Abstract
BACKGROUND
Little is known about the association between acute mental changes and adverse outcomes in hospitalized adults with COVID‐19.
OBJECTIVES
To investigate the occurrence of delirium in hospitalized patients with COVID‐19 and explore its association with adverse outcomes.
DESIGN
Longitudinal observational study.
SETTING
Tertiary university hospital dedicated to the care of severe cases of COVID‐19 in São Paulo, Brazil.
PARTICIPANTS
A total of 707 patients, aged 50 years or older, consecutively admitted to the hospital between March and May 2020.
MEASUREMENTS
We completed detailed reviews of electronic medical records to collect our data. We identified delirium occurrence using the Chart‐Based Delirium Identification Instrument (CHART‐DEL). Trained physicians with a background in geriatric medicine completed all CHART‐DEL assessments. We complemented our baseline clinical information using telephone interviews with participants or their proxy. Our outcomes of interest were in‐hospital death, length of stay, admission to intensive care, and ventilator utilization. We adjusted all multivariable analyses for age, sex, clinical history, vital signs, and relevant laboratory biomarkers (lymphocyte count, C‐reactive protein, glomerular filtration rate, D‐dimer, and albumin).
RESULTS
Overall, we identified delirium in 234 participants (33%). On admission, 86 (12%) were delirious. We observed 273 deaths (39%) in our sample, and in‐hospital mortality reached 55% in patients who experienced delirium. Delirium was associated with in‐hospital death, with an adjusted odds ratio of 1.75 (95% confidence interval = 1.15–2.66); the association held both in middle‐aged and older adults. Delirium was also associated with increased length of stay, admission to intensive care, and ventilator utilization.
CONCLUSION
Delirium was independently associated with in‐hospital death in adults aged 50 years and older with COVID‐19. Despite the difficulties for patient care during the pandemic, clinicians should routinely monitor delirium when assessing severity and prognosis of COVID‐19 patients.
Keywords: COVID‐19, delirium, prognosis, aged
INTRODUCTION
Many countries throughout the world have experienced an unprecedented healthcare crisis caused by the new severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection. 1 COVID‐19 is particularly concerning in older adults, whose underlying multimorbidity and vulnerability increase the risk of adverse outcomes following the infection. A recent report from the Centers for Disease Control and Prevention has indicated that geriatric patients account for almost half of hospital admissions and up to 80% of deaths associated with COVID‐19 acute respiratory syndrome, the most severe presentation of the disease. 2 The scenario is even more alarming in long‐term care facilities, where a combination of the oldest and frailest patients can be found living in close proximity, with a high susceptibility to transmissible diseases. 3
Fever and respiratory symptoms have been described as the most frequent manifestations of COVID‐19. 4 , 5 However, neurological symptoms have been commonly found in hospitalized patients. 6 In a case series completed in Wuhan, China, 36% of their patients had neurological complaints, and 8% had impaired consciousness. 7 Older age is also thought to be a risk factor for atypical manifestations of COVID‐19. 8 Recently, a case report from the United Kingdom described delirium, an acute neuropsychiatric syndrome characterized by inattention and fluctuating symptoms, as the sole manifestation of the disease in a frail nonagenarian. 9
Delirium has often been observed in the context of hospitalized patients with infectious diseases and identified as prominent clinical features in acute respiratory syndromes. 10 , 11 Their occurrence can be interpreted as the clinical translation of an acute brain failure, most often resulting from multiple precipitating factors (e.g., dehydration, use of psychoactive drugs, and infection) and leading to adverse outcomes, such as increased length of hospital stay, functional and cognitive decline, institutionalization, and death. 12 , 13 Given that COVID‐19 is an acute infectious and inflammatory disease, which in its more severe presentations can lead to respiratory distress syndrome and require mechanical ventilation, it is likely to be associated with delirium. The issue becomes particularly troubling in the context of social distancing and respiratory isolation. Notwithstanding their importance, such measures can become barriers to the prevention and detection of delirium in older adults. 14 , 15 Optimum nonpharmacologic management of delirious patients is also hindered by the limitations imposed on the presence of family members and caregivers in the hospital.
A recent investigation demonstrated that 26 of 40 patients with SARS‐CoV‐2 infection had suggestive clinical features for delirium. 6 Despite these preliminary findings, little is known about the clinical significance of delirium in SARS‐CoV‐2 infected patients. Previous analyses were limited by their small sample sizes, absence of prognostic estimations, 6 or unclear definitions of delirium. 16 Therefore, our aims were to investigate the occurrence of delirium in a cohort of older adults hospitalized with COVID‐19, and explore its association with adverse outcomes in this population.
METHODS
Study Design and Population
Our work is part of the ongoing CO‐FRAIL (COVID‐19 and Frailty) study, designed to investigate the association between frailty and adverse outcomes in SARS‐CoV‐2 infected patients. The CO‐FRAIL study is a cohort study recruiting patients at Hospital das Clinicas, a tertiary university hospital affiliated to the University of São Paulo Medical School, in Brazil. Hospital das Clinicas has become a major center for COVID‐19 treatment in São Paulo, the epicenter of the pandemic in Brazil. In March 2020, the main hospital building was converted to a COVID‐19–only facility, dedicating 900 beds to the care of infected patients. Admissions to the COVID‐19 unit are centrally managed by the Regulatory Central of the State of São Paulo, and severely ill patients are preferably referred to the hospital.
We assessed the eligibility of all patients who were consecutively admitted to the hospital between March 30, 2020, and May 18, 2020. We included COVID‐19 cases of candidates aged 50 years or older. COVID‐19 cases were defined according to the World Health Organization's recommendations and considered eligible when classified as probable (suggestive signs and symptoms of disease + computed tomography of the chest with suggestive alterations + no alternative diagnosis) or confirmed (detection of the new coronavirus using reverse transcription–polymerase chain reactions) cases of SARS‐CoV‐2 infection. 17
Our local institutional review board approved the study, and we adhered to the principles expressed in the Declaration of Helsinki. All patient‐identifiable information was stored in secure electronic servers, with access restricted to our researchers.
Data Collection
Trained medical investigators completed standardized electronic case report forms using Research Electronic Data Capture resources. 18 They retrospectively collected the study information, reviewing electronic medical records, nursing records, consulting notes, laboratory tests, and radiologic examinations from the complete hospitalizations. Whenever necessary, our investigators also performed structured telephone interviews with the participants or their representatives to complement or clarify their information.
Independent Variables
Our primary independent variable was the overall occurrence of delirium, which we defined using the Chart‐Based Delirium Identification Instrument (CHART‐DEL). 19 , 20 The presence of delirium is characterized by the CHART‐DEL instrument based on the documented evidence of acute mental change, combined with at least one of several key descriptors during hospital stay (e.g., delirium, confusional state, disorientation, hallucinations, or agitation). Medical records must be reviewed in their entirety to capture the occurrence of delirium, including medical and multidisciplinary notes, consultant notes, and admission and discharge summaries. CHART‐DEL is more accurate than other chart‐based methods, with an overall sensitivity of 74% and a specificity of 83% when performed by trained nurses. 19 In our study, the CHART‐DEL assessments were completed by trained physicians with a background in geriatric medicine. Our assessors were also instructed to review medical records in detail for any evidence of preexisting cognitive disorders. All patients were initially evaluated on admission by emergency physicians. Routine assessments in the emergency department included level of consciousness measurements, which we further reviewed for consistency during our study procedures. We also reviewed daily clinical notations to detect changes in level of consciousness from baseline. Delirium was defined as a binary event (yes or no), and recurrent episodes were not pondered in our analyses.
We also collected data on demographics (age, sex, race or ethnicity, marital status, and literacy), clinical history (previous diagnoses and medications, Charlson comorbidity index, physical examination, and supplemental oxygen), polypharmacy (five or more medications), premorbid functional status (Katz Activities of Daily Living Scale), and laboratory tests routinely collected on admission (complete blood cell count, D‐dimer, C‐reactive protein, urea, creatinine, and albumin). We defined the first available results from within the first 48 hours of hospitalization as the admission results of laboratory tests. Combining our detailed chart reviews with telephone interviews, we were able to work with a complete data set on our variables of interest.
Outcomes
Our primary outcome was in‐hospital death, which we retrieved from electronic medical records. Participants who survived were censored on hospital discharge. Secondary outcomes were length of hospital stay (days), admission to intensive care, and ventilator utilization.
Statistical Analysis
We reported descriptive results for the total sample, comparing the variables of interest according to the overall occurrence of delirium. We used the chi‐square test to compare categorical variables, and we used the Student t test (normal distribution) or the Wilcoxon rank‐sum test (nonnormal distribution) to compare numerical variables.
We used logistic regression models to explore the association between the overall occurrence of delirium and in‐hospital death, admission to intensive care, and ventilator utilization. We selected logistic regressions as our primary multivariable analysis method because we would be unable to determine precise dates of delirium onset using our current design. Even so, we performed a sensitivity analysis to examine the consistency of our primary outcome results using Cox proportional regression models. In this alternate approach, we approximated the onset of delirium using the earliest date on which the key descriptors of delirium were annotated, and defined length of hospital stay as our time‐dependent variable. The proportionality of hazards was checked using the Schoenfeld residuals test. Finally, we studied the association between delirium and length of hospital stay using negative binomial regression models.
In a secondary analysis, we investigated whether the association between delirium and adverse outcomes was modified according to age subgroups (50–64 vs ≥65 years). We assessed the uniformity of effect estimates across strata using chi‐square tests for heterogeneity and the Mantel‐Haenszel method to either calculate pooled adjusted estimates when effects were uniform or report stratum‐specific estimates in the presence of interactions (P < .05).
We adjusted all multivariable models for possible confounders, including age, sex, literacy, previous diagnoses, Charlson comorbidity index, polypharmacy, days of symptoms, supplemental oxygen, temperature, mean arterial pressure, lymphocyte count, C‐reactive protein, glomerular filtration rate, D‐dimer, and albumin. All statistical tests were two tailed, and an α error of up to 5% was accepted to define the statistical significance of any results. Statistical analyses were performed using Stata MP 16.1 (StataCorp).
RESULTS
We included 707 patients in our final analysis, with a mean (± standard deviation) age of 66 (±11) years and a predominance of the male sex (57%; N = 402). We identified delirium in 234 participants (33%), of which 86 (12%) already had descriptors of delirium on admission. Only 30 (4%) participants had a baseline diagnosis of dementia, of whom 22 (73%) experienced delirium during hospital stay. Patients with delirium also had a higher prevalence of other comorbidities, such as cerebrovascular disease, heart failure, and cancer (Table 1). Abnormal laboratory findings were more prominent in delirious patients, including higher C‐reactive protein and D‐dimer levels, more cases of lymphocytopenia, and lower albumin levels. Overall, in‐hospital mortality was 39% (N = 273), reaching 55% (N = 129) in patients who experienced delirium and 30% (N = 144) in those who did not (P < .001). Length of hospital stay, number of days in intensive care, and ventilator utilization were different across groups as well, with unfavorable estimates in the delirium group. Detailed descriptive findings are reported in Table 1 and supplementary materials (Supplementary Table S1).
Table 1.
Baseline Characteristics of Hospitalized Middle‐Aged and Older Adults with COVID‐19, According to Delirium Occurrence
| Characteristic | Total | No delirium | Delirium | P value |
|---|---|---|---|---|
| (N = 707) | (N = 473) | (N = 234) | ||
| Age, y | <.001 | |||
| 50–64 | 339 (48) | 258 (55) | 81 (35) | |
| 65–79 | 274 (39) | 176 (37) | 98 (42) | |
| ≥80 | 94 (13) | 39 (8) | 55 (24) | |
| Age, y | 66 (±11) | 64 (±10) | 70 (±11) | <.001 |
| Female sex | 303 (43) | 212 (45) | 91 (39) | .13 |
| Dementia | 30 (4) | 8 (2) | 22 (9) | <.001 |
| Diabetes mellitus | 299 (42) | 201 (42) | 98 (42) | .88 |
| Cerebrovascular disease | 52 (7) | 23 (5) | 29 (12) | <.001 |
| Coronary disease | 98 (14) | 69 (15) | 29 (12) | .43 |
| Hypertension | 483 (68) | 322 (68) | 161 (69) | .67 |
| Obesity | 182 (26) | 137 (29) | 45 (19) | .005 |
| Cancer | 104 (15) | 52 (11) | 52 (22) | <.001 |
| Chronic pulmonary disease | 70 (10) | 50 (11) | 20 (9) | .40 |
| Charlson score | 1 (0–4) | 1 (0–3) | 2 (1–5) | <.001 |
| Polypharmacy (≥5 medications) | 238 (34) | 157 (33) | 81 (35) | .18 |
| Previously independent | 517 (73) | 378 (80) | 139 (59) | <.001 |
| No. of typical symptoms | 3 (2–4) | 3 (3–5) | 3 (2–4) | <.001 |
| Supplemental oxygen | 513 (73) | 333 (70) | 180 (77) | .067 |
| MAP <70 mm Hg | 39 (6) | 19 (4) | 20 (9) | .012 |
| Lymphocytes, cells/mm3 | 900 (610–1,330) | 977 (650–1,374) | 835 (600–1,200) | .004 |
| C‐reactive protein (mg/L) | 132 (72–226) | 127 (68–218) | 149 (82–239) | .067 |
| GFR, mL/min | 70 (32–98) | 75 (38–104) | 56 (27–90) | <.001 |
| D‐dimer, ng/mL | 1,748 (838–7,244) | 1,485 (719–5,633) | 3,143 (1,142–13,728) | <.001 |
| Albumin, g/dL | 3.1 (2.8–3.4) | 3.2 (2.9–3.4) | 2.9 (2.6–3.2) | <.001 |
| Total time in ICU, d | 2 (0–10) | 0 (0–8) | 5 (0–12) | <.001 |
| Ventilator utilization | 289 (41) | 167 (35) | 122 (53) | .038 |
| Length of stay, d | 11 (6–16) | 10 (6–15) | 13 (8–20) | <.001 |
| In‐hospital death | 273 (39) | 144 (30) | 129 (55) | <.001 |
Note: Data are presented as mean (±standard deviation) or median (interquartile range) for continuous measures and number (percentage) for categorical measures.
Abbreviations: GFR, glomerular filtration rate; ICU, intensive care unit; MAP, mean arterial pressure.
We verified that the overall occurrence of delirium was independently associated with in‐hospital death (adjusted odds ratio (aOR) = 1.75; 95% confidence interval (CI) = 1.15–2.66) (Table 2). The association was confirmed in the Cox proportional hazards regression (Figure 1), with an adjusted hazard ratio of 1.39 (95% CI = 1.07–1.80). Delirium was also independently associated with length of hospital stay (adjusted incidence rate ratio = 1.36; 95% CI = 1.24–1.50), admission to intensive care (aOR = 3.32; 95% CI = 2.11–5.23), and ventilator utilization (aOR = 1.99; 95% CI = 1.30–3.05) (Table 2).
Table 2.
Association Between Delirium and Adverse Outcomes in Hospitalized Middle‐Aged and Older Adults with COVID‐19 (N = 707)
| Variable | Occurrence | Unadjusted estimates | Adjusted estimates | Adjusted |
|---|---|---|---|---|
| (95% CI) | (95% CI) a | P value | ||
| In‐hospital death | 129 (55) | 2.81 (2.03–3.88) | 1.75 (1.15–2.66) | .009 |
| Length of stay, d | 13 (8–20) | 1.34 (1.22–1.47) | 1.36 (1.24–1.50) | <.001 |
| Ventilator utilization | 122 (53) | 1.99 (1.45–2.74) | 1.99 (1.30–3.05) | .001 |
| Admission to intensive care | 165 (71) | 2.78 (1.99–3.89) | 3.32 (2.11–5.23) | <.001 |
Note: Occurrence results are presented as number (percentage) for categorical outcomes and median (interquartile range) for continuous outcomes.
Abbreviation: CI. confidence interval.
Estimates are presented as odds ratios for dichotomous outcomes and incidence rate ratios for length of stay. All multivariable analyses were adjusted for age, sex, literacy, previous diagnoses, Charlson comorbidity index, polypharmacy, days of symptoms, oxygen support, temperature, mean arterial pressure, lymphocyte count, C‐reactive protein, glomerular filtration rate, D‐dimer, and albumin.
Figure 1.
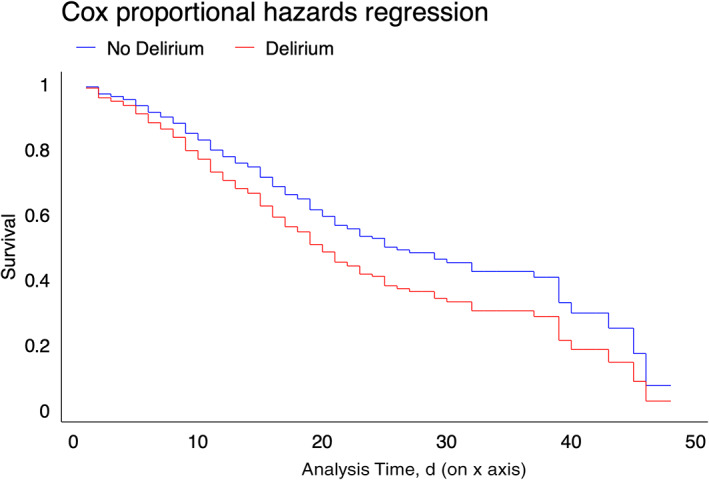
Cox proportional hazards regression curves for in‐hospital death, according to delirium occurrence, in middle‐aged and older adults with COVID‐19 (N = 707).
In our interaction analyses (Table 3), we verified that the association between delirium and in‐hospital death held both for participants aged 65 years or older (aOR = 2.33; 95% CI = 1.29–4.21) and for younger patients (aOR = 1.93; 95% CI = 1.01–3.68). The association between delirium and secondary outcomes was consistent across age subgroups as well, except for ventilator utilization, which was associated with delirium only in middle‐aged patients (Table 3).
Table 3.
Association Between Delirium Occurrence and Adverse Outcomes, According to Age Group, in Hospitalized Patients with COVID‐19 (N = 707)
| Outcome | Occurrence | Unadjusted estimates | Adjusted estimates | P value |
|---|---|---|---|---|
| (95% CI) | (95% CI) a | |||
| In‐hospital death | ||||
| 50–64 y | No delirium | 54 (21) | Ref. | Ref. | |
| 50–64 y | Delirium | 40 (49) | 3.69 (2.17–6.73) | 1.93 (1.01–3.68) | .046 |
| ≥65 y | No delirium | 90 (41) | 2.72 (1.82–4.07) | 1.40 (0.82–2.39) | .22 |
| ≥65 y | Delirium | 89 (58) | 5.25 (3.38–8.15) | 2.33 (1.29–4.21) | <.001 |
| Length of stay, d | ||||
| 50–64 y | No delirium | 10 (6–15) | Ref. | Ref. | |
| 50–64 y | Delirium | 15 (10–24) | 1.60 (1.38–1.86) | 1.47 (1.27–1.71) | <.001 |
| ≥65 y | No delirium | 10 (6–15) | 1.03 (0.92–1.15) | 1.04 (0.92–1.16) | .53 |
| ≥65 y | Delirium | 12 (7–19) | 1.22 (1.08–1.38) | 1.31 (1.15–1.50) | <.001 |
| Ventilator utilization | ||||
| 50–64 y | No delirium | 75 (29) | Ref. | Ref. | |
| 50–64 y | Delirium | 51 (63) | 4.15 (2.45–7.01) | 2.80 (1.45–5.38) | .002 |
| ≥65 y | No delirium | 92 (43) | 1.71 (1.18–2.46) | 1.05 (0.63–1.74) | .84 |
| ≥65 y | Delirium | 71 (46) | 2.11 (1.39–3.20) | 1.51 (0.84–2.70) | .16 |
| Admission to intensive care | ||||
| 50–64 y | No delirium | 105 (41) | Ref. | Ref. | |
| 50–64 y | Delirium | 67 (83) | 2.23 (3.72–13.1) | 6.19 (2.89–13.2) | <.001 |
| ≥65 y | No delirium | 116 (54) | 1.71 (1.18–2.46) | 1.01 (0.63–1.62) | .95 |
| ≥65 y | Delirium | 99 (65) | 2.67 (1.76–4.04) | 2.17 (1.24–3.82) | .007 |
Note: Occurrence results are presented as number (percentage) for categorical outcomes and median (interquartile range) for continuous outcomes.
Abbreviations: CI, confidence interval; Ref., reference.
Estimates are presented as odds ratios for dichotomous outcomes and incidence rate ratios for length of stay. All multivariable analyses were adjusted for age, sex, literacy, previous diagnoses, Charlson comorbidity index, polypharmacy, days of symptoms, oxygen support, temperature, mean arterial pressure, lymphocyte count, C‐reactive protein, glomerular filtration rate, D‐dimer, and albumin.
DISCUSSION
We found that one in three patients with COVID‐19 experienced delirium in the hospital. We observed high mortality rates in our cohort and verified that delirium was an independent predictor of in‐hospital death. Delirium was also independently associated with increased length of hospital stay, admission to intensive care, and ventilator utilization. In addition, we found that although delirium was particularly relevant in patients aged 65 years and older, its prognostic significance held for middle‐aged adults as well.
Previous studies have recognized the association between neurological manifestations of COVID‐19 and illness severity. 6 , 7 , 16 , 21 Most of these studies have either focused on impaired consciousness 7 , 21 or did not provide a clear construct of delirium as their main exposure. 16 In a cohort study of 214 adult patients, Mao et al7 (2020) reported that central nervous system (CNS) symptoms were present in 25% of the cases, the most common being dizziness (17%) and headache (13%). Impaired consciousness was detected in 8% in their sample but was six times more common in patients with severe forms of the disease (15% vs 2%). Despite these preliminary reports, their results were limited by smaller sample sizes and the absence of additional prognostic analyses. A recent prediction model for critical outcomes in patients with COVID‐19 included unconsciousness as one of the key risk factors to be evaluated on hospital admission, along with the presence of hemoptysis, cancer history, and X‐ray abnormalities. 21 Although these findings stress the relevance of impaired consciousness as a predictor of adverse outcomes in hospitalized patients with COVID‐19, the clinical meaning of delirium as a full syndrome was not addressed in the study.
In our study, we found consistent evidence that delirium is associated with adverse outcomes in hospitalized patients with COVID‐19. Delirium has been associated with postdischarge functional and cognitive decline, but its long‐term implications in COVID‐19, which frequently leads to severe cardiovascular and lung damage, are still unknown. However, we hope that our findings will stimulate health providers and researchers to work together and creatively overcome the present challenges to patient care to implement new and effective strategies to manage delirium and its complications.
The possible mechanisms behind the association between COVID‐19 and delirium are also intriguing. Although neurological manifestations can happen in a broad spectrum of acute and infectious diseases, 22 previous evidence suggests that the coronavirus family is especially neurotropic. 23 , 24 Coronaviruses have been shown to have neuroinvasive capacity and breach the CNS through either the olfactory nerve or the blood circulation and neuronal pathways. After invading the CNS, coronaviruses can cause direct brain damage by increasing demyelination, interleukin release, and the permeability of the blood‐brain barrier. 23 These neuroinflammatory pathways are known to participate in the pathogenesis of delirium and are possible windows to the understanding of delirium in the context of COVID‐19. 25 , 26 , 27 Besides plausible mechanistic pathways, significant environmental factors can be found to justify the occurrence of delirium in SARS‐CoV‐2 infected patients. Respiratory isolation and restrictions on family visits are standard practice in the hospital management of COVID‐19. Despite their importance, measures that limit patient access to caregivers and providers possibly increase the risk of delirium and delay its diagnosis. 15 , 28
We must also recognize that COVID‐19 is an acute respiratory disease and that its overall systemic effects could be sufficient to justify both the occurrence of delirium and adverse in‐hospital outcomes. In our study, we attempted to capture the systemic component of the disease by adjusting our analysis for important markers of acute illness, including C‐reactive protein, albumin, glomerular filtration rate, and vital signs. We found that even after these adjustments, delirium remained an independent predictor of adverse outcomes in the hospital, underlining its role as a prognostic marker in COVID‐19 patients.
Our study had limitations. We identified delirium using a chart‐based method, which could raise concerns regarding measurement bias. Misclassifications by the CHART‐DEL instrument mostly occur in three scenarios: (1) populations with a high risk of delirium (defined by the presence of at least three of the following factors: cognitive impairment, severe illness, visual impairment, and high serum urea nitrogen/creatinine ratio); (2) populations with a high prevalence of dementia; and (3) poor documentation of delirium signs and symptoms. 19 Although the first two contribute to the overestimation of delirium occurrence, the third leads to its underestimation. Most of our patients had a low‐to‐moderate baseline risk of delirium (96%), and the prevalence of preexisting cognitive disorders was low (4%). Therefore, we were at a higher risk of underestimating delirium, which would be more likely to drive our results toward the null, suggesting that the strengths of the associations we verified could be even higher than what we estimated. Moreover, all CHART‐DEL assessments were completed by trained medical investigators with a background in geriatric medicine, and the CHART‐DEL instrument has been demonstrated to have good accuracy for delirium detection, even when performed by nonmedical professionals. 19 Other limitations included the retrospective nature of our analyses, which prevented assessments regarding delirium duration, severity, and its temporal association with intensive care and other therapeutic measures. Finally, our study was performed in a single center dedicated to high‐complexity medical care and our results should be read with parsimony before being generalized to different populations.
The study also has notable strengths. We were able to collect detailed clinical data from a large sample of patients and provide new evidence indicating the prognostic relevance of delirium and impaired consciousness in COVID‐19. Unlike previous studies, we demonstrated the association between delirium not only with in‐hospital death but also with length of stay, intensive care admission, and ventilator utilization. We were powered to perform multivariable analyses and confirm our results in regression models adjusted for several possible confounders and in different subgroup analyses.
In a scenario with millions of persons infected by the new coronavirus, thousands of whom require hospital care, our study reinforces the clinical importance of delirium for patient care. Despite the challenges faced by health providers during the current pandemic, delirium is a key indicator of clinical severity and its detection should not be neglected. Even when detailed mental status assessments become impractical, be it because of time constraints or respiratory isolation, our results suggest that a straightforward delirium evaluation can provide valuable prognostic information. 29 , 30 Regardless of the chosen approach, clinicians should include mental status evaluations in their routine assessments of middle‐aged and older adults with COVID‐19. The prompt recognition of delirium is critical to ensure appropriate clinical care and prevent adverse outcomes in this population.
Supporting information
Supplementary Table S1: Complete Baseline Characteristics of the Study Population, According to the Overall Occurrence of Delirium.
ACKNOWLEDGMENTS
We thank members of the CO‐FRAIL (COVID‐19 and Frailty) Study Group for their efforts in collecting data for our work.
Financial Disclosure
Dr Garcez reports a research grant from the Network for Investigation of Delirium: Unifying Scientists (Subaward of Federal Award R24AG054259).
Conflict of Interest
The authors declare no conflict of interest in this study.
Author Contributions
Flavia B. Garcez had full access to all of the data and takes full responsibility for the integrity and accuracy of data analyses. All authors have contributed significantly to study design and/or data acquisition and were involved in the manuscriptʼs preparation and revision.
Sponsorʼs Role
The study did not receive any external funding.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12–March 16, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm. Accessed May 18, 2020.
- 3. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382:1‐7. 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80(6):656‐665. 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382:2268‐2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1‐9. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tay HS, Harwood R. Atypical presentation of COVID‐19 in a frail older person. Age Ageing. 2020;49:523‐524. 10.1093/ageing/afaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alkeridy WA, Almaghlouth I, Alrashed R, et al. A unique presentation of delirium in a patient with otherwise asymptomatic COVID‐19. J Am Geriatr Soc. 2020;68:1382‐1384. 10.1111/jgs.16536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munjal S, Ferrando SJ, Freyberg Z. Neuropsychiatric aspects of infectious diseases. Crit Care Clin. 2017;33(3):681‐712. 10.1016/j.ccc.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiSilvio B, Young M, Gordon A, Malik K, Singh A, Cheema T. Complications and outcomes of acute respiratory distress syndrome. Crit Care Nurs Q. 2019;42(4):349‐361. 10.1097/CNQ.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 12. Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443‐451. 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 13. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456‐1466. 10.1056/NEJMcp1605501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Hanlon S, Inouye SK. Delirium: a missing piece in the COVID‐19 pandemic puzzle. Age Ageing. 2020;49(4):497‐498. 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castro REV, Garcez FB, Avelino‐Silva TJ. Patient care during the COVID‐19 pandemic: do not leave delirium behind. Braz J Psychiatry. 2020; Published online May 20, 2020. 10.1590/1516-4446-2020-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020; Published online June 25, 2020. 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 15, 2020.
- 18. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inouye SK, Leo‐Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart‐based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312‐318. 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 20. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338‐1344. 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 21. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toovey S. Influenza‐associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114‐124. 10.1016/j.tmaid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desforges M, Le Coupanec A, Brison É, Meessen‐Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75‐96. 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33(11):1428‐1457. 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 26. van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009;9:21. 10.1186/1471-2377-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823‐832. 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCusker J, Cole M, Abrahamowicz M, Han L, Podoba JE, Ramman‐Haddad L. Environmental risk factors for delirium in hospitalized older people. J Am Geriatr Soc. 2001;49(10):1327‐1334. 10.1046/j.1532-5415.2001.49260.x. [DOI] [PubMed] [Google Scholar]
- 29. Mazumder H, Hossain MM, Das A. Geriatric care during public health emergencies: lessons learned from novel corona virus disease (COVID‐19) pandemic. J Gerontol Soc Work. 2020;63(4):257‐258. 10.1080/01634372.2020.1746723. [DOI] [PubMed] [Google Scholar]
- 30. LaHue SC, James TC, Newman JC, Esmaili AM, Ormseth CH, Ely EW. Collaborative delirium prevention in the age of COVID‐19. J Am Geriatr Soc. 2020;68(5):947‐949. 10.1111/jgs.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Complete Baseline Characteristics of the Study Population, According to the Overall Occurrence of Delirium.


