Abstract
Coronavirus disease 2019 (COVID‐19) pathogenesis remains under investigation. Growing evidence indicates the establishment of a hyperinflammatory response, characterized by sustained production of cytokines, such as IL‐1β. The release and maturation of this cytokine are dependent on the activation of a catalytic multiprotein complex, known as “inflammasome”. The most investigated is the NLRP3 inflammasome, which can be activated by various stimuli, such as the recognition of extracellular ATP by the P2X7 receptor. Based on the recent literature, we present evidence that supports the idea that the P2X7R/NLRP3 axis may be involved in the immune dysregulation caused by the SARS‐CoV‐2 infection.
Keywords: COVID‐19, immunopathogenesis, NLRP3 inflammasome, P2X7 receptor, SARS‐CoV‐2
1. INTRODUCTION
The emergence of the new coronavirus disease 2019 (COVID‐19) has become a significant global public health issue. 1 The disease is caused by the recently identified severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which belongs to the β‐coronaviruses subfamily, and it is genetically related to the SARS‐CoV. 2 Since, up to date, there is no effective vaccine or antiviral therapy, understanding the disease pathogenic mechanism may be crucial for the identification of pharmacological targets that can be explored for treatment strategies. The pathophysiology of SARS‐CoV‐2 infection has not been fully elucidated yet. However, growing evidence indicates a robust inflammatory response induced by viral replication as the main cause for the acute lung injury, observed in more critical patients. 3 , 4
2. SARS‐COV‐2‐INDUCED INFLAMMATORY RESPONSE
Clinical observations from COVID‐19 patients clearly indicate that the infection promotes an immune dysregulation, characterized by high levels of pro‐inflammatory cytokines and chemokines. 5 , 6 Furthermore, the blood count shows patterns compatible with leucopenia and lymphopenia. 6 , 7 Histological analysis of post‐mortem biopsy samples corroborate the establishment of a hyperinflammatory state with diffuse alveolar damage, pulmonary oedema with hyaline membrane formation, interstitial mononuclear infiltrate, presence of multinucleated giant cells and pneumocyte hyperplasia. 8 , 9
Although it might still be early to speculate which exact factors associated with SARS‐CoV‐2 infection triggers this exaggerated inflammatory response in susceptible hosts, some assumptions can be made in comparison to other respiratory virus infections. 10 , 11 After the infection of susceptible epithelial cells and several replication cycles, the virus reaches the lungs, where it will generate the most significant immune dysfunctions defining the disease severity. 12
As a result of this rapid viral replication in the lower respiratory tract, there can be massive alveolar epithelial cell (AEC) death and release of intracellular contents (known as damage‐associated molecular patterns—DAMPs) that, together with viral products, will trigger the release of inflammatory mediators. 13 , 14 These elements can cause the activation of resident macrophages and induce a shift to a more pro‐inflammatory phenotype. Another consequence is the influx of new inflammatory cells, such as blood monocytes and neutrophils, which can contribute to the disease's immunopathogenesis. 3 Furthermore, AEC type II can also contribute to the establishment of this pro‐inflammatory state by secreting and responding to cytokines and chemokine. 15
3. ROLE OF P2X7 RECEPTOR IN IMMUNE RESPONSES TO RESPIRATORY VIRAL INFECTIONS
Amongst the various DAMPs released in the context of tissue damage, adenosine triphosphate (ATP) acts as a potent inducer of the inflammatory response, activating a set of cell surface receptors, known as P2 receptors (P2R). 16 , 17 These receptors are classically divided into two subfamilies: P2X ligand‐gated ion channel receptors and P2Y G protein‐coupled receptors. Up to date, it has been identified seven members of P2X subfamily (P2X1‐7R) and eight members of P2Y subfamily (P2Y1R, 2R, 4R, 6R, 11‐14R). 18 , 19 Although ATP can bind and activate several P2R, the P2X7 receptor (P2X7R) is the most studied subtype regarding inflammatory responses. 20 , 21 This receptor is widely expressed by immune or non‐immune cells and has been implicated in the pathogenesis of several inflammatory diseases. 22
P2X7 activation can result in the expression and release of several pro‐inflammatory factors. 20 , 23 The main example is the cytokines IL‐1β and IL‐18, two essential promoters of acute phase responses. 24 , 25 Their expression and release are tightly regulated and involves the activation of a multiprotein complex named “inflammasome”. 26 The NLRP3 inflammasome is the best characterized subtype and the effector of P2X7R‐stimulated IL‐1β secretion. 27 The most accepted mechanism for ATP‐mediated NLRP3 inflammasome activation is the promotion of K+ efflux. 20 The P2X7/NLRP3 axis has also been implicated in pyroptosis, an inflammatory form of cell death dependent on caspase‐1 and caspase‐11 activation in mice (caspase‐1, caspase‐4 and caspase‐5 in humans) and characterized by osmotic lysis and release of pro‐inflammatory intracellular content. 28 , 29 , 30 Another P2X7R‐mediated mechanism involved in inflammation is the release of microparticles (MPs) by inflammatory cells. MPs are membrane‐coated vesicles released from activated or dying cells and that harbours membrane proteins, lipids from surface and other intracellular contents. 31 These MPs can accommodate IL‐1β and are considered a major secretory pathway for this cytokine from monocytes after P2X7R stimulation. 32 , 33
P2X7R‐mediated NLRP3 inflammasome activation and IL‐1β release has been associated to the immune dysfunction in viral‐induced ALI/ARDS pathogenesis. 34 , 35 , 36 In a study of acute lung inflammation caused by replication‐deficient adenoviral vectors, it was demonstrated the central role of ATP signalling through P2X7R in the regulation of inflammatory mechanisms associated with the development of ARDS. 37 Using co‐culture experiments, it was observed that ATP released from infected epithelial cells increased cytotoxicity and mediated production of inflammatory cytokines, nitric oxide and reactive oxygen species (ROS) in mouse macrophage through P2X7R activation. 37 Moreover, infection of epithelial cells also induced the release of IL‐1β and IL‐18 from the J774 cell line via NLRP3 activation mediated by P2X7R. In vivo experiments showed enhanced survival rates and reduced lung histological alterations in P2X7R or caspase‐1 KO mice or wild‐type mice after treatment with P2X7R antagonist, apyrase or caspase‐1 inhibitor, corroborating the hypothesis of the involvement of P2X7R/NLRP3 inflammasome axis in the dysregulated inflammatory response caused by adenovirus infection. 37 Similar results were observed in studies that investigated the hyperinflammatory response induced by Influenza virus infection. 38 , 39 Treatment of infected cells with AZ11645373, a P2X7R antagonist, reduced the viral replication in comparison with untreated cells. 38 This effect of P2X7 inhibition was also reproduced in experiments with P2X7R‐knockout (KO) mice. Reduced viral titres were detected in the lungs of KO mice. Interestingly, this effect was related to a better disease outcome, histological profile, and less production of inflammatory mediators in these mice. Additionally, the authors also observed the infiltration of activated macrophages and lymphocytes with immunoregulatory phenotype in infected KO mice. 38 In another study, it was demonstrated that inhibition of P2X7R with AZ11645373 and probenecid reduced IL‐1β in response to NLRP3 activation stimulated by the PB1‐F2 protein derived from pathogenic influenza A virus. Finally, treatment with these two substances in mice infected with a high dose of the influenza virus showed beneficial effects at different stages of infection, reducing the effects associated with hyperinflammation. 39 These findings suggest a protective effect of inhibition of the P2X7/NLRP3 pathway in the context of respiratory virus infections.
4. EVIDENCE OF THE INVOLVEMENT OF P2X7R‐MEDIATED RESPONSES IN COVID‐19 IMMUNOPATHOGENESIS
Although the available data on COVID‐19 are still incipient to make categorical assertions about the disease pathogenesis, we believe it is highly plausible the hypothesis of the involvement of P2X7R in the uncontrolled immune response associated with SARS‐CoV‐2 infection 91 . First, it is reasonable to assume that SARS‐CoV‐2, either by direct cytopathic effects or by indirect mechanism (e.g. induction of pyroptosis in cells susceptible), causes extracellular ATP accumulation in concentrations sufficient to activate P2X7R expressed by cells such as AEC type I or resident macrophage. In fact, extracellular ATP can be measured in alveolar space in pathological conditions associated to cell death, such as mechanical ventilation‐induced lung injury, smoke‐induced lung injury and idiopathic pulmonary fibrosis. 40 , 41 , 42
P2X7R is involved directly or indirectly in the production and release of several of inflammatory factors that are found elevated in the serum of COVID‐19 patients, such as pro‐inflammatory cytokines, chemokines and circulating membrane‐derived microparticles (MPs) found. 6 , 21 , 43 , 44 Amongst them, the cytokine IL‐1β has been pointed as satisfactory predictor of the disease prognosis. 45 , 46 The combination of viral products and sustained release of extracellular ATP can trigger NLRP3 inflammasome assembly and activation and release of IL‐1β. This hypothesis can be supported by studies that have demonstrated the ability of SARS‐CoV proteins to activate the NLRP3 inflammasome. 47 , 48 , 49 However, none of them has investigated the involvement of the P2X7R. Taking into account the high genetic identity between the new coronavirus and SARS‐CoV, it is possible to expect that its constituents are also able to activate the NLRP3 inflammasome.
Alveolar macrophages are probably the first immune innate cells to recognize the increase of extracellular ATP concentration and other PAMPs or DAMPs generated by SARS‐CoV‐2 infection. In the early stages of the infection, the activation of P2X7R on alveolar macrophage can lead to the establishment of a more pro‐inflammatory microenvironment through the assembly of the NLRP3 inflammasome and release of IL‐1β and IL‐18. Moreover, the activation of caspase‐1 in these cells can also trigger pyroptosis by the cleavage of the protein gasdermin D (Figure 1). 50
Figure 1.
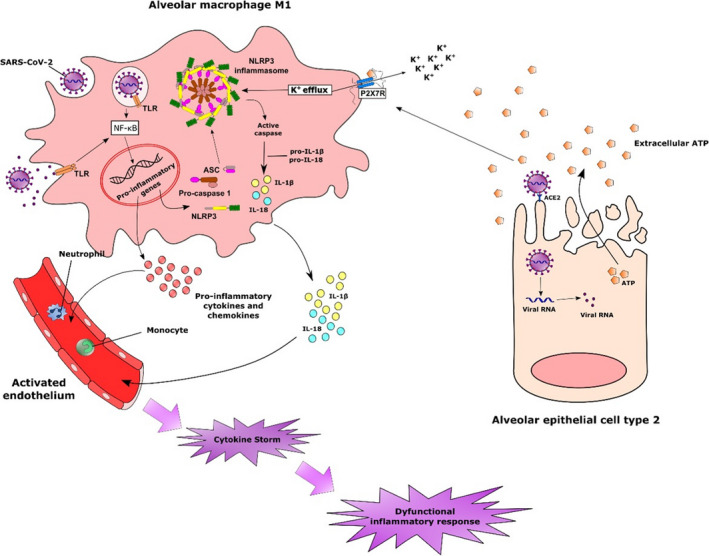
Possible involvement of alveolar macrophages in the inflammatory response triggered by SARS‐CoV‐2 infection. The rapid infection of susceptible cells can lead to sustained inflammatory response by the release of intracellular contents. The accumulation of extracellular ATP in concentrations sufficient to activate P2X7R associated with the recognition of viral products triggers several intracellular mechanisms that culminate with the expression of inflammatory genes, including those related to the NLRP3 inflammasome. The activation of P2X7R in alveolar macrophages with the consequent K+ efflux constitutes a potent signal for the assembly of NLRP3 inflammasome. The formation of this multiprotein complex leads to the activation of caspase‐1, which in turn cleaves pro‐IL‐1β and IL‐18 into their bioactive forms. Alternatively, active caspase‐1 can also cleave the protein gasdermin D, inducing pyroptosis. The release of IL1‐β and other cytokines and chemokines from activated macrophages results in the activation of the endothelium and infiltration of other inflammatory cells, such as monocytes and neutrophils, that will contribute to the amplification of the inflammatory response.
The sustained activation of resident macrophages by the release of DAMPs, such as ATP, can cause the influx of other inflammatory cells, such as monocytes and neutrophils, which will further intensify the lung injury. 51 , 52 Along with activated alveolar macrophages, infiltrating monocytes can also be another source of pro‐inflammatory mediators. In fact, depletion of circulating monocytes in experimental models of ALI/ARDS attenuated lung injury and production of inflammatory mediators. 53 In the presence of high concentration of ATP and P2X7R activation, it can be expected that these cells may release MPs containing pro‐inflammatory cytokines and other molecular mediators which can induce apoptosis of alveolar endothelial cells and promote dysfunction in the pulmonary microvasculature, another pathological characteristic that has been observed in patients with COVID‐19. 54 , 55 , 56 , 57 , 58 In fact, patients infected with SARS‐CoV‐2 possess higher blood levels of circulating MPs (cMPs). 44 Moreover, monocyte‐derived MPs may also activate the endothelium to produce chemoattractant factors or express adhesion molecules that facilitate the attraction of more inflammatory cells. 58 Emerging evidence from clinical studies suggests that endothelial activation and vascular dysfunction are also central elements in the COVID‐19 pathology. 54 , 59 Therefore, cMPs released from monocytes may also contribute to development of vascular disorders.
Another aspect of the innate immune response to SARS‐CoV‐2 infection that can be affected by the increase of extracellular ATP in alveolar space is the activity of infiltrating neutrophils. The accumulation of neutrophils in the microvasculature, interstitial and alveolar compartments is considered one of the leading causes of lung injury caused by various aetiologies. 60 At the inflammatory site, activated neutrophils will help in the elimination of invading pathogens, performing functions such as phagocytosis and release of granules containing toxic components. 61 Neutrophils can also release the so‐called neutrophil extracellular traps (NETs), web‐like structures consisting of chromatin fibres, DNA and histones. 62 However, these functions can also result in collateral tissue damage. Corroborating this harmful effect of neutrophil infiltration, it has been shown that the depletion of polymorphonuclear neutrophils in an experimental model of rat coronavirus infection resulted in a milder form of the disease with lower weight loss, clinical signs, mortality and prolonged pulmonary viral replication. 63 NET release was able to induce cell death of alveolar epithelial and endothelial cells in murine ARDS models, including those related to viral infections. 62 , 64 , 65
In this context, purinergic signalling can modulate several aspects of neutrophils functions (Figure 2). It has been shown that during migration towards a chemotactic gradient, neutrophils release ATP that acts in an autocrine manner activating the P2Y2R, amplifying chemotaxis signals and controlling cell orientation. 66 Extracellular ATP was also shown to regulate late stages of neutrophil recruitment to the lung in LPS‐induced ALI models. 67 Neutrophils express P2X7R and can release IL‐1β through ATP‐mediated NLRP3 inflammasome activation (Figure 2). 68 Moreover, it appears that, in certain conditions, neutrophils can also induce NLRP3 inflammasome activation in macrophages in a P2X7‐dependent manner, as demonstrated in a study of the role of NETs in systemic lupus erythematosus. 69 In this study, it was also shown that the release of IL‐1β and IL‐18 increased extracellular traps in infiltrating neutrophils, which can contribute to tissue damage. 69 Finally, in the context of ALI, ATP can stimulate the P2X7R on the surface of AEC type I and induce the release of a soluble form of VCAM‐1, which works as a neutrophil chemoattractant, also promoting infiltration of neutrophils (Figure 2). 70 Interestingly, clinical studies show increased blood neutrophil counts in COVID‐19 patients when compared to healthy subjects and in ICU patients when compared to non‐ICU patients. 6 , 7 , 71 Moreover, neutrophil‐to‐lymphocyte ratio was shown as a good early predictor for the disease severity. 72
Figure 2.
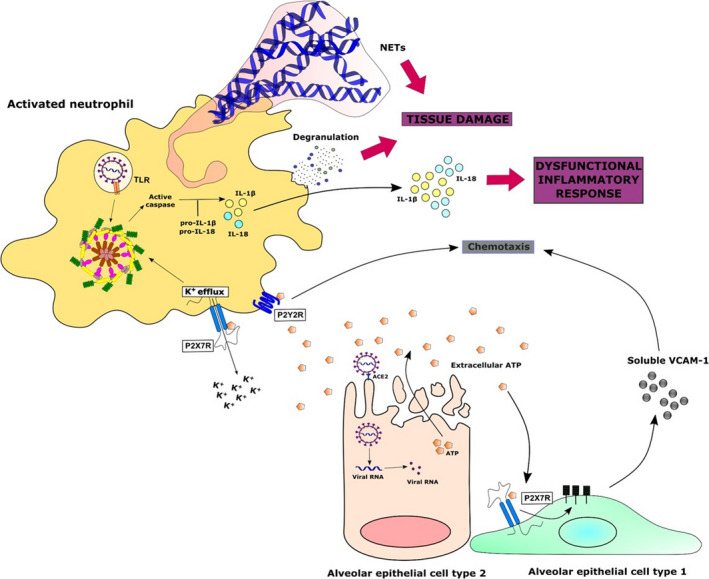
Potential role of the purinergic signalling in the activation of neutrophils against SARS‐CoV‐2 infection. Extracellular ATP released from SARS‐CoV‐2‐infected cells can modulate several functions of infiltrating neutrophils, such as chemotaxis through P2Y2 receptors. Stimulation of P2X7 receptors could stimulate the release of a soluble form of VCAM‐1 from alveolar epithelial cells type I and act as well as a chemoattractant. Similarly, ATP‐induced neutrophil P2X7R activation could also result in NLRP3 inflammasome assembly and IL‐1β and IL‐18 secretion, contributing more to the generation of an exacerbated inflammatory response. Finally, the accumulation of neutrophils in the infected tissue can lead to tissue damage due to a massive and sustained release of toxic contents from its granules and so‐called neutrophil extracellular traps (NETs)
In the late stages of the SARS‐CoV‐2 infection, the exacerbated release of IL‐1β can also shape the subsequent adaptive immune response and further contribute to the disease immunopathogenesis. In this sense, IL‐1β alone or in synergy with other cytokines (eg IL‐6 and IL‐23) can induce the differentiation of naive CD4+ T cells into pro‐inflammatory TH17 phenotype. 73 This cell subtype can contribute to the aggravation of the disease by the production of cytokines, such as IL‐17, that will further promote granulopoiesis and recruitment of more pathogenic neutrophils (Figure 3). 74 The development of a TH17 profile (as well as TH1) has been observed during the acute phase of some respiratory viruses infections such as by pandemic Influenza A and MERS‐CoV and is associated to a more intense alveolar inflammation and worst prognosis in ARDS. 75 , 76 , 77 Interestingly, P2X7 receptor has also been implicated in the differentiation of TH17 cells, since its pharmacological inhibition resulted in decrease of the production of these cells in an experimental model of type II collagen‐induced murine arthritis. 78 Another consequence of the establishment of a pro‐inflammatory state through P2X7R activation and release of pro‐inflammatory mediators is the suppression of Treg differentiation, a T helper phenotype essential for the control of the inflammatory response and restoration of the tissue homeostasis. 73 , 79 Furthermore, P2X7R activation on Treg cells inhibits their anti‐inflammatory properties, reduces their viability and favours their conversion into the TH17 phenotype (Figure 3). 80
Figure 3.
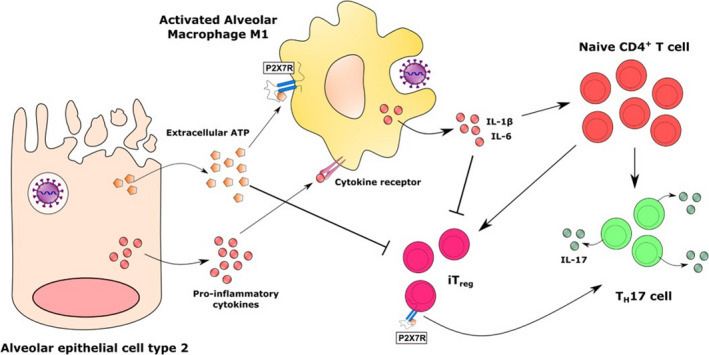
Effect of the P2X7 receptor activation on the adaptive immune response in COVID‐19. The release of ATP from dying alveolar epithelial cells or activated macrophages can shape the adaptive immune response by stimulating pro‐inflammatory lymphocyte profiles, such as TH17 (or TH1 cells). In addition, the activation of lymphocytes P2X7R in the presence of pro‐inflammatory cytokines can suppress the generation and decrease the viability of Treg cells, which could be essential for controlling the inflammatory response in the late stage of SARS‐CoV‐2 infection
5. CONCLUSIONS
Currently, the COVID‐19 outbreak has become a major concern in public health around the world, causing thousands of confirmed cases and fatalities. 81 The clinical presentations are diverse, ranging from asymptomatic or mildly symptomatic infection to acute respiratory distress syndrome (ARDS). Some cases it may also progress rapidly with disseminated intravascular coagulation (DIC), septic shock and multiple organ dysfunction syndrome (MODS). 71 , 81 The lack of efficient vaccines and specific antiviral therapy has forced several regions to take drastic measures of quarantine, social distancing, and isolation in an attempt to stop the spreading of the disease and prevent the collapse of health systems.
Although the exact mechanisms of pathogenesis are still under investigation, there is a growing perception in the literature that an exacerbated inflammatory response is responsible for the pathological alterations observed in critical patients. The administration of anti‐inflammatory therapy has been considered in the management of patients to avoid the exacerbation of lung injury. 4 Based on the cumulative knowledge of other human beta‐coronavirus infections and data derived from clinical studies with COVID‐19 patients, in the present work, we propose the involvement of P2X7R/NLRP3 inflammasome pathway in the immunopathogenesis of SARS‐CoV‐2 infection. The evidence presented and discussed now strongly suggests that SARS‐CoV‐2 infection may induce a massive release of ATP in the alveolar microenvironment in concentrations sufficient to activate the P2X7R. The combination of this purinergic stimulation with the PRR‐mediated recognition of viral products can lead to the NLRP3 inflammasome activation and release of large amounts of IL‐1β. This pleiotropic cytokine, in turn, will shape the subsequent innate and adaptive immune response, which may lead to harmful inflammatory responses. Moreover, we believe this pathway could also be related to the increased risk for patients with comorbidities, as in the case of diabetes and hypertension. P2X7R and NLRP3 inflammasome are emerging as therapeutic targets to alleviate the harmful effects of the inflammatory response associated with these disease. 82 , 83 , 84 , 85
In this sense, we believe that the P2X7/NLRP3 inflammasome pathway offers a possibility for therapeutic intervention, reducing or preventing severe lung pathology. Evidently, it would be necessary further basic and clinical studies to verify the effectiveness of this type of intervention in the outcome of SARS‐CoV‐2 infection.
Once this hypothesis has been confirmed, some pharmacological tools currently available could assist in the treatment of COVID‐19 patients, in order to control the progress of the inflammatory response, avoiding excessive tissue damage and allowing the appropriate restoration of pulmonary homeostasis. Two examples are the inhibitors AZ11645373 and probenecid that already have been shown beneficial effects in experimental models of ARDS induced by pandemic Influenza H1N1. 39 Regarding to clinical usage of these drugs, probenecid is already being evaluated in clinical trials (phase I) about toxicity (NCT01428284, NCT03296800, NCT01937026) and pharmacokinetics (NCT03138759, NCT00000706).
Another probable advantage of using P2X7R inhibitors for COVID‐19 treatment is modulation of CD147 expression. This glycoprotein was recently identified as a second receptor for SARS‐CoV‐2 invasion on host cells, becoming a new molecular target. 86 , 87 Pretreatment with A‐438079, a P2X7R antagonist, reduced CD147 and MMP9 expression in phorbol 12‑myristate 13‑acetate (PMA)‑induced macrophages through 5'‐AMP‐activated protein kinase (AMPK) and MAPK signalling. 88
Moreover, other strategy that has been evaluated for the management of critically ill COVID‐19 patients is the administration of the synthetic glucocorticoid dexamethasone. 89 This treatment has shown positive results reducing mortality amongst patients on ventilation support (NCT04381936). Interestingly, in a recent study, it was shown that dexamethasone exerted its anti‐allergic effects through the suppression of P2X7R receptor expression in peritoneal mast cells. 90 Therefore, we speculate whether the potential effect of dexamethasone, reducing the hyperinflammatory response in critically ill patients, could also involve the modulation of the P2X7R/NLRP3 inflammasome pathway.
Finally, we expect that all the information discussed throughout this work can open new investigation avenues and help in the elucidation of the COVID‐19 pathogenesis and in the identification of new therapeutic targets.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
PAF.P participated in the entire planning and writing of the manuscript. RXF participated in the entire planning, writing and revision of the manuscript.
Pacheco PAF, Faria RX. The potential involvement of P2X7 receptor in COVID‐19 pathogenesis: A new therapeutic target?. Scand J Immunol.2021;93:e12960. 10.1111/sji.12960
REFERENCES
- 1. Whitworth J. COVID‐19: a fast evolving pandemic. Trans R Soc Trop Med Hyg. 2020;114:241‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol. 2020;92:548‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020;35(3):266‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early‐Phase 2019 Novel Coronavirus (COVID‐19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15:700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID‐19 cases. J Clin Pathol. 2020;73:239‐242. [DOI] [PubMed] [Google Scholar]
- 10. Luyt C‐É, Combes A, Trouillet J‐L, Nieszkowska A, Chastre J. Virus‐induced acute respiratory distress syndrome: Epidemiology, management and outcome. Press Medicale. 2011;40:e561‐e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah RD, Wunderink RG. Viral Pneumonia and Acute Respiratory Distress Syndrome. Clin Chest Med. 2017;38:113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Liu S‐M, Yu X‐H, Tang S‐L, Tang C‐K. Coronavirus disease 2019 (COVID‐19): current status and future perspective. Int J Antimicrob Agents. 2019;2020:105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan Y‐X, Tan THP, Lee MJ‐R, et al. Induction of Apoptosis by the Severe Acute Respiratory Syndrome Coronavirus 7a Protein Is Dependent on Its Interaction with the Bcl‐XL Protein. J Virol. 2007;81:6346‐6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yue Y, Nabar NR, Shi C‐S, et al. SARS‐Coronavirus Open Reading Frame‐3a drives multimodal necrotic cell death. Cell Death Dis. 2018;9:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andreeva AV, Kutuzov MA, Voyno‐Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259‐271. [DOI] [PubMed] [Google Scholar]
- 16. Leo Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: A novel homeostatic pathway in inflammation. Front Biosci (Schol Ed). 2011;3:1443‐1456. [DOI] [PubMed] [Google Scholar]
- 17. Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5:e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burnstock G. The therapeutic potential of purinergic signalling. Biochem. Pharmacol. 2018;151:157‐165. [DOI] [PubMed] [Google Scholar]
- 19. Burnstock G. Purinergic signalling: Past, present and future. Braz J Med Biol Res. 2009;42:3‐8. [DOI] [PubMed] [Google Scholar]
- 20. Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234‐244. [DOI] [PubMed] [Google Scholar]
- 21. Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47:15‐31. [DOI] [PubMed] [Google Scholar]
- 22. Savio LEB, de Andrade Mello P, da Silva CG, Coutinho‐Silva R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front Pharmacol. 2018;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 Receptor: A Key Player in IL‐1 Processing and Release. J Immunol. 2006;176:3877‐3883. [DOI] [PubMed] [Google Scholar]
- 24. Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. The P2X7 receptor‐interleukin‐1 liaison. Front Pharmacol. 2017;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garlanda C, Dinarello CA, Mantovani A. The Interleukin‐1 Family: Back to the Future. Immunity. 2013;39:1003‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41:1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci. 2007;28:465‐472. [DOI] [PubMed] [Google Scholar]
- 28. Zha QB, Wei HX, Li CG, et al. ATP‐induced inflammasome activation and pyroptosis is regulated by AMP‐activated protein kinase in macrophages. Front Immunol. 2016;7:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang D, He Y, Muñoz‐Planillo R, Liu Q, Núñez G. Caspase‐11 Requires the Pannexin‐1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43:923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Li G, Liu ML. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics Proteomics Bioinforma. 2018;16:50‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacKenzie A, Wilson HL, Kiss‐Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin‐1β by microvesicle shedding. Immunity. 2001;15:825‐835. [DOI] [PubMed] [Google Scholar]
- 33. Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL‐1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages. J Immunol. 2007;179:1913‐1925. [DOI] [PubMed] [Google Scholar]
- 34. Zhao C, Zhao W. NLRP3 Inflammasome ‐ A Key Player in Antiviral Responses. Front Immunol. 2020;11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monção‐Ribeiro LC, Cagido VR, Lima‐Murad G, et al. Lipopolysaccharide‐induced lung injury: Role of P2X7 receptor. Respir Physiol Neurobiol. 2011;179:314‐325. [DOI] [PubMed] [Google Scholar]
- 36. Cicko S, Köhler TC, Ayata CK, et al. Extracellular ATP is a danger signal activating P2X7 Receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget. 2018;9:30635‐30648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee BH, Hwang DM, Palaniyar N, Grinstein S, Philpott DJ, Hu J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS One. 2012;7:e35812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leyva‐Grado VH, Ermler ME, Schotsaert M, et al. Contribution of the purinergic receptor P2X7 to development of lung immunopathology during influenza virus infection. MBio. 2017;8:e00229‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosli S, Kirby FJ, Lawlor KE, et al. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br J Pharmacol. 2019;176:3834‐3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rich PB, Douillet CD, Mahler SA, Husain SA, Boucher RC. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J Trauma. 2003;55:290‐297. [DOI] [PubMed] [Google Scholar]
- 41. Cicko S, Lucattelli M, Müller T, et al. Purinergic Receptor Inhibition Prevents the Development of Smoke‐Induced Lung Injury and Emphysema. J Immunol. 2010;185:688‐697. [DOI] [PubMed] [Google Scholar]
- 42. Riteau N, Gasse P, Fauconnier L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774‐783. [DOI] [PubMed] [Google Scholar]
- 43. Yang Y, Shen C, Li J, et al.Exuberant elevation of IP‐10, MCP‐3 and IL‐1ra during SARS‐CoV‐2 infection is associated with disease severity and fatal outcome. medRxiv 2020.
- 44. Guo D. Increased circulating microparticles and inflammatory factors aggravate coronavirus disease 2019 (COVID‐19). Res Sq. 2019;2019:1‐15. [Google Scholar]
- 45. Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS: Plasma IL‐1β and IL‐6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062‐1073. [DOI] [PubMed] [Google Scholar]
- 46. Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896‐1903. [DOI] [PubMed] [Google Scholar]
- 47. Siu K‐L, Yuen K‐S, Castano‐Rodriguez C, et al. Severe acute respiratory syndrome Coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3‐dependent ubiquitination of ASC. FASEB J. 2019;33:8865‐8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi CS, Nabar NR, Huang NN, Kehrl JH. SARS‐Coronavirus Open Reading Frame‐8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Torre‐Minguela C, Barberà‐Cremades M, Gómez AI, Martín‐Sánchez F, Pelegrín P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep. 2016;6:22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watkins TR. The monocyte and acute respiratory distress syndrome: Implicated, innocent bystander, or awash in research translation? Am J Respir Crit Care Med. 2013;188:407‐408. [DOI] [PubMed] [Google Scholar]
- 52. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jiang Z, Zhou Q, Gu C, Li D, Zhu L. Depletion of circulating monocytes suppresses IL‐17 and HMGB1 expression in mice with LPS‐induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L231‐L242. [DOI] [PubMed] [Google Scholar]
- 54. Leisman DE, Deutschman CS, Legrand M. Facing COVID‐19 in the ICU: vascular dysfunction, thrombosis, and dysregulated infammation. Intensive Care Med. 2020;28:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA , et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitra S, Wewers MD, Sarkar A. Mononuclear phagocyte‐derived microparticulate caspase‐1 induces pulmonary vascular endothelial cell injury. PLoS One. 2015;10:e0145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitra S, Exline M, Habyarimana F, et al. Microparticulate caspase 1 regulates gasdermin d and pulmonary vascular endothelial cell injury. Am J Respir Cell Mol Biol. 2018;59:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang J‐G, Williams JC, Davis BK, et al. Monocytic microparticles activate endothelial cells in an IL‐1β‐dependent manner. Blood. 2011;118:2366‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Escher R, Breakey N, Lämmle B. Severe COVID‐19 infection associated with endothelial activation. Thromb Res. 2020;190:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rebetz J, Semple JW, Kapur R. The Pathogenic Involvement of Neutrophils in Acute Respiratory Distress Syndrome and Transfusion‐Related Acute Lung Injury. Transfus Med Hemotherapy. 2018;45:290‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scott BNV, Kubes P. Death to the neutrophil! A resolution for acute respiratory distress syndrome? Eur Respir J. 2018;52:1801274. [DOI] [PubMed] [Google Scholar]
- 62. Twaddell SH, Baines KJ, Grainge C, Gibson PG. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest. 2019;156:774‐782. [DOI] [PubMed] [Google Scholar]
- 63. Haick AK, Rzepka JP, Brandon E, Balemba OB, Miura TA. Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology. J Gen Virol. 2014;95:578‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One. 2012;7:e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792‐1795. [DOI] [PubMed] [Google Scholar]
- 67. Shah D, Romero F, Stafstrom W, Duong M, Summer R. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L152‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome‐dependent IL‐1β secretion in response to ATP. Nat Commun. 2016;7:10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kahlenberg JM, Carmona‐Rivera C, Smith CK, Kaplan MJ. Neutrophil Extracellular Trap‐Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J Immunol. 2013;190:1217‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mishra A, Guo Y, Zhang L, et al. A Critical Role for P2X 7 Receptor‐Induced VCAM‐1 Shedding and Neutrophil Infiltration during Acute Lung Injury. J Immunol. 2016;197:2828‐2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang D, Hu BO, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. medRxiv 2020;807:2020.02.10.20021584. [DOI] [PMC free article] [PubMed]
- 73. Ikeda S, Saijo S, Murayama MA, Shimizu K, Akitsu A, Iwakura Y. Excess IL‐1 Signaling Enhances the Development of Th17 Cells by Downregulating TGF‐β–Induced Foxp3 Expression. J Immunol. 2014;192:1449‐1458. [DOI] [PubMed] [Google Scholar]
- 74. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 Cells. Annu Rev Immunol. 2009;27:485‐517. [DOI] [PubMed] [Google Scholar]
- 75. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mikacenic C, Hansen EE, Radella F, Gharib SA, Stapleton RD, Wurfel MM. Interleukin‐17A Is Associated with Alveolar Inflammation and Poor Outcomes in Acute Respiratory Distress Syndrome. Crit Care Med. 2016;44:496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bermejo‐Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13(6):R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fan Z‐D, Zhang Y‐Y, Guo Y‐H, et al. Involvement of P2X7 receptor signaling on regulating the differentiation of Th17 cells and type II collagen‐induced arthritis in mice. Sci. Rep. 2016;6:35804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235‐238. [DOI] [PubMed] [Google Scholar]
- 80. Schenk U, Frascoli M, Proietti M, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4(162):ra12. [DOI] [PubMed] [Google Scholar]
- 81. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krishnan SM, Ling YH, Huuskes BM, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt‐sensitive hypertension. Cardiovasc Res. 2019;115:776‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim Y, Wang W, Okla M, Kang I, Moreau R, Chung S. Suppression of NLRP3 inflammasome by γ ‐tocotrienol ameliorates type 2 diabetes. J Lipid Res. 2016;57:66‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ji X, Naito Y, Hirokawa G, et al. P2X 7 receptor antagonism attenuates the hypertension and renal injury in Dahl salt‐sensitive rats. Hypertens Res. 2012;35:173‐179. [DOI] [PubMed] [Google Scholar]
- 85. Solini A, Novak I. Role of the P2X7 receptor in the pathogenesis of type 2 diabetes and its microvascular complications. Curr Opin Pharmacol. 2019;47:75‐81. [DOI] [PubMed] [Google Scholar]
- 86. Ulrich H, Pillat MM. CD147 as a Target for COVID‐19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev. Reports 2020. [DOI] [PMC free article] [PubMed]
- 87. Wang K, Chen W, Zhou Y‐S, et al. SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv [Internet] 2020;2020.03.14.988345. https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1.
- 88. Lin L, Huang S, Zhu Z, et al. P2X7 receptor regulates EMMPRIN and MMP‐9 expression through AMPK/MAPK signaling in PMA‐induced macrophages. Mol Med Rep. 2018;18:3027‐3033. [DOI] [PubMed] [Google Scholar]
- 89. Selvaraj V, Dapaah‐Afriyie K, Finn A, Flanigan T. Short‐Term Dexamethasone in Sars‐CoV‐2 Patients. R I Med J. 2020;103:39‐43. [PubMed] [Google Scholar]
- 90. Yoshida K, Ito M, Hoshino Y, Matsuoka I. Effects of dexamethasone on purinergic signaling in murine mast cells: Selective suppression of P2X7 receptor expression. Biochem Biophys Res Commun. 2017;493:1587‐1593. [DOI] [PubMed] [Google Scholar]
- 91. Francesco DV, Yong T, Alba CS, Marco R. A rationale for targeting the P2X7 receptor in Coronavirus disease 19. Br J Pharmacol. 2020:1–5. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.15138 [DOI] [PMC free article] [PubMed] [Google Scholar]


