ABSTRACT
We report a case of a pregnant woman with COVID‐19 who developed coagulopathy in the absence of severe clinical symptoms. A polymerase chain reaction test of a vaginal swab was positive for SARS‐CoV‐2 RNA, suggesting a possibility of perinatal transmission. Cesarean delivery was performed because of a non‐reassuring fetal heart rate; the placenta showed increased perivillous fibrin deposition and intervillositis. Moreover, placental infection with SARS‐CoV‐2 was demonstrated by placental immunostaining. The findings suggest a possible relationship between placental fibrin deposition and chronic and acute intervillositis, non‐reassuring fetal heart rate and coagulopathy in pregnant women with COVID‐19. © 2020 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: coagulopathy, COVID‐19, placental pathology, pregnancy, SARS‐CoV‐2
CASE REPORT
A 27‐year‐old woman (gravida 2, para 1) presented to the obstetric outpatient clinic at a gestational age (GA) of 31 + 4 weeks, with complaints of headache, malaise, coughing, shortness of breath for 10 days, fever and decreased fetal movements. She had a history of Type‐1 diabetes controlled with low‐dose insulin, and pre‐eclampsia in her previous pregnancy. She was taking prophylactic acetylsalicylic acid (80 mg/day). Her body mass index was 22.4 kg/m2.
The woman's vital signs were normal, with respiratory rate of 15 breaths per min, peripheral oxygen saturation of 98%, blood pressure of 142/76 mmHg, heart rate of 105 bpm and body temperature of 37.6° C. Cardiotocography (CTG) showed fetal tachycardia (heart rate, 165 bpm), but no additional abnormalities. Estimated fetal weight (2051 g, 78th percentile) and the volume of amniotic fluid were normal. Laboratory results showed signs of maternal infection (Table 1).
Table 1.
Laboratory findings from admission to discharge in pregnant woman with COVID‐19
| Variable | Normal range | Day 1 (admission) | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 (delivery) | Day 6 (postpartum) | Day 8 (discharge) |
|---|---|---|---|---|---|---|---|---|---|
| C‐reactive protein (mg/L) | < 5 | 49 | 63 | 48 | — | 14 | 9.8 | — | — |
| Hemoglobin (mmol/L) | 7.5–10.0 | 5.5 | 5.8 | 6.1 | 6.5 | 6.2 | 6.4 | 4.4 | 5.7 |
| Hematocrit (L/L) | 0.35–0.45 | 0.29 | 0.31 | 0.33 | 0.35 | 0.34 | 0.35 | 0.23 | — |
| Mean corpuscular volume (fL) | 80–100 | 77 | 77 | 77 | 77 | 77 | 78 | 77 | 80 |
| White blood cell count (109/L) | 4.0–10.0 | 4.8 | 3.9 | 5.1 | — | 8.5 | 9.3 | 10.3 | — |
| Platelet count (109/L) | 150–400 | 150 | 131 | 104 | 123 | 118 | 131 | 109 | 170 |
| APTT (s) | 25–34 | — | — | 45 | 39 | 38 | 36 | 34 | 35 |
| Prothrombin time (s) | 12.1–15.6 | — | — | 16.7 | 16.8 | 17.4 | 16.8 | 17 | 15.7 |
| Fibrinogen (g/L) | 2.0–4.0 | — | — | 1.2 | 1.0 | 0.7 | 0.9 | 1.7 | 4.2 |
| D‐dimer (mg/L) | < 0.50 | — | — | 27 | 20 | 9.4 | — | 2.6 | 0.41 |
| Creatinine (µmol/L) | 49–90 | 45 | 50 | 46 | 46 | 52 | 50 | 50 | — |
| eGFR (mL/m/1.73 m2) | > 90 | > 90 | > 90 | > 90 | > 90 | > 90 | > 90 | > 90 | — |
| Urate (mmol/L) | 0.12–0.34 | 0.18 | 0.21 | 0.22 | 0.22 | 0.24 | 0.23 | 0.25 | — |
| Aspartate aminotransferase (U/L) | < 31 | 24 | 35 | 37 | 36 | 29 | 32 | 62 | — |
| Alanine aminotransferase (U/L) | < 34 | 9 | 9 | 9 | 8 | 9 | 14 | 17 | — |
| Lactate dehydrogenase (U/L) | < 247 | 220 | 310 | 410 | 460 | 450 | 430 | 360 | — |
| Haptoglobin (g/L) | 0.37–2.21 | — | — | 1.72 | — | — | — | — | — |
| Protein–creatinine ratio (urine) (mg/mmol) | < 30 | 21 | — | — | < 11 | — | — | — | — |
| Albumin (g/L) | 35–50 | — | — | — | — | — | — | 28 | 29 |
| Corrected calcium (mmol/L) | 2.15–2.55 | — | — | — | — | — | — | 2.14 | 2.39 |
APTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate.
The woman was admitted for fetal and maternal monitoring and cared for in isolation. COVID‐19 was confirmed by a positive nasopharyngeal SARS‐CoV‐2 RNA polymerase chain reaction (PCR) test, which was performed using primers and probes as described by Corman et al. 1 .
At a GA of 31 + 5 weeks, despite an absence of clinical symptoms and normal vital signs, the woman developed thrombocytopenia, anemia and increased lactate dehydrogenase and aspartate aminotransferase. A diagnosis of HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome was excluded due to normal urate and haptoglobin levels. Disseminated intravascular coagulation (DIC) was suspected. A (partial) placental abruption as the cause of DIC was deemed unlikely because of an absence of symptoms and a normal clinical examination, CTG and placental ultrasound evaluation. There were no signs of bacterial superinfection. Corticosteroids were administered. A PCR test of a vaginal swab was positive for SARS‐CoV‐2 RNA. Group‐B streptococcus was not detected in a rectovaginal swab.
At a GA of 32 + 1 weeks, CTG showed a decrease in variability and an incidental deceleration. The patient did not report any symptoms and had normal vital signs. Ultrasound examination showed oligohydramnios. Umbilical arterial Doppler was normal (pulsatility index, 0.8). Preterm prelabor rupture of the membranes was suspected based on a fern‐like pattern in a vaginal swab.
An emergency Cesarean delivery was performed. The total blood loss was 1370 mL. Three units of fresh frozen plasma, 2 g of fibrinogen and two units of red blood cells were administered. The patient had a swift recovery and was discharged from the hospital 2 days postoperatively. A neonate weighing 1920 g (58th percentile) was delivered with Apgar scores of 3, 6 and 10 at 1, 5 and 10 min, respectively. Arterial and venous umbilical cord pH values were 7.28 and 7.29, respectively, with corresponding base excess values of −4.9 mmol/L and −5.0 mmol/L. The neonate was admitted to the neonatal intensive care unit in isolation. Both the mother and father visited the baby only 72 h after all COVID‐19‐associated complaints resolved.
Empirical antibiotics were stopped after 48 h in the absence of signs of infection. PCR tests on neonatal nasopharyngeal swabs (days 4 and 7) were negative for SARS‐CoV‐2 RNA. The neonate had an uneventful course and was discharged from the hospital after 31 days.
The placenta was highly abnormal on macroscopic examination. The parenchyma showed an increase in perivillous fibrin (> 30−40%), extending from the chorial plate to the decidual plate. The intervillous space was reduced by 30−50%. Possible elements of infarction were seen. The villi were clumped together and trophoblast tissue was mostly no longer visible, consistent with fibrin depositions leading to trophoblast necrosis. Additionally, extensive intervillositis without villitis was noted. The intervillositis had a chronic (histiocytes) and an acute (granulocytes) component. Fetal thrombotic vasculopathy or microthrombi were not seen. In the small spared areas, there was increased maturation with terminal villi already forming (Figure 1). There was no sign of chorioamnionitis. A PCR test of the swab of the fetal side of the placenta tested positive for SARS‐CoV‐2 RNA. The presence of SARS‐CoV‐2 immunoreactive cells (trophoblasts and stromal cells) was observed after immunostaining.
Figure 1.
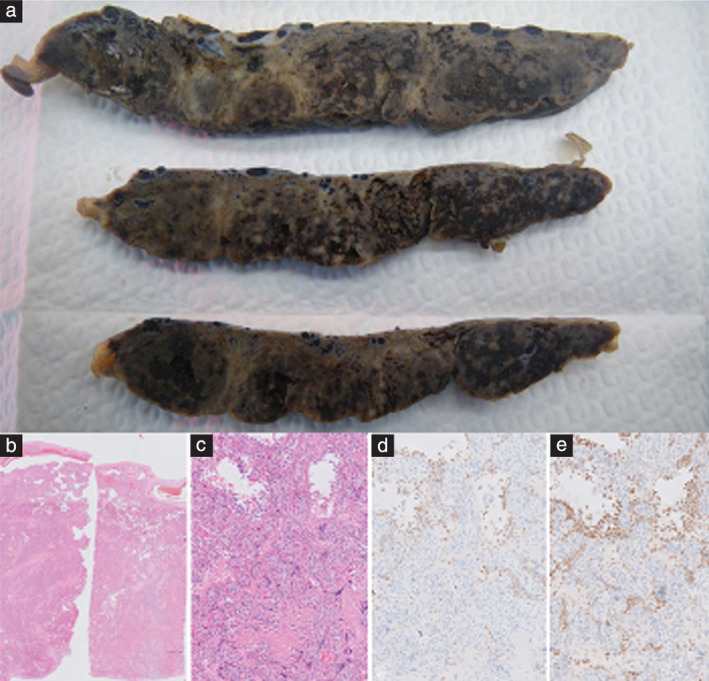
Placental abnormalities in patient diagnosed with COVID‐19. (a) Cross‐sections of placenta, showing multiple pale areas. (b) Microscopic overview of placenta, showing increased perivillous fibrin deposition and intervillositis. (c) Hematoxylin and eosin‐stained section showing fibrin around villi and infiltrate. (d) CD68‐stained section showing histiocytes (brown areas). (e) Myeloperoxidase‐stained section showing granulocytes (brown areas).
DISCUSSION
Given the observed positive vaginal SARS‐CoV‐2 PCR test, as well as the placental infection in the case reported here, it could be suggested that perinatal transmission of SARS‐CoV‐2 is possible. In this case, preterm prelabor rupture of the membranes was diagnosed prior to Cesarean delivery. The neonate may thus have been exposed to SARS‐CoV‐2 RNA present in the vagina. However, the neonate tested negative for SARS‐CoV‐2 RNA.
Studies have evaluated the risk of perinatal transmission of SARS‐CoV‐2 by testing the placenta, vaginal secretion, cord blood and amniotic fluid. The results of all samples obtained in these studies were negative for SARS‐CoV‐2 2 , 4 . Up to now, only one case report has demonstrated transplacental transmission, while others have suggested vertical transmission without compelling evidence 5 , 6 . In the present case, in the abnormal areas of the placenta, the trophoblast tissue had mostly disappeared. Placental immunostaining demonstrated placental infection with SARS‐CoV‐2, but in view of the negative neonatal test results and the absence of signs of infection, we determined that no transmission had taken place. Moreover, there was no sign of chorioamnionitis.
Signs of fetal distress in pregnant women with COVID‐19 have been reported 3 , without a clear cause. We describe a placenta with increased perivillous fibrin depositions and chronic and acute intervillositis in a woman with COVID‐19. Fibrin depositions and chronic intervillositis may coexist 7 . In the presence of acute intervillositis, however, fibrin depositions have been described only once before, namely in the first described case of proven transplacental transmission of SARS‐CoV‐2 6 . In that case, maternal thrombocytopenia and prolonged activated partial thromboplastin time, followed by non‐reassuring fetal heart rate and an emergency Cesarean delivery, were also noted 6 . A pathologic immune reaction has been proposed as reason for extensive perivillous fibrin depositions and chronic intervillositis, whilst a relationship with viral infections in pregnancy has been described only sporadically 8 , 9 . Extensive fibrin depositions are associated with adverse obstetric outcome 10 and have also been linked to coagulation disorders 11 , which is consistent with the findings in the current case. The acute placental intervillous pathologies and especially the extensive fibrin depositions, possibly causing a non‐reassuring fetal heart rate tracing, might be related to maternal illness (diabetes, COVID‐19 and possible DIC). Due to pre‐existing diabetes, our patient might have been more susceptible to a more severe course.
A study on COVID‐19 in a non‐pregnant population suggested a possible prothrombogenic component associated with COVID‐19 12 . COVID‐19 can also be associated with coagulation disorders (COVID‐19‐associated coagulation disorder (CAC)). It has been suggested that CAC may be distinguished from other coagulopathies by a predominant increase in D‐dimer and other fibrinogen degradation products. The features of DIC and CAC may, however, partly overlap 13 . CAC was not a described entity at the time of presentation of the current case early in the COVID‐19 pandemic.
In non‐pregnant COVID‐19 patients, it has been suggested that fibrin deposition in the pulmonary microvasculature is one of the causes of more severe acute respiratory distress syndrome in some patients 14 . A similar pathological process caused by COVID‐19 might occur in the placenta, leading to CAC and a non‐reassuring fetal heart rate.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, Veer B, Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance 2020; 25: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C, Zhou Y‐H, Yang H‐X, Poon LC. Intrauterine vertical transmission of SARS‐CoV‐2: what we know so far. Ultrasound Obstet Gynecol 2020; 55: 724–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, Nie X, Huang B. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020; 49: 418–423. [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis 2020; 71: 853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun 2020; 11: 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parant O, Capdet J, Kessler S, Aziza J, Berrebi A. Chronic intervillositis of unknown etiology (CIUE): Relation between placental lesions and perinatal outcome. Eur J Obstet Gynecol Reprod Biol 2008; 143: 9–13. [DOI] [PubMed] [Google Scholar]
- 8. Yu W, Tellier R, Wright J. Coxsackie Virus A16 Infection of Placenta with Massive Perivillous Fibrin Deposition Leading to Intrauterine Fetal Demise at 36 Weeks Gestation. Pediatr Dev Pathol 2015; 18: 331–334. [DOI] [PubMed] [Google Scholar]
- 9. Ordi J, Ismail M, Ventura P, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive Chronic Intervillositis of the Placenta Associated with Malaria Infection. Am J Surg Pathol 1998; 22: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 10. Devisme L, Chauvière C, Franquet‐Ansart H, Chudzinski A, Stichelbout M, Houfflin‐Debarge V, Subtil D. Perinatal outcome of placental massive perivillous fibrin deposition: a case–control study. Prenat Diagn 2017; 37: 323–328. [DOI] [PubMed] [Google Scholar]
- 11. Faye‐Petersen OM, Ernst LM. Maternal Floor Infarction and Massive Perivillous Fibrin Deposition. Surg Pathol Clin 2013; 6: 101–114. [DOI] [PubMed] [Google Scholar]
- 12. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. J Thromb Thrombolysis 2020. DOI: 10.1007/s11239‐020‐02105‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID‐19 coagulopathy. Crit Care 2020; 24: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox S, Akmatbekov A, Harbert J, Li G, Brown J, vd Heide R. Pulmonary and Cardiac Pathology in Covid‐19: The First Autopsy Series from New Orleans. medRxiv, 2020. DOI: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


