26 They also demonstrated that their Negative Airway Pressure Respirator (NAPR) was able to prevent aerosol escape. However, their image analysis differed in that they selected one image two thirds of the way through their scenarios for threshold analysis. In an effort to capture all of the particles emitted over each scenario, we stacked all of the captured images to form a composite. This allowed for greater sensitivity and analysis for any leaks that would be potentially missed by assessing only a single time point. Despite this, we demonstrated the effective capture of aerosolized particles with a similar mask, provided a suction circuit was in use.
Workman et al demonstrated significant fluorescein droplet spread in ESS, particularly when using powered drills on the sphenoid rostrum and frontal beak for 10‐second intervals. 20 They did not demonstrate external spread with the powered microdebrider, and our experience concurs with this. In a similar cadaveric study, Sharma et al. again showed droplet spread with powered drill use but also up to 6 cm using a microdebrider for 10 minutes. Gross contamination up to 13 cm was shown on external burr activation for 10 seconds. 16 We did not believe it necessary to repeat this scenario but were able to demonstrate that drill activation external to the nares, but within the mask instrument aperture, did not result in droplet spread beyond the confines of the mask, regardless of whether the suction circuit was activated. Furthermore, Helman et al. constructed a bespoke 3D‐printed mask using a cut surgical glove placed over the aperture as an instrument port, which successfully reduced fluorescein droplet spread by 86% when drilling in the anterior nasal cavity. 22 Depending on the size of the mask aperture, this study suggests that covering it may not be necessary as no smoke or fluorescein spread were observed when the instrument valve was removed.
This study has some limitations. It proved difficult to fully eliminate camera movement in our setup during each smoke scenario, resulting in minor motion artefact (manifest as a fine white outline around the cadaver/mask in the subtraction images). This could be reduced with more robust equipment. The mask tested was not sterile, although it could easily be sterilized preoperatively. We only tested a 4‐minute period of powered instrument activation rather than a full‐duration ESS. The smoke model did not allow for real‐time assessment of droplet nuclei generation during surgical instrumentation with the mask. Repeating the experiment using an optical particle sizer would allow assessment of sub 10‐μm particles generated during ESS and, as such, would allow the analysis of mask effectiveness at preventing external spread.
CONCLUSION
As global elective otolaryngology services resume, managing the risk of aerosolized coronavirus is paramount. This study demonstrates the effectiveness of a simple negative‐pressure mask in reducing droplets and respiratory nuclei generated during endoscopic sinus surgery, thus reducing potential exposure for both operator and theatre staff.
ACKNOWLEDGEMENTS
The authors thank Dr David Walker & Ellen Adams of the Centre for Learning Anatomical Sciences at University of Southampton for their support and specimen preparation; Simon Charters of the Medical Photography department at Queen Alexandra Hospital, Portsmouth Photography Dept for his postprocessing advice; and Richard Towler, Hospital Sales Director Freelance Surgical Ltd, for supplying the VBM mask samples.
Editor's Note: This Manuscript was accepted for publication on August 10, 2020.
None of the authors have conflicts to declare.
All authors contributed to and reviewed the manuscript.
BIBLIOGRAPHY
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease 2019. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed June 28, 2020.
- 3. Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses: Methods and Protocols. New York: Springer; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modes of transmission of virus causing COVID‐19: Implications for IPC precaution recommendations. Available at: https://www.who.int/news‐room/commentaries/detail/modes‐of‐transmission‐of‐virus‐causing‐covid‐19‐implications‐for‐ipc‐precaution‐recommendations. Accessed June 22, 2020.
- 5. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis 2020;20:411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Natural ventilation for infection control in health‐care settings. Published online 2009. Available at: https://www.ncbi.nlm.nih.gov/books/NBK143284/. Accessed June 28, 2020. [PubMed]
- 9. Harding H, Broom A, Broom J. Aerosol generating procedures and infective risk to healthcare workers: SARS‐CoV‐2 ‐ the limits of the evidence. J Hosp Infect 2020;105:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Gerven L, Hellings PW, Cox T, et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID‐19 outbreak. Rhinol J 2020;58:289–294. [DOI] [PubMed] [Google Scholar]
- 11. Taha MA, Hall CA, Rathbone RF, et al. Rhinologic procedures in the era of COVID‐19: health‐care provider protection protocol. Am J Rhinol Allergy 2020;34:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard BE, Lal D. Rhinologic practice special considerations during COVID‐19: visit planning, personal protective equipment, testing, and environmental controls. Otolaryngol Head Neck Surg USA Published online 2020. 10.1177/0194599820933169. [DOI] [PubMed] [Google Scholar]
- 13.Nasal endoscopy and laryngoscopy examination of ENT patients. Available at: https://www.entuk.org/nasal-endoscopy-and-laryngoscopy-examination-ent-patients. Accessed June 16, 2020.
- 14.New recommendations regarding urgent and nonurgent patient care | American Academy of Otolaryngology‐Head and Neck Surgery. https://www.entnet.org/content/new‐recommendations‐regarding‐urgent‐and‐nonurgent‐patient‐care‐0. Accessed June 16, 2020.
- 15. Workman AD, Jafari A, Welling DB, et al. Airborne aerosol generation during Endonasal procedures in the era of COVID‐19: risks and recommendations. Otolaryngol Head Neck Surg USA 2020;163:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma D, Rubel KE, Ye MJ, et al. Cadaveric simulation of endoscopic Endonasal procedures: analysis of droplet splatter patterns during the COVID‐19 pandemic. Otolaryngol Head Neck Surg USA 2020;163:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleeman MJ, Schauer JJ, Cass GR. Size and composition distribution of fine particulate matter emitted from wood burning, meat charbroiling, and cigarettes. Environ Sci Tech 1999;33:3516–3523. [Google Scholar]
- 18. Singh NP, Roberts DN. An inexpensive blue filter for fluorescein‐assisted repair of cerebrospinal fluid rhinorrhea. Laryngoscope 2014;124:1103–1105. [DOI] [PubMed] [Google Scholar]
- 19. Khoury T, Lavergne P, Chitguppi C, et al. Aerosolized particle reduction: a novel cadaveric model and a negative airway pressure respirator (NAPR) system to protect health care workers from COVID‐19. Otolaryngol Head Neck Surg USA 2020;163:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol 2020;10:798–805. [DOI] [PubMed] [Google Scholar]
- 21.Exiting the pandemic 3: a graduated return to elective ENT within the COVID‐19 pandemic. Available at: https://www.entuk.org/graduated-return-elective-ent-within-covid-19-pandemic. Accessed June 16, 2020.
- 22. Helman SN, Soriano RM, Tomov ML, et al. Ventilated upper airway endoscopic Endonasal procedure mask: surgical safety in the COVID‐19 era. Operat Neurosurg 2020;19:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. David AP, Jiam NT, Reither JM, Gurrola JG, Aghi M, El‐Sayed IH. Endoscopic Skull Base and Transoral surgery during the COVID‐19 pandemic: minimizing droplet spread with a negative‐pressure otolaryngology viral isolation drape (NOVID). Head Neck 2020;42:1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spock T, Kessler R, Lerner D, et al. Endoscopic Skull Base surgery protocol from the frontlines: Transnasal surgery during the COVID‐19 pandemic. Otolaryngol Head Neck Surg USA 2020;163:482–490. [DOI] [PubMed] [Google Scholar]
- 25. Narwani V, Kohli N, Lerner MZ. Application of a modified endoscopy face mask for flexible laryngoscopy during the COVID‐19 pandemic. Otolaryngol Head Neck Surg USA 2020;163:107–109. [DOI] [PubMed] [Google Scholar]
- 26. Yu CC, Hung IF. Incense smoke: characterization and dynamics in indoor environments. Aerosol Sci Tech 1995;23:271–281. [Google Scholar]


