Abstract
Objective
Preliminary data from different cohorts of small sample size or with short follow‐up indicate poorer prognosis in people with obesity compared with other patients. This study aims to precisely describe the strength of association between obesity in patients hospitalized with coronavirus disease 2019 (COVID‐19) and mortality and to clarify the risk according to usual cardiometabolic risk factors in a large cohort.
Methods
This is a prospective cohort study including 5,795 patients aged 18 to 79 years hospitalized from February 1 to April 30, 2020, in the Paris area, with confirmed infection by severe acute respiratory syndrome coronavirus 2. Adjusted regression models were used to estimate the odds ratios (ORs) and 95% CIs for the mortality rate at 30 days across BMI classes, without and with imputation for missing BMI values.
Results
Eight hundred ninety‐one deaths had occurred at 30 days. Mortality was significantly raised in people with obesity, with the following ORs for BMI of 30 to 35 kg/m2, 35 to 40 kg/m2, and >40 kg/m2: 1.89 (95% CI: 1.45‐2.47), 2.79 (95% CI: 1.95‐3.97), and 2.55 (95% CI: 1.62‐3.95), respectively (18.5‐25 kg/m2 was used as the reference class). This increase holds for all age classes.
Conclusions
Obesity doubles mortality in patients hospitalized with COVID‐19.
Study Importance.
What is already known?
-
►
Preliminary data from different cohorts with a small sample size (fewer than 400 patients) with short follow‐up or with poorly described BMI indicate a poorer prognosis in people with obesity compared with other patients.
What does this study add?
-
►
This cohort study of 5,795 patients aged 18 to 79 years hospitalized in Paris shows a doubling for mortality risk in patients hospitalized for severe acute respiratory syndrome coronavirus 2 infection independently of cardiometabolic risk factors.
How might these results change the focus of clinical practice?
-
►
People with obesity in the coronavirus disease 2019 pandemic context require personalized treatment.
Introduction
Since its emergence from China at the end of 2019, the coronavirus disease 2019 (COVID‐19) pandemic has become the main worldwide public health threat and it is responsible for lockdown measures affecting millions of people. COVID‐19 symptoms include a wide variety of clinical presentations, from no symptoms to severe respiratory symptoms leading to death (1).
Obesity, and especially its most extreme forms, is a source of stigma (2), high emergency care use (3), higher morbidity, and increased mortality (4). In the context of infectious disease, a high BMI has been recognized as a risk factor for nosocomial, skin, and respiratory disease infections (5). About 10 years ago, against the backdrop of the H1N1 influenza epidemic, it was clearly pointed out in a meta‐analysis of more than 3,000 individuals that people with severe obesity had a twofold increased risk for intensive care unit (ICU) admission and mortality, compared with counterparts without obesity (6). In two single‐center studies, the risk for need of invasive mechanical ventilation in patients with COVID‐19–related severe acute respiratory syndrome was higher in patients with severe obesity than in patients with normal weight (7) but was not higher for those with class I obesity (BMI of 30 to 35 kg/m2) (8).
Preliminary data from different small‐sample‐size (fewer than 400 patients) cohorts of patients with COVID‐19 with short follow‐up or with poorly described BMI values indicate poorer prognosis in people with obesity than in other patients. For instance, one study showed a higher mortality frequency in people with severe obesity admitted to the ICU compared with people with less‐severe obesity (9). However, it is not possible to conclude from these results that obesity is an independent factor of mortality for patients with COVID‐19 because of the small sample sizes of these studies, nor is it possible to have a precise estimate of the obesity effect size because of the absence of BMI categories and incomplete follow‐up. These results need, therefore, to be confirmed in a large cohort, with available BMI categories and adequate follow‐up. To further investigate the topic, we conducted an analysis of the association between BMI and risk for mortality at 30 days after hospitalization for COVID‐19 in all Paris area–based public university hospitals.
Methods
Data source
The Assistance Publique–Hôpitaux de Paris (AP‐HP) is the largest hospital entity in Europe, with 39 hospitals (22,474 beds) mainly located in the Greater Paris area and 1.5 million hospitalizations per year (10% of all hospitalizations in France). Since 2014, the AP‐HP has been building an analytics platform based on a clinical data repository (CDR), aggregating day‐to‐day clinical data from 8.8 million patients captured by clinical databases (10). The clinical data repository has received authorization from the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés, number 1980120). From the beginning of the COVID‐19 epidemic, the "Entrepôt de Données de Santé COVID" (EDS‐COVID) database stemmed from this initiative. The later database retrieves electronic health records from all AP‐HP facilities and aggregates them into a clinical data warehouse following the Observational Medical Outcomes Partnership common data model (11). Our analysis follows recommendations provided by the Reporting of Studies Conducted Using Observational Routinely Collected Health Data Statement (12).
This study was approved by the institutional review board (authorization number IRB 00011591) of the scientific and ethical committee of the AP‐HP. All subjects included in this study were informed about the reuse of their data for research, and subjects who objected to the reuse of their data were excluded from this study, in accordance with French legislation.
Patients
We retrieved all patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by polymerase chain reaction (PCR) between February 1 and April 30 and hospitalized in one of the AP‐HP hospitals from the EDS‐COVID database. Patients with no bioclinical information (n = 580) were excluded (i.e., those with no hospitalization reports or recorded vital signs). For included patients, follow‐up was recorded until May 30.
BMI values were collected from a dedicated clinical form including height, weight, and BMI, and when these values were unavailable directly from hospitalization reports, they were calculated using a dedicated natural language processing (NLP) pipeline (“COVID‐19–AP‐HP–NLP pipeline”) that extracted height, weight, and BMI and categorized them according to the standard World Health Organization classes (<18.5 [18.5‐25, 25‐30, 30‐35, and 35‐40] kg/m2 and >40 kg/m2). When either height or weight was unavailable, BMI information was considered as missing.
Smoking status was defined as being a current smoker or having a history of smoking using the formerly mentioned COVID‐19–AP‐HP–NLP pipeline. Comorbidities were extracted from the International Classification of Diseases, Tenth Revision (ICD‐10) codes of previous and current hospitalization: I10 for hypertension, N18 for chronic kidney disease, G473 for sleep apnea, E78 for dyslipidemia, and C00 to D48 for malignancies. Heart failure was defined as having an I50 ICD‐10 code in a previous hospitalization. Diabetes was defined as having an E11 ICD‐10 code of diabetes or having an glycated hemoglobin level greater than 6.5% in any previous hospitalization.
Indirect information concerning BMI values was also retrieved for BMI imputation in patients with missing BMI. Using four‐digit E66 ICD‐10 codes, the following variables were created: E6603, E6613, E6683, and E6693 composed the ICD‐10 BMI of 25 to 30 class; E6604, E6614, E6624, E6684, and E6694 composed the ICD‐10 BMI of 30 to 35 class; E6605, E6615, E6625, E6685, and E6695 composed the ICD‐10 BMI of 35 to 40 class; E6606, E6616, E6626, E6686, and E6696 composed the ICD‐10 BMI of 40 to 50 class; and E6607, E6617, E6627, E6687, and E6697 composed the ICD‐10 BMI > 50 class. Malnutrition was extracted using E43 to E46 ICD‐10 codes. Mentions of obesity in free‐text reports were also retrieved using the formerly mentioned COVID‐19–AP‐HP–NLP pipeline.
Age at admission, sex, ICU admission, and death during hospitalization were extracted from hospital administrative data.
Outcome
The considered outcome was death during hospitalization at 30 days after a positive SARS‐CoV‐2 PCR test result. Outcomes were retrieved from administrative hospital data.
Statistical methods
Patients’ characteristics were defined according to BMI classes and sex using medians and interquartile ranges for continuous variables and proportions for binary variables, both before and after missing BMI imputation.
We imputed missing BMI categories using predictive mean matching, considering the following as explanatory variables: comorbidities (hypertension, diabetes, sleep apnea, dyslipidemia, chronic kidney disease, heart failure, and/or cancer), smoking status, sex, age, and indirect information regarding BMI values (obesity from free‐text reports, variables extracted from four‐digit E66 ICD‐10 codes, and malnutrition ICD‐10 codes). To assess the predictive ability of these variables, we performed a regression analysis on BMI using the same explanatory variables on the complete data set. To account for imputation variability, we generated five imputed samples.
Multivariate odds ratios (ORs) (95% CIs) were estimated according to BMI classes, with adjustment for comorbidities, smoking status, age, and sex using logistic regressions, both including and excluding patients with missing BMI and with stratification on age class. For analysis including patients with imputed BMI, variation across imputed data sets was taken into account by incorporating sample variability in the estimated CIs. All analyses were performed using R 4.0.2 software (R Foundation for Statistical Computing, Vienna Austria), and the MICE package was used for multiple imputations process.
Results
Demographic and clinical characteristics
During the period of February 1 through April 30, 2020, a total of 8,671 patients with a PCR‐confirmed SARS‐CoV‐2 infection were hospitalized in 1 of the 39 hospitals (Figure 1). Among them, 5,795 patients were between 18 and 80 years old and had available bioclinical data, of whom 4,056 had available BMI values (2,597 extracted from free‐text reports and 1,459 extracted from clinical signs). The mean (SD) age was 58.9 (14.7) years for women (n = 2004) and 60.3 (13.0) years for men (n = 3,791) (Table 1). The mean BMI was 29.3 (7.5) kg/m2 and 27.2 (6) kg/m2 in women and men, respectively. Comorbidities were frequent and increased with BMI classes. People with class III obesity aggregated the most risk factors. ICU admission rates increased with BMI classes. Use of mechanical ventilation did not follow an obvious trend across BMI classes. BMI was imputed for 1,739 patients, and the main BMI predictors in the imputation model were variables derived from indirect information on BMI from hospitalization reports and ICD‐10 codes (see regression coefficients and significance in Supporting Information Table S1). The correlation coefficient for the regression model used to assess the ability to predict BMI was 63%; therefore, the available indirect information on BMI was relevant to predict BMI.
Figure 1.
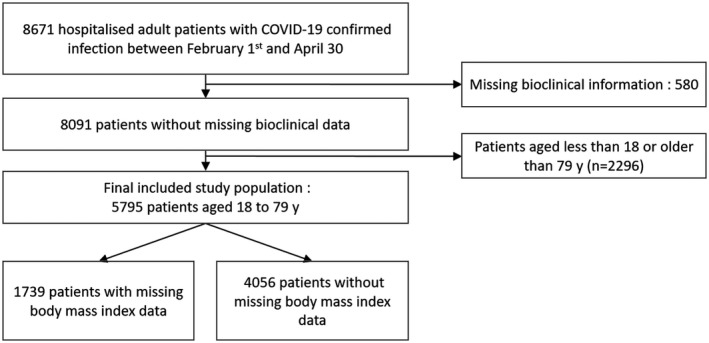
Study population flowchart.
TABLE 1.
Characteristics of the patients at baseline according to sex and BMI classes before imputation for missing values
| All BMI classes | N/A | <18.5 kg/m2 | 18.5‐25 kg/m2 | 25‐30 kg/m2 | 30‐35 kg/m2 | 35‐40 kg/m2 | 40+ kg/m2 | |
|---|---|---|---|---|---|---|---|---|
| Female patients | ||||||||
| n | 2,004 | 617 | 71 | 329 | 395 | 318 | 170 | 104 |
| Deceased | 262 (13) | 65 (11) | 11 (15) | 33 (10) | 49 (12) | 55 (17) | 32 (19) | 17 (16) |
| Intensive care | 581 (29) | 122 (20) | 13 (18) | 83 (25) | 135 (34) | 124 (39) | 65 (38) | 39 (37.5) |
| Mechanical ventilation | 590 (29) | 97 (16) | 22 (31) | 110 (33) | 123 (31) | 126 (40) | 68 (40) | 44 (42) |
| Age, mean (SD), y | 59 (15) | 56 (15.5) | 60 (17) | 61 (15) | 61.3 (14) | 58.9 (13) | 58 (13) | 58.7 (14) |
| Hypertension | 1,042 (52) | 234 (38) | 30 (42) | 161 (49) | 216 (55) | 208 (65) | 118 (69) | 75 (72) |
| Sleep apnea | 99 (5) | 10 (2) | 4 (6) | 9 (3) | 12 (3) | 18 (6) | 24 (14) | 22 (21) |
| Dyslipidemia | 140 (7) | 11 (2) | 7 (10) | 20 (6) | 39 (10) | 30 (9) | 15 (9) | 18 (17) |
| Diabetes | 777 (39) | 153 (25) | 25 (35) | 109 (33) | 158 (40) | 174 (55) | 94 (55) | 64 (62) |
| Heart failure | 86 (4) | 8 (1) | 8 (11) | 19 (6) | 18 (5) | 18 (6) | 6 (4) | 9 (9) |
| Chronic kidney disease | 165 (8) | 14 (2) | 6 (8) | 43 (13) | 36 (9) | 37 (12) | 18 (11) | 11 (11) |
| Cancer | 244 (12) | 24 (4) | 18 (25) | 70 (21) | 72 (18) | 34 (11) | 17 (10) | 9 (9) |
| Smoking | 153 (8) | 20 (3) | 16 (23) | 44 (13) | 33 (8) | 23 (7) | 11 (6) | 6 (6) |
| C‐reactive protein, mean (SD), mg/L | 87 (82) | 86 (82) | 62 (74) | 75 (81) | 87 (84) | 95 (82) | 100 (76) | 102 (87) |
| Male patients | ||||||||
| n | 3,791 | 1,122 | 105 | 845 | 1,047 | 474 | 126 | 72 |
| Deceased | 629 (17) | 161 (14) | 17 (16) | 121 (14) | 190 (18) | 90 (19) | 31 (25) | 19 (26) |
| Intensive care | 1,565 (41) | 331 (30) | 25 (24) | 337 (40) | 509 (49) | 253 (53) | 68 (54) | 42 (58.3) |
| Mechanical ventilation | 1,394 (37) | 218 (19) | 47 (45) | 340 (40) | 474 (45) | 226 (48) | 57 (45.2) | 32 (44.4) |
| Age, mean (SD), y | 60 (13) | 60 (14) | 61 (16) | 61 (13) | 61 (12) | 59 (12) | 56 (13) | 52.3 (15) |
| Hypertension | 2,100 (55) | 497 (44) | 54 (51) | 479 (57) | 643 (61) | 301 (64) | 79 (63) | 47 (65) |
| Sleep apnea | 177 (5) | 18 (2) | 3 (3) | 16 (2) | 54 (5) | 51 (11) | 18 (14) | 17 (24) |
| Dyslipidemia | 330 (9) | 40 (4) | 11 (10) | 78 (9) | 129 (12) | 48 (10) | 18 (14) | 6 (8) |
| Diabetes | 1,696 (45) | 406 (36) | 49 (47) | 381 (45) | 509 (49) | 248 (52) | 61 (48) | 42 (58) |
| Heart failure | 178 (5) | 16 (1) | 9 (9) | 55 (7) | 60 (6) | 26 (5) | 8 (6) | 4 (6) |
| Chronic kidney disease | 378 (10) | 34 (3) | 17 (16) | 135 (16) | 127 (12) | 46 (10) | 12 (10) | 7 (10) |
| Cancer | 412 (11) | 50 (4) | 33 (31) | 143 (17) | 138 (13) | 36 (8) | 9 (7) | 3 (4) |
| Smoking | 633 (17) | 95 (8) | 36 (34) | 176 (21) | 217 (21) | 77 (16) | 23 (18) | 9 (12.5) |
| C‐reactive protein, mean (SD), mg/L | 117 (94) | 117 (91) | 85 (86) | 113 (96) | 121 (95) | 121 (97) | 111 (95) | 117 (94) |
Values are n (%), except when specified otherwise.
N/A, not available.
BMI and mortality risk
Mortality was significantly higher in people with obesity when taking into account age groups, sex, smoking history, and comorbidities (Figure 2), with the following adjusted ORs for BMI of 30 to 35, 35 to 40, and >40 before missing BMI imputation: 1.89 (95% CI: 1.45‐2.47), 2.79 (95% CI: 1.95‐3.97), and 2.55 (95% CI: 1.62‐3.95), respectively, compared with BMI of 18.5 to 25 as the reference class. This association remained similar in all age classes (Figure 3), with ORs increasing with older ages. The results were comparable after imputation with the following adjusted ORs: 1.75 (95% CI: 1.37‐2.25), 2.69 (95% CI: 1.91‐3.77), and 2.38 (95% CI: 1.52‐3.67), respectively (Supporting Information Figure S1).
Figure 2.
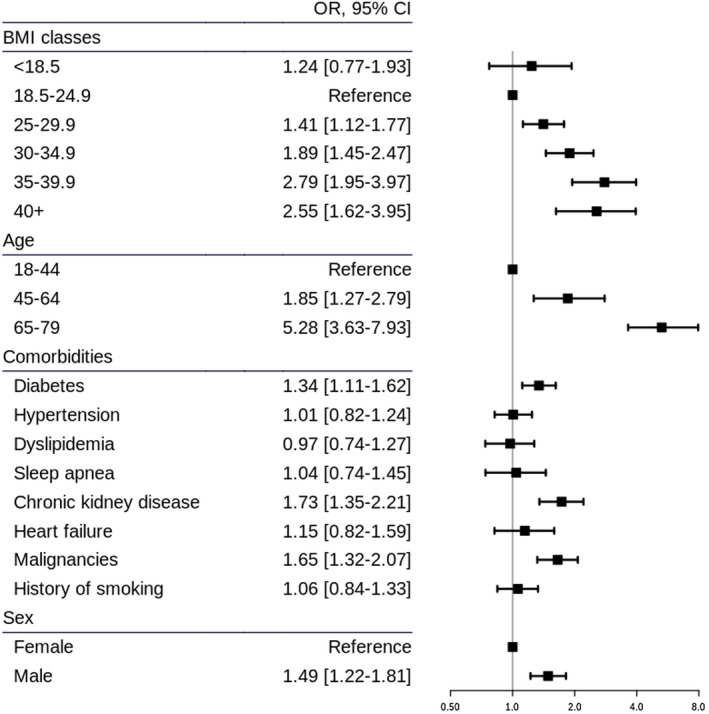
Adjusted ORs (95% CIs) for mortality according to BMI from the multivariate regression analysis. OR, odds ratio.
Figure 3.
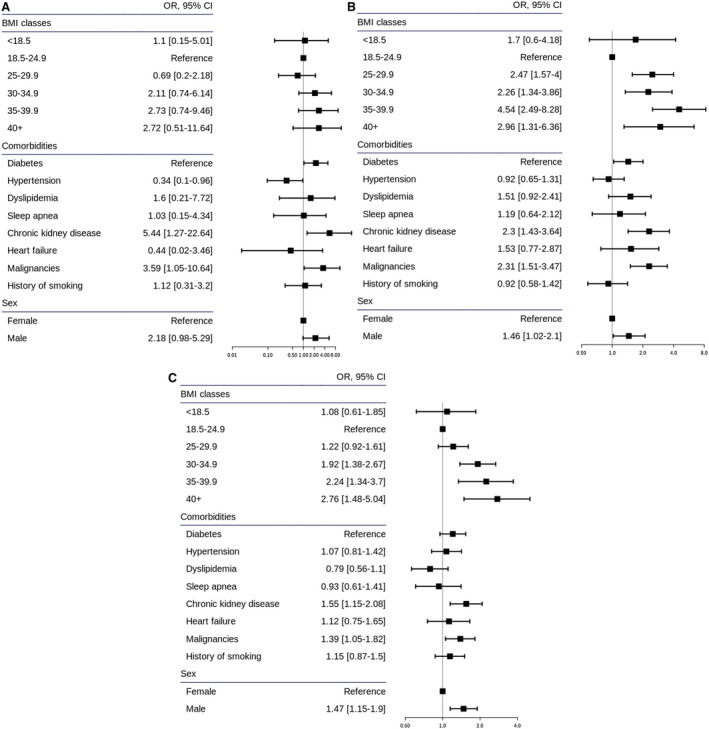
Adjusted ORs (95% CIs) for mortality according to BMI for age class: (A) 18‐44, (B) 45‐64, and (C) 65‐79 years. OR, odds ratio.
Discussion
This large study investigates the role of obesity in mortality risk at 30 days in patients hospitalized with COVID‐19 in any of the 39 university public hospitals in the Paris region (France). We have shown that obesity was a major prognostic factor, independent of known chronic comorbidities.
Several hypotheses can be made to explain a worse survival rate in people with obesity compared with people without obesity. First, obesity is characterized by an increased low‐grade inflammatory state that relates to a dysfunctional adipose microenvironment (13). The adipose cells are responsible for the secretion of pro‐inflammatory adipokines, such as tumor necrosis factor alpha (TNF‐alpha) and interleukin‐6, lower adiponectin, and increased leptin. The dysregulated cytokine environment may be the early biological step that mediates multiple organ failure (14). Second, obesity is associated with several respiratory disorders, such as obstructive sleep apnea syndrome, asthma, restrictive respiratory syndrome, and obesity hypoventilation syndrome (15). People with obesity are at particular risk of acute respiratory distress syndrome (ARDS), whatever the etiology of the syndrome (16). One explanation for the high prevalence of ARDS in people with obesity may be the very specific pulmonary mechanics of such patients, characterized mainly by excessively high pleural pressures with generally preserved chest‐wall compliance. Such a pattern leads to the frequent occurrence of negative transpulmonary pressures favoring a greater incidence of atelectasis (17). One suggested means to counteract such phenomenon is to use high positive end‐expiratory pressure settings, ideally based on esophageal monitoring (18).
An important result of the study is the poorer vital prognosis observed in people with obesity and COVID‐19. Such a result contrasts with the general findings of a similar or even better prognosis than in the population with ARDS without obesity (19). However, one should keep in mind the worse vital prognosis previously observed in people with obesity and H1N1 infection (6). A specific detrimental influence of the viral insult in people with obesity is, therefore, conceivable. In addition, the design of the study did not allow for precise assessment of the ventilator settings used in people with obesity and COVID‐19, compared with those without obesity. BMI data were missing in about a third of included patients and were, therefore, imputed when missing. Of note, we benefitted from a large amount of indirect information regarding missing BMI values using free‐text reports and ICD‐10 codes, but this was not sufficient to accurately predict BMI values. However, ORs before and after imputation were similar.
This study was considerably facilitated by the EDS‐COVID database, which retrieves electronic health records from all AP‐HP facilities and aggregates them into a clinical data warehouse. This clinical data warehouse allowed real‐time retrieval of a large set of data to deeply characterize our study population. This approach was secured by a data‐quality program, ensuring a high standard of quality for this database (10). Furthermore, even if we had been able to collect a large sample size, BMI does not capture body composition or even variations in weight. Indeed, our data indicate poorer prognosis with aging, both for undernutrition and severe obesity, which strongly relates to muscle‐mass loss and sarcopenia in the context of an hypercatabolic state related to COVID‐19 infection (20). It has been previously shown that sarcopenic obesity is associated with a longer hospital stay and a less successful recovery after an ICU stay (21). Our results might be limited by the fact that only mortality during hospitalization was considered. However, it is unlikely that patients hospitalized for COVID‐19 died of their infection after being discharged from hospital. Therefore, the subsequent underestimation of mortality due to this potential bias is likely to be limited.
Conclusion
In summary, our data show for the first time in a large multicenter setting that obesity is related to mortality in patients hospitalized with COVID‐19. The presence or absence of cardiometabolic risk factors did not modify the increased mortality risk. In the context of a global COVID‐19 pandemic lockdown, the detrimental effect of an increasingly sedentary lifestyle and increased food intake will worsen quality of life, depression risk (22), and global mortality in fragile patients with severe obesity. Thus, people with obesity in the COVID‐19 pandemic context require a personalized treatment.
Disclosure
SC reports an honorarium from Novo Nordisk for board participation and conferences. All other authors declared no conflict of interest.
Author contributions
SC and ASJ designed the study, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. SC is the corresponding author and takes responsibility for the decision to submit the manuscript for publication. SC drafted the paper with the help of ASJ, CR‐L, TP, EG, J‐SH, J‐LD, SK, NB, and CC. ASJ and NB conducted the analyses. Data were collected from all Assistance Publique–Hôpitaux de Paris hospitals. All authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published.
Funding agencies
The study was funded by the Assistance Publique–Hôpitaux de Paris. The study sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of this report.
Supporting information
Supplementary Material
Acknowledgments
The authors thank the EDS AP‐HP COVID‐19 consortium integrating the AP‐HP Health Data Warehouse team as well as all the AP‐HP staff and volunteers who contributed to the implementation of the EDS‐COVID database and to the operating solutions for this database (list in the Appendix Table A1).
Appendix 1.
Table A1. List of collaborators
| Name | Prénom | Affiliation | Contribution |
|---|---|---|---|
| Ancel | Pierre‐Yves | AP‐HP Paris University Center | Local CDW coordinator |
| Bauchet | Alain | AP‐HP Saclay University | |
| Benoit | Vincent | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Bernaux | Mélodie | Strategy and transformation department, APHP Greater Paris University Hospital | Medical coordination of data analysis |
| Bellamine | Ali | WIND Department APHP Greater Paris University Hospital | Data engineer, data scientist |
| Bey | Romain | WIND Department APHP Greater Paris University Hospital | Data engineer, data scientist, regulatory assessment |
| Bourmaud | Aurélie | APHP Paris University North | Local CDW coordinator |
| Bréant | Stéphane | WIND Department APHP Greater Paris University Hospital | Coordination of clinical research informatics |
| Burgun | Anita | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Medical and scientific coordination |
| Carrat | Fabrice | APHP Sorbonne University | |
| Caucheteux | Charlotte | Université Paris‐Saclay, Inria, CEA | Data integration and analysis |
| Champ | Julien | INRIA Sophia‐Antipolis – ZENITH team, LIRMM, Montpellier, France | Data integration and analysis |
| Cormont | Sylvie | WIND Department APHP Greater Paris University Hospital | Data standardization |
| Daniel | Christel | WIND Department APHP Greater Paris University Hospital UMRS1142 INSERM | Medical director of data standardization and clinical research informatics |
| Dubiel | Julien | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Ducloas | Catherine | APHP Paris Seine Saint Denis Universitary Hospital | Local CDW coordinator |
| Esteve | Loic | SED/SIERRA, Inria Centre de Paris | Data engineer, data scientist |
| Frank | Marie | APHP Saclay University | Local CDW coordinator |
| Garcelon | Nicolas | Imagine Institute | Data engineer, data scientist |
| Gramfort | Alexandre | Université Paris‐Saclay, Inria, CEA | Data engineer, data scientist |
| Griffon | Nicolas | WIND Department APHP Greater Paris University Hospital, UMRS1142 INSERM | Data standardization |
| Grisel | Olivier | Université Paris‐Saclay, Inria, CEA | Data engineer, data scientist |
| Guilbaud | Martin | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Hassen‐Khodja | Claire | Direction of the Clinical Research and Innovation, AP‐HP | Medical coordination of data‐driven research |
| Hemery | François | APHP Henri Mondor University Hospital | Local CDW coordinator |
| Hilka | Martin | WIND Department APHP Greater Paris University Hospital | Director of big data platform |
| Lambert | Jerome | APHP Paris University North | Local CDW coordinator |
| Layese | Richard | APHP Henri Mondor University Hospital | |
| Leblanc | Judith | Clincial Research Unit, Saint Antoine Hospital, APHP Greater Paris University Hospital | Data scientist |
| Lebouter | Léo | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Lemaitre | Guillaume | Université Paris‐Saclay, Inria, CEA | Data engineer, data scientist |
| Leprovost | Damien | Clevy.io | Data engineer, data scientist |
| Lerner | Ivan | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Data engineer, data scientist |
| Levi Sallah | Kankoe | APHP Paris University North | |
| Maire | Aurélien | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Mamzer | Marie‐France | President of the AP‐HP IRB | President of the AP‐HP IRB |
| Martel | Patricia | APHP Saclay University | Data scientist |
| Mensch | Arthur | ENS, PSL University | Data engineer, data scientist |
| Moreau | Thomas | Université Paris‐Saclay, Inria, CEA | Data engineer, data scientist |
| Neuraz | Antoine | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Data engineer, data scientist |
| Orlova | Nina | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Paris | Nicolas | WIND Department APHP Greater Paris University Hospital | Data engineer, data scientist |
| Rance | Bastien | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Data engineer, data scientist |
| Ravera | Hélène | WIND Department APHP Greater Paris University Hospital | Data engineer |
| Rozes | Antoine | APHP Sorbonne University | |
| Salamanca | Elisa | WIND Department APHP Greater Paris University Hospital | Director of the Data & Innovation department |
| Sandrin | Arnaud | WIND Department APHP Greater Paris University Hospital | Director of the National Rare Diseases Database |
| Serre | Patricia | WIND Department APHP Greater Paris University Hospital | Data engineer, data standardisation |
| Tannier | Xavier | Sorbonne University | Data engineer, data scientist |
| Treluyer | Jean‐Marc | APHP Paris University Center | Local CDW coordinator |
| Van Gysel | Damien | APHP Paris University North | Local CDW coordinator |
| Varoquaux | Gael | Université Paris‐Saclay, Inria, CEA, Montréal Neurological Institute, McGill University | Data engineer, data scientist |
| Vie | Jill Jen | SequeL, Inria Lille | Data engineer, data scientist |
| Wack | Maxime | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Data engineer, data scientist |
| Wajsburt | Perceval | Sorbonne University | Data engineer, data scientist |
| Wassermann | Demian | Université Paris‐Saclay, Inria, CEA | Data engineer, data scientist |
| Zapletal | Eric | Department of Biomedical Informatics, HEGP, APHP Greater Paris University Hospital | Data engineer |
Collégiales of AP‐HP: anesthésie‐réanimation, médecine intensive réanimation, infectiologie, virologie, nutrition
Contributor Information
Sébastien Czernichow, Email: sebastien.czernichow@aphp.fr.
AP‐HP / Universities / INSERM COVID‐19 research collaboration and AP‐HP COVID CDR Initiative:
Pierre‐Yves Ancel, Alain Bauchet, Nathanael Beeker, Nathanael Beeker, Vincent Benoit, Mélodie Bernaux, Ali Bellamine, Romain Bey, Aurélie Bourmaud, Stéphane Bréant, Anita Burgun, Fabrice Carrat, Charlotte Caucheteux, Julien Champ, Sylvie Cormont, Christel Daniel, Julien Dubiel, Catherine Ducloas, Loic Esteve, Marie Frank, Nicolas Garcelon, Alexandre Gramfort, Nicolas Griffon, Olivier Grisel, Martin Guilbaud, Claire Hassen‐Khodja, François Hemery, Martin Hilka, Anne Sophie Jannot, Jerome Lambert, Richard Layese, Judith Leblanc, Léo Lebouter, Guillaume Lemaitre, Damien Leprovost, Ivan Lerner, Kankoe Levi Sallah, Aurélien Maire, Marie‐France Mamzer, Patricia Martel, Arthur Mensch, Thomas Moreau, Antoine Neuraz, Nina Orlova, Nicolas Paris, Bastien Rance, Hélène Ravera, Antoine Rozes, Elisa Salamanca, Arnaud Sandrin, Patricia Serre, Xavier Tannier, Jean‐Marc Treluyer, Damien Van Gysel, Gael Varoquaux, Jill Jen Vie, Maxime Wack, Perceval Wajsburt, Demian Wassermann, and Eric Zapletal
References
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med 2020;26:485‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feral‐Pierssens A‐L, Carette C, Rives‐Lange C, et al. Obesity and emergency care in the French CONSTANCES cohort. PLoS One 2018;13:e0194831. doi: 10.1371/journal.pone.0194831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tobias DK, Pan A, Jackson CL, et al. Body‐mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014;370:233‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dossett LA, Dageforde LA, Swenson BR, et al. Obesity and site‐specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009;10:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta‐analysis. Obes Rev 2011;12:653‐659. [DOI] [PubMed] [Google Scholar]
- 7. Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID‐19. Obesity (Silver Spring) 2020;28:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID‐19 in critically ill patients in the Seattle region ‐ case series. N Engl J Med 2020;382:2012‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daniel C, Serre P, Orlova N, Bréant S, Paris N, Griffon N. Initializing a hospital‐wide data quality program. The AP‐HP experience. Comput Methods Programs Biomed 2019;181:104804. doi: 10.1016/j.cmpb.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 11. Hripcsak G, Duke JD, Shah NH, et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015;216:574‐578. [PMC free article] [PubMed] [Google Scholar]
- 12. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh M, Benencia F. Inflammatory processes in obesity: focus on endothelial dysfunction and the role of adipokines as inflammatory mediators. Int Rev Immunol 2019;38:157‐171. [DOI] [PubMed] [Google Scholar]
- 14. Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID‐19 infection: multiple potential mechanisms. Circulation 2020;142:4‐6. [DOI] [PubMed] [Google Scholar]
- 15. Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med 2019;45:757‐769. [DOI] [PubMed] [Google Scholar]
- 16. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010;65:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diehl J‐L, Vimpere D, Guérot E. Obesity and ARDS: opportunity for highly personalized mechanical ventilation? Respir Care 2019;64:1173‐1174. [DOI] [PubMed] [Google Scholar]
- 18. Florio G, Ferrari M, Bittner EA, et al. A lung rescue team improves survival in obesity with acute respiratory distress syndrome. Crit Care 2020;24:4. doi: 10.1186/s13054-019-2709-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Jong A, Verzilli D, Jaber S. ARDS in People with obesity: specificities and management. Crit Care 2019;23:74. doi: 10.1186/s13054-019-2374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tieland M, van Dronkelaar C, Boirie Y. Sarcopenic obesity in the ICU. Curr Opin Clin Nutr Metab Care 2019;22:162‐166. [DOI] [PubMed] [Google Scholar]
- 21. Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta‐analysis. Crit Care Med 2008;36:151‐158. [DOI] [PubMed] [Google Scholar]
- 22. Pan A, Sun Q, Czernichow S, et al. Bidirectional association between depression and obesity in middle‐aged and older women. Int J Obes (Lond) 2012;36:595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


